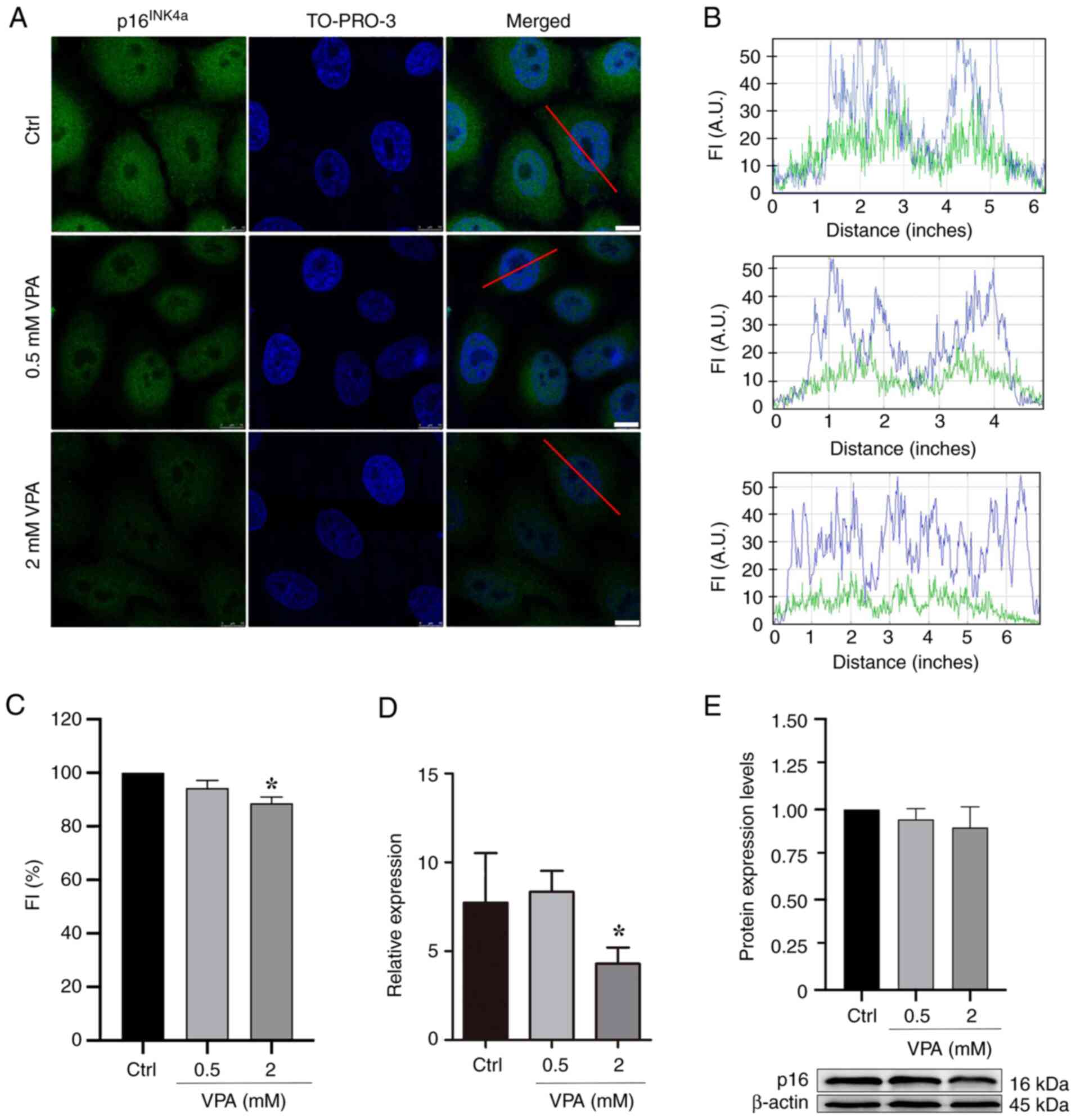
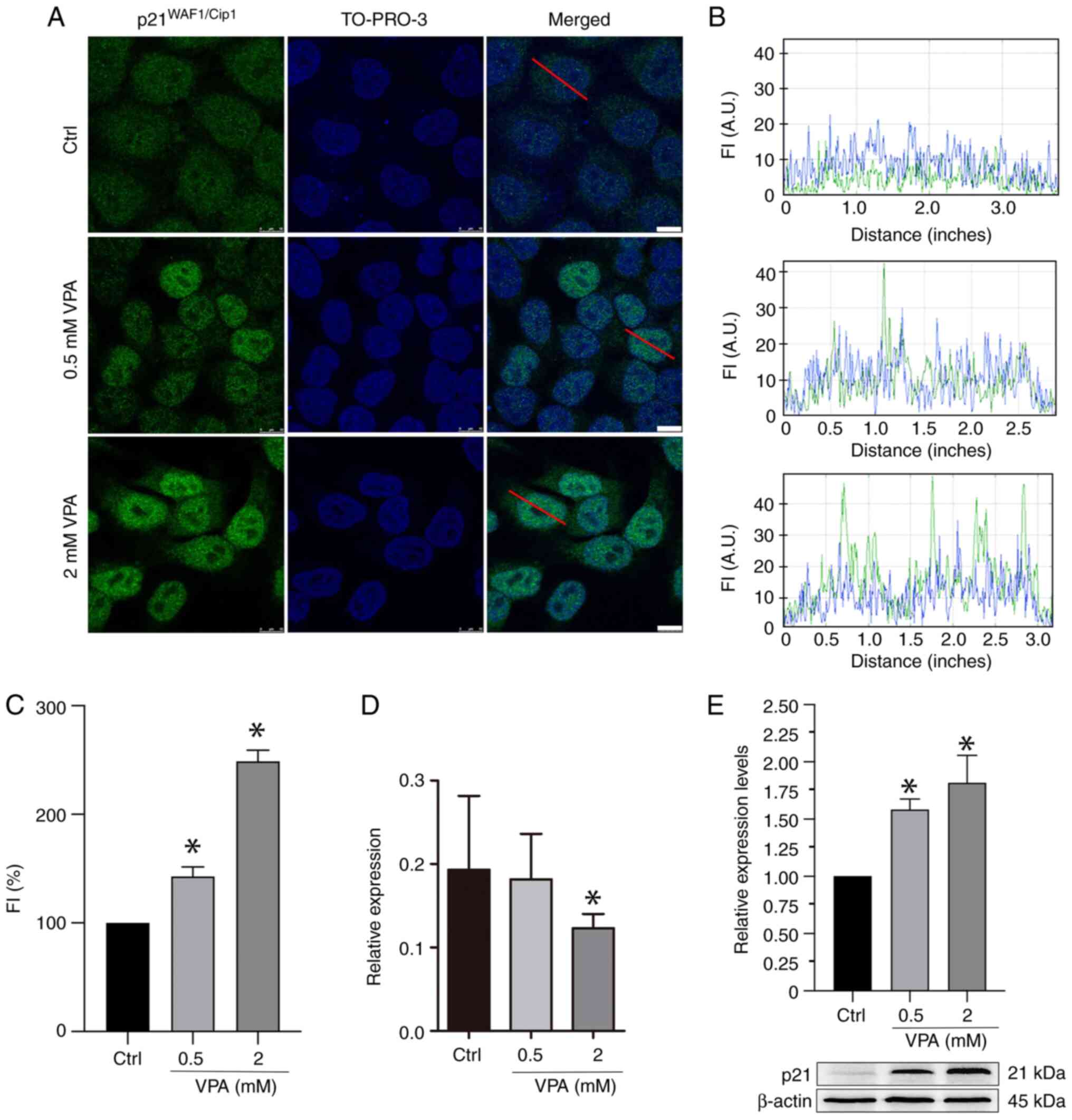
