Introduction
Oral cavity cancer ranks sixth among the most
commonly diagnosed cancer types; the most prevalent pathological
subtype is oral squamous cell carcinoma (OSCC), accounting for
>90% of all reported diagnoses (1–3). OSCC
is associated with various factors, including tobacco consumption,
alcohol abuse, exposure to human papillomavirus and genetic
predisposition (4). Surgery is the
preferred radical treatment option for OSCC. However, surgery in
patients with recurrent/metastatic (R/M) OSCC has limited
feasibility, and systemic therapy for palliation with active drugs,
such as cetuximab, platinum, 5-fluorouracil and paclitaxel, is
recognized as the standard treatment (5–7).
Despite the use of standard treatment, a proportion of patients
with R/M OSCC develop further tumor progression, resulting in a
dismal prognosis of the disease (5). Therefore, the exploration of certain
different treatment options with the potency to improve the
treatment response or survival of patients with R/M OSCC is
urgent.
Programmed cell death 1 (PD-1) inhibitor binds to
the PD-1 checkpoint receptor to avoid its interaction with the
programmed cell death ligand 1 (PD-L1), which restores the
recognition and cytotoxic effect of immune cells, preventing the
immune escape of tumor cells and inhibiting tumor progression
(8,9). Available evidence suggests that the
use of PD-1 inhibitors is effective for certain tumor types. In
recent years, the efficacy of the PD-(L)1 inhibitors (including
camrelizumab, nivolumab, pembrolizumab and durvalumab) in OSCC has
been reported in several studies (10–14).
For instance, a previous study has shown that nearly 60% of
patients with recurrent/unresectable/metastatic OSCC treated with
pembrolizumab achieve objective response (10). An additional study has revealed that
the 1-year progression-free survival (PFS) rate is 25.4% in
nivolumab-treated patients with R/M OSCC (11). Another study showed that durvalumab
was able to achieve numerically higher 12-, 18- and 24-month
survival rates compared to standard of care in R/M head and neck
squamous cell carcinoma (HNSCC) (lacking statistical significance)
(13). However, certain patients do
not have a durable clinical benefit and the efficacy of the PD-1
inhibitors can be improved by rational combination with other
therapies, which results in overcoming potential resistance
(10,11,15–18).
To date, only one study has suggested that PD-1 inhibitor combined
with concurrent chemoradiotherapy treatment is able to achieve
satisfactory efficacy [median overall survival (OS), 19 months] for
Chinese patients with R/M HNSCC (19). However, the previous study is
single-arm and the potential of PD-1 inhibitor + chemotherapy in
Chinese patients with R/M OSCC requires further exploration
(19).
In this light, the present study intended to assess
the efficacy and safety of PD-1 inhibitors (pembrolizumab,
camrelizumab and nivolumab) + chemotherapy as first-line treatment
compared with the standard treatment in Chinese patients with R/M
OSCC.
Materials and methods
Study population and treatment
In the present retrospective cohort study, 51
patients with R/M OSCC who were treated with PD-1 inhibitor +
chemotherapy or standard treatment as the first-line treatment from
August 2020 to February 2023 were included. The inclusion criteria
were the following: i) Patients diagnosed as R/M OSCC by
histopathology; ii) age >18 years; iii) patients who received
PD-1 inhibitor + chemotherapy or standard treatment as the
first-line treatment; iv) patients who had at least one clinical
response result and follow-up data. If the patients had active
autoimmune disease with serious lesions in important organs, such
as the heart, lungs and kidneys, they were excluded. Females during
pregnancy or lactation were also excluded. All patients received
first-line PD-1 inhibitor + chemotherapy or standard treatment. The
regimen details of PD-1 inhibitor + chemotherapy included the
following: Pembrolizumab (200 mg per cycle) + platinum +
5-fluorouracil (5-FU); camrelizumab (200 mg per cycle) + platinum +
5-FU; and nivolumab (3 mg/kg every 2 weeks) + platinum + 5-FU. PD-1
inhibitors were continued until disease progression, death or
toxicity-based intolerance. The chemotherapy lasted 4 to 6 cycles
with a 3-week cycle and the conventional doses used were identical
to those reported in a previous study (18). The regimen of standard treatment
involved the following: Cetuximab + platinum + 5-FU; cetuximab +
cisplatin + docetaxel; platinum + 5-FU; and cetuximab + paclitaxel.
The doses of the drugs used were based on the Chinese Society of
Clinical Oncology diagnosis and treatment guidelines for head and
neck cancer 2018 (7).
Data collection and assessment
Age, gender, current or former smoking status,
Eastern Cooperative Oncology Group performance status (ECOG PS)
score (20), primary tumor location
according to the 4th edition of the World Health Organization
Classification of Head and Neck Tumors (21), disease status and PD-L1 combined
positive score (CPS) were collected. PD-L1 expression was
determined by immunohistochemical staining (22), and the staining was performed
according to the manufacturer's protocol. Antibodies used included
PD-L1 polyclonal antibody (cat. no. PA5-88105; dilution, 1:100;
Thermo Fisher Scientific, Inc.) and goat anti-rabbit IgG (H+L)
secondary antibody, HRP (cat. no. 31460; dilution, 1:100; Thermo
Fisher Scientific, Inc.). The PD-L1 CPS was calculated via the
following formula (23):
PD-L1 CPS <1 was defined as PD-L1-negative
(Fig. S1A), while PD-L1 CPS ≥1 was
defined as PD-L1-positive (Fig.
S1B). In addition, the clinical response results were collected
and assessed via the response evaluation criteria in solid tumors
version 1.1 (24). The disease
progression or death statuses were obtained as well. In addition,
adverse events (AEs) were recorded.
