Introduction
Cervical cancer is the fourth most common type of
cancer in women, with an incidence of 570,000 estimated new cases
and 311,000 resultant deaths worldwide in 2018 (1). Currently, laparoscopic radical
hysterectomy with pelvic lymph node dissection remains a common
treatment option for early cervical cancer. Yet the perioperative
period may promote metastasis and recurrence of malignant tumor
cells through a variety of mechanisms, such as surgery stress,
inhibition of the immune system, modulation of angiogenesis and
growth factors (2,3). In addition, perioperative immune
dysfunction is associated with the occurrence of postoperative
complications, such as infection, delayed wound healing and septic
events (4–6). Therefore, perioperative interventions
that preserve or enhance immune function during the postoperative
period may improve patient outcome. Opioids have long been used in
the treatment of perioperative severe acute pain, but it has
dose-dependent adverse reactions that contribute to poor patient
health outcomes (7). In addition,
the opioid-induced immunosuppression should not be ignored. Opioid
abuse is widespread and poses a serious threat to public health.
Since both pain and opioid analgesics can cause immunosuppression
(5), it is important to effectively
and safely relieve postoperative pain and avoid immune
dysfunction.
As a highly selective α2-adrenoceptor agonist,
dexmedetomidine (DEX) has sedative, anti-anxiety and analgesic
effects, while reducing opioid use and alleviating
immunosuppression (8). A
meta-analysis showed that perioperative administration of DEX as an
adjunct to general anesthesia can significantly reduce the levels
of inflammatory cytokines, such as interleukin-6 (IL-6), IL-8 and
tumor necrosis factor-α (TNF-α) in patients, thus ameliorating the
impaired immune function (9).
Ketorolac is a non-steroidal anti-inflammatory drug (NSAID) with
analgesic, anti-inflammatory and anti-pyretic effects. NSAIDs
perform these functions by blocking prostaglandin synthesis through
the inhibition of the cyclooxygenase (COX) enzyme (10). Ketorolac may reduce demand of opioid
and opioid-related side effects postoperatively (11). Furthermore, postoperative ketorolac
analgesia has been shown to contribute to the preservation of
natural killer cell cytotoxicity and have a favorable effect on
immune function (4).
In addition to immune function, tumor recurrence and
metastasis are closely associated with angiogenesis. VEGF levels
are closely associated with tumor angiogenesis, tumor proliferation
and distant metastasis (12).
Studies have shown that opioids may upregulate VEGF and promote
angiogenesis (13,14). The µ-opioid receptor antagonist
methylnaltrexone has been found to block opioid-induced
angiogenesis and exert intracellular effects to inhibit
angiogenesis (15).
Although DEX and ketorolac are increasingly common
components of multimodal analgesia (16), their effects on perioperative
immunity and tumor promotion while controlling postoperative
analgesia are rarely studied. Therefore, the present study was
conducted to determine the influence of two postoperative analgesic
methods on the changes of postoperative serum VEGF levels, T helper
cell 1 (Th1) cytokine interferon-γ (IFN-γ) and Th2 cytokine IL-4,
as well as to estimate the balance of Th1/Th2 (IFN-γ/IL-4 ratio)
(17,18). The potential influence of DEX
combined with ketorolac on tumor recurrence and growth following
radical cervical cancer resection was also studied.
Materials and methods
Participants
The present study was conducted according to the
Declaration of Helsinki and the Guidelines on Good Clinical
Practice (19). Approval for the
present study was provided by the Ethics Committee of Tangshan
Maternity and Child Healthcare Hospital (approval no. 2019-031-01;
Tangshan, China). This trial was registered prior to participant
enrollment at www.chictr.org.cn (registration no. ChiCTR1900027979).
Written informed consent was obtained from each patient from
October 2020 to April 2022. The inclusion criteria were as follows:
i) Female patients with newly diagnosed cervical cancer; ii) age,
25–65 years; iii) American Society of Anesthesiologists physical
status I or II; and iv) scheduled to undergo elective laparoscopic
radical hysterectomy with pelvic lymphadenectomy (20). The exclusion criteria were as
follows: i) Body mass index >30; ii) history of severe viscera
system or immune system diseases, gastrointestinal ulcer or
bleeding; iii) history of chemotherapy, radiation or
immunosuppressive therapy; iv) history of chronic pain or substance
abuse; v) leukocytosis (>10,000/ml) or high level of C-reactive
protein; vi) history of allergies to DEX or NSAIDs.
Randomization and blinding
A total of 70 adult female patients were enrolled
(age, 25–65 years) and randomly allocated to either the sufentanil
(SUF) or the DK group using sequentially-numbered sealed envelopes
through a random number generator. The allocation ratio between the
two groups was 1:1. An assistant not involved in the study prepared
and distributed the envelopes. One anesthesiologist blinded to the
allocation status was responsible for administering anesthesia and
perioperative care. Based on the randomized sequence, the
postoperative patient-controlled analgesia (PCA) pumps were
prepared on the day of surgery by an independent nurse anesthetist.
PCA pumps were sealed and then transferred to the intraoperative
anesthesiologist blinded to their contents. Similarly, each surgery
was performed by the same group of surgeons. The anesthesia
follow-ups were conducted by another anesthesiologist, who was also
blinded to the treatment regimen and not allowed to be involved in
data analysis.
Anesthesia and pain management
Upon arrival at the operating room, each patient was
monitored using electrocardiography and blood pressure, pulse
oxygen saturation, pressure of end-tidal carbon dioxide
(PETCO2), and bispectral index (BIS)
measurements were taken. Patients were administered 0.5 mg
penehyclidine and 1–2 mg midazolam intravenously (IV) prior to the
induction of anesthesia. Anesthesia was induced with 2–3 mg/kg
propofol, 0.3–0.5 µg/kg sufentanil and 0.2 mg/kg cisatracurium.