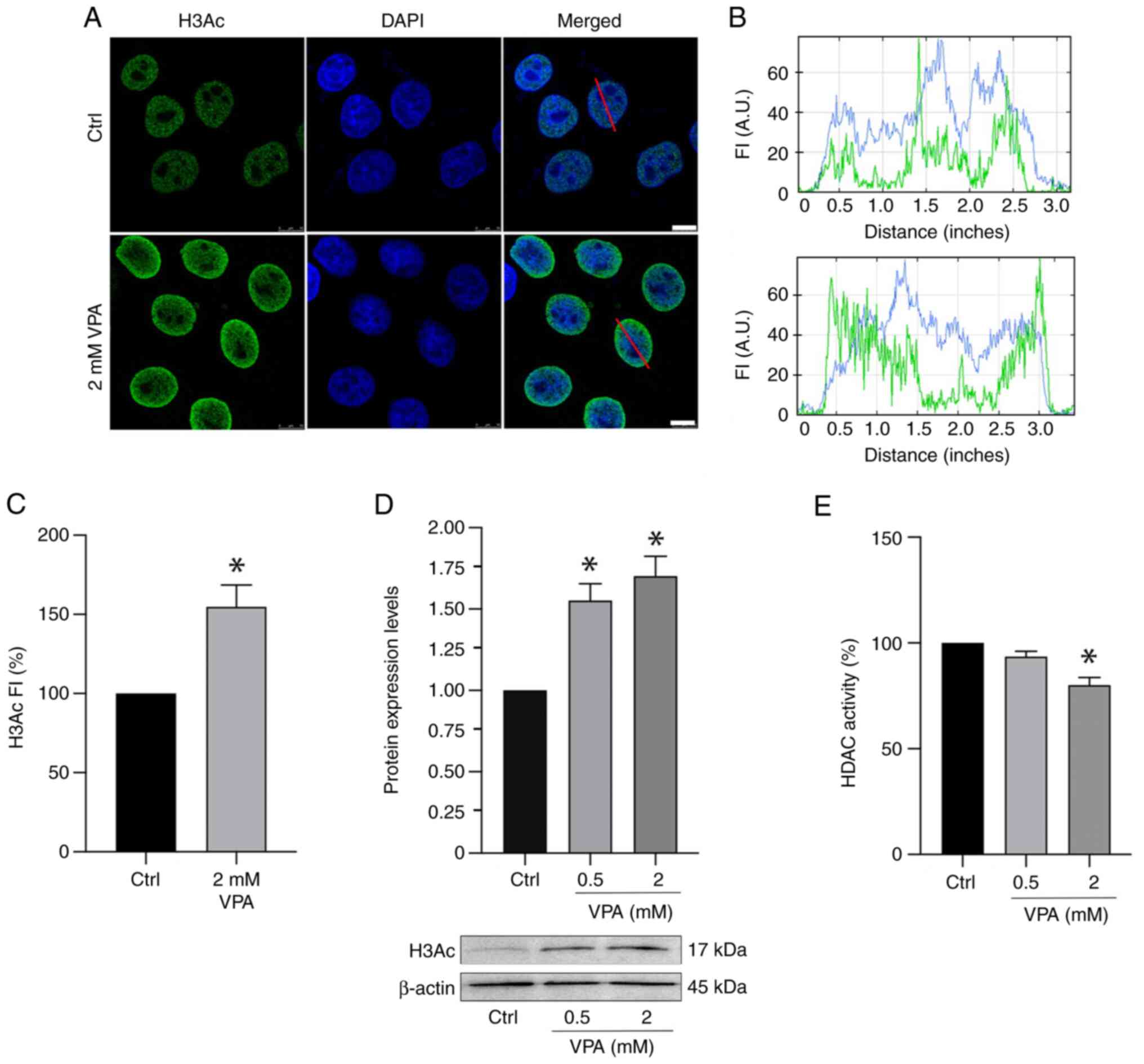
|
1
|
Chen YQ, Cipriano SC, Arenkiel JM and
Miller FR: Tumor suppression by p21WAF1. Cancer Res. 55:4536–4539.