The objective response rate (ORR) and disease
control rate (DCR) were calculated according to the clinical
response results after 4 cycles (~2.8 months) of treatment. The PFS
and OS rates were calculated based on the disease statuses and
survival durations. PFS was defined as the time from the start of
treatment in the study until disease progression or death of any
cause; OS was defined as the time from the start of treatment in
the study until any-cause death of any cause. AEs were graded via
the Common Terminology Criteria for Adverse Events (v.5.0)
(25).
Statistical analysis
SPSS v.26.0 (IBM Corp.) was used for data analyses.
Student's t-test, the χ2 test or Fisher's exact test
were applied for comparisons as appropriate. Kaplan-Meier curves
were plotted to illustrate PFS and OS of the groups and the
log-rank test was used for statistical comparison. Enter-method
multi-variable Cox regression analyses were performed to identify
factors affecting PFS and OS in patients with OSCC, in which PD-1
inhibitor plus chemotherapy, age, male sex, current or former
smoking, ECOG PS score, primary tumor location, and disease status
were included in the analyses. P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient characteristics
The mean age of the PD-1 inhibitor + chemotherapy
group and the standard treatment group was 59.9±11.9 years and
61.8±10.0 years (P=0.524), respectively. There were 17 (81.0%)
males in the PD-1 inhibitor + chemotherapy group and 21 (70.0%)
males in the standard treatment group (P=0.377). A total of 10
(47.6%), 1 (4.8%) and 5 (23.8%) patients in the PD-1 inhibitor +
chemotherapy group had a history of surgery, chemotherapy and
radiotherapy, respectively. In comparison, in the standard
treatment group, 13 (43.3%), 8 (26.7%) and 12 (40.0%) patients had
a history of surgery, chemotherapy and radiotherapy, respectively.
In the PD-1 inhibitor + chemotherapy group, all 21 (100%) patients
were rated as PD-L1 CPS ≥1, while in the standard treatment group,
8 (26.7%) and 9 (30.0%) patients were classified as PD-L1 CPS <1
and ≥1, respectively; the PD-L1 CPS status of the remaining 13
(43.3%) patients in that group was unknown (P<0.001). In
addition, current or former smoking, primary tumor location,
disease status and ECOG PS score did not exhibit any differences
between the two groups (all P>0.050). The specific information
is displayed in Table I.
 | Table I.Clinical features of patients with
oral squamous cell carcinoma. |
Table I.
Clinical features of patients with
oral squamous cell carcinoma.
| Item | PD-1 inhibitor plus
chemotherapy (n=21) | Standard treatment
(n=30) | P-value |
|---|
| Age, years | 59.9±11.9 | 61.8±10.0 | 0.524 |
| Male sex | 17 (81.0) | 21 (70.0) | 0.377 |
| Current or former
smoker | 16 (76.2) | 20 (66.7) | 0.463 |
| History of
surgery | 10 (47.6) | 13 (43.3) | 0.783 |
| History of
chemotherapy | 1 (4.8) | 8 (26.7) | 0.064 |
| History of
radiotherapy | 5 (23.8) | 12 (40.0) | 0.366 |
| ECOG PS score |
|
| 0.530 |
|
0 | 6 (28.6) | 11 (36.7) |
|
|
1 | 14 (66.7) | 19 (63.3) |
|
|
2 | 1 (4.8) | 0 (0.0) |
|
|
Differentiation |
|
| 0.604 |
|
Poor | 4 (19.0) | 9 (30.0) |
|
|
Moderate | 13 (61.9) | 18 (60.0) |
|
|
Well | 4 (19.0) | 3 (10.0) |
|
| Primary tumor
location |
|
| 0.672 |
|
Tongue | 8 (38.1) | 16 (53.3) |
|
|
Gingiva | 8 (38.1) | 9 (30.0) |
|
|
Mouth floor | 3 (14.3) | 4 (13.3) |
|
|
Others | 2 (9.5) | 1 (3.3) |
|
| Disease status |
|
| 0.568 |
|
Metastatic disease
only | 11 (52.4) | 14 (46.7) |
|
|
Recurrent disease
only | 4 (19.0) | 10 (33.3) |
|
|
Recurrent
metastatic disease | 6 (28.6) | 6 (20.0) |
|
| Metastatic
site |
|
|
|
|
Lymph nodes | 7 (33.3) | 16 (53.3) | 0.253 |
|
Lung | 9 (42.9) | 11 (36.7) | 0.773 |
|
Bone | 7 (33.3) | 5 (16.7) | 0.196 |
|
Liver | 1 (4.8) | 6 (20.0) | 0.217 |
|
Other | 5 (23.8) | 7 (23.3) | >0.999 |
| Recurrence
site |
|
| 0.886 |
|
Tongue | 5 (50.0) | 7 (43.8) |
|
|
Gingiva | 3 (30.0) | 4 (25.0) |
|
|
Mouth floor | 2 (20.0) | 5 (31.3) |
|
| PD-L1 CPS |
|
| <0.001 |
|
<1 | 0 (0.0) | 8 (26.7) |
|
|
≥1 | 21 (100.0) | 9 (30.0) |
|
|
Unknown | 0 (0.0) | 13 (43.3) |
|
Treatment information
In the PD-1 inhibitor + chemotherapy group, 14
(66.7%) patients, 4 (19.0%) patients and 3 (14.3%) patients
received pembrolizumab + platinum + 5-FU, nivolumab + platinum +
5-FU and camrelizumab + platinum + 5-FU, respectively. In the
standard treatment group, 20 (66.7%), 4 (13.3%), 5 (16.7%) patients
and 1 (3.3%) patient were treated with cetuximab + platinum + 5-FU,
cetuximab + cisplatin + docetaxel, platinum + 5-FU and cetuximab +
paclitaxel, respectively (Table
II).
 | Table II.Treatment regimens for patients with
oral squamous cell carcinoma. |
Table II.