Propofol and remifentanil were administered to the patients through
a target-controlled infusion during surgery to maintain hemodynamic
stability intraoperatively. Mechanical ventilation was performed
with 8 ml/kg tidal volume, and ventilator frequency was adjusted to
maintain PETCO2 at 35–40 mmHg. According to
surgical requirements, cisatracurium was administered
intermittently to promote muscle relaxation. The depth of
anesthesia was also monitored using a BIS monitor (Aspect Medical
System, Inc.), which was maintained at a value of 40–60. A total of
10 min before the end of surgery, each group was administered 6 ml
of the corresponding PCA pump drug. PCA in each group was initiated
at the end of the surgery and maintained for up to 48 h
postoperatively.
Both groups of participants were treated with an IV
PCA pump for postoperative pain management. The SUF group received
SUF via IV PCA. The SUF PCA was composed of 1.5 µg/kg SUF mixed
with normal saline to a total volume of 100 ml. The DK group
received DEX and ketorolac through IV PCA, which was composed of 2
µg/kg DEX and 3 mg/kg ketorolac mixed with normal saline to a total
volume of 100 ml. The bolus dose was set to 0.5 ml at a basal
infusion rate of 2 ml/h, with a lockout interval of 15 min. At the
end of the surgery, residual muscle relaxation was antagonized with
1 mg neostigmine and 0.5 mg atropine. Following extubation, the
patient was sent to the post-anesthesia care unit for further
monitoring.
Observation indexes
An 11-point numerical rating scale (NRS; range,
0–10, 0 indicating no pain and 10 indicating the worst pain
imaginable) was performed to assess the pain intensity at rest and
while coughing 30 min before induction (T0), 4 h after
surgery (T1), 12 h after surgery (T2), 24 h
after surgery (T3) and 48 h after surgery
(T4). To ensure the accuracy of the assessment, the use
of the NRS was explained in detail to each patient. The
participants were advised to press the PCA bolus when NRS >3.
The valid PCA pressing times and total consumption of the PCA pump
within 48 h from surgery were recorded. Furthermore, main
postoperative side effects and complications, such as postoperative
nausea and vomiting (PONV), pruritus, respiratory depression and
dizziness were observed.
A total of 5 ml peripheral venous blood sample of
each participant was collected 30 min before induction
(T0), 24 h after surgery and 48 h after surgery for
detecting cytokine concentration. To determine cytokine levels, the
blood samples were centrifuged at 2,200 × g for 10 min at 4°C.
Next, the supernatant serum was removed and stored at −20°C for
further analysis. Serum concentrations of IFN-γ (cat. no. DIF50C;
Human IFN-γ Quantikine ELISA Kit; R&D Systems, Inc.), IL-4
(cat. no. D4050; Human IL4 Quantikine ELISA Kit; R&D Systems,
Inc.) and VEGF (cat. no. DVE00; Human VEGF Quantikine ELISA Kit;
R&D Systems, Inc.) were measured using ELISA. A Spectra Max 190
microplate reader (Molecular Devices) was used to read the
absorbance at 450 nm. To determine the balance of Th1/Th2, the
IFN-γ/IL-4 ratio was also calculated. All steps are in accordance
with the manufacturer's instructions. All cytokines were detected
within 7 days from serum separation, and the procedure was repeated
at least 3 times.
Statistical analysis
Statistical analysis was performed using the SPSS
statistical software (version 20.0; IBM Corp.). Data were tested
for normality using the Shapiro-Wilk test. Continuous variables are
presented as the mean ± standard deviation or median (interquartile
range), and continuous variables were analyzed using an independent
t-test or Mann Whitney U test, where appropriate. The comparisons
of cytokine concentrations between groups and within groups were
analyzed using a mixed two-way ANOVA followed by Bonferroni
correction. Categorical variables were described as numbers or
frequencies and analyzed by Fisher's exact test or χ2
test, where appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
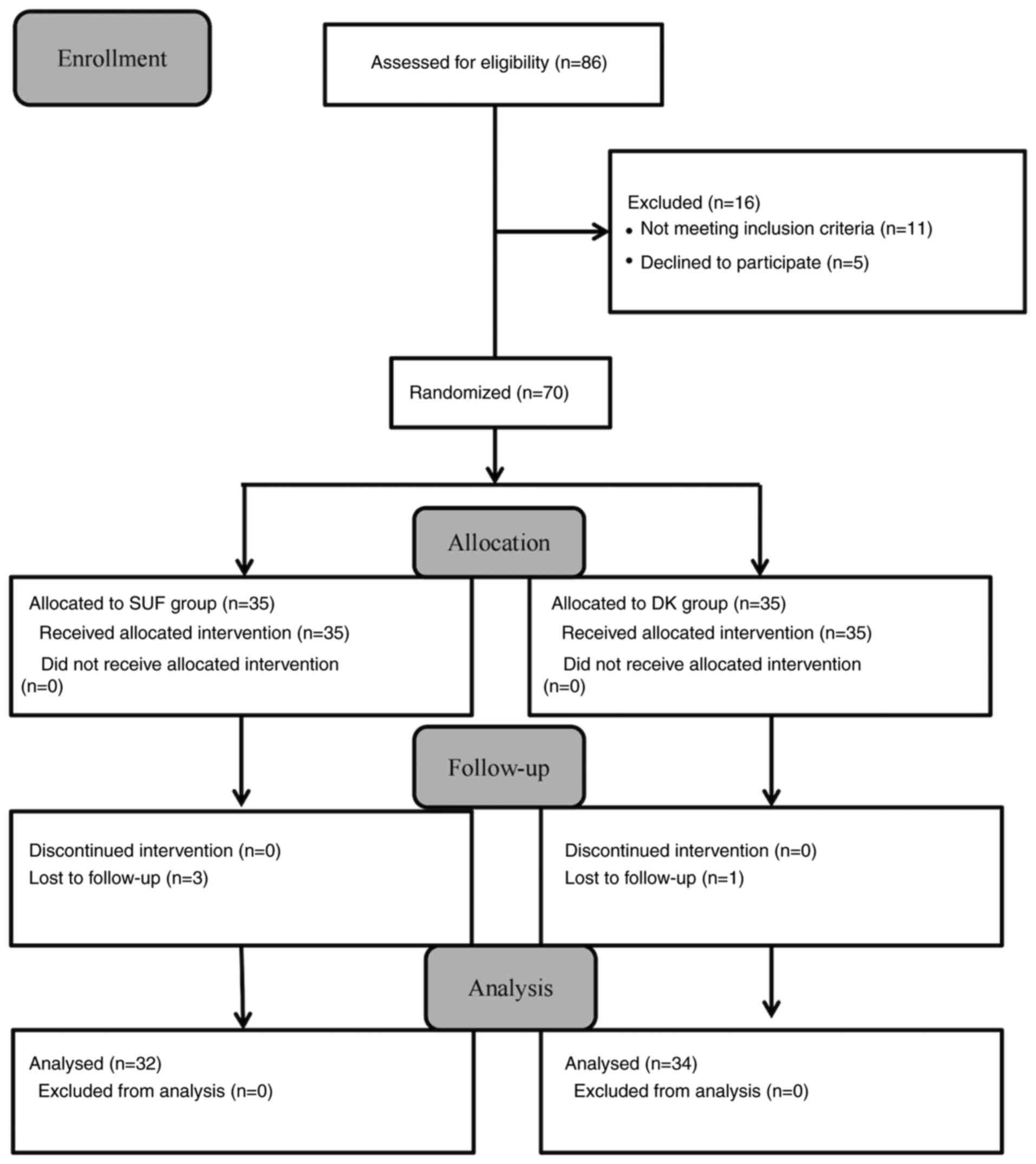
Participant enrollment
A total of 86 participants were initially recruited.