1995.PubMed/NCBI
|
|
2
|
Yang ZY, Perkins ND, Ohno T, Nabel EG and
Nabel GJ: The p21 cyclin-dependent kinase inhibitor suppresses
tumorigenicity in vivo. Nat Med. 1:1052–1056. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim YT, Cho NH, Park SW and Kim JW:
Underexpression of cyclin-dependent kinase (CDK) inhibitors in
cervical carcinoma. Gynecol Oncol. 71:38–45. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YT and Zhao M: Aberrant cell cycle
regulation in cervical carcinoma. Yonsei Med J. 46:597–613. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huo W, Zhai S, Wang Y, Qiang X, Na R, Gui
H, Wu N, Cao Y and Bai H: Relevance research between the expression
of p16INK4a, Notch1, and hTERC genes: The development of
HPV16-positive cervical cancer. J Clin Lab Anal. 34:e232072020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Medema RH, Herrera RE, Lam F and Weinberg
RA: Growth suppression by p16ink4 requires functional
retinoblastoma protein. Proc Natl Acad Sci USA. 92:6289–6293. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Israels ED and Israels LG: The cell cycle.
Stem Cells. 19:88–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pei XH and Xiong Y: Biochemical and
cellular mechanisms of mammalian CDK inhibitors: A few unresolved
issues. Oncogene. 24:2787–2795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nehls K, Vinokurova S, Schmidt D, Kommoss
F, Reuschenbach M, Kisseljov F, Einenkel J, von Knebel Doeberitz M
and Wentzeusen N: p16 methylation does not affect protein
expression in cervical carcinogenesis. Eur J Cancer. 44:2496–2505.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin CK, Liu ST, Chang CC and Huang SM:
Regulatory mechanisms of fluvastatin and lovastatin for the p21
induction in human cervical cancer HeLa cells. PLoS One.
14:e02144082019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Yang J, Liu K, Yang J, Zhan X, Wang
L, Shen X, Chen J and Mao Z: p16 promotes proliferation in cervical
carcinoma cells through CDK6-HuR-IL1A axis. J Cancer. 11:1457–1467.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klaes R, Friedrich T, Spitkovsky D, Ridder
R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D and von Knebel
Doeberitz M: Overexpression of p16(INK4A) as a specific marker for
dysplastic and neoplastic epithelial cells of the cervix uteri. Int
J Cancer. 92:276–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van de Putte G, Holm R, Lie AK, Tropé CG
and Kristensen GB: Expression of p27, p21, and p16 protein in early
squamous cervical cancer and its relation to prognosis. Gynecol
Oncol. 89:140–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Volgareva G, Zavalishina L, Andreeva Y,
Frank G, Krutikova E, Golovina D, Bliev A, Spitkovsky D, Ermilova V
and Kisseljov F: Protein p16 as a marker of dysplastic and
neoplastic alterations in cervical epithelial cells. BMC Cancer.
4:582004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bahnassy AA, Zekri AR, Alam El-Din HM,
Aboubakr AA, Kamel K, El-Sabah MT and Mokhtar NM: The role of
cyclins and cyclins inhibitors in the multistep process of
HPV-associated cervical carcinoma. J Egypt Natl Cancer Inst.
18:292–302. 2006.PubMed/NCBI
|
|
17
|
Yoruker EE, Mert U, Bugra D, Yamaner S and
Dalay N: Promoter and histone methylation and p16(INK4A) gene
expression in colon cancer. Exp Ther Med. 4:865–870. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang CY, Bao W and Wang LH:
Downregulation of p16(ink4a) inhibits cell proliferation and
induces G1 cell cycle arrest in cervical cancer cells. Int J Mol
Med. 33:1577–1585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu H, Zhang J and Shi H: Expression of
cancer stem markers could be influenced by silencing of p16 gene in
HeLa cervical carcinoma cells. Eur J Gynaecol Oncol. 37:221–225.
2016.PubMed/NCBI
|
|
20
|
Merlo A, Herman JG, Mao L, Lee DJ,
Gabrielson E, Burger PC, Baylin SB and Sidransky D: 5′ CpG island
methylation is associated with transcriptional silencing of the
tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med.