Treatment regimens for patients with
oral squamous cell carcinoma.
| Treatment | N (%) |
|---|
| Programmed cell
death 1 inhibitor plus chemotherapy |
|
|
Pembrolizumab +
platinum + 5-FU | 14 (66.7) |
|
Nivolumab +
platinum + 5-FU | 4 (19.0) |
|
Camrelizumab +
platinum + 5-FU | 3 (14.3) |
| Standard
treatment |
|
|
Cetuximab +
platinum + 5-FU | 20 (66.7) |
|
Cetuximab +
cisplatin + docetaxel | 4 (13.3) |
|
Platinum +
5-FU | 5 (16.7) |
|
Cetuximab +
paclitaxel | 1 (3.3) |
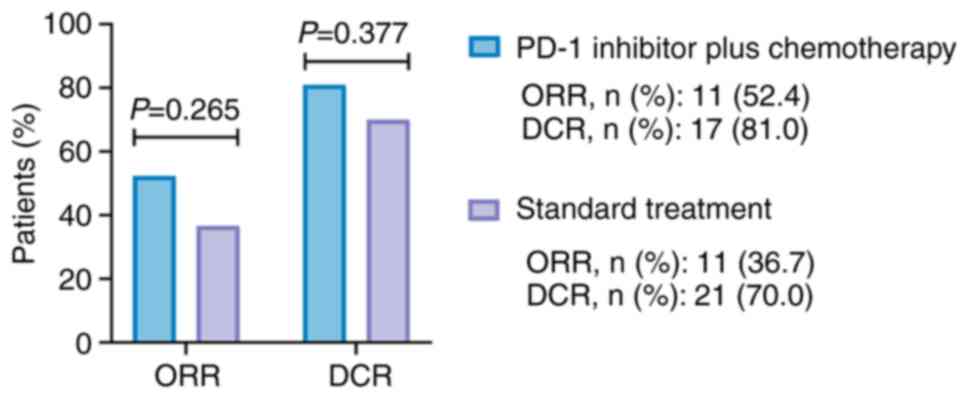
ORR and DCR
The ORR was numerically elevated (without
statistical significance) in the PD-1 inhibitor + chemotherapy
group compared with that in the standard treatment group (52.4 vs.
36.7%, P=0.265). In addition, the DCR exhibited an elevated trend
(lacking statistical significance) in the PD-1 inhibitor +
chemotherapy group compared with that in the standard treatment
group (81.0 vs. 70.0%, P=0.377; Fig.
1).
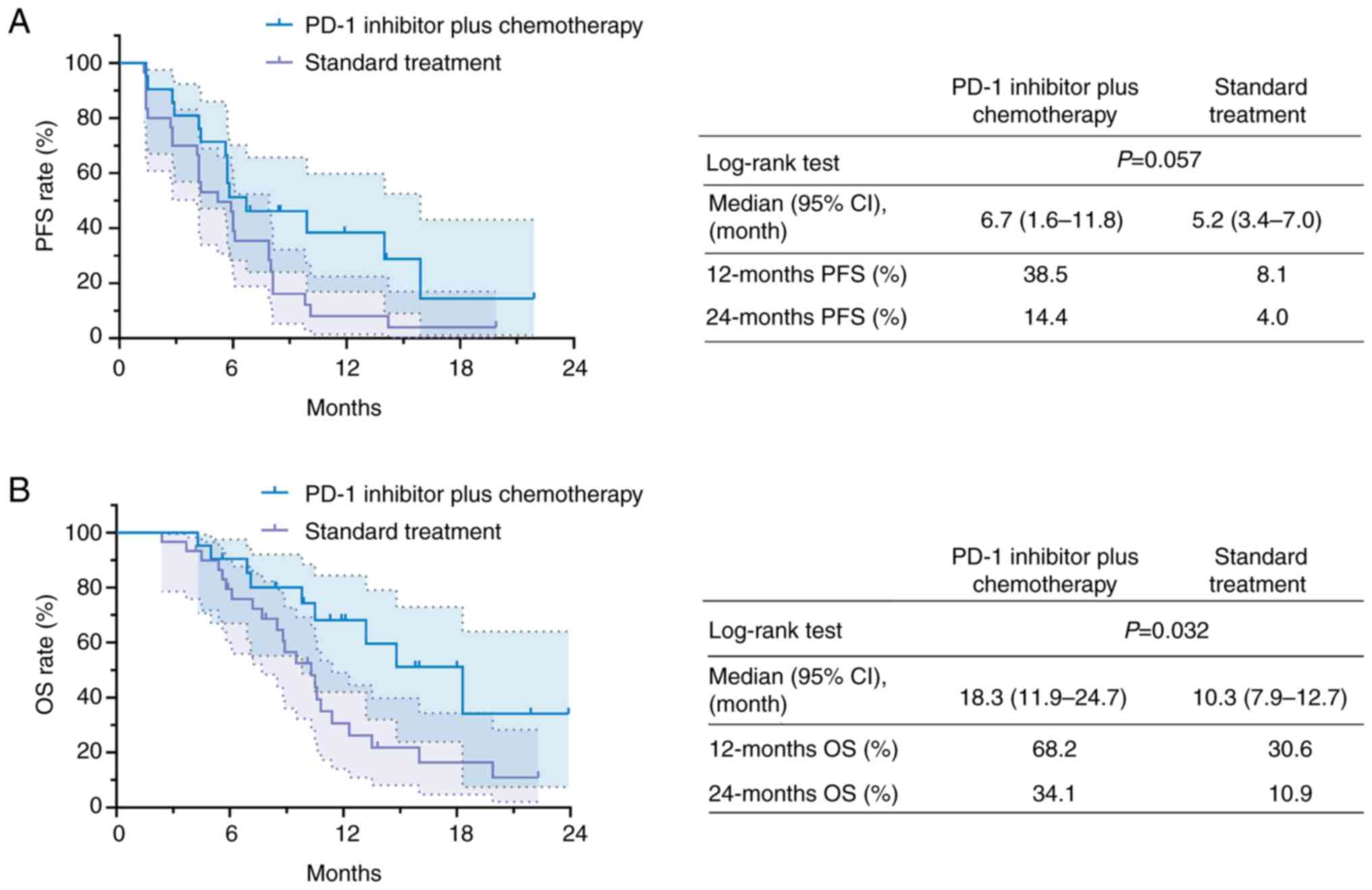
PFS and OS
The PFS exhibited a prolonged trend (lacking
statistical significance) in the PD-1 inhibitor + chemotherapy
group compared with that in the standard treatment group (P=0.057).