However, 11 participants (12.79%) were excluded due to meeting the
exclusion criteria and 5 participants (5.81%) declined to
participate. Subsequently, 2 participants (3.49%) in the SUF group
and 1 participant (1.16%) in the DK group were lost to follow-up
due to refusing to complete blood drawing at any time-point.
Consequently, available data from 66 participants (76.74%) were
included in the analysis (Fig.
1).
Demographics and surgery details
No significant differences were observed between the
groups regarding the baseline demographic characteristics of
patients. The details of surgery and anesthesia, in terms of
duration, fluid infusion volume, blood loss and urine output were
comparable between the two groups (Table I).
 | Table I.Clinical characteristics of patients
between two groups. |
Table I.
Clinical characteristics of patients
between two groups.
| Variables | SUF | DK | P-value |
|---|
| Average age,
years | 46.2±9.3 | 48.0±9.2 | 0.453 |
| Body mass index,
kg/m2 | 23.8±3.4 | 24.0±3.8 | 0.768 |
| ASA physical
status, I/II | 20/12 | 19/15 | 0.585 |
| Anesthesia
duration, min | 253.1±39.2 | 244.3±35.4 | 0.342 |
| Operation duration,
min | 215.7±37.8 | 210.6±32.3 | 0.557 |
| Blood loss, ml | 215.4±58.0 | 205.7±57.8 | 0.499 |
| Infusion volume,
ml | 1564.2±260.2 | 1532.8±210.9 | 0.591 |
| Urine output,
ml | 301.5±51.8 | 313.5±56.7 | 0.371 |
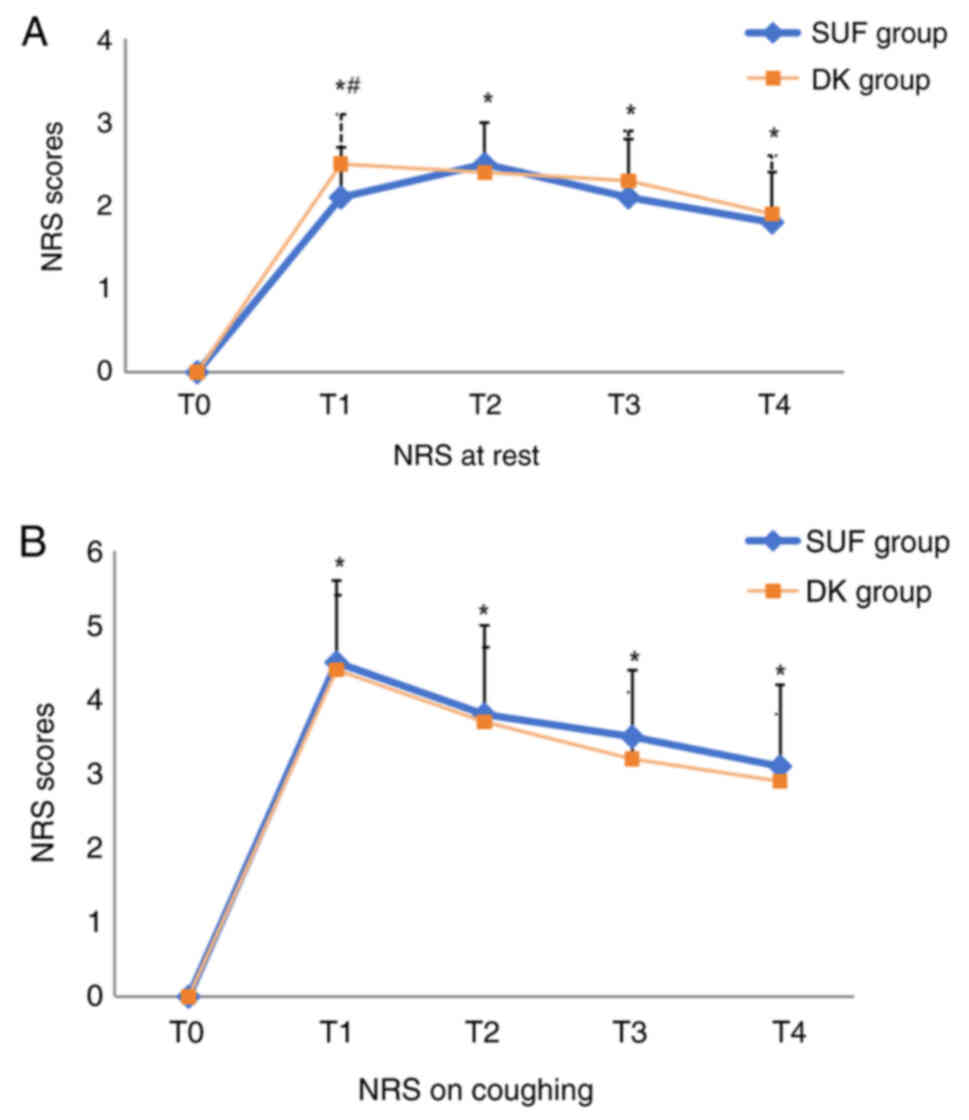
Postoperative analgesia indexes
Compared with T0, the resting and
coughing NRS score of both groups increased at each time point
postoperatively. The postoperative resting NRS scores at
T1 in the SUF group were significantly lower compared
with those in the DK group (2.1±0.6 vs. 2.5±0.6; P=0.009), but the
resting NRS score in both groups at different postoperative time
points was <4 (Fig. 2A). There
was no difference in the coughing NRS score between the two groups
at any times point (Fig. 2B). There
was no significant difference in valid PCA pressing times between
the two groups (Table II).
