Introduction
Hypertension is a common risk factor for
cardiovascular diseases (1). A
previous multi-center prospective cohort study that included 17,712
patients from the US population with prostate cancer, respiratory
cancer, breast cancer, digestive system cancers, gynecological
cancers, urinary system cancers, and head and neck cancers reported
that 37% of the patients with cancer were diagnosed with
hypertension and needed to take both anti-cancer drugs and
antihypertensive drugs (2).
Previous studies have shown that the use of antihypertensive drugs
during cancer treatment is correlated to a certain extent with
prognosis and may affect the occurrence and development of tumors
(3,4). The renin-angiotensin-aldosterone
system (RAAS) serves a critical role in the maintenance of
cardiovascular homeostasis and the RAAS of local tissues may also
be involved in the development of tumors (5). Lung, thyroid, breast, stomach and
colorectal cancer have been reported to express components of the
RAAS, renin and angiotensin (Ang) II receptors (6,7).
According to the classical viewpoint, angiotensin II (Ang II) is
the main element of the RAAS generated by angiotensin-converting
enzyme (ACE), and its various effects, mainly mediated by the
angiotensin type 1 (AT1) receptor, include vasoconstriction,
detrimental remodeling, and oxidative stress in various tissues
(8). It has been reported that Ang
II can also serve as a growth factor, promoting tumor cell
proliferation through paracrine signal transduction, and can
facilitate angiogenesis by stimulating VEGF expression through
activation of the angiotensin I receptor (AT1) (9). Angiotensin-converting enzyme
inhibitors (ACEIs) and AT1 receptor antagonists (ARBs), which
inhibit the generation of angiotensin II, are classical inhibitors
of the RAAS (10). Previous
experimental and clinical investigations have reported the
potential impact of these medications on tumor development and
progression. For example, in a mouse model of colon cancer with
liver metastases, the co-administration of ACEI, captopril, and the
angiotensin receptor blocker (ARB) irbesartan, reduced the size of
metastatic foci (11). However, in
clinical studies, results regarding the relationship between the
use of ACEIs and cancer prognosis in randomized trials and
observational studies are contradictory. In a 2003 clinical trial
the impact of ARB (candesartan) on the morbidity and mortality of
patients with heart failure reported that the cancer incidence
increased in patients using candesartan compared with those treated
with placebo (12). By contrast, a
retrospective study of 287 patients conducted in 2009 reported that
the addition of an ACEI or an ARB to platinum-based chemotherapy
could extend the survival of patients with advanced lung cancer
(13). This indicates that the
application of RAAS inhibitors, such as ACEI/ARB drugs, may have
certain effects on the survival and prognosis of tumor
patients.
Therefore, the present meta-analysis analyzed cohort
and case-control studies that investigated the relationship of the
use of ACEIs and ARBs, and the prognosis of patients with cancer
and aimed to evaluate whether the use of ACEIs and ARBs alone or in
combination can influence the overall survival (OS) of patients
with cancer.
Materials and methods
Protocol registration and
guidance
The study protocol was registered in the PROSPERO
database (registration no. CRD42023487852; http://www.crd.york.ac.uk/prospero). The present study
was performed in accordance with the Cochrane handbook (14) and Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (15).
Literature retrieval
Literature published in English was retrieved from
the Embase (https://www.embase.com), PubMed
(https://pubmed.ncbi.nlm.nih.gov) and Web
of Science databases (https://access.clarivate.com) and studies that
examined the association between the use of ACEIs and ARBs and the
OS of patients with cancer were selected. The retrieval period was
from the establishment of the aforementioned databases to July 10
2023. Databases with the following search algorithm: (‘ACE
inhibitor’ or ‘angiotensin converting enzyme inhibiting agent’ or
‘angiotensin converting enzyme inhibitor’ or ‘angiotensin
converting enzyme inhibitors’ or ‘angiotensin I converting enzyme
inhibitor’ or ‘angiotensin-converting enzyme inhibitors’ or
‘converting enzyme inhibitor’ or ‘angiotensin II type 1 receptor
blockers’ or ‘angiotensin-converting enzyme inhibitors’ or
‘captopril’ or ‘cilazapril’ or ‘enalapril’ or ‘fosinopril’ or
‘imidapril’ or ‘lisinopril’ or ‘moexipril’ or ‘perindopril’ or
‘perindopril’ or ‘quinapril’ or ‘ramipril’ or ‘trandolapril’ or
‘eprosartan’ or ‘irbesartan’ or ‘irbesartan’ or ‘Olmesartan’ or
‘telmisartan’ or ‘valsartan’ or ‘candesartan’ or ‘ARB’ or
‘angiotensin receptor antagonists’) and (‘cancer’ or ‘cancers’ or
‘malignant neoplasia’ or ‘malignant neoplastic disease’ or
‘malignant tumor’ or ‘malignant tumour’ or ‘neoplasia’ or
‘malignan’ or ‘neoplasmic malignancy’ or ‘neoplastic malignancy’ or
‘oncologic malignancy’ or ‘oncological malignancy’ or ‘tumor’ or
‘malignant’ or ‘tumoral malignancy’ or ‘tumorous malignancy’ or
‘tumour’ or ‘malignant’ or ‘malignant neoplasm’).
