Introduction
Radical cystectomy and pelvic lymphadenectomy are
the standard treatments for muscle-invasive bladder cancer (BC)
(1), and the presence of lymph node
(LN) metastases is associated with poor survival outcomes (2). In the 8th American Joint Committee on
Cancer/International Union Against Cancer tumor (T) node (N)
metastasis (M) staging system, patients with positive LNs are
stratified into three stages (N1, N2 and N3) based on the number of
positive LNs and the site of metastatic LNs (3).
The number of positive LNs, LN density (defined as
the number of positive LNs divided by the total number of removed
LNs), maximum diameter of metastatic LNs and extranodal extension
(ENE) are independent prognostic factors in BC (4–9). A
previous multivariable model that included LN density, number of
removed LNs, number of positive LNs and ENE, demonstrated that ENE
is a strong independent prognostic factor (7).
The International Collaboration on Cancer Reporting
currently recommends that the extranodal spread in regional LN
status be included in the pathology reporting of the carcinoma of
the bladder/cystectomy (10).
Although ENE in metastatic LNs is associated with worse
cancer-specific survival (CSS), an ENE assessment is not included
in the current TNM staging system due to limited published data.
Therefore, LN evaluation methods that include ENE are required.
In a previous study, the prognostic impact of cancer
invasion levels in sentinel LNs was reported in melanoma (11). In this assessment, sentinel node
invasion level (SNIL) was defined as follows: SNIL 1, tumor cells
confined to lymphatic vessels and subcapsular sinus or transverse
sinuses; SNIL 2, tumor cells infiltrating the cortex or paracortex;
and SNIL 3, tumor cells infiltrating the medulla or capsule
(11). The SNIL status stratified
three independent risk groups of patients who were SN-positive.
This is a simple histology-based assessment of metastasis using the
anatomic localization of LNs without distance measurements with a
microscope (12). Nevertheless, the
prognostic impact of LN invasion levels in anatomically- and
immunologically-defined substructures has not been assessed in
BC.
In the present study, the clinical significance of
LN invasion levels in BC was assessed and the prognostic accuracy
of the generated model pathological (p)T stage and LN invasion
level] and the conventional model (pT and pN stages) in patients
who underwent radical cystectomy and pelvic lymphadenectomy was
compared.
Materials and methods
Case selection
After receiving ethics approval from Institutional
Review Board of Kansai Medical University Hospital (Osaka, Japan;
approval no. 2021226), data from 131 patients with BC (≥pT1) who
underwent radical cystectomy at Kansai Medical University Hospital
between January 2006 and December 2017 were extracted from the
institutional database. An opt-out approach was used to obtain
informed consent on the hospital website. A total of 33 patients
were excluded, including eight patients with nonurothelial
carcinoma (small cell carcinoma, n=2; urachal carcinoma, n=2; and
undifferentiated carcinoma, n=4), 14 patients with unremoved LNs,
and 11 patients treated with neoadjuvant chemotherapy (NAC). Thus,
98 patients who underwent radical cystectomy and pelvic
lymphadenectomy were selected for analysis. Patients with TNM
clinical stage III or higher in the present study underwent an
extended pelvic lymphadenectomy.
Histological evaluation
All surgical specimens were processed according to
standard pathology procedures. Hematoxylin and eosin
(H&E)-stained slides prepared for routine histological
examination were re-evaluated by a urologic pathologist (CO) using
the 2016 World Health Organization classification (13) and the 2017 Union for International
Cancer Control TNM staging system (3). Clinicopathological characteristics
such as pathological TNM stage, grade and histological subtypes of
urothelial carcinoma, including divergent differentiation/subtypes,
lymphovascular invasion and surgical margin, were reviewed.
Furthermore, the association between inflammatory status and lymph
node metastasis was assessed using histology-based tumor-associated
immune cell status (TAICs), a methodology previously reported by
our group (14).
The resected LNs of pelvic lymphadenectomy during
radical cystectomy were evaluated using H&E-stained slides.
Based on best practice guidelines for the routine pathology
evaluation of the immune system (15), pN was assessed based on the TNM
staging system (3): pN0, no cancer
cells in any LNs; pN1, single regional LN metastasis in the true
pelvis (perivesical, obturator, internal and external iliac, or
sacral LN); pN2, multiple regional LN metastases in the true
pelvis; and pN3, LN metastasis in the common iliac LNs.