 | Table II.Pressing times and incidence of
adverse events between two groups. |
Table II.
Pressing times and incidence of
adverse events between two groups.
| Variables | SUF | DK | P-value |
|---|
| Valid pressing,
times | 4.3±1.7 | 4.9±1.6 | 0.135 |
| Dizziness | 6 | 4 | 0.505 |
| Pruritis | 4 | 2 | 0.420 |
| PONV | 8 | 2 | 0.041a |
| Hypotension | 1 | 1 | 1.000 |
| Bradycardia | 0 | 2 | 0.493 |
The incidence of PONV was significantly higher in
the SUF group compared with the DK group. The incidence of
dizziness and pruritus did not differ between the two groups, and
no respiratory depression or gastrointestinal hemorrhage was
observed. Although there was 1 case of hypotension and 2 cases of
bradycardia in the DK group and 1 case of hypotension in the SUF
group, there was no statistical difference between the two groups
(Table II).
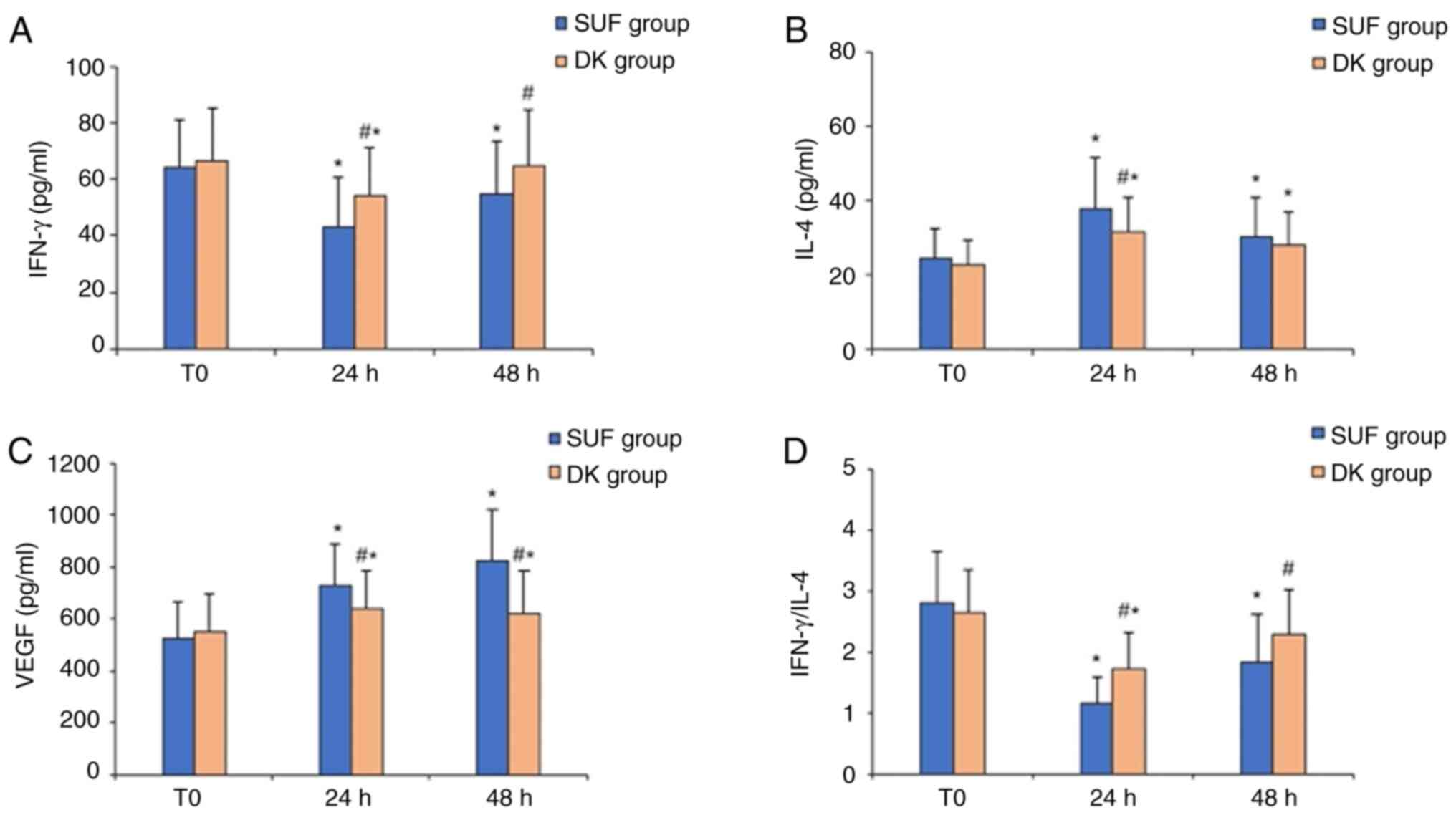
Immunological indexes
Since the pain control effectiveness of the two
different pain management strategies were roughly similar, the
possible influence of varying pain intensity on postoperative
immune function in the two groups could be excluded. The baseline
levels of IFN-γ/IL-4 and VEGF at T0 were similar between
the two groups. Compared with baseline levels at T0, the
serum concentration of IFN-γ and the ratio of IFN-γ/IL-4 were
significantly decreased at 24 and 48 h after surgery in the SUF
group, whereas the serum levels of IL-4 and VEGF were significantly
increased at all postoperative time points. Similarly, in the DK
group, a lower serum concentration of IFN-γ, a downregulated
IFN-γ/IL-4 ratio, and higher serum levels of IL-4 and VEGF were
detected at 24 h after surgery compared with T0.