In detail, the median [95% confidence interval (CI)] PFS duration
of the PD-1 inhibitor + chemotherapy group and the standard
treatment group was 6.7 (1.6–11.8) months and 5.2 (3.4–7.0) months,
respectively. The 12-month PFS rate in the PD-1 inhibitor +
chemotherapy group and in the standard treatment group was 38.5 and
8.1%, respectively. Furthermore, the 24-month PFS rate in the two
groups was 14.4 and 4.0%, respectively (Fig. 2A).
The OS was prolonged in the PD-1 inhibitor +
chemotherapy group compared with that in the standard treatment
group (P=0.032). Specifically, the median (95% CI) OS duration of
the PD-1 inhibitor + chemotherapy group and that of the standard
treatment group was 18.3 (11.9–24.7) months and 10.3 (7.9–12.7)
months, respectively. Furthermore, the 12-month OS rate of the PD-1
inhibitor + chemotherapy group and that of the standard treatment
group was 68.2 and 30.6%, respectively, while the 24-month OS rate
was 34.1 and 10.9% in the corresponding groups (Fig. 2B).
In addition, neither PFS (P=0.869; Fig. S2A) nor OS (P=0.834; Fig. S2B) was different among R/M OSCC
patients with different primary tumor locations.
Subgroup analysis
In patients who received treatment between 2020 and
2022, PFS exhibited a prolonged trend (without statistical
significance) in the PD-1 inhibitor + chemotherapy group in
comparison with that in the standard treatment group (P=0.054;
Fig. S3A). OS was prolonged in the
PD-1 inhibitor + chemotherapy group compared to the standard
treatment group (P=0.031; Fig.
S3B). However, in patients who received treatment in 2023,
neither PFS (P=0.918; Fig. S3C)
nor OS (P=0.705; Fig. S3D) was
different between the two groups.
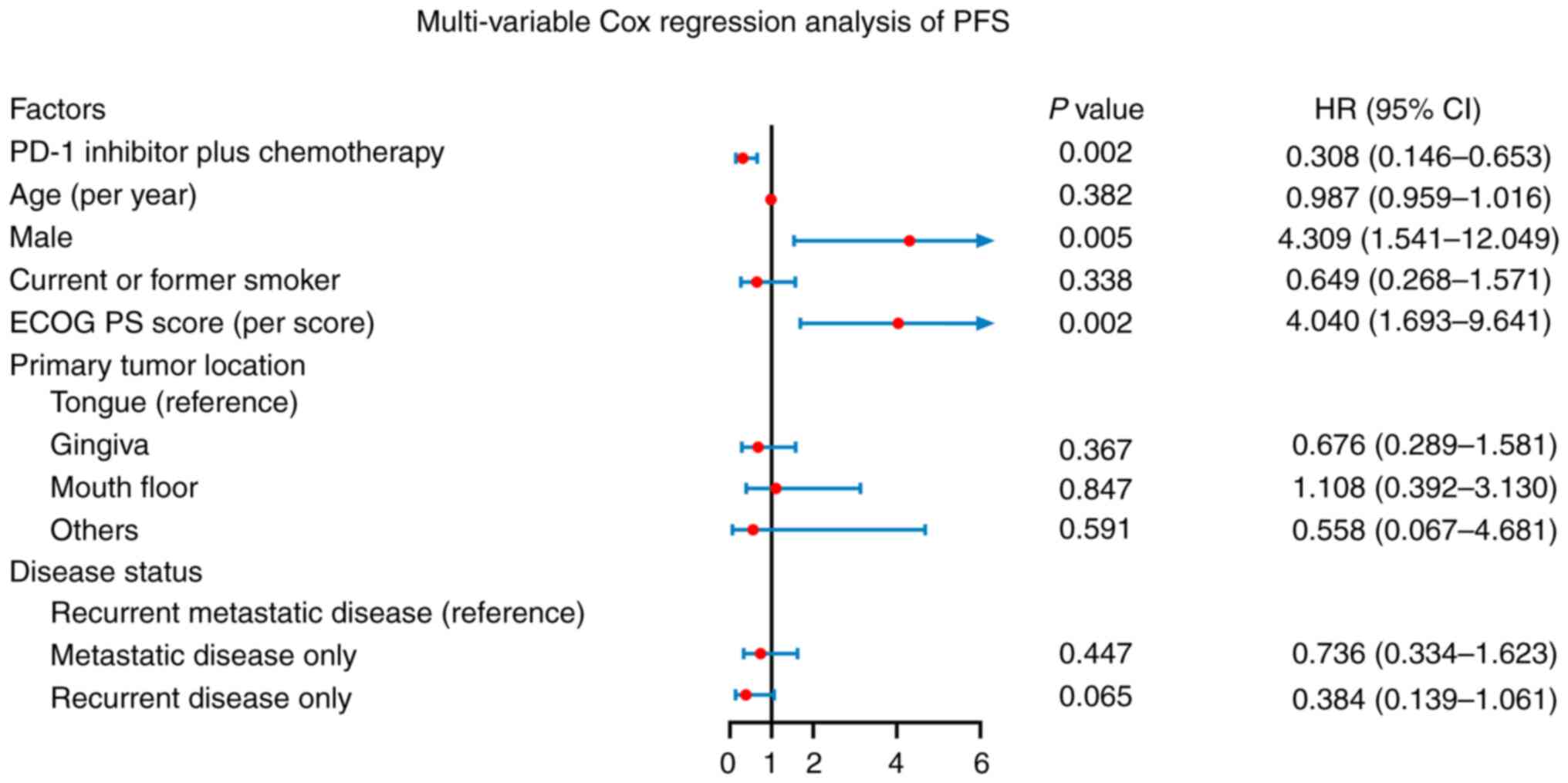
Independent factors affecting PFS and
OS
PD-1 inhibitor in addition to chemotherapy was
independently associated with longer PFS [hazard ratio (HR): 0.308,
P=0.002]. In addition, male sex (HR: 4.309, P=0.005) and a higher
ECOG PS score (HR: 4.040, P=0.002) were independently related to
reduced PFS. Whereas higher age, current or former smoking, primary
tumor location in the gingiva (vs. tongue), mouth floor (vs.