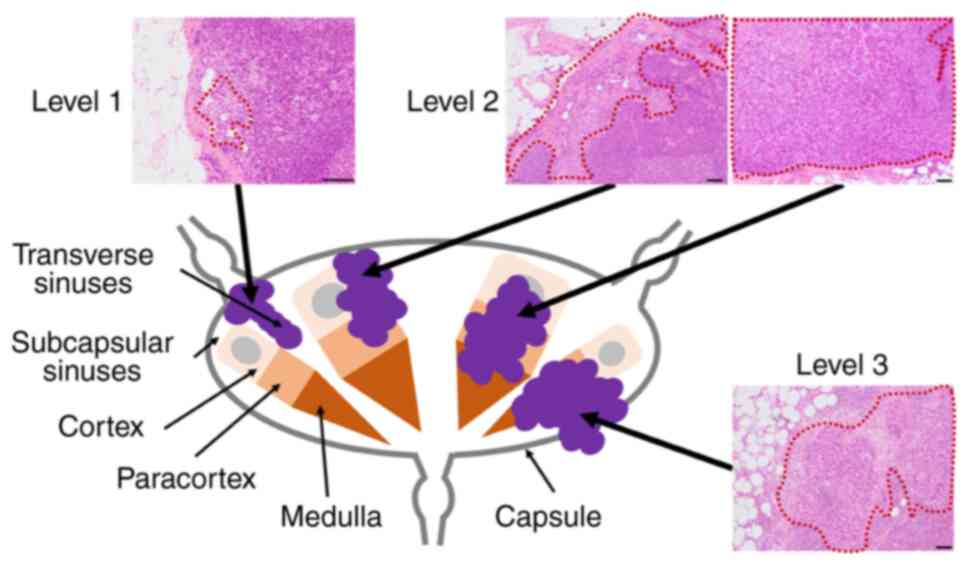
The LN invasion level in each case was evaluated in
the most highly invasive LNs with reference to the previous
methodology (11): Level 0, no
cancer cell within the resected LNs; Level 1, cancer cells confined
to intracapsular lymph vessels and subcapsular or transverse
sinuses; Level 2, cancer cells infiltrating the cortex, paracortex,
or medulla; and Level 3, cancer cells infiltrating or beyond the LN
capsule, corresponding to the ENE (Fig.
1). Histological evaluations of LNs were performed by two
independent pathologists (JI and CO) blinded to clinical outcomes,
and discordant patterns were resolved by consensus. The LN invasion
level was evaluated using the H&E-stained slides only.
Statistical analysis
Continuous data are presented as median and
interquartile range (IQR). Fisher's exact test and the Mann-Whitney
U test were used for comparisons between two groups.
Recurrence-free survival (RFS), CSS and overall survival (OS) were
assessed using the Kaplan-Meier method and the log-rank test.
Harrell's concordance index (c-index) was used to compare the
predictive accuracy of the Cox models. Multivariate Cox
proportional hazard models were assessed to determine hazard ratio
(HR). All statistical analyses were performed using EZR version
1.55 (Saitama Medical Center, Jichi Medical University) (16). P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient characteristics
As shown in Table I,
of the 98 patients, 16 (16.3%) were female and 82 (83.7%) were
male, with a median age of 71 (IQR, 67.0-77.0) years. Pathology
examination revealed the proportions of pT1, pT2, pT3 and pT4 as
9.2% (9/98), 34.7% (34/98), 42.9% (42/98), and 13.3% (13/98),
respectively. Divergent differentiation/subtypes on urothelial
histology were observed in 33 patients (33.7%). The number of
removed LNs was 22.5 (IQR, 14.3-29.5). A total of 26 (26.5%)
patients experienced recurrence and 22 (22.5%) patients died due to
BC. The median follow-up time was 99.0 (IQR, 72.2-129.0) months.
The number of metastatic lymph nodes at each lymph node invasion
level is presented in Table SI.
Furthermore, a total of 12 patients (12.24%) with intravesical
Bacillus Calmette-Guérin therapy were included.
 | Table I.Patient characteristics (n=98). |
Table I.
Patient characteristics (n=98).