A comprehensive literature search of published
studies was performed in January 2016 based on PubMed, Web of
Science, and the Chinese National Knowledge Infrastructure (CNKI)
databases with the following search algorithm: (‘hypertension’ or
‘blood pressure’ or ‘systolic pressure’ or ‘diastolic pressure’)
and (‘prostate cancer’ or ‘prostate neoplasm’) and (‘cohort’ or
‘case control’ or ‘case-control’). In addition, the lists of
references from retrieved articles and reviews were also checked to
identify any additional eligible studies. No limitations on
language or publication date were applied. This systematic review
and meta-analysis was designed, performed, and reported based on
the standards of quality for reporting meta-analyses.
Inclusion and exclusion criteria
The present meta-analysis included studies in which:
i) Participants were diagnosed with tumors by pathological
examination; ii) the relationship between ACEI and ARB use and the
OS of patients with cancer with hazard ratios (HR) and 95% CIs were
reported, or studies in which the HRs and CIs could be calculated
from the data provided in the studies; and iii) cohort and
case-control studies.
The following studies were excluded: i) Reviews,
conference minutes, case reports and systematic reviews; ii) The
use of non-standard scoring criteria for outcome indicators; iii)
studies in which the participants had distant metastasis or other
malignant tumors at the time of diagnosis; and iv) studies for
which the full text could not be accessed.
Literature screening and data
extraction
The literature was independently screened by two
investigators, who extracted the data and cross-checked the
results. In cases of disagreement, a third party was consulted to
arbitrate discrepancies and, when possible, the authors of the
included studies were contacted to supplement missing information.
Literature was screened through the removal of duplicate studies,
reading of the titles and abstracts to exclude publications
irrelevant to the research topic and evaluation of the full text to
determine whether each study should be included. The extracted data
included: Author(s), tumor type, country, year, sex, sample size,
follow-up duration, types of drugs used, study type, HRs and 95%
CIs. The quality of the screened literature was assessed using the
Newcastle-Ottawa scale (NOS) (16)
with a total score of 9 points, which included assessments on the
selection of study groups (4 points), comparability between groups
(2 points) and outcome measures (3 points). Studies with a score of
≥6 points were considered to be of high quality.
Data analysis
The STATA software (version 16.0; StataCorp LP) was
used to perform the meta-analysis. The HR and 95% CI of each
outcome measure were weighted and combined by calculating the log
HR and SElog HR, which used the general inverse variance method to
construct a forest plot. The heterogeneity of the studies were
assessed quantitatively based on I2 values. Due to the
potential heterogeneity in the intervention effects across
different populations and geographic locations, a random effects
model was used and subgroup analyses was performed. Egger's funnel
plots and linear regression tests were used to assess publication
bias. The robustness and reliability of the obtained results were
tested through sensitivity analysis. All statistical tests
performed were two-sided and P<0.05 was considered to indicate a
statistically significant difference.
