Introduction
Cutaneous squamous cell carcinoma (cSCC) is the
second most common type of non-melanoma skin cancer, after basal
cell carcinoma. cSCC constitutes ~20% of all skin malignancies and
is responsible for >75% of deaths related to non-melanoma skin
cancer (1). The prevalence of this
illness is increasing rapidly, mostly due to the aging population
and the focus on screening for skin cancer. cSCC develops from the
aberrant proliferation of keratinocytes in the epidermis, perhaps
as a result of an extended period of intraepidermal dysplasia
(2). Tumor development is a known
progressive process that involves numerous histological and
pathologically defined stages, starting with actinic keratosis (AK)
and progressing to invasive cSCC (3,4). cSCC
has a tendency to cause local cutaneous damage affecting the soft
tissues, cartilage and bone; however, metastasis is uncommon
(5). Generally, the prognosis for
cSCC is positive, with a 5-year survival rate of ≥90%. The
etiopathogenesis of the condition is mainly impacted by risk
factors such as exposure to UV radiation, chronic photoaging,
increasing age, the male sex, immunosuppression, smoking and
certain genetic variables (6,7).
Surgery remains the main treatment option for cSCC
(8). Lesions on the eyelid, lip or
ear need particular attention for tissue preservation. Surgery may
not be an appropriate choice as it may lead to disappointing
cosmetic outcomes. Hence, it is essential to explore non-surgical
approaches. The use of cryotherapy, imiquimod and 5-fluorouracil
has been associated with negative outcomes and a high chance of the
condition coming back (9). By
contrast, photodynamic therapy (PDT) has significant effectiveness,
producing pleasing cosmetic outcomes and showing a low rate of
recurrence (10).
PDT is a treatment method for skin cancer that
relies on the combined effect of a photosensitizer, light and
oxygen (11). Tumor cells and
vascular endothelial cells inside the body have a greater
attraction to photosensitizers than other cell types. Specifically,
tumor cell surface proteins, or receptors, may interact better with
certain substances and they may absorb photosensitizers better
(12). Vascular endothelial cells
line bodily blood vessels, and they are necessary for blood flow,
immunological reactions and other functions (13). Cancer and inflammation increase
tumor surface molecule expression and blood arterial permeability.
These changes may increase vascular endothelial cell
photosensitizer attraction. This means tumor and vascular
endothelial cells are more likely to absorb photosensitizers,
making them more photodynamically vulnerable. Photosensitizers may
capture light energy and transmit it to nearby triplet oxygen
molecules. This mechanism results in the generation of reactive
oxygen species (ROS). ROS may harm tumor cells and vascular
endothelial cells by directly triggering the necrosis and apoptosis
pathways (14). An antitumor immune
response is initiated due to the harmful consequences caused by
ROS. This immune response aims to efficiently eliminate tumor cells
and hinder the development of tumor blood vessels, hence starving
the tumor of necessary nutrition. Recently, there has been a
greater emphasis on studying the immunological response in PDT for
cancer treatment, exceeding the attention previously devoted to the
vascular effects (14,15). Cell death induced by PDT has been
well researched in laboratory settings and living organisms
(16,17). However, there is a lack of
information about the immunological changes after PDT for cSCC, and
the particular differences in the inflammatory infiltrates after
PDT are yet unknown (18). The
major aim of the present study was to investigate and compare the
immune cell composition before and after PDT therapy for cSCC to
assess the changes in immune cells caused by the treatment.
Patients and methods
Patients enrolled
A total of 10 patients aged between 65 and 70 years
who were diagnosed with cSCC, as determined by histological
examination at Daping Hospital of Army Medical University
(Chongqing, China), were randomly selected for the investigation
(Table I). Patients were recruited
between November 2023 and March 2024. The 10 included individuals
remained in the study for >4 months. The inclusion criterion was
a histological diagnosis of cSCC and the exclusion of any other
tumor tissue. The exclusion criteria were the presence of non-cSCC,
anogenital SCC, Marjolin ulcers and genetic abnormalities that
predisposed patients to cSCC. All patients were provided with
comprehensive information on aminolevulinic acid (ALA)-PDT,
including its indications, treatment principles, therapeutic
effects and potential problems. Patients provided written informed
consent to engage in this research and for the publication of their
relevant information.
 | Table I.Clinical data of 10 patients with
cutaneous squamous cell carcinoma. |
Table I.
Clinical data of 10 patients with
cutaneous squamous cell carcinoma.