However, the serum level of IFN-γ and the ratio of IFN-γ/IL-4 at 48
h after surgery were similar to those at baseline in the DK group,
and the serum levels of IL-4 and VEGF were still upregulated at 48
h after surgery (Fig. 3). In the DK
group, the serum concentrations of IFN-γ and the IFN-γ/IL-4 ratio
at 24 and 48 h after surgery were higher compared with the SUF
group. Conversely, the serum concentrations of IL-4 at 24 h after
surgery and VEGF at 24 and 48 h after surgery were significantly
lower (Fig. 3).
Discussion
The present study revealed that the administration
of DEX combined with ketorolac for PCA following laparoscopic
radical resection of cervical cancer could effectively alleviate
postoperative pain intensity, inhibit the perioperative
immunosuppressive state by adjusting the inflammatory factor levels
and inhibiting Th1/Th2 drift and reduce the expression of VEGF and
angiogenesis, which could potentially reduce tumor recurrence.
Cancer surgery and anesthesia can inhibit cellular
immune function, release catecholamines and prostaglandin
E2 (PGE2) and induce the release of a variety
of inflammatory cytokines and tumorigenic factors, including TNF-α,
IL-1β, IL-6 and VEGF (21,22). Surgical trauma and postoperative
acute pain can contribute to the release of inflammatory cytokines,
leading to immune dysfunction (4–6). DEX
and non-steroidal anti-inflammatory drugs can reduce the
consumption of opioids, enhance the analgesic effect, reduce the
incidence of adverse drug reactions and improve clinical safety
(8,11). In particular, ketorolac can be used
to control cancer-related pain and as an analgesic following cancer
surgery (23,24). In the present study, within 48 h
from laparoscopic radical resection of cervical cancer, DEX
combined with ketorolac administered for controlled analgesia had
significant analgesic effects with limited adverse reactions,
avoiding the related side effects caused by opioid analgesia.
Secondly, in addition to pain management,
maintaining immune balance is also important for patient recovery
and early discharge following surgery, even for long-term tumor
recurrence. Th cells can be differentiated into Th1 or Th2
subtypes. Under normal conditions, Th1 and Th2 cells are in a
relative balance state, which is important for maintaining immune
balance. Cancer has been shown to disrupt the Th1/Th2 balance,
which is called ‘balance shifting’ (18,25).
Since the balance drift from Th1 to Th2 cells seems to be
associated with immunosuppression and the progression of cancer
(26), Th1/Th2 balance is critical
for cancer patients. Cytokines secreted by Th-1 and Th-2 are
closely associated with immune response and immune regulation. Th-1
cells promote cell-mediated immune responses and mainly release
IL-2, IFN-γ and TNF-β, which are essential for antineoplastic and
anti-inflammatory processes (27,28).
On the other hand, the pro-inflammatory cytokines secreted by Th-2
cells are mainly composed of IL-4, IL-5, IL-6 and IL-10,
facilitating humoral immunity but suppressing particular types of
cell-mediated immune responses (29). IFN-γ and IL-4 are key cytokines
produced by Th1 and Th2 cells, respectively, so Th1/Th2 balance can
be estimated by calculating the IFN-γ/IL-4 ratio (17,18).
The implication of the surgery-induced upregulation of Th2 or
downregulation of the Th1/Th2 ratio increases susceptibility to
infection and tumor progression in the patients with postoperative
cellular immunosuppression (30).
The main outcome of the present study was that
postoperative DEX combined with ketorolac analgesia significantly
alleviated the decrease of the IFN-γ/IL-4 ratio in patients
undergoing surgery and anesthesia stress. These results indicated
that postoperative DEX combined with ketorolac for PCA may shift
the Th1/Th2 balance toward Th1, thereby contributing to
immunomodulatory effects. DEX has been shown to reduce the
secretion of pro-inflammatory cytokines, thereby modulating harmful
inflammatory responses due to surgery, which was consistent with
another study in mice with concanavalin A-induced liver injury
(31,32). In a randomized controlled trial, the
intraoperative administration of DEX could shift the Th1/Th2
imbalance toward Th1 in a dose-dependent manner in patients
subjected to surgical and anesthetic stress, exhibiting
immunomodulatory effects (33).
This is consistent with another study of intraoperative DEX in
patients with gastric cancer undergoing gastrectomy, which
demonstrated immunomodulatory properties, with a decline in IL-6
and TNF and increase in the Th1/Th2 ratio observed (8). Not only as an analgesic, ketorolac has
been shown to improve survival in cancer patients. A retrospective
study that included 327 women with breast cancer reported that,
compared with other analgesics (SUF, clonidine and ketamine), the
intraoperative use of ketorolac reduced the risk of breast cancer
relapse (34). Unlike other NSAIDs,
ketorolac is a 1:1 racemic mixture of two different independent
pharmacological enantiomers (S- and R-ketorolac) (23). S-ketorolac is considered the active
component in pain management with selective activity against COX
enzymes; while R-ketorolac has an activity as an inhibitor of
Ras-related C3 botulinum toxin substrate and cell division control
protein 42 GTPases, which are recognized as attractive therapeutic
targets in cancer, thereby promoting anti-cancer activity (23,35).