Results
Literature retrieval
A total of 19,480 articles were obtained during the
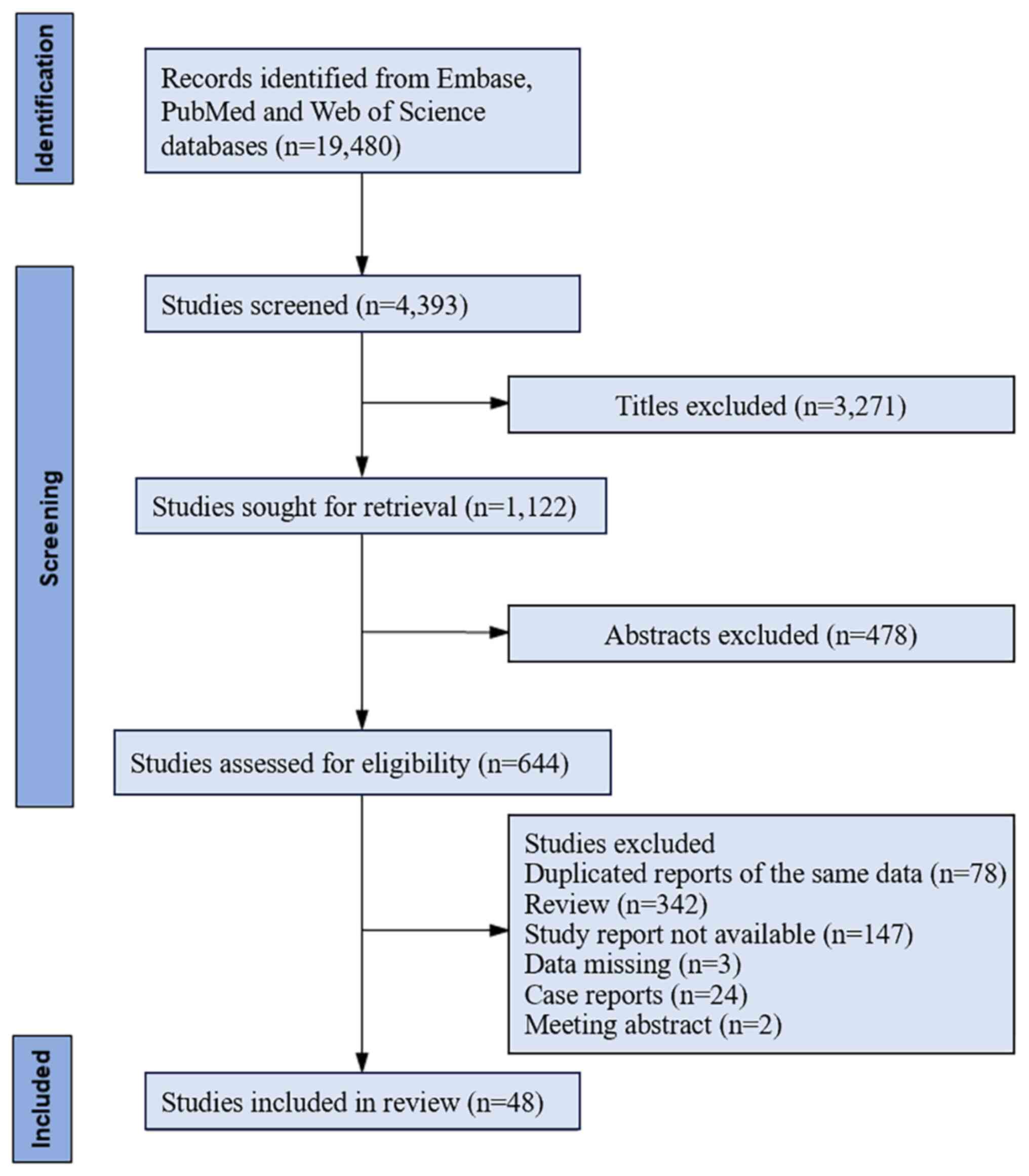
initial retrieval (Fig. 1).
Following the initial screening process, 4,393 articles were
identified for the present meta-analysis. Subsequently, 644
articles were selected after reading the titles and abstracts.
After reading the full texts, 48 articles that met the inclusion
and exclusion criteria were included in the present
meta-analysis.
Characteristics of the included
studies
The present meta-analysis included a total of 48
studies, which involved 923,134 participants (13,17–63).
All included studies reported the HRs and 95% CIs which were
calculated using the Cox regression model. The included studies
were published between 2011 and 2023. Among these, 12 studies were
conducted in the United States, 7 in Japan, 7 in Finland, 6 in
China, 2 in England, 2 in Canada, 2 in the Czech Republic, 3 in
Italy and 1 each in Denmark, Germany, South Korea, North Korea,
Norway, Oman and Poland (Table I).