| Variable | Value |
|---|
| Age, years | 71 (67.0–77.0) |
| Sex |
|
|
Female | 16 (16.3) |
| Male | 82 (83.7) |
| Tumor grade |
|
| Low | 0 (0.0) |
| High | 98 (100.0) |
| Divergent
differentiation/subtypea |
|
|
Absent | 65 (66.3) |
|
Present | 33 (33.7) |
| pT stage |
|
| 1 | 9 (9.2) |
| 2 | 34 (34.7) |
| 3 | 42 (42.9) |
| 4 | 13 (13.3) |
| pN stage |
|
| 0 | 69 (70.4) |
| 1 | 12 (12.2) |
| 2 | 15 (15.3) |
| 3 | 2 (2.0) |
| Surgical margin |
|
|
Negative/X | 91 (92.8) |
|
Positive | 7 (7.1) |
| Lymphovascular
invasion |
|
|
Absent | 22 (22.5) |
|
Present | 76 (77.6) |
| Number of removed
LNs | 22.5
(14.3–29.5) |
| Adjuvant
chemotherapy |
|
| No | 64 (65.3) |
|
Yes | 34 (34.7) |
| Recurrence | 26 (26.5) |
| Cancer-specific
mortality | 22 (22.5) |
| Overall
mortality | 40 (40.8) |
| Follow-up time,
months | 99
(72.2–129.0) |
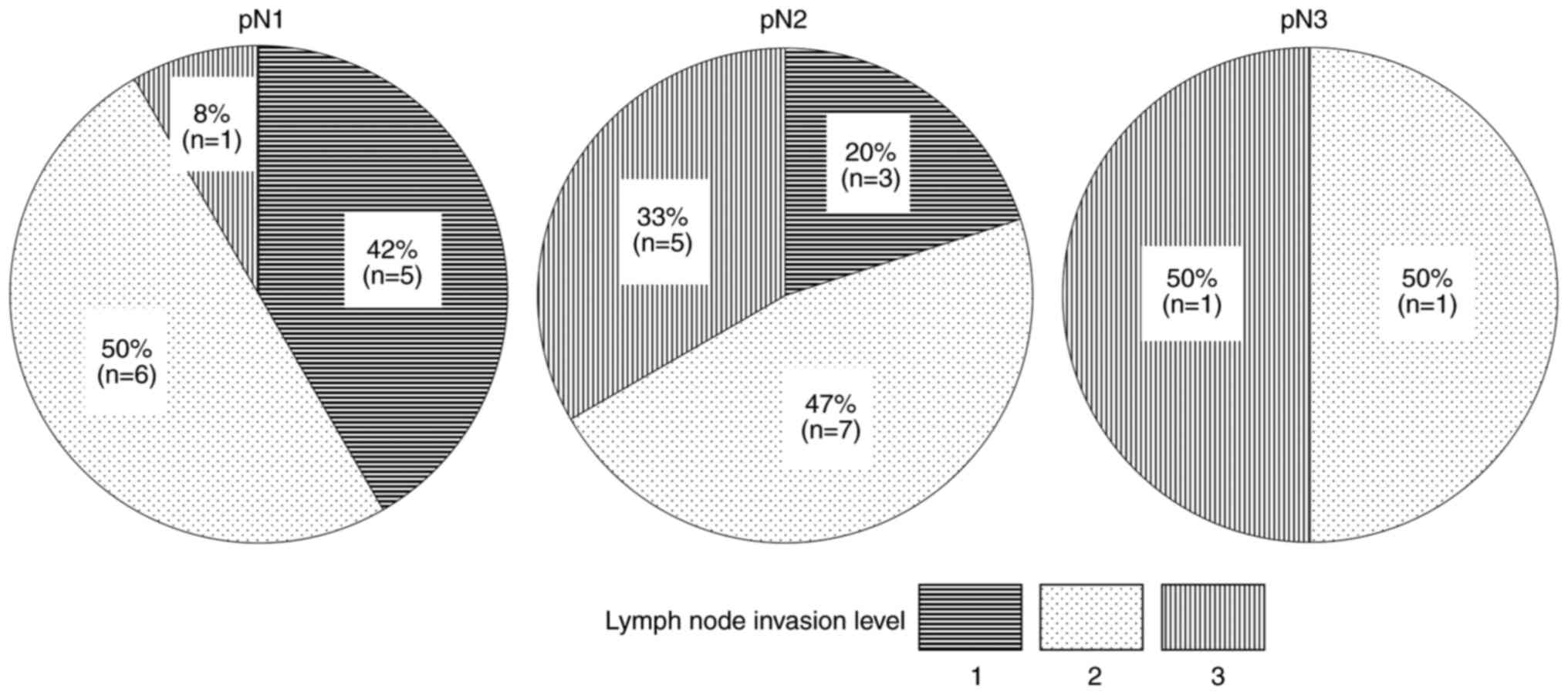
Association of LN invasion level with
pN stage, and TAICs with pN stage
The proportion of pN0, pN1, pN2 and pN3 was 70.4%
(69/98), 12.2% (12/98), 15.3% (15/98) and 2.0% (2/98), respectively
(Table I). Level 2 accounted for
the majority of cases in pN1 and pN2, and Levels 2 and 3 accounted
for 50% of the cases in pN3 (Fig.
2). TAICs was significantly associated with pN stages (P=0.03;
Table SII).