|
|
Patient
no. |
|---|
|
|
|
|---|
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|
| Age, years | 89 | 74 | 69 | 80 | 66 | 70 | 70 | 70 | 70 | 70 |
| Sex | M | F | F | M | M | F | M | F | M | F |
| Tumor location | Head | Face | Vulva | Face | Scrotum | Face | Face | Face | Head | Vulva |
| Size, cm | 0.5×0.5 | 3.5×5 | 5×5 | 2.5×3 | 6×5 | 0.5×0.5 | 0.5×0.5 | 0.5×0.5 | 0.5×0.5 | 0.5×0.5 |
| Number of follow-up
visits | 1 | 2 | 2 | 3 | 1 | 2 | 1 | 2 | 1 | 1 |
| Length of follow-up
time, years | 1.2 | 0.8 | 1.5 | 1.1 | 1.2 | 0.7 | 1.1 | 1.2 | 0.9 | 1.0 |
| VAS score of
patientsa |
|
|
|
|
|
|
|
|
|
|
| 1st PDT
session | 4 | 4 | 3 | 4 | 5 | 3 | 4 | 3 | 3 | 5 |
| 2nd PDT
session | 4 | 5 | 4 | 3 | 3 | 4 | 4 | 3 | 5 | 3 |
Histopathological biopsy and
immunohistochemistry
Patients meeting the inclusion criteria and
suspected of malignancy were managed by the Department of Plastic
and Cosmetic Surgery, which conducted necessary surgical
interventions. Subsequently, biopsy or resected specimens were
analyzed by the Department of Pathology. The surgical procedure was
carried out following recognized guidelines, and histology slides
were generated.
The surgical specimens were processed for
hematoxylin and eosin staining. Briefly, the obtained tissue was
fixed overnight in a 4% paraformaldehyde solution at room
temperature. Fixed tissue samples were dehydrated using a series of
graded alcohol solutions (70, 95 and 100% ethanol) to remove water
from the tissues. Dehydrated tissues were cleared using xylene to
remove the alcohol and make the tissues transparent, and then the
tissue was placed in paraffin after embedding. Thin sections (4- to
5-µm thick) were cut from the paraffin block using a sharp blade
and transferred onto glass slides. Rehydrated tissue sections were
stained with hematoxylin for 5 min and then counterstained with
eosin for 3 min at room temperature. The approach is concisely
summarized as follows: The investigation included analyzing the
tissue specimen at a magnification of ×40 using a fluorescence
microscope, and then doing a more detailed review at a
magnification of ×100 using a fluorescence microscope. Two
pathologists, both skilled and working independently, examined the
tissue specimens on the slides.
For immunohistochemistry, first, the tissue slices
prepared as aforementioned were blocked with a 10% bovine serum
albumin-purified solution (cat. no. 37520; Thermo Fisher
Scientific, Inc.) at room temperature for ~1 h. Next, incubation
was conducted using the following primary antibodies: Rabbit
anti-CD3 (cat. no. 78588; 1:200; Cell Signaling Technology, Inc.)
and rabbit anti-CD56 (cat. no. 99746; 1:200; Cell Signaling
Technology, Inc.). Tissue slices were deparaffinized and then
incubated with antibodies overnight at 4°C. The samples were washed
with PBS and then incubated with donkey anti-rabbit IgG antibodies
(cat. no. A-21206; 2 µg/ml; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h.
ALA-PDT procedure
Once the patient consent for treatment was obtained,
the tumor tissue underwent one session of PDT. The tumor tissue was
surgically removed 24 h after confirming the patient had no
apparent adverse effects, followed by immediate PDT therapy on the
afflicted region. Specifically, the lesions that were positioned in
close proximity to the region of interest, extending 0.5 cm beyond
the apparent lesions, were exposed to cleaning using benzalkonium
bromide. A solution containing 20% ALA (0.5 ml: 118 mg; Shanghai
Fudan Zhangjiang Biomedical, Co., Ltd.) in saline was applied to
the wound. The specified area was covered with a plastic film and
protected from any light exposure for 4 h. After removing the
plastic layer, a diode laser type XD-635AB (Xingda Photoelectricity
Medical Equipment Corp.) generated laser beams with a wavelength of
635 nm. The laser beams were aimed toward the therapeutic region,
ensuring a constant energy density of 120 J/cm2. The
exposure period for each spot size of 3 cm2 was set at
15 min. For big lesions, numerous spots were employed to light the
afflicted region. The power output was set at 100
mW/cm2. The light exposure was adjusted to provide a
constant vertical distance of 5 cm from the lesions. The PDT
treatment protocol remained consistent both pre- and
post-operatively.
Visual analogue scale
The Visual Analog Scale (VAS) (19) was used to assess patient discomfort
throughout the PDT session. VAS is a technique used to measure pain
severity. The process uses distance in centimeter on a
10-centimeter line to represent pain, where each centimeter
corresponds to one unit, between the ‘no pain’ reference point and
the mark reported by the patient. The approach produces ratings
that range from 0 to 10.
Results
All 10 patients with cSCC were identified by
histological investigation, with additional MRI or X-ray scans
conducted as needed. Tumors in all patients had diameters ranging
from 4–6 cm. No major wound infection was apparent in the patients,
and no drug-resistant bacteria such as Pseudomonas, Citrobacter
freundii, Staphylococcus aureus or Serratia marcescens
were found in the secretions.