Furthermore, there were 34 cohort studies and 14 case-control
studies among the included studies. Of the total included studies,
7 reported the use of ACEIs alone, 8 on the use of ARBs alone and
33 on the combined use of ACEIs and ARBs. Based on the NOS, all
included studies scored ≥6 points, which indicated the included
studies were of high quality.
 | Table I.Characteristics of the studies
included in the present meta-analysis. |
Table I.
Characteristics of the studies
included in the present meta-analysis.
| First author,
year | Type of cancer | Patient sex | Median age, years ±
SD (range) | Country of
residence | Study population,
n | Diagnosis
period | Follow-up
period | Treatment | Study design | Newcastle-Ottawa
quality score | (Refs.) |
|---|
| Anderson et
al, 2021 | Lung | Mix | 60.2±15.1 | United States | 187,060 | 1996-2018 | 7.1 years | ACEI/ARB | Cohort | ≥6 | (17) |
| Aydiner et
al, 2015 | Non-small cell
lung | Mix | 61±1 (42–75) | Japan | 117 | 2003-2011 | 18.9 months | ACEI/ARB | Case-control | ≥6 | (18) |
| Balkrishnan et
al, 2021 | Colorectal | Mix |
(365) | United States | 13,982 | 2007-2012 | 6.0 years | ACEI/ARB | Cohort | ≥6 | (19) |
| Botteri et
al, 2013 | Breast | Female | 62 (48–80) | Italy | 800 | 1997-2008 | 72 months | ARB | Case-control | ≥6 | (20) |
| Busby et al,
2017 |
Gastro-esophageal | Mix | - | England | 5,124 | 1998-2012 | 1.4 years | ARB | Cohort | ≥6 | (21) |
| Cardwell et
al, 2014 | Multi-cancer | Mix | - | England | 51,507 | 1998-2006 | 6.0 years | ACEI/ARB | Cohort | ≥6 | (22) |
| Chae et al,
2013 | Breast | Female | 58 | United States | 1,449 | 1995-2007 | 55 months | ACEI | Case-control | ≥6 | (23) |
| Cho et al,
2020 | Ovarian | Female | - | Korea | 878 | 2001-2014 | 120 months | ARB | Cohort | ≥6 | (24) |
| Cui et al,
2019 | Multi-cancer | Mix | (40–74) | China | 2,891 | 1996-2006 | 3.4 years | ACEI/ARB | Cohort | ≥6 | (25) |
| Engineer et
al, 2013 | Colorectal | Mix | 66.48
(318) | United States | 262 | 2000-2009 | 4,364 days | ACEI/ARB | Cohort | ≥6 | (26) |
| Eskelinen et
al, 2022 | Renal | Mix | - | Finland | 13,873 | 1995-2012 | 6.2 years | ACEI/ARB | Cohort | ≥6 | (27) |
| Fiala et al,