tongue), other locations (vs. tongue), metastatic disease only (vs.
recurrent metastatic disease) and recurrent disease only (vs.
recurrent metastatic disease) were not independently associated
with PFS (all P>0.050) (Fig. 3).
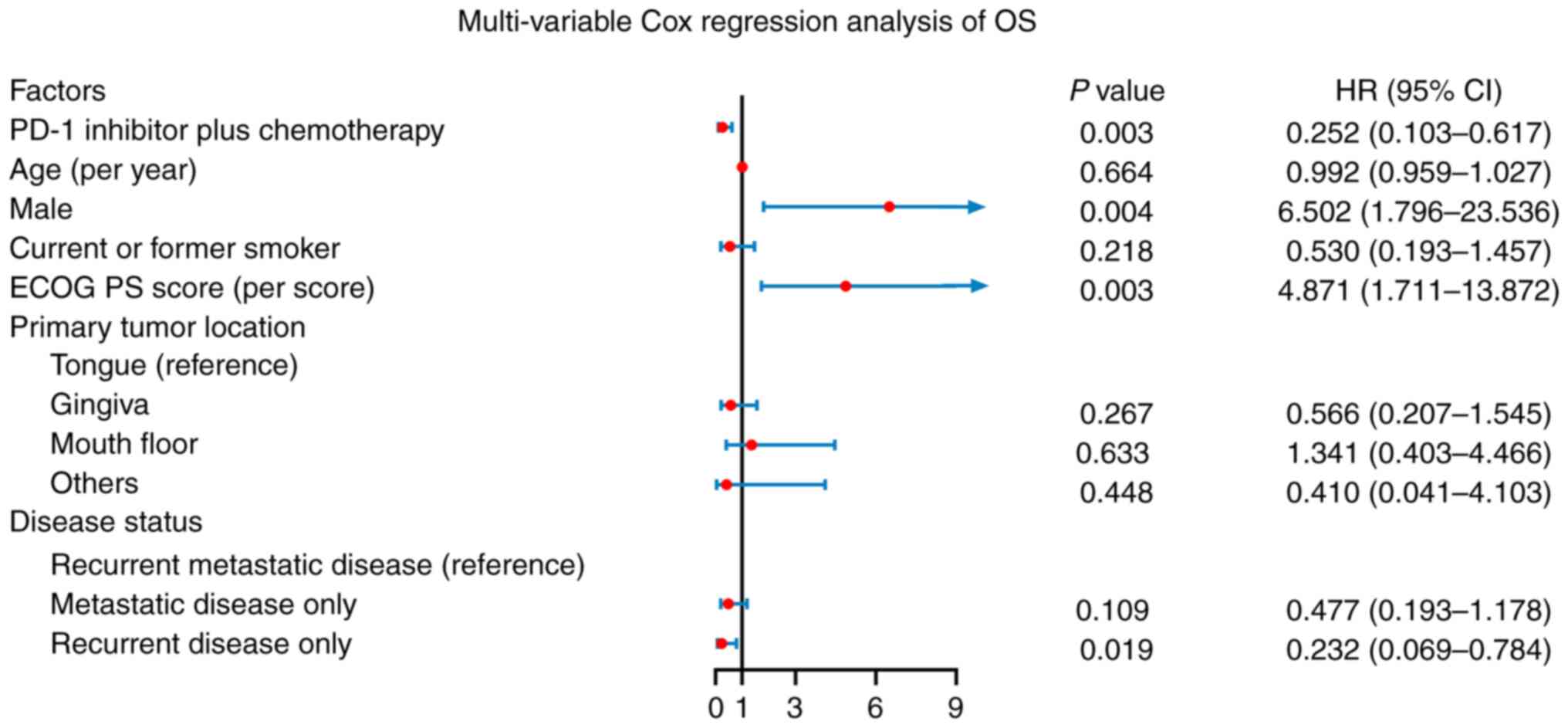
Furthermore, PD-1 inhibitor in addition to chemotherapy (HR: 0.252,
P=0.003) and recurrent disease only (vs. recurrent metastatic
disease; HR: 0.232, P=0.019) were independently associated with
longer OS, whereas male sex (HR: 6.502, P=0.004) and higher ECOG PS
score (HR: 4.871, P=0.003) were independently associated with
reduced OS. However, higher age, current or former smoking, primary
tumor location in gingiva (vs. tongue), mouth floor (vs. tongue),
other locations (vs. tongue) and metastatic disease only (vs.
recurrent metastatic disease) were not independent related factors
for OS (Fig. 4).