All 10 patients received surgical excision of the
tumor tissues, and adjuvant PDT was provided before and after
surgery. All surgical wounds healed well without any cases of tumor
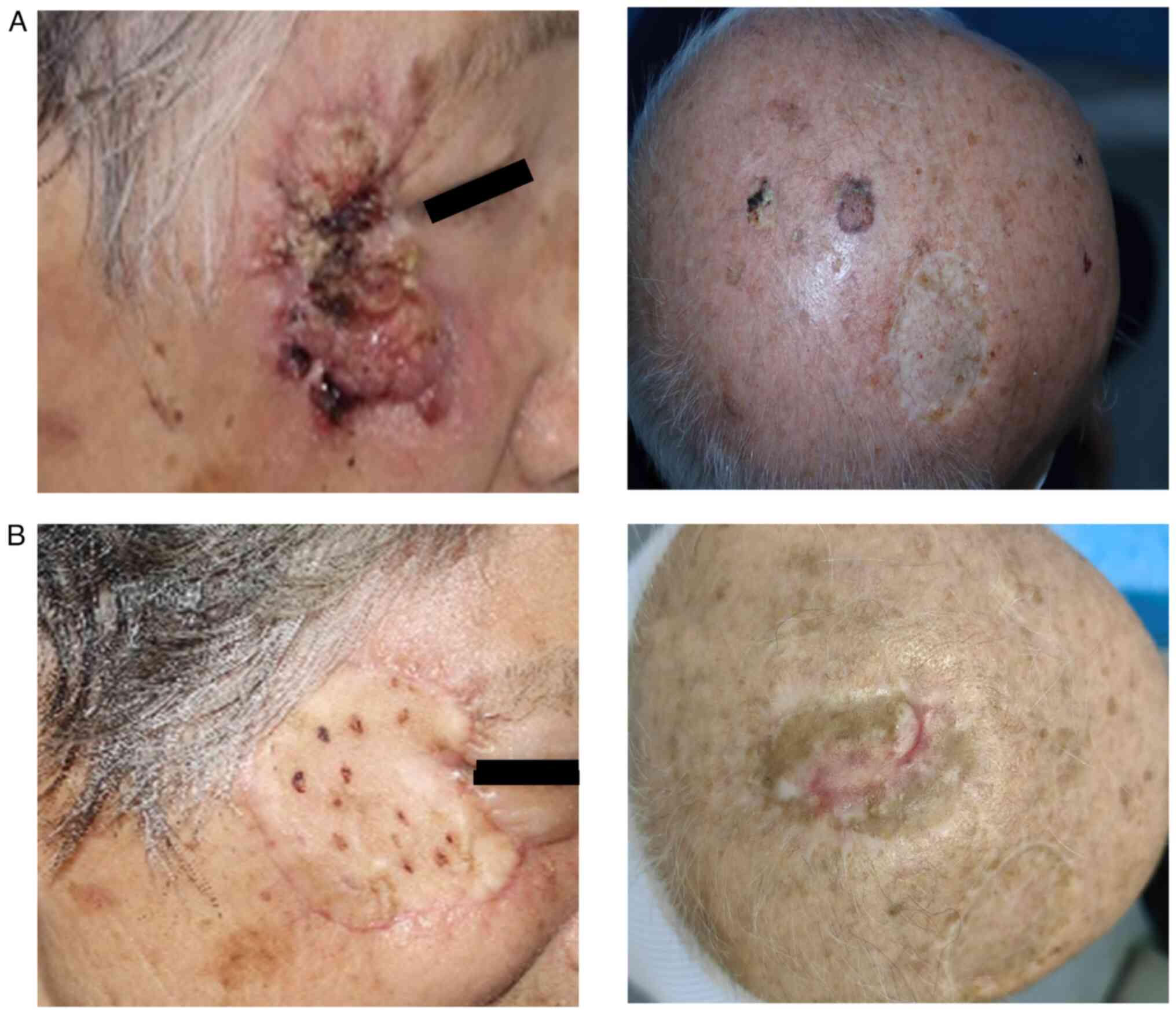
recurrence or wound infections. Fig.
1 shows two representative cases of cSCC, one on the head and
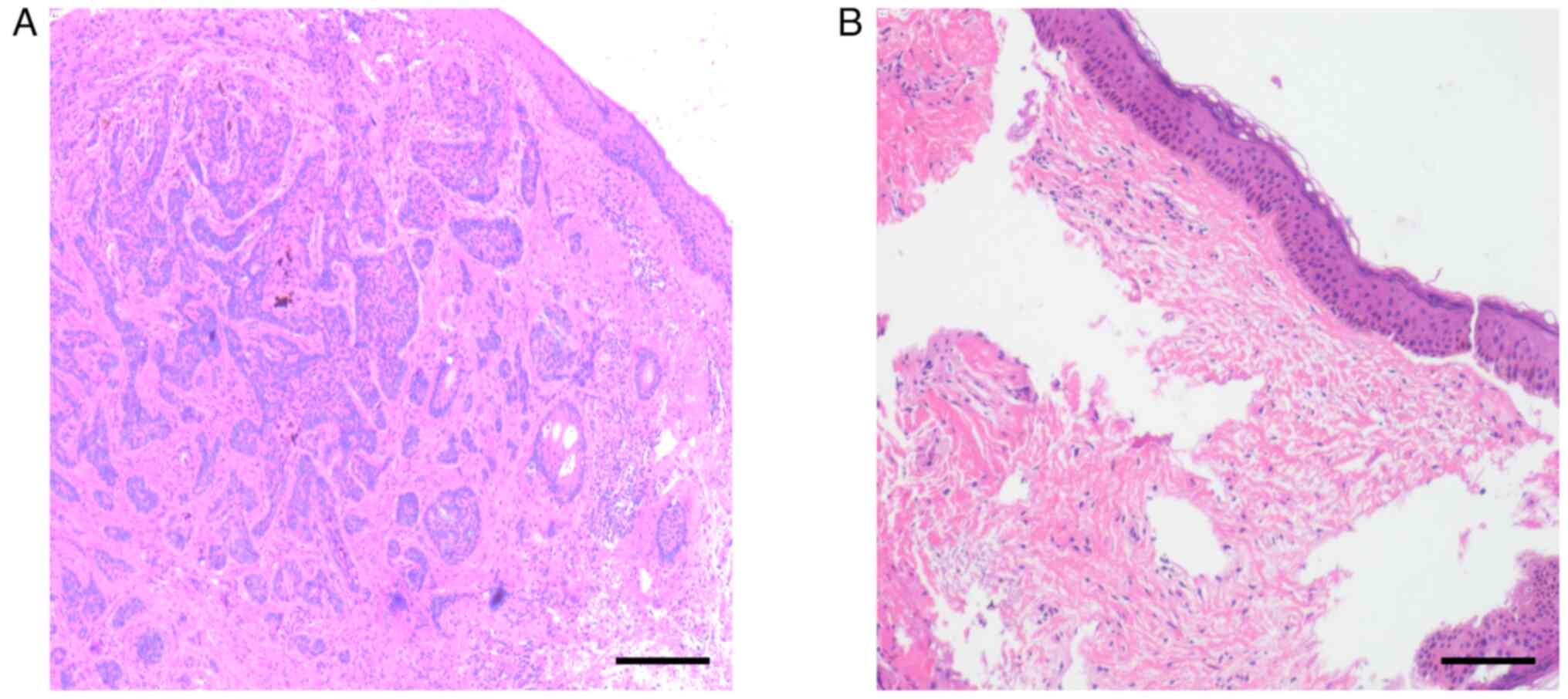
the other on the face. The pathological results showed that there
were no cells with heterotrophic hyperplasia after use of PDT on
the tumors (Fig. 2). Therefore, the
cases that underwent the described technique showed positive