1:686–692. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Z, Gao M, Zhang X, Kim YS, Lee ES, Kim
HK and Kim I: The hypermethylation and protein expression of p16
INK4A and DNA repair gene O6-methylguanine-DNA
methyltransferase in various uterine cervical lesions. J Cancer Res
Clin Oncol. 131:364–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beyer S, Zhu J, Mayr D, Kuhn C, Schulze S,
Hofmann S, Dannecker C, Jeschke U and Kost BP: Histone H3 acetyl K9
and histone H3 tri methyl K4 as prognostic markers for patients
with cervical cancer. Int J Mol Sci. 18:4772017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santos-Rosa H, Schneider R, Bannister AJ,
Sherriff J, Bernstein BE, Tolga Emre NC, Schreiber SL, Mellor J and
Kouzarides T: Active genes are tri-methylated at K4 of histone H3.
Nature. 419:407–411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai Y, Zhang Y, Loh YP, Tng JQ, Lim MC,
Cao Z, Raju A, Aiden EL, Li S, Manikandan L, et al: H3K27me3-rich
genomic regions can function as silencers to repress gene
expression via chromatin interactions. Nature Commun. 12:7192021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McLaughlin-Drubin ME, Crum CP and Münger
K: Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B
histone demethylase expression and causes epigenetic reprogramming.
Proc Natl Acad Sci USA. 108:2130–2135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McLaughlin-Drubin ME, Park D and Munger K:
Tumor suppressor p16INK4A is necessary for survival of cervical
carcinoma cell lines. Proc Natl Acad Sci USA. 110:16175–16180.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yokoyama Y, Takahashi Y, Morishita S,
Hashimoto M and Tamaya T: Introduction of p21(Waf1/Cip1) gene into
a carcinoma cell line of the uterine cervix with inactivated p53.
Cancer Lett. 116:233–239. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang JY and Lu YY: Effects of histone
acetylation and DNA methylation on p21(WAF1) regulation. World J
Gastroenterol. 8:400–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YX, Fang JY, Lu R and Qiu DK:
Expression of p21(WAF1) is related to acetylation of histone H3 in
total chromatin in human colorectal cancer. World J Gastroenterol.
13:2209–2213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karmodiya K, Krebs AR, Oulad-Abdelghani M,
Kimura H and Tora L: H3K9 and H3K14 acetylation co-occur at many
gene regulatory elements, while H3K14ac marks a subset of inactive
inducible promoters in mouse embryonic stem cells. BMC Genomics.
13:4242012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sami S, Höti N, Xu HM, Shen Z and Huang X:
Valproic acid inhibits the growth of cervical cancer both in vitro
and in vivo. J Biochem. 144:357–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsai C, Leslie JS, Franko-Tobin LG,
Prasnal MC, Yang T, Vienna Mackey L, Fuselier JA, Coy DH, Liu M, Yu
C and Sun L: Valproic acid suppresses cervical cancer tumor
progression possibly via activating Notch1 signaling and enhances
receptor-targeted cancer chemotherapeutic via activating
somatostatin receptor type II. Arch Gynecol Obstetr. 288:393–400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mawatari T, Ninomiya I, Inokuchi M, Harada
S, Hayashi H, Oyama K, Makino I, Nakagawara H, Miyashita T, Tajima
H, et al: Valproic acid inhibits proliferation of HER2-expressing
breast cancer cells by inducing cell cycle arrest and apoptosis
through Hsp70 acetylation. Int J Oncol. 47:2073–2081. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lipska K, Filip A and Gumieniczek A: The
impact of chlorambucil and valproic acid on cell viability,
apoptosis, and expression of p21, HDM2, BCL2 and MCL1
genes in chronic lymphocytic leukemia. Cells. 10:10882021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luna-Palencia GR, Correa-Basurto J,
Trujillo-Ferrara J, Meraz-Ríos MA and Vásquez-Moctezuma I:
Epigenetic evaluation of N-(2-hydroxyphenyl)-2-propylpentanamide, a
valproic acid aryl derivative with activity against HeLa cells.