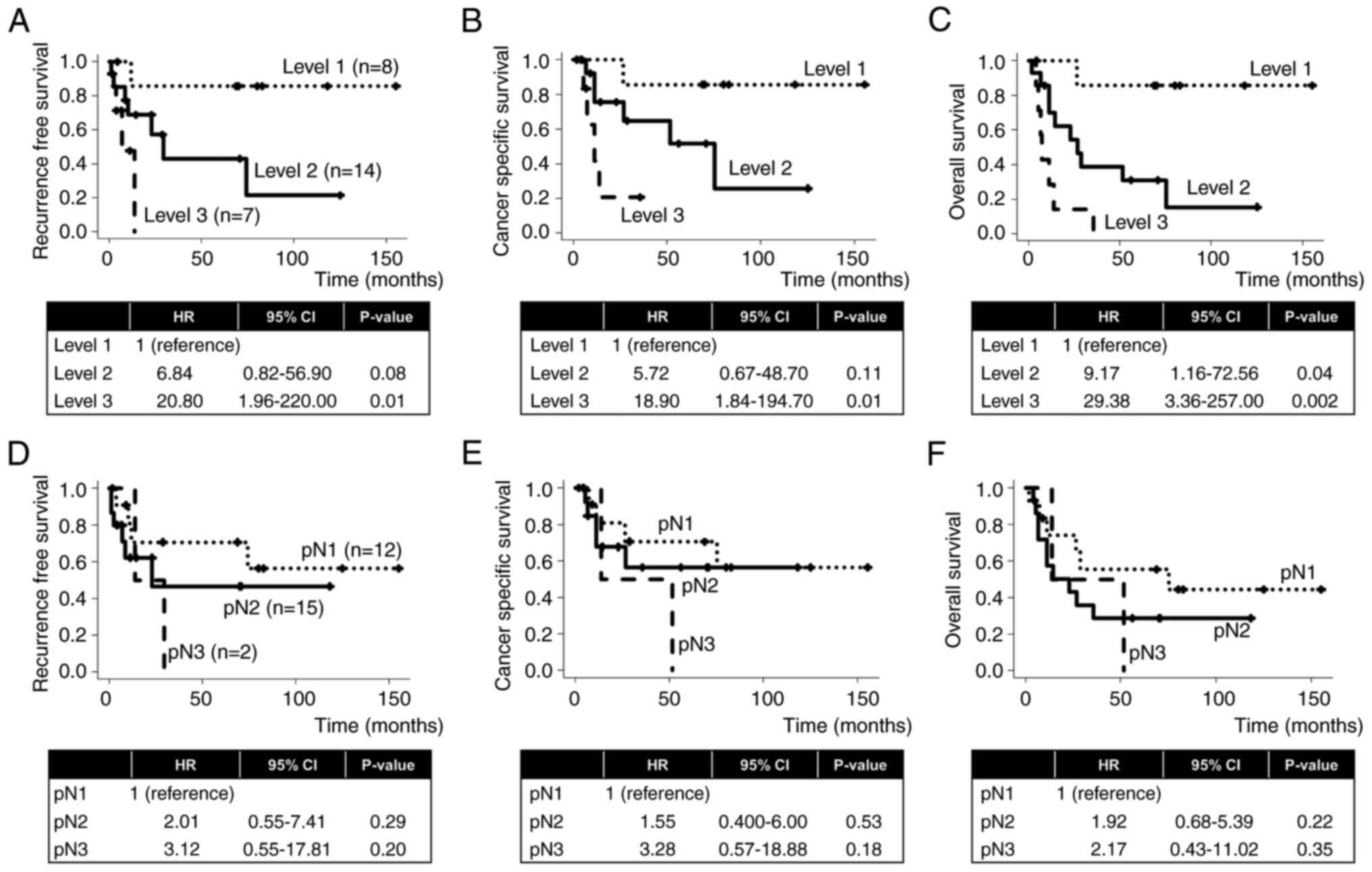
Comparison of prognostic significance
between LN invasion level and pN stage
The Kaplan-Meier survival curves of RFS, CSS and OS
in LN invasion Levels 1–3 and pN1-3 are presented in Fig. 3. In comparison with Level 1, LN
invasion Levels 2 and 3 were associated with a higher risk of RFS
(HR, 6.84; P=0.08 and HR, 20.80; P=0.01, respectively; Fig. 3A), CSS (HR, 5.72; P=0.11 and HR,
18.90; P=0.01, respectively; Fig.
3B) and OS (HR, 9.17; P=0.04 and HR, 29.38; P=0.002,
respectively; Fig. 3C). In
contrast, in comparison with pN1, pN2 and pN3 were associated with
a higher risk of RFS (HR, 2.01; P=0.29 and HR, 3.12; P=0.20,
respectively; Fig. 2D), CSS (HR,
1.55; P=0.53 and HR, 3.28; P=0.18, respectively; Fig. 3E) and OS (HR, 1.92; P=0.22 and HR,
2.17; P=0.35, respectively; Fig.
3F), but without statistical significance. Cases with low TAICs
were associated with a significantly worse RFS rate (HR, 5.13;
P<0.001; Fig. S1A), CSS rate
(HR, 3.64; P=0.008; Fig. S1B) and
OS rate (HR, 2.29; P=0.01; Fig.
S1C) than those with high TAICs. Furthermore, 20/29 of patients
with LN metastasis received adjuvant chemotherapy, which did not
significantly improve CSS or OS (HR, 0.70; P=0.60 and HR, 0.52;
P=0.20, respectively).