2019 | Colorectal | Mix | 62.3
(28.0–86.1) | Czech Republic | 514 | 2005-2019 | 22.3 months | ACEI | Cohort | ≥6 | (28) |
| Fiala et al,
2021 | Renal cell
carcinoma | Mix | 37.5–83.1 | Czech Republic | 343 | 2007-2020 | 96 months | ACEI/ARB | Cohort | ≥6 | (29) |
| Fryzek et
al, 2005 | Renal cell
carcinoma | Mix | 62 (30–85) | Denmark | 113,298 | 1989-2002 | 10.0 years | ACEI/ARB | Cohort | ≥6 | (30) |
| Ganz et al,
2011 | Breast | Female | - | United States | 1779 | 1997-2000 | 8.2 years | ACEI | Cohort | ≥6 | (31) |
| Harding et
al, 2019 | Ovarian | Female |
(366) | United States | 2,195 | 2007-2012 | 12 months | ACEI | Cohort | ≥6 | (32) |
| Holmes et
al, 2013 | Multi-cancer | Mix |
(365) | Canada | 15,582 | 2004-2008 | 6.0 years | ACEI/ARB | Cohort | ≥6 | (33) |
| Huang et al,
2021 | Ovarian | Female | - | United States | 743 | 1994-2017 | 1.0 years | ACEI | Cohort | ≥6 | (34) |
| Keith et al,
2022 | Pancreatic | Mix | 74.4
(66.3–81.5) | Italy | 8,158 | 2003-2011 | 6.2 months | ACEI/ARB | Cohort | ≥6 | (35) |
| Keizman et
al, 2011 | Renal cell
carcinoma | Mix | 66 (47–79) | United States | 127 | 2004-2010 | 60 months | ARB | Case-control | ≥6 | (36) |
| Kim et al,
2012 | Gastric | Mix | 67 (37–85) | South Korea | 63 | 2002-2010 | 60 months | ACEI/ARB | Case-control | ≥6 | (37) |
| Iede et al,
2022 | Pancreatic | Mix | 73 (42–80) | Japan | 56 | 2015-2020 | 50 months | ACEI/ARB | Case-control | ≥6 | (38) |
| Li et al,
2022 |
Gastro-esophageal | Mix |
(350) | China | 4,577 | 2008-2016 | 10.0 years | ACEI/ARB | Cohort | ≥6 | (39) |
| Lin et al,
2021 | Nasopharyngeal
carcinoma | Mix | - | China | 927 | 2008-2017 | 5.0 years | ARB | Cohort | ≥6 | (40) |
| Lorona et
al, 2021 | Breast | Female | (20–69) | United States | 4,557 | 2004-2015 | 5.0 years | ACEI | Cohort | ≥6 | (41) |
| Mafiana et
al, 2019 | Colorectal | Mix | - | Oman | 301 | 2006-2014 | 8.0 years | ACEI/ARB | Case-control | ≥6 | (42) |
| Ho et al,
2018 | Hepatocellular
carcinoma | Mix | - | China | 15,597 | 2005-2014 | 9.0 years | ACEI/ARB | Cohort | ≥6 | (43) |
| Morris et
al, 2016 | Rectal | Mix | - | United States | 216 | 1999-2012 | 4.1 years | ACEI/ARB | Case-control | ≥6 | (44) |
| Nakai et al,