Curr Mol Pharmacol. 14:570–578. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Richon VM, Sandhoff TW, Rifkind RA and
Marks PA: Histone deacetylase inhibitor selectively induces p21WAF1
expression and gene-associated histone acetylation. Proc Natl Acad
Sci USA. 97:10014–10019. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Minucci S and Pelicci PG: Histone
deacetylase inhibitors and the promise of epigenetic (and more)
treatments for cancer. Nat Rev Cancer. 6:38–51. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin YC, Lin JH, Chou CW, Chang YF, Yeh SH
and Chen CC: Statins increase p21 through inhibition of histone
deacetylase activity and release of promoter-associated HDAC1/2.
Cancer Res. 68:2375–2383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee S, Park JR, Seo MS, Roh KH, Park SB,
Hwang JW, Sun B, Seo K, Lee YS, Kang SK, et al: Histone deacetylase
inhibitors decrease proliferation potential and multilineage
differentiation capability of human mesenchymal stem cells. Cell
Prolif. 42:711–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aizawa S and Yamamuro Y: Valproate
administration to mice increases hippocampal p21 expression by
altering genomic DNA methylation. Neuroreport. 26:915–920. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo Q, Li X, Han H, Li C, Liu S, Gao W and
Sun G: Histone lysine methylation in TGF-β1 mediated p21 gene
expression in rat mesangial cells. Biomed Res Int.
2016:69272342016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Li C, Li X, Cui P, Li Q, Guo Q, Han
H, Liu S and Sun G: Involvement of histone lysine methylation in
p21 gene expression in rat kidney in vivo and rat mesangial cells
in vitro under diabetic conditions. J Diabetes Res.
2016:38532422016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Göttlicher M, Minucci S, Zhu P, Krämer OH,
Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG
and Heinzel T: Valproic acid defines a novel class of HDAC
inhibitors inducing differentiation of transformed cells. EMBO J.
20:6969–6978. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Phiel CJ, Zhang F, Huang EY, Guenther MG,
Lazar MA and Klein PS: Histone deacetylase is a direct target of
valproic acid, a potent anticonvulsant, mood stabilizer, and
teratogen. J Biol Chem. 276:36734–36741. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peterson GM and Naunton M: Valproate: A
simple chemical with so much to offer. J Clin Pharm Therap.
30:417–421. 2005. View Article : Google Scholar
|
|
46
|
Terbach N and Williams RSB:
Structure-function studies for the panacea, valproic acid. Biochem
Soc Trans. 37:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tomson T, Battino D and Perucca E:
Valproic acid after five decades of use in epilepsy: Time to
reconsider the indications of a time-honoured drug. Lancet Neurol.
15:210–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Makarević J, Rutz J, Juengel E, Maxeiner
S, Tsaur I, Chun FKH, Bereiter-Hahn J and Blaheta RA: Influence of
the HDAC inhibitor valproic acid on the growth and proliferation of
temsirolimus-resistant prostate cancer cells in vitro. Cancers
(Basel). 11:5662019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Romoli M, Mazzocchetti P, D'Alonzo R,
Siliquini S, Rinaldi VE, Verrotti A, Calabresi P and Costa C:
Valproic acid and epilepsy: From molecular mechanisms to clinical
evidences. Curr Neuropharmacol. 17:926–946. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Y, Zhang Y, Li M, Meng F, Yu Z, Chen
Y and Cui G: Combination of SB431542, CHIR99021 and PD0325901 has a
synergic effect on abrogating valproic acid-induced
epithelial-mesenchymal transition and stemness in HeLa, 5637 and
SCC-15 cells. Oncol Rep. 41:3545–3554. 2019.PubMed/NCBI
|
|
51
|
Han W, Yu F, Wang R, Guan W and Zhi F:
Valproic acid sensitizes glioma cells to luteolin through induction
of apoptosis and autophagy via Akt signaling. Cell Mol Neurobiol.