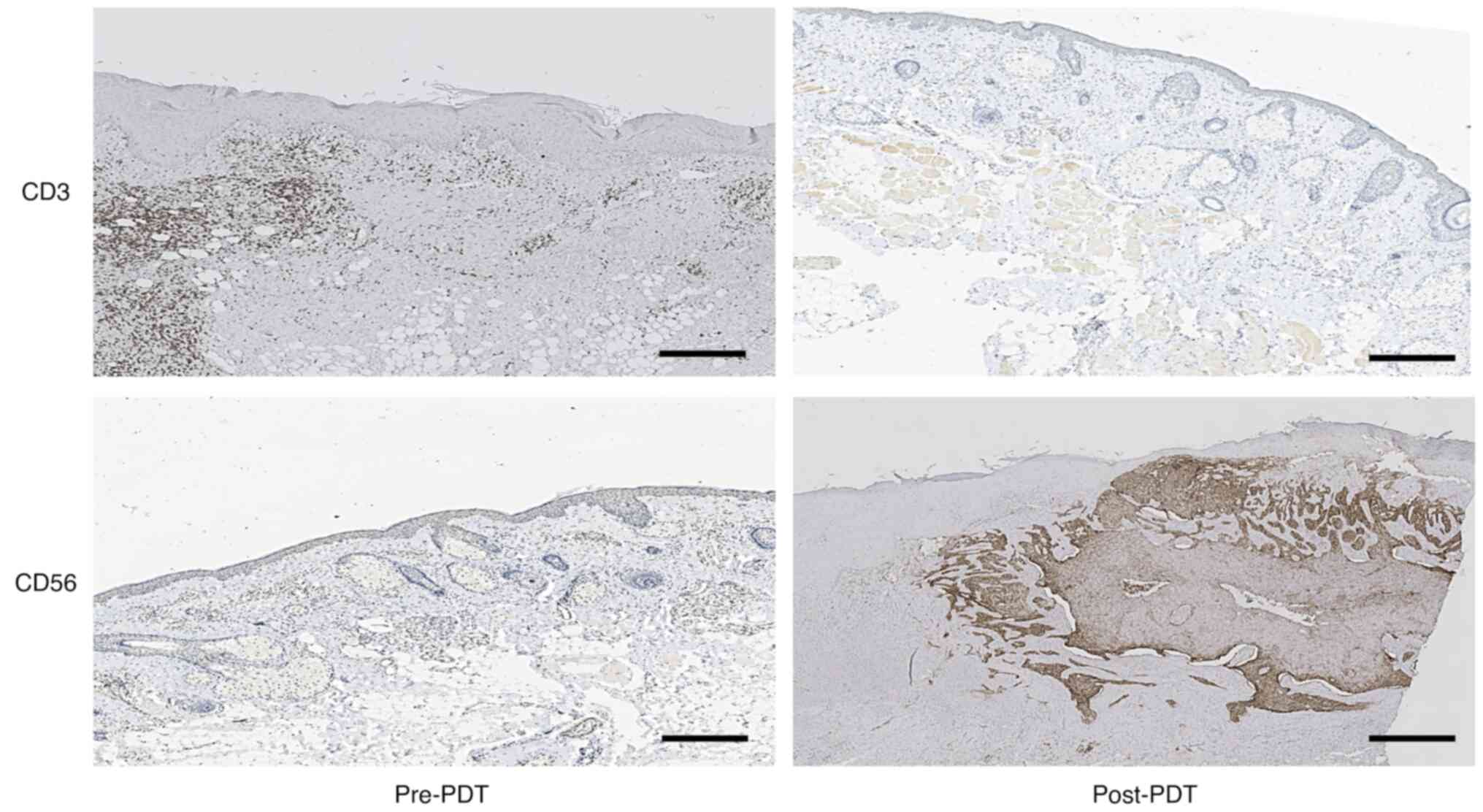
treatment outcomes with significant effects. It is well known that
CD56 serves as a distinctive cell surface marker of natural killer
(NK) cells (20), while CD3 is
recognized as a typical cell-surface marker of T lymphocytes
(21). The immunohistochemistry
results from the tissues in the present study indicated that the
expression of CD56 increased and that the expression of CD3 was not
different after PDT. The results also indirectly indicated that the
overall count of NK cells in the 10 patients increased, while there
was no difference in T lymphocyte count (Fig. 3).