2010 | Pancreatic | Mix | 71 (53–87) | Japan | 155 | 2001-2009 | 9.5 months | ACEI/ARB | Case-control | ≥6 | (45) |
| Nakai et al,
2015 | Pancreatic | Mix | 67 (39–89) | Japan | 349 | 2001-2014 | 9.6 months | ACEI/ARB | Cohort | ≥6 | (46) |
| Nayan et al,
2018 | Kidney | Mix | (≥65) | Canada | 9,124 | 1997-2013 | 16.0 years | ACEI/ARB | Cohort | ≥6 | (47) |
| Osumi et al,
2015 | Colorectal | Mix | 61.5 (38–75) | Japan | 181 | 2007-2010 | 70 months | ARB | Case-control | ≥6 | (48) |
| Ozawa et al,
2019 | Colorectal | Mix | - | Japan | 461 | 2009-2014 | 57 months | ACEI/ARB | Case-control | ≥6 | (49) |
| Santala et
al, 2019 | Urothelial | Mix | 75 (44–96) | Finland | 14,065 | 1995-2012 | 4.1 years | ACEI/ARB | Cohort | ≥6 | (50) |
| Santala et
al, 2020 | Breast | Female | 67 (27–102) | Finland | 73,170 | 1995-2013 | 20.0 years | ACEI/ARB | Cohort | ≥6 | (51) |
| Santala et
al, 2019 | Prostate | Male | 63 (40–93) | Finland | 14,422 | 1995-2013 | 9.9 years | ACEI/ARB | Cohort | ≥6 | (52) |
| Siltari et
al, 2020 | Prostate | Male | 68 (64–72) | Finland | 8,253 | 1996-2016 | 7.6 years | ACEI/ARB | Cohort | ≥6 | (53) |
| Santala et
al, 2021 | Ovarian | Female | 70 (18–101) | Finland | 12,122 | 1995-2013 | 19.0 years | ACEI/ARB | Cohort | ≥6 | (54) |
| Siltari et
al, 2018 | Prostate | Male | 59 (55–63) | Finland | 78,615 | 1996-2015 | 20.0 years | ACEI/ARB | Cohort | ≥6 | (55) |
| Støer et al,
2021 | Pancreatic
adenocarcinoma | Mix | 67 (60–74) | Norway | 2,614 | 2007-2015 | 7.0 years | ARB | Cohort | ≥6 | (56) |
| Stokes et
al, 2021 | Head and neck | Mix |
(366) | United States | 5,000 | 2008-2015 | 24 months | ACEI/ARB | Cohort | ≥6 | (57) |
| Tamburrino et
al, 2021 | Pancreatic
adenocarcinoma | Mix | - | Italy | 430 | 2015-2019 | 3.0 years | ACEI/ARB | Cohort | ≥6 | (13) |
| Wilk et al,
2021 | Prostate | Male | 69 (43–88) | Poland | 93 | 2014-2018 | 9.8 months | ACEI/ARB | Case-control | ≥6 | (58) |
| Wilop et al,
2009 | Non-small cell
lung | Mix | 62 (31–83) | Germany | 287 | 1996-2007 | 8.1 months | ACEI/ARB | Cohort | ≥6 | (59) |
| Wu et al,
2021 | Oral squamous cell
carcinoma | Mix | 58 (51.7–66) | China | 714 | 2007-2018 | 11.0 years | ARB | Case-control | ≥6 | (60) |