AEs
The most common AEs of any grade in the PD-1
inhibitor + chemotherapy group were fatigue (42.9%), anemia
(28.6%), neutropenia (28.6%), nausea (28.6%), leukopenia (23.8%)
and liver dysfunction (23.8%). In addition, the incidence of grade
3–5 AEs in the PD-1 inhibitor + chemotherapy group was relatively
low, including neutropenia (9.5%), fatigue (4.8%), anemia (4.8%),
nausea (4.8%), leukopenia (4.8%) and liver dysfunction (4.8%). In
the standard treatment group, fatigue (36.7%), nausea (33.3%),
anemia (26.7%), diarrhea (26.7%), rash (26.7%), thrombocytopenia
(23.3%), neutropenia (20%), leukopenia (20.0%), liver dysfunction
(20.0%) and vomiting (20.0%) were common AEs of any grade. Grade
3–5 AEs in the standard treatment group included neutropenia
(6.7%), fatigue (3.3%), nausea (3.3%), leukopenia (3.3%), liver
dysfunction (3.3%), diarrhea (3.3%) and vomiting (3.3%). Overall,
the incidence of each AE of any grade was not significantly
different between the PD-1 inhibitor + chemotherapy and the
standard treatment group (all P>0.050; Table III).
 | Table III.AEs [n (%)]. |
Table III.
AEs [n (%)].
|
| Programmed cell
death 1 inhibitor plus chemotherapy (n=21) | Standard treatment
(n=30) |
|
|---|
|
|
|
|
|
|---|
| AE | Any grade | Grade 1-2 | Grade 3-5 | Any grade | Grade 1-2 | Grade 3-5 | P-value |
|---|
| Fatigue | 9 (42.9) | 8 (38.1) | 1 (4.8) | 11 (36.7) | 10 (33.3) | 1 (3.3) | 0.656 |
| Anemia | 6 (28.6) | 5 (23.8) | 1 (4.8) | 8 (26.7) | 8 (26.7) | 0 (0.0) | 0.881 |
| Neutropenia | 6 (28.6) | 4 (19.0) | 2 (9.5) | 6 (20.0) | 4 (13.3) | 2 (6.7) | 0.518 |
| Nausea | 6 (28.6) | 5 (23.8) | 1 (4.8) | 10 (33.3) | 9 (30.0) | 1 (3.3) | 0.718 |
| Leukopenia | 5 (23.8) | 4 (19.0) | 1 (4.8) | 6 (20.0) | 5 (16.7) | 1 (3.3) | 0.744 |
| Liver
dysfunction | 5 (23.8) | 4 (19.0) | 1 (4.8) | 6 (20.0) | 5 (16.7) | 1 (3.3) | 0.744 |
|
Thrombocytopenia | 4 (19.0) | 4 (19.0) | 0 (0.0) | 7 (23.3) | 7 (23.3) | 0 (0.0) | >0.999 |
| Stomatitis | 4 (19.0) | 4 (19.0) | 0 (0.0) | 5 (16.7) | 5 (16.7) | 0 (0.0) | >0.999 |
| Diarrhea | 4 (19.0) | 4 (19.0) | 0 (0.0) | 8 (26.7) | 7 (23.3) | 1 (3.3) | 0.739 |
| Hypothyroidism | 3 (14.3) | 3 (14.3) | 0 (0.0) | 1 (3.3) | 1 (3.3) | 0 (0.0) | 0.293 |
| Vomiting | 3 (14.3) | 3 (14.3) | 0 (0.0) | 6 (20.0) | 5 (16.7) | 1 (3.3) | 0.720 |
| Pyrexia | 2 (9.5) | 2 (9.5) | 0 (0.0) | 3 (10.0) | 3 (10.0) | 0 (0.0) | >0.999 |
| Rash | 2 (9.5) | 2 (9.5) | 0 (0.0) | 8 (26.7) | 8 (26.7) | 0 (0.0) | 0.167 |
| Pneumonitis | 1 (4.8) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.412 |
| Renal
dysfunction | 1 (4.8) | 1 (4.8) | 0 (0.0) | 2 (6.7) | 2 (6.7) | 0 (0.0) | >0.999 |
Discussion
Various studies have reported on the utilization of
PD-1 inhibitors in patients with R/M HNSCC (26–28).
For instance, a previous study revealed that patients with R/M
HNSCC receiving nivolumab exhibited an ORR of 13.3%, which was
superior to that noted in the standard therapy group (5.8%)
(26). An additional study
demonstrated that pembrolizumab caused a numerical elevation in the
ORR (14.6 vs. 10.1%) compared with methotrexate, docetaxel or
cetuximab following platinum drug treatment in patients with R/M
HNSCC (28). However, the efficacy
of the application of PD-1 inhibitor + chemotherapy lacked
sufficient evidence. In the present study, the results did not
demonstrate a statistically significant benefit of treatment
response in patients with PD-1 inhibitor + chemotherapy compared to
standard treatment as the first-line treatment in patients with R/M
OSCC, which may be due to the relatively small sample size.
However, the ORR (52.4 vs. 36.7%) and DCR (81.0 vs. 70.0%) were
numerically elevated in patients with R/M OSCC treated with PD-1
inhibitor + chemotherapy compared with those receiving standard
treatment. The potential explanations may be as follows: PD-1
inhibitor was assumed to potentiate the cytotoxic effect of
chemotherapy on tumor cells by altering the tumor microenvironment
(29–31), and to facilitate death of tumor
cells through reviving the cytotoxic activity of T lymphocytes
(32). Therefore, PD-1 inhibitor +
chemotherapy achieved a numerically higher ORR and DCR in patients
with R/M OSCC compared with those on standard treatment; however,
the findings require further validation in large-scale studies. In
addition, it is worth mentioning that although the salvage
operation was an optimal choice for locally recurrent OSCC, certain
patients were not suitable for salvage operation due to overload
tumor burden or rejection of salvage operation for various reasons.
Thus, for this population in the present study, conservative
treatment was chosen. Furthermore, the treatment decision is made
by the oncological board, which is based on the Chinese Society of
Clinical Oncology guidelines (7)
combined with the patient's condition and choice.
The application of PD-1 inhibitors has improved the
survival profile of patients with HNSCC, including cancer of the
hypopharynx, larynx, oral cavity and oropharynx (18,33,34).
For instance, a previous study indicated that the OS was increased
in patients with R/M HNSCC receiving pembrolizumab + chemotherapy
(13.0 months) compared with those receiving cetuximab +
chemotherapy (10.7 months) (18).