NSAIDs are well-known immunomodulators. NSAIDs increase mRNA
expression and production of cytokines produced by Th1 cells (TNF,
IFN-γ and IL-2), but decrease that of IL-4 and IL-6 produced by Th2
cells both at the mRNA and protein levels (36). This regulation may be partially
independent of the inhibition of PGE2 synthesis in T
cell clones (36). A recent study
has suggested that the combination of DEX and NSAIDs can reduce
postoperative IL-2 inhibition, restrain the secretion of serum
TNF-α and IL-6 and improve the levels of B and T lymphocytes
(37).
Surgery-induced sympathetic nervous system
activation and anesthetic-associated cell-mediated
immunosuppression may also promote angiogenesis by increasing the
release of cytokines such as VEGF (38). VEGF levels are closely associated
with tumor angiogenesis, tumor proliferation and distant metastasis
(12). Moreover, studies have found
that VEGF concentration is associated with clinical stage, tumor
size and their role in monitoring the disease process (3,39).
VEGF has been found to be overexpressed in cervical cancer cells
and the serum levels of VEGF are often elevated in patients with
cervical cancer (40,41). Kotowicz et al (42) revealed that the elevated serum VEGF
concentration in patients with cervical cancer may be an important
prognostic factor at the early stage of clinical development.
Moreover, the serum VEGF level of patients with cervical cancer was
increased, and decreased significantly following successful
treatment (43). Therefore,
VEGF-mediated angiogenesis has become a new target for anti-cancer
therapy in recent years, and different intervention methods have
been explored to block tumor angiogenesis (44).
As a monoclonal antibody for VEGF, Bevacizumab has
been approved to inhibit tumor angiogenesis and improve overall
survival in patients with certain types of tumors (45). Mirzaei et al (46) revealed that simvastatin combined
with arsenic trioxide inhibits cell proliferation and has
antiangiogenesis properties, which may be achieved by
downregulating VEGF expression. In addition, a recent study has
demonstrated that miR-503-5p inhibits tumorigenesis and
angiogenesis in colon cancer by downregulating the expression of
VEGF-AA (47).
VEGF has been implicated in tumorigenesis and tumor
growth due to its ability to promote angiogenesis, mitotic activity
and vascular permeability-enhancing activity (12,40).
In addition, PGE2, the synthesized product of
cyclooxygenase-2, can induce tissues to create VEGF, which in turn
leads to a gradual increase in VEGF expression (48). NSAIDs inhibit the synthesis of
PGE2 by inhibiting the COX enzyme, showing antitumor and
anti-angiogenic properties (49,50).
Furthermore, Ji et al (48)
found that surgeries without any perioperative drugs for analgesia
caused marked increases in serum PGE2 and VEGF in rats,
which were inhibited by NSAIDs. A randomized controlled study
suggests that the use of COX-2 inhibitors for pain management in
cancer patients may decrease the expression of VEGF, thereby
reducing the risk of cancer recurrence and metastasis (22). In lung cancer surgery, the
administration of DEX can induce the proliferation of monocytic
myeloid-derived suppressor cells (MDSCs) which have the ability to
promote angiogenesis (51).
Treatment with DEX also increases monocytic MDSC in mice and
promotes tumor metastasis by increasing VEGF production (51). In the present study, there was a
significantly decreased level of VEGF in the DK group compared with
the SUF group at 24 and 48 h after surgery, although the VEGF
levels were not restored to the preoperative level at 48 h after
surgery. The present study showed that DEX and ketorolac patient
controlled-analgesia can effectively inhibit the increase of VEGF
serum concentration induced by cervical cancer surgery.
The present study was not without its limitations.
First, since it was a single-center study with a strictly defined
population, the results may not apply to other institutions,
despite the high homogeneity of the two groups. Secondly, it may
not be accurate to estimate Th1/Th2 cell balance by detecting serum
cytokine concentrations, since not all IFN-γ and IL-4 expression in
the sera originates solely from Th1 and Th2 cells. Thirdly, the
present study was unable to determine the relationship between
changes in cytokine levels and clinical outcomes, such as
short-term effects on wound infection or length of hospital stay,
and long-term effects on cancer metastasis or recurrence. Finally,
it cannot be concluded whether the results were due to the
elimination of opioids or the use of COX inhibitors and DEX, and
their contribution to the results. Therefore, further studies with
larger sample sizes and multiple indicators are warranted to focus
on clinical outcomes in terms of perioperative morbidity and
long-term metastatic development in patients undergoing cancer
surgery.
Briefly, the present prospective, randomized study
demonstrated that the combination of DEX and ketorolac for PCA
significantly improved postoperative pain following laparoscopic
radical resection for cervical cancer, decreased the serum levels
of VEGF and enhanced immune function by shifting the Th1/Th2
balance to Th1. The anesthetics decreased the serum VEGF
concentration, which may be associated with angiogenesis in
cervical cancer. This finding may offer opportunities for
postoperative pain management to improve cancer patient
outcomes.
Acknowledgements
Not applicable.
Funding
This research was funded by The Tianjin Key Medical Discipline
(Specialty) Construction Project (grant no. TJYXZDXK-045A).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LA and JS drafted the manuscript. LA and WY
developed the study protocol and LA carried out patient
recruitment. JS collected individual data and JG performed
statistical analysis. WY and HD contributed to the study conception
and reviewed the manuscript. All authors read and approved the
final version of the manuscript. LA and WY confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Human Research of Tangshan Maternity and Child
Healthcare Hospital (approval no. 2019-031-01). The trial was also
registered prior to participant enrollment at www.chictr.org.cn (registration no. ChiCTR1900027979).
Written informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hiller JG, Perry NJ, Poulogiannis G,
Riedel B and Sloan EK: Perioperative events influence cancer
recurrence risk after surgery. Nat Rev Clin Oncol. 2018.15:205–218.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manders P, Beex LV, Tjan-Heijnen VC, Span
PN and Sweep CG: Vascular endothelial growth factor is associated
with the efficacy of endocrine therapy in patients with advanced
breast carcinoma. Cancer. 98:2125–2132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho JS, Lee MH, Kim SI, Park S, Park HS,
Oh E, Lee JH and Koo BN: The effects of perioperative anesthesia
and analgesia on immune function in patients undergoing breast
cancer resection: A prospective randomized study. Int J Med Sci.