41:1625–1634. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Johannessen CU and Johannessen SI:
Valproate: Past, present, and future. CNS Drug Rev. 9:199–216.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chateauvieux S, Morceau F, Dicato M and
Diederich M: Molecular and therapeutic potential and toxicity of
valproic acid. J Biomed Biotechnol. 2010:4793642010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mello MLS: Sodium valproate-induced
chromatin remodeling. Front Cell Dev Biol. 9:6455182021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sargolzaei J, Rabbani-Chadegani A, Mollaei
H and Deezagi A: Spectroscopic analysis of the interaction of
valproic acid with histone H1 in solution and in chromatin
structure. Int J Biol Macromol. 99:427–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
de Campos Vidal B and Mello MLS: Sodium
valproate (VPA) interactions with DNA and histones. Int J Biol
Macromol. 163:219–231. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Baumann C, Zhang X, Zhu L, Fan Y and De La
Fuente R: Changes in chromatin accessibility landscape and histone
H3 core acetylation during valproic acid-induced differentiation of
embryonic stem cells. Epigenetics Chromatin. 14:582021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vidal BC and Mello MLS: Data on FTIR
spectra of mixtures of sodium valproate (VPA) and histones H1 and
H3. Latin Amer Data Sci. 1:102–109. 2022. View Article : Google Scholar
|
|
59
|
Gurvich N, Tsygankova OM, Meinkoth JL and
Klein PS: Histone deacetylase is a target of valproic acid-mediated
cellular differentiation. Cancer Res. 64:1079–1086. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dejligbjerg M, Grauslund M, Litman T,
Collins L, Qian X, Jeffers M, Lichenstein H, Jensen PB and Sehested
M: Differential effects of class I isoform histone deacetylase
depletion and enzymatic inhibition by belinostat or valproic acid
in HeLa cells. Mol Cancer. 7:702008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Felisbino MB, Tamashiro WMSC and Mello
MLS: Chromatin remodeling, cell proliferation and cell death in
valproic acid-treated HeLa cells. PLoS One. 6:e291442011.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Veronezi GMB, Felisbino MB, Gatti MSV,
Mello MLS and Vidal BC: DNA methylation changes in valproic
acid-treated HeLa cells as assessed by image analysis,
immunofluorescence and vibrational microspectroscopy. PLoS One.
12:e01707402017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rocha MA, Veronezi GMB, Felisbino MB,
Gatti MSV, Tamashiro WMSC and Mello MLS: Sodium valproate and
5-aza-2′-deoxycytidine differentially modulate DNA demethylation in
G1 phase-arrested and proliferative HeLa cells. Sci Rep.
9:182362019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rocha MA, Vidal BC and Mello MLS: Sodium
valproate modulates the methylation status of lysine residues 4, 9
and 27 in histone H3 of HeLa cells. Curr Mol Pharmacol. 16:197–210.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tringler B, Gup CJ, Singh M, Groshong S,
Shroyer AL, Heinz DE and Shroyer KR: Evaluation of p16INK4a and pRb
expression in cervical squamous and glandular neoplasia. Hum
Pathol. 35:689–696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rocha MA, Oliveira CBM and Mello MLS:
Sodium valproate cytotoxicity effects as assessed by the MTT assay.
Repositório de Dados de Pesquisa da Unicamp; version 2, . 2021
|
|
67
|
Han BR, You BR and Park WH: Valproic acid
inhibits the growth of HeLa cervical cancer cells via
caspase-dependent apoptosis. Oncol Rep. 30:2999–3005. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hashemi N, Zoshk MY, Rahidian A, Laripour
R, Fasihi H, Hami Z and Chamanara M: Anti-proliferative and
apoptotic effects of valproic acid on HeLa cells. Int J Cancer
Manag. 15:e1202242022. View Article : Google Scholar
|
|
69
|
Kondo Y, Shen L and Issa JPJ: Critical
role of histone methylation in tumor suppressor gene silencing in
colorectal cancer. Mol Cell Biol. 23:206–215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sanmukh SG, Dos Santos NJ, Barquilha CN,
Cucielo MS, de Carvalho M, Dos Reis PP, Delella FK, Carvalho HF and
Felisbino SL: Bacteriophages M13 and T4 increase the expression of
anchorage-dependent survival pathway genes and down regulate
androgen receptor expression in LNCaP prostate cell line. Viruses.