Prognostic significance of LN invasion
level
The associations of clinicopathological factors with
RFS, CSS and OS after radical cystectomy are presented in Table II, Table III, Table IV. In the univariate analysis, pT4,
pN3 and LN invasion level 3 were significantly associated with RFS
(P=0.026, P=0.009 and P<0.001, respectively), CSS (P=0.042,
P=0.004 and P<0.001, respectively) and OS (P=0.023, P=0.017 and
P<0.001, respectively). In multivariate analysis, two models
were evaluated: Model 1 (conventional model) with pT and pN stages;
and Model 2 (proposed model) with pT stage and LN invasion level.
In comparison with Model 1, Model 2 demonstrated a higher accuracy
in predicting RFS (c-index, 0.703 and 0.723, respectively), CSS
(c-index, 0.694 and 0.710, respectively) and OS (c-index, 0.692 and
0.725, respectively) than Model 1.
 | Table II.Cox regression analysis of prognostic
factors for predicting recurrence-free survival. |
Table II.
Cox regression analysis of prognostic
factors for predicting recurrence-free survival.
|
| Univariate | Model 1 | Model 2 |
|---|
|
|
|
|
|
|---|
| Variable | P-value | c-index | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 0.418 | 0.542 |
|
|
|
|
| Sex (female vs.
male) | 0.411 | 0.533 |
|
|
|
|
| pT stage |
| 0.687 |
|
|
|
|
| 1 | - |
| 1 (reference) |
| 1 (reference) |
|
| 2 | 0.800 |
| 1.16
(0.13–10.07) | 0.892 | 1.29
(0.15–11.16) | 0.815 |
| 3 | 0.270 |
| 2.11
(0.26–17.04) | 0.486 | 2.25
(0.28–18.11) | 0.446 |
| 4 | 0.026 |
| 7.53
(0.88–64.42) | 0.065 | 5.73
(0.66–50.00) | 0.115 |
| pN stage |
| 0.646 |
|
|
|
|
| 0 | - |
| 1 (reference) |
|
|
|
| 1 | 0.159 |
| 2.00
(0.64–6.26) | 0.235 |
|
|
| 2 | 0.004 |
| 2.34
(0.81–6.78) | 0.116 |
|
|
| 3 | 0.009 |
| 6.90
(1.39–34.18) | 0.018 |
|
|
| Lymph node invasion
level |
| 0.672 |
|
|
|
|
| 0 | - |
|
|
| 1 (reference) |
|
| 1 | 0.713 |
|
|
| 0.71
(0.09–5.47) | 0.740 |
| 2 | 0.001 |
|
|
| 2.90
(1.06–7.96) | 0.038 |
| 3 | <0.001 |
|
|
| 7.25
(1.94–27.15) | 0.003 |
 | Table III.Cox regression analysis of prognostic
factors for predicting cancer-specific survival. |
Table III.
Cox regression analysis of prognostic
factors for predicting cancer-specific survival.
|
| Univariate | Model 1 | Model 2 |
|---|
|
|
|
|
|
|---|
| Variable | P-value | c-index | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 0.100 | 0.600 |
|
|
|
|
| Sex (female vs.
male) | 0.145 | 0.565 |
|
|
|
|
| pT stage |
| 0.675 |
|
|
|
|
| 1 | - |
| 1 (reference) |
| 1 (reference) |
|
| 2 | 0.815 |
| 1.06
(0.12–9.24) | 0.958 | 1.23
(0.14–10.65) | 0.850 |
| 3 | 0.423 |
| 1.29
(0.15–11.08) | 0.816 | 1.38
(0.16–11.73) | 0.769 |
| 4 | 0.042 |
| 5.90
(0.66–52.68) | 0.112 | 4.08
(0.44–37.53) | 0.215 |
| pN stage |
| 0.665 |
|
|
|
|
| 0 | - |
| 1 (reference) |
|
|
|
| 1 | 0.070 |
| 3.01
(0.92–9.88) | 0.070 |
|
|
| 2 | 0.007 |
| 2.35
(0.72–7.66) | 0.157 |
|
|
| 3 | 0.004 |
| 10.81
(2.05–57.08) | 0.005 |
|
|
| Lymph node invasion
level |
| 0.688 |
|
|
|
|
| 0 | - |
|
|
| 1 (ref.) |
|
| 1 | 0.878 |
|
|
| 0.93
(0.12–7.35) | 0.946 |
| 2 | 0.002 |
|
|
| 3.37
(1.09–10.43) | 0.035 |
| 3 | <0.001 |
|
|
| 13.04
(3.31–51.37) | <0.001 |
 | Table IV.Cox regression analysis of prognostic
factors for predicting overall survival. |
Table IV.