Following ALA-PDT, some patients may have temporary
local side effects, such as skin burning and heightened wound
exudation, often linked to the execution of the procedure. Table I displays the VAS scores of all
patients after each ALA-PDT therapy. A flurbiprofen axetil (5 ml:50
mg) injection was administered if the VAS score exceeded 3.
Discussion
cSCC includes AK and keratoacanthomas (KA), and is a
type of non-melanoma skin cancer that develops from keratinocytes
in the epidermis. AK is often considered a precursor to cSCC. KA
may be classified as a variant of cSCC or a benign tumor with
histological resemblances to well-differentiated cSCC. KA typically
regresses spontaneously in most cases, unlike cSCC. Dermatologists
generally consider that KA does not have the potential to
metastasize (22–25). The variables that are causing the
differences in clinical behavior are not yet understood. One such
discrepancy might be the participation of T cells. In the present
study, a comparative investigation was performed on the clinical
data from 10 cSCC lesions.
In the present study, the patients with cSCC showed
no difference in T cell number after PDT within 24 h, which seems
to be a contradicting result. Studies have shown that PDT may
trigger immune responses that contribute to its therapeutic
effectiveness (26,27). PDT is linked to several important
immunological effects. PDT often triggers an inflammatory response
within the specific area it targets (28). Inflammation may help attract immune
cells like macrophages and T lymphocytes to a particular site,
aiding in the removal of abnormal cells and leftover
photosensitizers (29). PDT may
directly activate immune cells, increasing their ability to respond
aggressively. This intervention helps strengthen the ability of the
immune system to recognize and eliminate abnormal cells, such as
those linked to cancer. PDT may induce tumor cell death, resulting
in the release of tumor-specific antigens. The immune system may
recognize antigens as foreign substances, triggering both humoral
(including antibodies) and cellular (using T-cells) immune
responses (30). PDT may also
enhance immunological memory. Successful PDT may help improve the
capacity of the immune system to recognize and respond to abnormal
cell growth in the future. Based on the parameters discussed, we
consider that the decrease in the total number of T lymphocytes in
cSCC after early PDT may be due to a number of reasons, including
the precise location of tumor growth, the degree of tumor
penetration, the different reactions of people to photosensitizing
drugs and the possible existence of immunosuppressive diseases in
patients. In a previous study, although there was a decrease in the
total T lymphocyte count, the ratio of
CD4+/CD8+ T lymphocytes varied across the
patient group, with some showing an increase and others a decrease
(31).
The possibility of utilizing PDT just for treating
cSCC should be considered. PDT appears to be a feasible alternative
to surgery. Both PDT and PDT with surgery are effective modalities
for managing cSCC. The choice between the two treatments should be
based on individual patient-specific characteristics. PDT is a
non-invasive treatment that uses a photosensitizer and precise
light wavelengths to target and destroy tumor tissue by producing
active chemicals such as oxygen-free radicals, which eliminate
cancer cells (32,33). PDT offers benefits such as minimum
trauma, faster recuperation and fewer side effects, while its
effectiveness may not match that of surgery. PDT is used initially
to target and kill some cancer cells, followed by surgical excision
of the tumor. This combined method offers powerful therapeutic
effectiveness but comes with the drawbacks of increased trauma,
delayed recovery and a higher likelihood of adverse effects
(34,35). The present study aimed to
investigate the impact of PDT on NK cells in patients with cSCC,
since, to the best of our knowledge, little information is
available on this topic. Additionally, it was observed that the
white blood cell count of each patient had increased following PDT.
Possibly due to the inflammation from wound healing, infection and
the patients' underlying conditions (data not shown). In the
future, we aim to expand our patient recruitment efforts for this
research study. The results necessitate a larger dataset for
validation. Over 4 months, only 10 patients were enrolled in the
present study due to several factors: Primarily, the disruptive
impact of the COVID-19 pandemic; secondly, a dearth of eligible
candidates within the local vicinity; and lastly, an increasing
trend of patient preference for alternative treatment facilities.
Therefore, the next step will be to design an animal experiment to
further explore the specific mechanism of PDT for skin SCC,
especially the change in characteristics of lymphocytes in the
peripheral blood.