| Yoshida et
al, 2017 | Bladder | Mix | 70 (39–91) | Japan | 269 | 1995-2014 | 44.5 months | ACEI/ARB | Case-control | ≥6 | (61) |
| Zhang et al,
2022 | Colorectal | Mix | - | United States | 2,343 | 1976-2014 | 28.0 years | ACEI | Cohort | ≥6 | (62) |
| Yang et al,
2022 | Multi-cancer | Mix | - | China | 253,491 | 2002-2019 | 6.5 years | ACEI/ARB | Cohort | ≥6 | (63) |
Impact of ACEI and ARB use on the OS
of patients with cancer
The use of ACEIs or ARBs alone and in combination on
the OS of patients with cancer over the past decade was analyzed in
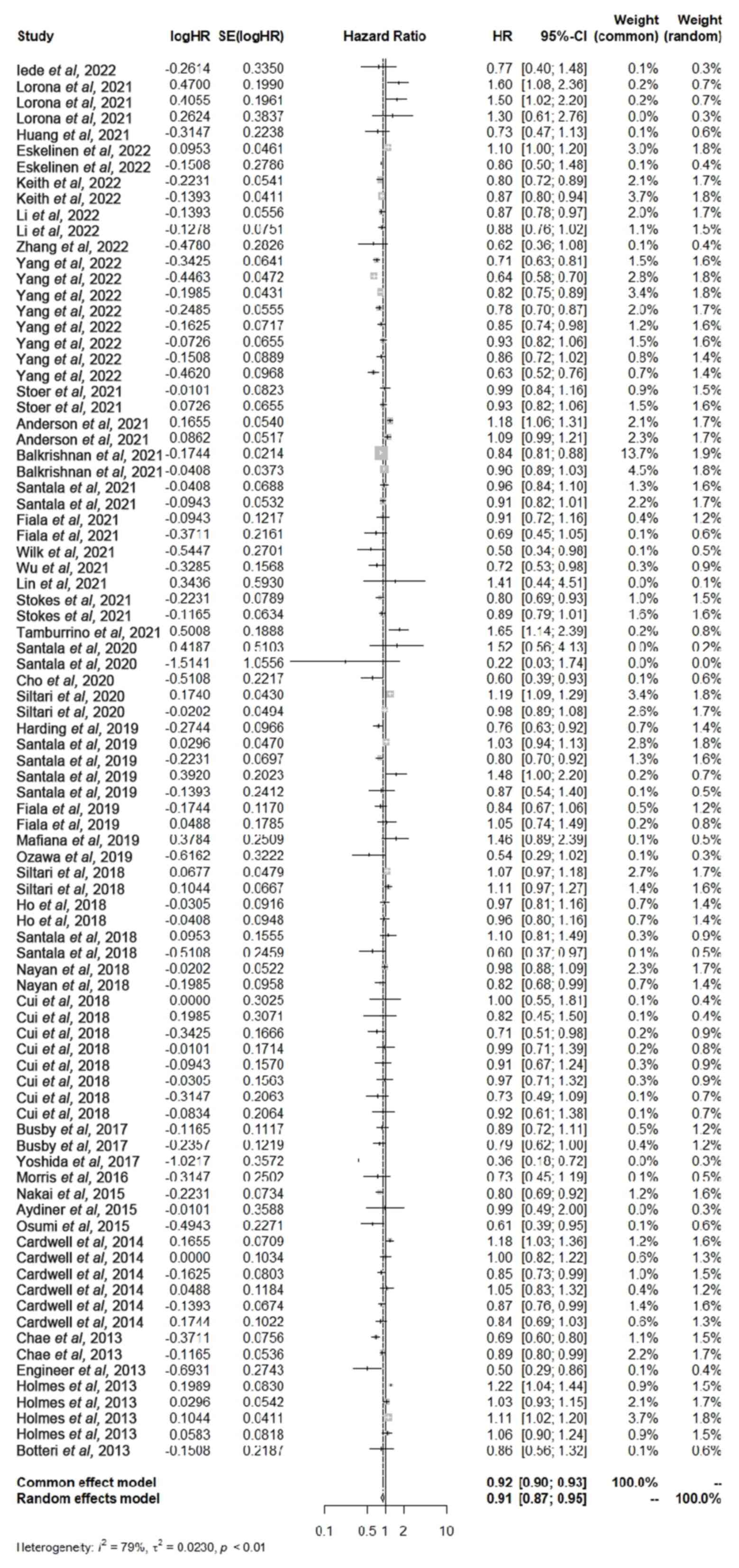
the 48 included studies (Fig. 2).
Meta-analysis indicated that patients who used ACEIs or ARBs,
either alone or in combination, had a significantly increased OS
compared with that of patients with cancer who did not use ACEI or
ARB drugs (HR, 0.91; 95% CI; 0.87–0.95; P<0.01).
Impact of ACEI and ARB use on the OS
of patients in specific types of cancer
A subgroup analysis was performed on the data from
the included studies according to the site of tumor (Fig. 3). Among patients with ovarian (HR,
0.85; 95% CI, 0.74–0.971; P<0.01), pancreatic (HR, 0.86; 95% CI,
0.73–1.01; P<0.01), prostate (HR, 0.98; 95% CI, 0.89–1.07;
P<0.01), hepatocellular carcinoma (HR, 0.86; 95% CI, 0.70–1.06;
P<0.01), lung (HR, 0.93; 95% CI, 0.77–1.12; P<0.01),
esophageal (HR, 0.88; 95% CI, 0.78–1.00; P<0.01), gastric (HR,
0.84; 95% CI, 0.77–0.92; P<0.01), colonic (HR, 0.87; 95% CI,
0.81–0.93; P<0.01), nasopharyngeal (HR, 0.79, 95% CI, 0.50–1.24;
P<0.01), head and neck (HR, 0.85; 95% CI, 0.77–0.94; P<0.01),
gallbladder (HR, 0.74; 95% CI, 0.44–1.23; P<0.01) and rectal
(HR, 0.91; 95% CI, 0.87–0.95; P<0.01) cancers, patients who used
ACEIs or ARBs, either alone or in combination, had a significantly
increased OS compared with that of patients who did not use the
aforementioned drugs. By contrast, the use of ACEIs or ARBs, either
alone or in combination, did not show no significant benefit in the
OS of patients with renal cancer (HR, 1.00; 95% CI, 0.83–1.20;
P<0.01) and significantly decreased the OS of patients with
breast cancer (HR, 1.04; 95%CI, 0.90–1.19; P<0.01).