14:970–976. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Snyder GL and Greenberg S: Effect of
anaesthetic technique and other perioperative factors on cancer
recurrence. Br J Anaesth. 105:106–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogawa K, Hirai M, Katsube T, Murayama M,
Hamaguchi K, Shimakawa T, Naritake Y, Hosokawa T and Kajiwara T:
Suppression of cellular immunity by surgical stress. Surgery.
127:329–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shafi S, Collinsworth AW, Copeland LA,
Ogola GO, Qiu T, Kouznetsova M, Liao IC, Mears N, Pham AT, Wan GJ
and Masica AL: Association of opioid-related adverse drug events
with clinical and cost outcomes among surgical patients in a large
integrated health care delivery system. JAMA Surg. 153:757–763.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Xu X, Liu H and Ji F: Effects of
dexmedetomidine on patients undergoing radical gastrectomy. J Surg
Res. 194:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li B, Li Y, Tian S, Wang H, Wu H, Zhang A
and Gao C: Anti-inflammatory effects of perioperative
dexmedetomidine administered as an adjunct to general anesthesia: A
meta-analysis. Sci Rep. 5:123422015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rainsford KD: Ibuprofen: Pharmacology,
efficacy and safety. Inflammopharmacology. 17:275–342. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cepeda MS, Carr DB, Miranda N, Diaz A,
Silva C and Morales O: Comparison of morphine, ketorolac, and their
combination for postoperative pain. Anesthesiology. 103:1225–1232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foekens JA, Peters HA, Grebenchtchikov N,
Look MP, Meijer-van Gelder ME, Geurts-Moespot A, van der Kwast TH,
Sweep CG and Klijn JG: High tumor levels of vascular endothelial
growth factor predict poor response to systemic therapy in advanced
breast cancer. Cancer Res. 61:5407–5414. 2001.PubMed/NCBI
|
|
13
|
Wang Z, Jiang L, Wang J, Chai Z and Xiong
W: Morphine promotes angiogenesis by activating PI3K/Akt/HIF-1α
pathway and upregulating VEGF in hepatocellular carcinoma. J
Gastrointest Oncol. 12:1761–1772. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singleton PA and Moss J: Effect of
perioperative opioids on cancer recurrence: A hypothesis. Future
Oncol. 6:1237–1242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singleton PA, Garcia JG and Moss J:
Synergistic effects of methylnaltrexone with 5-fluorouracil and
bevacizumab on inhibition of vascular endothelial growth
factor-induced angiogenesis. Mol Cancer Ther. 7:1669–1679. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martinez L, Ekman E and Nakhla N:
Perioperative opioid-sparing strategies: Utility of Conventional
NSAIDs in Adults. Clin Ther. 41:2612–2628. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adurthi S, Mukherjee G, Krishnamurthy H,
Sudhir K, Bafna UD, Umadevi K and Jayshree RS: Functional tumor
infiltrating TH1 and TH2 effectors in large early-stage cervical
cancer are suppressed by regulatory T cells. Int J Gynecol Cancer.
22:1130–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma J, Liu H and Wang X: Effect of ginseng
polysaccharides and dendritic cells on the balance of Th1/Th2 T
helper cells in patients with non-small cell lung cancer. J Tradit
Chin Med. 34:641–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guideline for Good Clinical Practice
E6(R1) 1996, . International Conference on Harmonization of
Technical Requirements for Registration of Pharmaceuticals for
Human Use. ICH Harmonized Tripartite Guideline. Accessed May 12,
2015. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf
|
|
20
|
American Society of Anesthesiologists, .
ASA physical status classification system. November
23–2018https://www.asahq.org/resources/AmericanSociety of
Anesthesiologists.
|
|
21
|
Green JS and Tsui BC: Impact of anesthesia
for cancer surgery: Continuing professional development. Can J
Anaesth. 60:1248–1269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wen Y, Wang M, Yang J, Wang Y, Sun H, Zhao
J, Liu W, Zhou Z, Deng H, Castillo-Pedraza C, et al: A comparison
of fentanyl and flurbiprofen axetil on serum VEGF-C, TNF-α, and
IL-1ß concentrations in women undergoing surgery for breast cancer.
Pain Practice. 15:530–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hudson LG, Cook LS, Grimes MM, Muller CY,
Adams SF and Wandinger-Ness A: Dual actions of ketorolac in
metastatic ovarian cancer. Cancers (Basel). 11:10492019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Butcher B, Hutchings E, Fazekas B, Clark
K, Rowett D and Currow D: Opioid-sparing effects of ketorolac in
palliative care patients receiving opioids for chronic
cancer-related pain: A systematic literature review. Palliat Med.
36:71–80. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Pan Y, Gou P, Zhou C, Ma L, Liu
Q, Du Y, Yang J and Wang Q: Effect of xanthohumol on Th1/Th2
balance in a breast cancer mouse model. Oncol Rep. 39:280–288.
2018.PubMed/NCBI
|
|
26
|
Hao CJ, Li J, Liu P, Li XL, Hu YQ, Sun JC
and Wei Y: Effects of the balance between type 1 and type 2 T
helper cells on ovarian cancer. Genet Mol Res. 15:2016. View Article : Google Scholar
|
|
27
|
Webster NR and Galley HF: Immunomodulation
in the critically ill. Br J Anaesth. 103:70–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao W, Lin JX and Leonard WJ: IL-2 family
cytokines: New insights into the complex roles of IL-2 as a broad
regulator of T helper cell differentiation. Curr Opin Immunol.