An additional study suggested that nivolumab effectively improved
the 24-month OS rate of patients with R/M HNSCC compared with that
of patients treated with methotrexate, docetaxel or cetuximab (16.9
vs. 6.0%) (33). Another study
found that PD-1 inhibitor in combination with paclitaxel and
cisplatin achieved a 12-month PFS rate of 80.4% and 12-month OS
rate of 94.1% in patients with locally advanced laryngeal and
hypopharyngeal squamous cell carcinoma (34). In the current study, PFS indicated a
numerically elevated trend and OS was prolonged in patients with
R/M OSCC who underwent PD-1 inhibitor + chemotherapy compared with
those who underwent standard treatment. In addition, PD-1 inhibitor
+ chemotherapy was independently related to higher PFS and OS of
patients with R/M OSCC. The possible explanation may be as follows:
Inhibition of PD-1 had a favorable anti-tumor effect and its
combination with chemotherapy further impeded the progression of
OSCC, contributing to improved survival (16). Consequently, the PD-1 inhibitor +
chemotherapy combination was an independent factor for prolonged
PFS and OS (vs. standard treatment) of patients with R/M OSCC. Of
note, in comparison with previous studies (18,33),
patients administered with PD-1 inhibitor + chemotherapy in the
current study achieved a relatively longer OS, which may be
explained by the following etiology: Clinically, PD-1 inhibitors
are recommended for patients with PD-L1 CPS≥1 (35); in addition, all of the patients
receiving PD-1 inhibitor + chemotherapy in the present study were
classified as PD-L1 CPS≥1 and thus benefited more from the
immunotherapy. It is interesting to note that male sex was
independently associated with worse PFS and OS in the present
study, which was consistent with a previous study (36).
In line with previous studies (18,37),
fatigue, nausea, neutropenia and anemia were the most common AEs
associated with PD-1 inhibitor + chemotherapy in the present study.
Furthermore, fatigue, nausea, anemia, diarrhea and rush were the
most common AEs in the standard treatment group of the current
study, which was similar to the results noted in previous studies
(18,38). More importantly, the incidences of
all AEs did not vary between patients who received PD-1 inhibitor +
chemotherapy and standard treatment. In addition, the majority of
AEs in the present study were of grade 1-2; the incidence of grade
3–5 AEs was relatively low, indicating that the systematic toxicity
of both PD-1 inhibitor + chemotherapy and standard treatment in
patients with R/M OSCC was controllable.
Certain limitations were inevitable in the present
study: First, the current retrospective study was conducted at a
single center and it was difficult to avoid selection bias.
Furthermore, this was a retrospective study; therefore, the
documentation of AEs may have been inadequate, which may have
potentially led to an underestimation of AEs in both groups. In
addition, the present study had a relatively small number of
enrolled patients and it was difficult to summarize useful
information, such as the association of various tumor localizations
with treatment response. Finally, the follow-up duration of the
present study was not adequate and the findings required more
verification in studies with a longer follow-up period.
In conclusion, the present study indicated that PD-1
inhibitor + chemotherapy achieved numerically elevated treatment
response and was an independent factor for prolonged PFS and OS
with comparable safety compared to standard treatment in patients
with R/M OSCC. The findings suggest that PD-1 inhibitor +
chemotherapy may serve as a potential first-line therapeutic
regimen for patients with R/M OSCC. Further validation through
multi-center randomized, controlled studies with a large sample
size is necessary.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LC, CC, YL and JJ contributed to the study
conception and design. Material preparation, data collection and
analysis were performed by LC, CC and YL. The first draft of the
manuscript was written by LC. LC, CC, YL and JJ commented on
previous versions of the manuscript. YL and JJ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Handan Central Hospital
(Handan, China) gave approval for this study (approval date,
2023/02/10; no approval number was provided). Oral informed consent
for analysis and publication of personal information was obtained
from each patient via telephone. Besides, the PD-L1 IHC staining
was performed for treatment purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xie L and Shang ZJ: Oral cancer incidence,
mortality, and mortality-to-incidence ratio are associated with
human development index in China, 1990-2019. Biomed Res Int.
2022:64578402022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
3
|
Sarode G, Maniyar N, Sarode SC, Jafer M,
Patil S and Awan KH: Epidemiologic aspects of oral cancer. Dis Mon.
66:1009882020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chamoli A, Gosavi AS, Shirwadkar UP,
Wangdale KV, Behera SK, Kurrey NK, Kalia K and Mandoli A: Overview
of oral cavity squamous cell carcinoma: Risk factors, mechanisms,
and diagnostics. Oral Oncol. 121:1054512021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow LQM: Head and neck cancer. N Engl J
Med. 382:60–72. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chinese society of clinical oncology
(CSCO) diagnosis and treatment guidelines for head and neck cancer
2018 (english version). Chin J Cancer Res. 31:84–98. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bagchi S, Yuan R and Engleman EG: Immune
checkpoint inhibitors for the treatment of cancer: Clinical impact
and mechanisms of response and resistance. Annu Rev Pathol.
16:223–249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y,
Wu W, Han L and Wang S: The role of PD-1/PD-L1 and application of
immune-checkpoint inhibitors in human cancers. Front Immunol.
13:9644422022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, He Z, Tao Y, Yang X, Ge S, Xu H,
Shang W and Song K: Efficacy and safety of pembrolizumab
monotherapy for recurrent/unresectable/metastatic oral squamous
cell carcinoma: A single-center study in China. J Oncol.
2022:72839462022.PubMed/NCBI
|
|
11
|
Yamakawa N, Umeda M, Yoshii Y, Mitsudo K,
Noguchi M, Kusukawa J, Katakura A, Nakayama H, Sasaki M, Noguchi T,
et al: Multicenter retrospective study of nivolumab for
recurrent/metastatic oral squamous cell carcinoma. Oral Dis.