The present results showed an increase in the total
number of NK cells after early PDT for cSCC. NK cells are a type of
innate cytotoxic lymphoid cell that plays a crucial role in tumor
surveillance (36). Activated NK
cells destroy tumor cells by releasing cytotoxic cytokines and
producing granules containing perforin and granzyme B. Perforin and
granzyme B are recognized for their ability to compromise the
structural integrity of tumor cell membranes and trigger the onset
of apoptosis. Unlike T cells (37),
NK cells do not need prior antigen sensitization to start cytolytic
activity (38). NK cells possess
cytotoxic capabilities, rendering them a valuable asset for
immunotherapy (39). The
partnership of NK cells and Langerhans cells has been observed to
successfully prevent the growth of
1,12-dimethylbenz(a)anthracene-induced cSCC tumors in mice
(40). NK cells significantly
impede tumor growth, especially in the initial phases of cSCC
(41). As the disease progresses,
there is a possibility of functional impairment of the NK cells.
The reduced activity of NK cells in patients with advanced cSCC may
be caused by the tumor microenvironment (42). Prolonged exposure of NK cells to the
cSCC tumor environment leads to reduced NK cell activity (43). NK cells have shown great
effectiveness in both laboratory studies and medical experiments,
establishing them as a valuable treatment strategy for preventing
tumor growth (44).
A previous study has shown the significant potential
of PDT in treating cSCC; PDT selectively kills cancer cells using a
photosensitizing chemical and light, and when combined with
surgical intervention, it is the most ideal treatment for cSCC.
Cancer cells preferentially absorb the photosensitizing drug, which
is triggered by certain wavelengths of light. This specific
activation destroys malignant cells without harming healthy tissue.
PDT offers fewer side effects and scars than radiation therapy
(45). Further research is needed
to confirm this assertion; however, it is important to highlight
the fact that NK cells have shown potential in both preclinical
studies and clinical trials, establishing them as a crucial
strategy for inhibiting cSCC. Specifically, these results showed
that NK cells may directly kill cSCC cells and suppress tumor
development, supporting therapeutic trials (46). Meanwhile, clinical studies using NK
cell-based treatments for cSCC have shown tumor shrinkage, disease
stability and better patient outcomes (47), so the innate cytotoxicity and
tumor-targeting ability of NK cells may be a selective and
effective therapy.
In conclusion, the present study demonstrated that
the combination of ALA-PDT and surgery is efficacious in the
treatment of cSCC. The upregulation of NK cells, as evidenced by
increased expression of CD56, may represent one of the mechanisms
underlying the effectiveness of PDT in treating cSCC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Medical Research Project of
Chongqing Health Commission (grant no. 2023WSJK077).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HZ and JZ conceived and designed the experiments. XZ
and YR contributed new reagents and conducted the experiments. HZ
and HK wrote the manuscript. HZ, JZ, XZ, YR and HK analyzed and
discussed the results, and reviewed the manuscript. HZ and XZ
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Human skin tissue samples were collected from
consenting patients at Daping Hospital, Army Medical University
(Chongqing, China). The research was conducted in accordance with
the Helsinki Declaration and the Guidelines for the Care and Use of
Laboratory Animals of the Chinese Institute of Health. The study
was authorized by the Research Committee and Ethics Committee of
the General Hospital (Daping Hospital) of the Army Medical
University (approval no. DP2019-46). All patients who took part in
this study provided written informed consent.
Patient consent for publication
All patients consented to the publishing of this
paper, and provided writtem informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gallego-Rentero M, Gutiérrez-Pérez M,
Fernández-Guarino M, Mascaraque M, Portillo-Esnaola M, Gilaberte Y,
Carrasco E and Juarranz Á: TGFβ1 secreted by cancer-associated
fibroblasts as an inductor of resistance to photodynamic therapy in
squamous cell carcinoma cells. Cancers (Basel). 13:56132021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hao Y, Chen Y, He X, Yang F, Han R, Yang
C, Li W and Qian Z: Near-infrared responsive 5-fluorouracil and
indocyanine green loaded MPEG-PCL nanoparticle integrated with
dissolvable microneedle for skin cancer therapy. Bioact Mater.
5:542–552. 2020.PubMed/NCBI
|
|
3
|
Que SKT, Zwald FO and Schmults CD:
Cutaneous squamous cell carcinoma: Incidence, risk factors,
diagnosis, and staging. J Am Acad Dermatol. 78:237–247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martincorena I, Roshan A, Gerstung M,
Ellis P, van Loo P, McLaren S, Wedge DC, Fullam A, Alexandrov LB,
Tubio JM, et al: Tumor evolution. High burden and pervasive
positive selection of somatic mutations in normal human skin.
Science. 348:880–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Skulsky SL, O'Sullivan B, McArdle O,
Leader M, Roche M, Conlon PJ and O'Neill JP: Review of high-risk
features of cutaneous squamous cell carcinoma and discrepancies
between the American Joint Committee on Cancer and NCCN Clinical
Practice Guidelines In Oncology. Head Neck. 39:578–594. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Work Group and Invited Reviewers, . Kim
JYS, Kozlow JH, Mittal B, Moyer J, Olenecki T and Rodgers P:
Guidelines of care for the management of cutaneous squamous cell
carcinoma. J Am Acad Dermatol. 78:560–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiang F, Lucas R, Hales S and Neale R:
Incidence of nonmelanoma skin cancer in relation to ambient UV
radiation in white populations, 1978–2012: Empirical relationships.