13:17542021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Muller PY, Janovjak H, Miserez AR and
Dobbie Z: Processing of gene expression data generated by
quantitative real-time RT-PCR. Biotechniques. 32:1372–1374.
13761378–1379. 2002.PubMed/NCBI
|
|
72
|
Simon P: Q-Gene: Processing quantitative
real-time RT-PCR data. Bioinformatics. 19:1439–1440. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Matheu A, Klatt P and Serrano M:
Regulation of the INK4a/ARF locus by histone deacetylase
inhibitors. J Biol Chem. 280:42433–42441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yewdell JW: Not such a dismal science: The
economics of protein synthesis, folding, degradation and antigen
processing. Trends Cell Biol. 11:294–297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sun Y, Chen J, Huang SYN, Su YP, Wang W,
Agama K, Saha S, Jenkins LM, Pascal JM and Pommier Y: PARylation
prevents the proteasomal degradation of topoisomerase I DNA-protein
crosslinks and induces their deubiquitylation. Nat Commun.
12:50102021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Block MF, Delley CL, Keller LML,
Stuehlinger TT and Weber-Ban E: Electrostatic interactions guide
substrate recognition of the prokaryotic ubiquitin-like protein
ligase PafA. Nat Commun. 14:52662023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kinger S, Jagtap YA, Dubey AR, Kumar P,
Choudhary A, Karmakar S, Lal G, Prajapti VK, Jha HC, Gutti RK and
Mishra A: Valproate mediated proteasome dysfunctions induce
apoptosis. Adv Therap. 23004212024. View Article : Google Scholar
|
|
79
|
Zupkovitz G, Grausenburger R, Brunmeir R,
Senese S, Tischler J, Jurkin J, Rembold M, Meunier D, Egger G,
Lagger S, et al: The cyclin-dependent kinase inhibitor p21 is a
crucial target for histone deacetylase 1 as a regulator of cellular
proliferation. Mol Cell Biol. 30:1171–1181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fan J, Lou B, Chen W, Zhang J, Lin S, Lv
FF and Chen Y: Down-regulation of HDAC5 inhibits growth of human
hepatocellular carcinoma by induction of apoptosis and cell cycle
arrest. Tumor Biol. 35:11523–11532. 2014. View Article : Google Scholar
|
|
81
|
Chun SM, Lee JY, Choi J, Lee JH, Hwang JJ,
Kim CS, Suh YA and Jang SJ: Epigenetic modulation with HDAC
inhibitor CG200745 induces anti-proliferation in non-small cell
lung cancer cells. PLoS One. 10:e01193792015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Han JW, Ahn SH, Park SH, Wang SY, Bae GU,
Seo DW, Kwon HK, Hong S, Lee HY, Lee YW and Lee HW: Apicidin, a
histone deacetylase inhibitor, inhibits proliferation of tumor
cells via induction of p21WAF1/Cip1 and gelsolin. Cancer Res.
60:6068–6074. 2000.PubMed/NCBI
|
|
83
|
Kim YB, Ki SW, Yoshida M and Horinouchi S:
Mechanism of cell cycle arrest caused by histone deacetylase
inhibitors in human carcinoma cells. J Antibiot (Tokyo).
53:1191–1200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Psilopatis I, Garmpis N, Garmpi A, Vrettou
K, Sarantis P, Koustas E, Antoniou EA, Dimitroulis D, Kourakis G,
Karamouzis MV, et al: The emerging role of histone deacetylases
inhibitors in cervical cancer therapy. Cancers (Basel).
15:22222023. View Article : Google Scholar : PubMed/NCBI
|