30:247–258. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ju WT, Xia RH, Zhu DW, Dou SJ, Zhu GP,
Dong MJ, Wang LZ, Sun Q, Zhao TC, Zhou ZH, et al: A pilot study of
neoadjuvant combination of anti-PD-1 camrelizumab and VEGFR2
inhibitor apatinib for locally advanced resectable oral squamous
cell carcinoma. Nat Commun. 13:53782022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferris RL, Haddad R, Even C, Tahara M,
Dvorkin M, Ciuleanu TE, Clement PM, Mesia R, Kutukova S, Zholudeva
L, et al: Durvalumab with or without tremelimumab in patients with
recurrent or metastatic head and neck squamous cell carcinoma:
EAGLE, a randomized, open-label phase III study. Ann Oncol.
31:942–950. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsieh RW, Borson S, Tsagianni A and
Zandberg DP: Immunotherapy in recurrent/metastatic squamous cell
carcinoma of the head and neck. Front Oncol. 11:7056142021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Upadhaya S, Neftelino ST, Hodge JP, Oliva
C, Campbell JR and Yu JX: Combinations take centre stage in
PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov.
20:168–169. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meric-Bernstam F, Larkin J, Tabernero J
and Bonini C: Enhancing anti-tumour efficacy with immunotherapy
combinations. Lancet. 397:1010–1022. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oliva M, Spreafico A, Taberna M, Alemany
L, Coburn B, Mesia R and Siu LL: Immune biomarkers of response to
immune-checkpoint inhibitors in head and neck squamous cell
carcinoma. Ann Oncol. 30:57–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Chen L, Yan L, Guo Y, Li F, Fan M,
Lan M, Lai X, Zhou J, Huang Y, et al: Initial analysis of the
synergy of programmed cell death-1 (PD-1) inhibitor and concurrent
chemoradiotherapy treatment for recurrent/metastatic head and neck
squamous cell carcinoma patients. Radiat Oncol. 18:1092023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Naggar AK, Chan JKC, Grandis JR, Takata
T and Slootweg PJ: WHO classification of head and neck tumours.
IARC Press; Lyon: pp. 203–260. 2017
|
|
22
|
Cho JH, Sorensen SF, Choi YL, Feng Y, Kim
TE, Choi H, Georgsen JB, Dolled-Filhart M, Emancipator K, Meldgaard
P, et al: Programmed death ligand 1 expression in paired non-small
cell lung cancer tumor samples. Clin Lung Cancer. 18:e473–e479.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kulangara K, Zhang N, Corigliano E,
Guerrero L, Waldroup S, Jaiswal D, Ms MJ, Shah S, Hanks D, Wang J,
et al: Clinical utility of the combined positive score for
programmed death ligand-1 expression and the approval of
pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med.
143:330–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Sousa LG and Ferrarotto R:
Pembrolizumab in the first-line treatment of advanced head and neck
cancer. Expert Rev Anticancer Ther. 21:1321–1331. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cohen EEW, Soulieres D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab
for recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xue Y, Gao S, Gou J, Yin T, He H, Wang Y,
Zhang Y, Tang X and Wu R: Platinum-based chemotherapy in
combination with PD-1/PD-L1 inhibitors: Preclinical and clinical
studies and mechanism of action. Expert Opin Drug Deliv.
18:187–203. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saleh K, Khalifeh-Saleh N, Kourie HR, Nasr
F and Chahine G: Do immune checkpoint inhibitors increase
sensitivity to salvage chemotherapy? Immunotherapy. 10:163–165.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Melo-Alvim C, Neves ME, Santos JL,
Abrunhosa-Branquinho AN, Barroso T, Costa L and Ribeiro L:
Radiotherapy, chemotherapy and immunotherapy-current practice and
future perspectives for recurrent/metastatic oral cavity squamous
cell carcinoma. Diagnostics (Basel). 13:992022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wakabayashi G, Lee YC, Luh F, Kuo CN,
Chang WC and Yen Y: Development and clinical applications of cancer
immunotherapy against PD-1 signaling pathway. J Biomed Sci.
26:962019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE,
Even C, et al: Nivolumab vs investigator's choice in recurrent or
metastatic squamous cell carcinoma of the head and neck: 2-year
long-term survival update of checkMate 141 with analyses by tumor
PD-L1 expression. Oral Oncol. 81:45–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang Q, Xu P, Cao F, Wu D and Liu X: PD-1
Inhibitors combined with paclitaxel (albumin-bound) and cisplatin
for larynx preservation in locally advanced laryngeal and
hypopharyngeal squamous cell carcinoma: A retrospective study.
Cancer Immunol Immunother. 72:4161–4168. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cohen EEW, Bell RB, Bifulco CB, Burtness
B, Gillison ML, Harrington KJ, Le QT, Lee NY, Leidner R, Lewis RL,
et al: The society for immunotherapy of cancer consensus statement
on immunotherapy for the treatment of squamous cell carcinoma of
the head and neck (HNSCC). J Immunother Cancer. 7:1842019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oh LJ, Asher R, Veness M, Smee R,
Goldstein D, Gopalakrishna Iyer N, Balasubramanian D, Low TH, Palme
CE, Gupta R and Clark J: Effect of age and gender in non-smokers
with oral squamous cell carcinoma: Multi-institutional study. Oral
Oncol. 116:1052102021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hato SV, Khong A, de Vries IJ and
Lesterhuis WJ: Molecular pathways: The immunogenic effects of
platinum-based chemotherapeutics. Clin Cancer Res. 20:2831–2837.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pontes F, Garcia AR, Domingues I, João
Sousa M, Felix R, Amorim C, Salgueiro F, Mariano M and Teixeira M:
Survival predictors and outcomes of patients with recurrent and/or
metastatic head and neck cancer treated with chemotherapy plus
cetuximab as first-line therapy: A real-world retrospective study.
Cancer Treat Res Commun. 27:1003752021. View Article : Google Scholar : PubMed/NCBI
|