23:598–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurosawa S and Kato M: Anesthetics, immune
cells, and immune responses. J Anesth. 22:263–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cardinale F, Chinellato I, Caimmi S,
Peroni DG, Franceschini F, Miraglia Del Giudice M and Bernardini R:
Perioperative Period: Immunological Modifications. Int J
Immunopathol Pharmacol. 24 (3 Suppl):S3–S12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ueki M, Kawasaki T, Habe K, Hamada K,
Kawasaki C and Sata T: The effects of dexmedetomidine on
inflammatory mediators after cardiopulmonary bypass. Anaesthesia.
69:693–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Hu B, Zou Y, Bo L, Wang J, Li J
and Luo Y: Dexmedetomidine premedication attenuates concanavalin
A-induced hepatitis in mice. J Toxicol Sci. 39:755–764. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee JM, Han HJ, Choi WK, Yoo S, Baek S and
Lee J: Immunomodulatory effects of intraoperative dexmedetomidine
on T helper 1, T helper 2, T helper 17 and regulatory T cells
cytokine levels and their balance: A prospective, randomised,
double-blind, dose-response clinical study. BMC Anesthesiol.
18:1642018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Forget P, Vandenhende J, Berliere M,
MacHiels JP, Nussbaum B, Legrand C and De Kock M: Do intraoperative
analgesics influence breast cancer recurrence after mastectomy? A
retrospective analysis. Anesth Analg. 110:1630–1635. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo Y, Kenney SR, Muller CY, Adams S,
Rutledge T, Romero E, Murray-Krezan C, Prekeris R, Sklar LA, Hudson
LG and Wandinger-Ness A: R-Ketorolac Targets Cdc42 and Rac1 and
alters ovarian cancer cell behaviors critical for invasion and
metastasis. Mol Cancer Ther. 14:2215–2227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsuboi I, Tanaka H, Nakao M, Shichijo S
and Itoh K: Nonsteroidal anti-inflammatory drugs differentially
regulate cytokine production in human lymphocytes: Up-regulation of
TNF, IFN-gamma and IL-2, in contrast to down-regulation of IL-6
production. Cytokine. 7:372–379. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma XD, Li BP, Wang DL and Yang WS:
Postoperative benefits of dexmedetomidine combined with
flurbiprofen axetil after thyroid surgery. Exp Ther Med.
14:2148–2152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang EV, Kim SJ, Donovan EL, Chen M, Gross
AC, Webster Marketon JI, Barsky SH and Glaser R: Norepinephrine
upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor
cell lines: Implications for stress-related enhancement of tumor
progression. Brain Behav Immun. 23:267–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gadducci A, Tana R, Cosio S and Genazzani
AR: The serum assay of tumour markers in the prognostic evaluation,
treatment monitoring and follow-up of patients with cervical
cancer: A review of the literature. Crit Rev Oncol Hematol.
66:10–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mitsuhashi A, Suzuka K, Yamazawa K, Matsui
H, Seki K and Sekiya S: Serum vascular endothelial growth factor
(VEGF) and VEGF-C levels as tumor markers in patients with cervical
carcinoma. Cancer. 103:724–730. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Trappen PO, Steele D, Lowe DG, Baithun
S, Beasley N, Thiele W, Weich H, Krishnan J, Shepherd JH, Pepper
MS, et al: Expression of vascular endothelial growth factor
(VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different
stages of cervical carcinogenesis. J Pathol. 201:544–554. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kotowicz B, Fuksiewicz M, Jonska-Gmyrek J,
Bidzinski M and Kowalska M: The assessment of the prognostic value
of tumor markers and cytokines as SCCAg, CYFRA 21.1, IL-6, VEGF and
sTNF receptors in patients with squamous cell cervical cancer,
particularly with early stage of the disease. Tumour Biol.
37:1271–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moon HS, Kim SC, Ahn JJ and Woo BH:
Concentration of vascular endothelial growth factor (VEGF) and
transforming growth factor-beta1 (TGF-beta1) in the serum of
patients with cervical cancer: Prediction of response. Int J
Gynecol Cancer. 10:151–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mabeta P and Steenkamp V: The VEGF/VEGFR
axis revisited: Implications for cance therapy. Int J Mol Sci.
23:155852022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garcia J, Hurwitz HI, Sandler AB, Miles D,
Coleman RL, Deurloo R and Chinot OL: Bevacizumab
(Avastin®) in cancer treatment: A review of 15 years of
clinical experience and future outlook. Cancer Treat Rev.
86:1020172020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mirzaei A, Rashedi S, Akbari MR, Khatami F
and Aghamir SMK: Combined anticancer effects of simvastatin and
arsenic trioxide on prostate cancer cell lines via downregulation
of the VEGF and OPN isoforms genes. J Cell Mol Med. 26:2728–2740.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wei L, Sun C, Zhang Y, Han N and Sun S:
miR-503-5p inhibits colon cancer tumorigenesis, angiogenesis, and
lymphangiogenesis by directly downregulating VEGF-A. Gene Ther.
29:28–40. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ji C, Xiong Y, Pan X, Guo X, Li Z, Qian S,
Xu C, Yu DH and Liao WQ: Effect of non-steroidal anti-inflammatory
drugs on the increasing the incidence of colonic anastomosis in
rats. Int J Clin Exp Pathol. 8:6126–6134. 2015.PubMed/NCBI
|
|
49
|
Farooqui M, Li Y, Rogers T, Poonawala T,
Griffin RJ, Song CW and Gupta K: COX-2 inhibitor celecoxib prevents
chronic morphine-induced promotion of angiogenesis, tumour growth,
metastasis and mortality, without compromising analgesia. Br J
Cancer. 97:1523–1531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Davies G, Martin LA, Sacks N and Dowsett
M: Cyclooxygenase-2 (COX-2), aromatase and breast cancer:a possible
role for COX-2 inhibitors in breast cancer chemoprevention. Ann
Oncol. 13:669–678. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Su X, Fan Y, Yang L, Huang J, Qiao F, Fang
Y and Wang J: Dexmedetomidine expands monocytic myeloid-derived
suppressor cells and promotes tumour metastasis after lung cancer
surgery. J Transl Med. 16:3472018. View Article : Google Scholar : PubMed/NCBI
|