Cox regression analysis of prognostic
factors for predicting overall survival.
|
| Univariate | Model 1 | Model 2 |
|---|
|
|
|
|
|
|---|
| Variable | P-value | c-index | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 0.019 | 0.610 |
|
|
|
|
| Sex (female vs.
male) | 0.110 | 0.542 |
|
|
|
|
| pT stage |
| 0.660 |
|
|
|
|
| 1 | - |
| 1 (reference) |
| 1 (reference) |
|
| 2 | 0.784 |
| 1.04
(0.23–4.79) | 0.962 | 1.21
(0.26–5.55) | 0.804 |
| 3 | 0.275 |
| 1.45
(0.32–6.55) | 0.632 | 1.34
(0.29–6.14) | 0.708 |
| 4 | 0.023 |
| 3.36
(0.66–17.00) | 0.143 | 2.50
(0.48–12.94) | 0.273 |
| pN stage |
| 0.661 |
|
|
|
|
| 0 | - |
| 1 (reference) |
|
|
|
| 1 | 0.071 |
| 2.20
(0.87–5.59) | 0.100 |
|
|
| 2 | <0.001 |
| 3.41
(1.45–8.05) | 0.005 |
|
|
| 3 | 0.017 |
| 5.60
(1.21–25.82) | 0.027 |
|
|
| Lymph node invasion
level |
|
|
|
|
|
|
| 0 | - | 0.702 |
|
| 1 (reference) |
|
| 1 | 0.450 |
|
|
| 0.47
(0.06–3.54) | 0.465 |
| 2 | <0.001 |
|
|
| 3.81
(1.60–9.06) | 0.003 |
| 3 | <0.001 |
|
|
| 14.81
(5.13–42.73) | <0.001 |
Discussion
The present study demonstrated that a histological
assessment of LN invasion levels in BC better stratified patient
outcome using Levels 0, 1, 2 and 3 in comparison with the pN stage
of the TNM staging system. In addition, the LN invasion level
better predicted RFS, CSS and OS compared with the pN stage, and
multivariate analysis revealed that the predictive accuracy of the
proposed model was greater than that of the conventional model.
Thus, LN invasion levels may provide a better prognostic prediction
for patients with BC after radical cystectomy and lymphadenectomy.
Furthermore, LN invasion level may be a useful criterion for
implementing adjuvant therapy.
Recently, inflammation and nodal state have
attracted attention in muscle invasive BC, and new biomarkers have
been identified (17). Although the
number of positive LNs and the site of metastatic LNs are the
standard methods of evaluation in the TNM staging system (3), several LN parameters, such as the
number of positive LNs, LN density, the maximum diameter of
metastatic LNs and ENE, are also prognostic indicators (4–9).
Consistent with previous studies (7), Level 3 corresponding to the ENE was
significantly associated with a worse prognosis compared with other
levels in RFS, CSS and OS in the present study. In addition to the
prognostic impact of ENE the present study demonstrated that LN
invasion levels could be used to classify patients into three
independent risk groups according to the degree of cancer
infiltration in metastatic LNs.
To the best of our knowledge, LN invasion levels
have not been previously assessed in BC. Although a similar
evaluation method was first proposed for metastatic sentinel nodes
in melanoma (11), the definition
of Levels 2 and 3 in the present study also integrated the ENE.
Notably, the present study demonstrated that LN invasion levels
were more significantly associated with prognosis compared with pN
stages. By using LN invasion levels, the present study evaluated
the metastatic infiltration in their anatomically and
immunologically defined substructures. Thus, the results of the
present study are consistent with the assessment that the natural
route of metastatic spread in LNs reflects a more accurate
prognosis compared with the current pN stage (11).
Anatomically, LNs are surrounded by capsules, and
multiple lymph lobules are demarcated by lymph-filled sinuses. The
superficial cortex is composed of follicles and the interfollicular
cortex of all adjacent lobules, and the paracortex is formed by
their deep cortical units. The medulla is composed of the
paracortex and its medullary cords and sinuses (18). In the first step of LN metastasis,
cancer cells enter the LNs through the subcapsular sinus; from
there, they invade the cortex to the medulla and break the LN
capsule (19). The leakage of tumor
cells through holes in the LN capsule into nearby soft tissues
results in the ENE (20–22). Due to the metastatic process in LNs,
it was reasonable to expect that a higher LN invasion level was
significantly associated with poor prognosis.
Univariate analysis revealed that there were no
significant differences between invasion levels 0 and 1 (Table II, Table III, Table IV); cancer cells confined to
intracapsular lymph vessels and subcapsular or transverse sinuses
corresponded to no parenchymal metastasis in LNs. Contrary to the
findings in the present study, the cause-specific survival rate,
which is defined as the mortality rate owing to bladder cancer in
patients with molecularly detected micrometastases, has been
reported to be markedly lower than in those without
micrometastases, independent of pathologically-positive LNs
(23). In contrast, it has been
reported that there is no notable difference in survival between
the pN micro+ and pN0 groups of patients with
immunohistochemically-detected micrometastases in LNs, nor between
the pN micro+ and pN+ groups (24,25).
The issue is controversial, and larger studies are needed to
evaluate the clinical significance of micrometastases and
parenchymal metastasis in LNs as the number of patients with Level
1 LN metastases remains too small for a definitive conclusion to be
reached.