JAMA Dermatol. 150:1063–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marsidi N, Ottevanger R, Bouwes Bavinck
JN, Krekel-Taminiau NMA, Goeman JJ and Genders RE: Risk factors for
incomplete excision of cutaneous squamous cell carcinoma: A large
cohort study. J Eur Acad Dermatol Venereol. 36:1229–1234. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Curiel-Lewandrowski C, Myrdal CN, Saboda
K, Hu C, Arzberger E, Pellacani G, Legat FJ, Ulrich M, Hochfellner
P, Oliviero MC, et al: In Vivo reflectance confocal microscopy as a
response monitoring tool for actinic keratoses undergoing
cryotherapy and photodynamic therapy. Cancers (Basel). 13:54882021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keyal U, Bhatta AK, Zhang G and Wang XL:
Present and future perspectives of photodynamic therapy for
cutaneous squamous cell carcinoma. J Am Acad Dermatol. 80:765–773.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XL, Wang HW, Guo MX and Xu SZ: T
reatment of skin cancer and pre-cancer using topical ALA-PDT -a
single hospital experience. Photodiagnosis Photodyn. Ther.
5:127–133. 2008.
|
|
12
|
Jaworski S, Biniecka P, Bugajska Ż,
Daniluk K, Dyjak S, Strojny B, Kutwin M, Wierzbicki M, Grodzik M
and Chwalibog A: Analysis of the cytotoxicity of hierarchical
nanoporous graphenic carbon against human glioblastoma grade IV
cells. Int J Nanomedicine. 12:3839–3849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilson C, Zhang X, Buckley C, Heathcote
HR, Lee MD and McCarron JG: Increased vascular contractility in
hypertension results from impaired endothelial calcium signaling.
Hypertension. 74:1200–1214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castano AP, Mroz P and Hamblin MR:
Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer.
6:535–545. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Ji J, Zhang H, Fan Z, Zhang L, Shi
L, Zhou F, Chen WR, Wang H and Wang X: Stimulation of dendritic
cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget.
6:44688–44702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Li J, Lv T, Tu Q, Huang Z and Wang
X: Therapeutic and immune effects of 5-aminolevulinic acid
photodynamic therapy on UVB-induced squamous cell carcinomas in
hairless mice. Exp Dermatol. 22:362–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakaseko H, Kobayashi M, Akita Y, Tamada Y
and Matsumoto Y: Histological changes and involvement of apoptosis
after photodynamic therapy for actinic keratosis. Br J Dermatol.
148:122–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gellén E, Fidrus E, Péter M, Szegedi A,
Emri G and Remenyik É: Immunological effects of photodynamic
therapy in the treatment of actinic keratosis and squamous cell
carcinoma. Photodiagnosis Photodyn Ther. 24:342–348. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Souwer IH, Bor JH, Smits P and
Lagro-Janssen AL: Nifedipine vs placebo for treatment of chronic
chilblains: A randomized controlled trial. Ann Fam Med. 14:453–459.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou F, Lu L, Liu J, Xia B, Zhang W, Hu Q,
Liu W, Zhang Y, Lin Y, Jing S, et al: Engineered triple inhibitory
receptor resistance improves anti-tumor CAR-T cell performance via
CD56. Nat Commun. 10:41092019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang X, Qin Y, Sheng X, Xing J and Zhan W:
Characterization of CD3+ T lymphocytes of Japanese flounder
(Paralichthys olivaceus) and its response after immunization with
formalin-inactivated Edwardsiella tarda. Fish Shellfish Immunol.
63:220–227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gleich T, Chiticariu E, Huber M and Hohl
D: Keratoacan-thoma: A distinct entity? Exp Dermatol. 25:85–91.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwiek B and Schwartz RA: Keratoacanthoma
(KA): An update and review. J Am Acad Dermatol. 74:1220–1233. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Selmer J, Skov T, Spelman L and Weedon D:
Squamous cell carcinoma and keratoacanthomas are biologically
distinct and can be diagnosed by light microscopy: A review.
Histopathology. 69:535–541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cassarino DS, Derienzo DP and Barr RJ:
Cutaneous squamous cell carcinoma: A comprehensive
clinicopathologic classification-part two. J Cutan Pathol.
33:261–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao Y, Gu Z, Yu Z, Schomann T, Sayedipour
S, Aguilar JC, Ten Dijke P and Cruz LJ: Photodynamic therapy in
combination with the hepatitis B core virus-like particles (HBc
VLPs) to prime anticancer immunity for colorectal cancer treatment.
Cancers (Basel). 14:27242022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mossakowska BJ, Shahmoradi Ghahe S,
Cysewski D, Fabisiewicz A, Tudek B and Siedlecki JA: Mechanisms of
resistance to photodynamic therapy (PDT) in vulvar cancer. Int J
Mol Sci. 23:41172022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Z, Hu X, Zhou L, He Y, Zhang X, Yang
J, Ju Z, Liou YC, Shen HM, Luo G, et al: Photodynamic therapy
accelerates skin wound healing through promoting
re-epithelialization. Burns Trauma. 9:tkab0082021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Y, Wang C, Zhuge Y, Zhang J, Xu K,
Zhang Q, Zhang H, Chen H, Chu M and Jia C: Photodynamic antifungal
activity of hypocrellin a against candida albicans. Front
Microbiol. 10:18102019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Zhao T, Han F, Hu Y and Li Y:
Photothermal and gene therapy combined with immunotherapy to
gastric cancer by the gold nanoshell-based system. J
Nanobiotechnology. 17:802019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Q, Wu B, Weng Q, Hu F, Lin Y, Xia C,
Peng H, Wang Y, Liu X, Liu L, et al: Regeneration of
immunocompetent B lymphopoiesis from pluripotent stem cells guided
by transcription factors. Cell Mol Immunol. 19:492–503. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Algarin YA, Jambusaria-Pahlajani A, Ruiz E
and Patel VA: Advances in topical treatments of cutaneous
malignancies. Am J Clin Dermatol. 24:69–80. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao H, Sun J and Yang Y: Research
progress of photodynamic therapy in wound healing: A literature
review. J Burn Care Res. 44:1327–1333. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao Z, Wu Y, Zhou Z, Zhao Y, Sun X, Hu C,
Wang X and Zhang G: ALA-PDT successfully treated multiple cSCC in
situ and AK in a patient with Epidermodysplasia verruciformis.
Photodiagnosis Photodyn Ther. 35:1023952021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aggarwal I, Puyana C, Chandan N, Jetter N
and Tsoukas M: Field cancerization therapies for the management of
actinic keratosis: An updated review. Am J Clin Dermatol.
25:391–405. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heipertz EL, Zynda ER, Stav-Noraas TE,
Hungler AD, Boucher SE, Kaur N and Vemuri MC: Current perspectives
on ‘Off-The-Shelf’ allogeneic NK and CAR-NK cell therapies. Front
Immunol. 12:7321352021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morvan MG and Lanier LL: NK cells and
cancer: You can teach innate cells new tricks. Nat Rev Cancer.
16:7–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bryceson YT, March ME, Barber DF,
Ljunggren HG and Long EO: Cytolytic granule polarization and
degranulation controlled by different receptors in resting NK
cells. J Exp Med. 202:1001–1012. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gonçalves-Maia M, Gache Y, Basante M,
Cosson E, Salavagione E, Muller M, Bernerd F, Avril MF, Schaub S,
Sarasin A, et al: NK cell and Fibroblast-Mediated regulation of
skin squamous cell carcinoma invasion by CLEC2A is compromised in
Xeroderma pigmentosum. J Invest Dermatol. 140:1723–1732. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ortner D, Tripp CH, Komenda K, Dubrac S,
Zelger B, Hermann M, Doppler W, Tymoszuk PZ, Boon L, Clausen BE and
Stoitzner P: Langerhans cells and NK cells cooperate in the
inhibition of chemical skin carcinogenesis. Oncoimmunology.
6:e12602152016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen X, Chen X, Gao J, Yang H, Duan Y,
Feng Y, He X, Gong X, Wang H, Wu X and Chang J: Astragaloside III
enhances anti-tumor response of NK cells by elevating NKG2D and
IFN-γ. Front Pharmacol. 10:8982019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shi J, Wu P, Sheng L, Sun W and Zhang H:
Ferroptosis-related gene signature predicts the prognosis of
papillary thyroid carcinoma. Cancer Cell Int. 21:6692021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gill S, Vasey AE, De Souza A, Baker J,
Smith AT, Kohrt HE, Florek M, Gibbs KD Jr, Tate K, Ritchie DS and
Negrin RS: Rapid development of exhaustion and down-regulation of
eomesodermin limit the antitumor activity of adoptively transferred
murine natural killer cells. Blood. 119:5758–5768. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oyer JL, Gitto SB, Altomare DA and Copik
AJ: PD-L1 blockade enhances anti-tumor efficacy of NK cells.
Oncoimmunology. 7:e15098192018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luci C, Bihl F, Bourdely P, Khou S, Popa
A, Meghraoui-Kheddar A, Vermeulen O, Elaldi R, Poissonnet G, Sudaka
A, et al: Cutaneous squamous cell carcinoma development is
associated with a temporal infiltration of ILC1 and NK cells with
immune dysfunctions. J Invest Dermatol. 141:2369–2379. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fu XQ, Liu B, Wang YP, Li JK, Zhu PL, Li
T, Tse KW, Chou JY, Yin CL, Bai JXL, et al: Activation of STAT3 is
a key event in TLR4 signaling-mediated melanoma progression. Cell
Death Dis. 11:2462020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vuong NL, Cheung KW, Periaswamy B, Vi TT,
Duyen HTL, Leong YS, Binte Hamis ZN, Gregorova M, Ooi EE, Sessions
O, et al: Hyperinflammatory syndrome, natural killer cell function,
and genetic polymorphisms in the pathogenesis of severe dengue. J
Infect Dis. 226:1338–1347. 2022. View Article : Google Scholar : PubMed/NCBI
|