The present study has several limitations; i) The
present study was a retrospective, single-center study with a small
sample size. Therefore, validation analysis across multiple
institutions is needed; ii) LN invasion levels were assessed in the
most highly invasive LNs, not the sentinel LNs, using
H&E-stained slides prepared for routine pathology examinations;
iii) lymphadenectomy was performed by different surgeons, although
a pelvic lymphadenectomy was performed and the mean number of
resected LNs was 22.5, which was comparable to the number of
dissected lymph nodes reported previously (26,27).
If the dissection range is determined in advance and the procedure
is performed reliably, there should be little difference between
surgeons; iv) no patients treated with NAC were included in the
present study. Lymph node status may change when NAC is
administered, and the pN+ status after NAC may have a negative
impact compared with a pN+ status without NAC (28). Therefore, the present study excluded
patients who received NAC to adjust LN status. Del Bene et
al (28) reported that NAC
reduces pN+ status and improves prognosis; therefore, further
investigation in to the association between LN invasion levels and
prognosis after NAC treatment is needed; and v) sentinel LNs were
not detected in Kansai Medical University Hospital. For a more
detailed assessment of sentinel LNs, indocyanine green fluorescence
should be considered (29). Despite
these limitations, the present study demonstrated the clinical
impact of a novel LN assessment in BC considering the natural route
of metastatic spread in LNs.
In conclusion, the present study demonstrated the
usefulness of the histological assessment of LN invasion levels,
considering their anatomical and immunological substructures. The
proposed model integrating pT and LN invasion levels accurately
predicted the prognosis of patients with BC undergoing radical
cystectomy and pelvic lymphadenectomy.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JI, CO and TY designed the present study. JI, CO, TY
and TN performed data collection and pathological assessments. JI
and CO confirm the authenticity of all the raw data. JI performed
the statistical analyses. JI, CO, TY, RS, KT and HK interpreted the
data. JI and CO drafted the manuscript. JI, CO, TY, TN, RS, KT and
HK critically revised the manuscript for important intellectual
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Kansai Medical University Hospital (Osaka, Japan;
approval no. 2021226). Kansai Medical University Hospital is one of
the affiliated medical facilities of Kansai Medical University.
Informed consent was obtained as an opt-out on the Kansai Medical
University Hospital website (https://hp.kmu.ac.jp/about/research/diagnostic_pathology/).
No patients expressed a refusal.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LN
|
lymph node
|
|
ENE
|
extranodal extension
|
|
BC
|
bladder cancer
|
References
|
1
|
Margulis V, Lotan Y, Montorsi F and
Shariat SF: Predicting survival after radical cystectomy for
bladder cancer. BJU Int. 102:15–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karl A, Carroll PR, Gschwend JE, Knüchel
R, Montorsi F, Stief CG and Studer UE: The impact of
lymphadenectomy and lymph node metastasis on the outcomes of
radical cystectomy for bladder cancer. Eur Urol. 55:826–835. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brierley JD: TNM classification of
malignant tumours. 8th edition. Union for International Cancer
Control; 2017
|
|
4
|
Stein JP, Cai J, Groshen S and Skinner DG:
Risk factors for patients with pelvic lymph node metastases
following radical cystectomy with en bloc pelvic lymphadenectomy:
Concept of lymph node density. J Urol. 170:35–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
May M, Herrmann E, Bolenz C, Tiemann A,
Brookman-May S, Fritsche HM, Burger M, Buchner A, Gratzke C,
Wülfing C, et al: Lymph node density affects cancer-specific
survival in patients with lymph node-positive urothelial bladder
cancer following radical cystectomy. Eur Urol. 59:712–718. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stephenson AJ, Gong MC, Campbell SC,
Fergany AF and Hansel DE: Aggregate lymph node metastasis diameter
and survival after radical cystectomy for invasive bladder cancer.
Urology. 75:382–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fajkovic H, Cha EK, Jeldres C, Robinson
BD, Rink M, Xylinas E, Chromecki TF, Breinl E, Svatek RS, Donner G,
et al: Extranodal extension is a powerful prognostic factor in
bladder cancer patients with lymph node metastasis. Eur Urol.
64:837–845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masson-Lecomte A, Vordos D, Hoznek A, Yiou
R, Allory Y, Abbou CC, de la Taille A and Salomon L: External
validation of extranodal extension and lymph node density as
predictors of survival in node-positive bladder cancer after
radical cystectomy. Ann Surg Oncol. 20:1389–1394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fleischmann A, Thalmann GN, Markwalder R
and Studer UE: Extracapsular extension of pelvic lymph node
metastases from urothelial carcinoma of the bladder is an
independent prognostic factor. J Clin Oncol. 23:2358–2365. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Compérat E, Srigley JR, Brimo F, Delahunt
B, Koch M, Lopez-Beltran A, Reuter V, Samaratunga H, Shanks JH,
Tsuzuki T, et al: Dataset for the reporting of carcinoma of the
bladder-cystectomy, cystoprostatectomy and diverticulectomy
specimens: Recommendations from the International Collaboration on
Cancer Reporting (ICCR). Virchows Arch. 476:521–534. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kretschmer L, Mitteldorf C, Hellriegel S,
Leha A, Fichtner A, Ströbel P, Schön MP and Bremmer F: The sentinel
node invasion level (SNIL) as a prognostic parameter in melanoma.
Mod Pathol. 34:1839–1849. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van der Ploeg AP, van Akkooi AC, Schmitz
PI, Koljenovic S, Verhoef C and Eggermont AM: EORTC Melanoma Group
sentinel node protocol identifies high rate of submicrometastases
according to Rotterdam Criteria. Eur J Cancer. 46:2414–2421. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moch H, Humphrey PA, Ulbright TM and
Reuter VE: WHO classification of tumours of the urinary system and
male genital organs. International Agency for Research on Cancer;
Lyon: 2016
|
|
14
|
Ikeda J, Ohe C, Yoshida T, Kuroda N, Saito
R, Kinoshita H, Tsuta K and Matsuda T: Comprehensive pathological
assessment of histological subtypes, molecular subtypes based on
immunohistochemistry, and tumor-associated immune cell status in
muscle-invasive bladder cancer. Pathol Int. 71:173–182. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haley P, Perry R, Ennulat D, Frame S,
Johnson C, Lapointe JM, Nyska A, Snyder P, Walker D and Walter G;
STP Immunotoxicology Working Group, : STP position paper: Best
practice guideline for the routine pathology evaluation of the
immune system. Toxicol Pathol. 33:404–407; discussion 408. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russo P, Palermo G, Iacovelli R, Ragonese
M, Ciccarese C, Maioriello G, Fantasia F, Bizzarri FP, Marino F,
Moosavi K, et al: Comparison of PIV and other immune inflammation
markers of oncological and survival outcomes in patients undergoing
radical cystectomy. Cancers (Basel). 16:6512024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Willard-Mack CL: Normal structure,
function, and histology of lymph nodes. Toxicol Pathol. 34:409–424.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nathanson SD: Insights into the mechanisms
of lymph node metastasis. Cancer. 98:413–423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Margaris KN and Black RA: Modelling the
lymphatic system: Challenges and opportunities. J R Soc Interface.
9:601–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bazigou E, Wilson JT and Moore JE Jr:
Primary and secondary lymphatic valve development: Molecular,
functional and mechanical insights. Microvasc Res. 96:38–45. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao YA, Chiang CJ, Lee WC, Zhuang BZ,
Chen CH and Pu YS: Extranodal extension predicts poor survival
outcomes among patients with bladder cancer. Cancers (Basel).
13:41082021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurahashi T, Hara I, Oka N, Kamidono S,
Eto H and Miyake H: Detection of micrometastases in pelvic lymph
nodes in patients undergoing radical cystectomy for locally
invasive bladder cancer by real-time reverse transcriptase-PCR for
cytokeratin 19 and uroplakin II. Clin Cancer Res. 11:3773–3777.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumoto R, Takada N, Abe T, Minami K,
Harabayashi T, Nagamori S, Hatanaka KC, Miyajima N, Tsuchiya K,
Maruyama S, et al: Prospective mapping of lymph node metastasis in
Japanese patients undergoing radical cystectomy for bladder cancer:
Characteristics of micrometastasis. Jpn J Clin Oncol. 45:874–880.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jensen JB, Høyer S and Jensen KM:
Incidence of occult lymph-node metastasis missed by standard
pathological examination in patients with bladder cancer undergoing
radical cystectomy. Scand J Urol Nephrol. 45:419–424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weingärtner K, Ramaswamy A, Bittinger A,
Gerharz EW, Vöge D and Riedmiller H: Anatomical basis for pelvic
lymphadenectomy in prostate cancer: Results of an autopsy study and
implications for the clinic. J Urol. 156:1969–1971. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu W and Zhang X: Laparoscopic and
robotic-assisted extended pelvic lymph node dissection for invasive
bladder cancer: A review. Bladder (San Franc).
10:e212000042023.PubMed/NCBI
|
|
28
|
Del Bene G, Calabrò F, Giannarelli D,
Plimack ER, Harshman LC, Yu EY, Crabb SJ, Pal SK, Alva AS, Powles
T, et al: Neoadjuvant vs. adjuvant chemotherapy in muscle invasive
bladder cancer (MIBC): Analysis from the RISC database. Front
Oncol. 8:4632018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loverro M, Bizzarri N, Capomacchia FM,
Watrowski R, Querleu D, Gioè A, Naldini A, Santullo F, Foschi N,
Fagotti A, et al: Indocyanine green fluorescence applied to
gynecologic oncology: Beyond sentinel lymph node. Int J Surg.
110:3641–3653. 2024.PubMed/NCBI
|