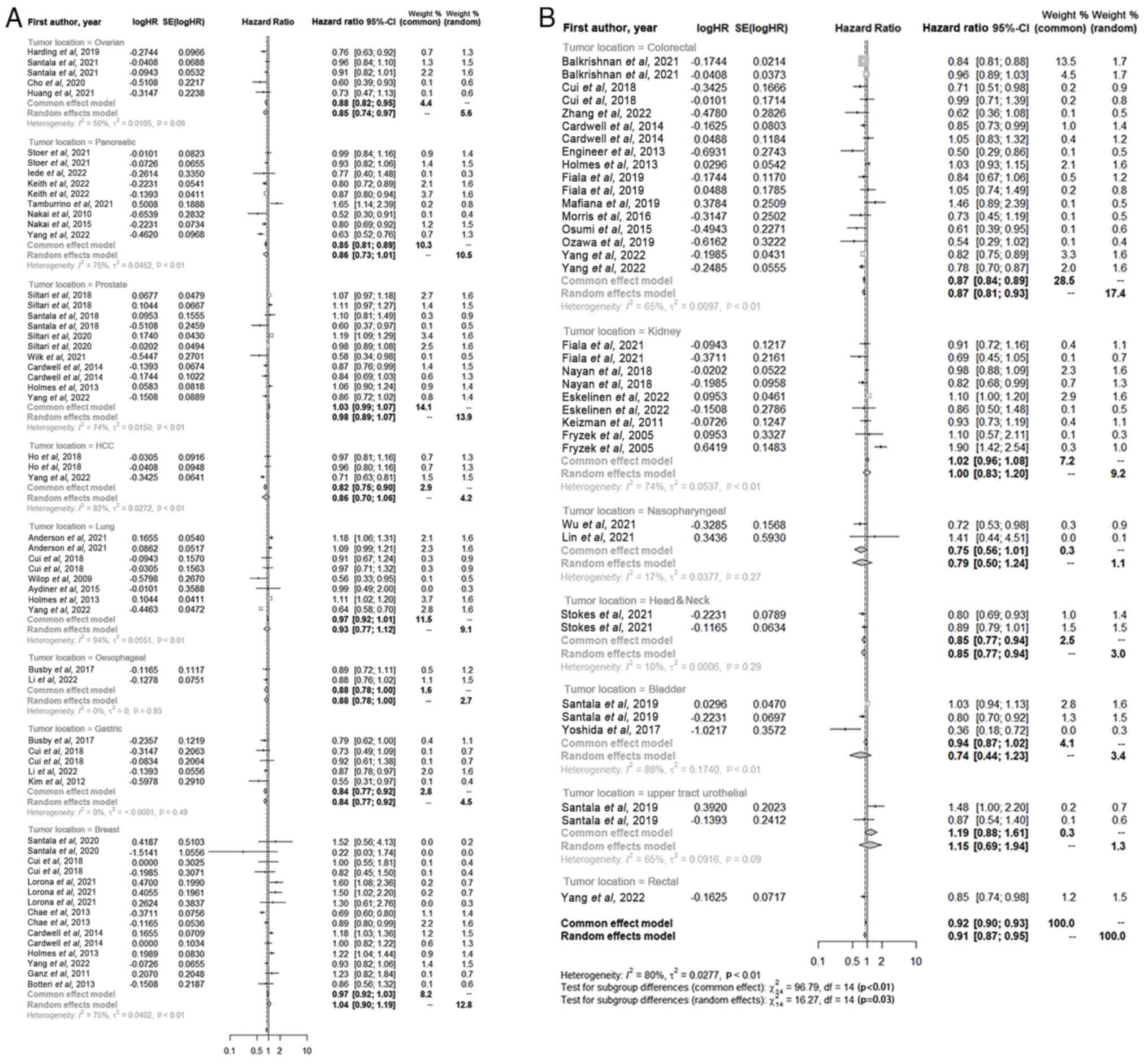 | Figure 3.Forest plot of the impact of the use
of angiotensin-converting enzyme inhibitors/angiotensin receptor
blockers on the overall survival of patients with different tumors.
(A) Outlines the impact of the use of ACEI/ARB drugs on the overall
survival of patients with ovarian, pancreatic, prostate, HCC, lung,
oesophageal, gastric and breast cancers. (B) Outlines the impact of
the use of ACEI/ARB drugs on the overall survival of patients with
colorectal cancer, kidney cancer, nasopharyngeal, head&neck,
bladder, upper tract urothelial and rectal cancers. Within this
graphical representation, each block corresponds to an individual
study, with the size of the block reflecting its relative weight in
the analysis. The horizontal line through each block represents the
95% CI for the observed effect. At the bottom of the plot, the
diamond represents the pooled effect calculated across all included
studies, with the width of the diamond indicating the 95% CI. HR,
hazard ratio; CI, confidence interval; SE, standard error. |
Impact of ACEI and ARB use alone or in
combination on the OS of patients with cancer
Subgroup analysis was performed on the included
studies according to the use of the drugs, either alone or in
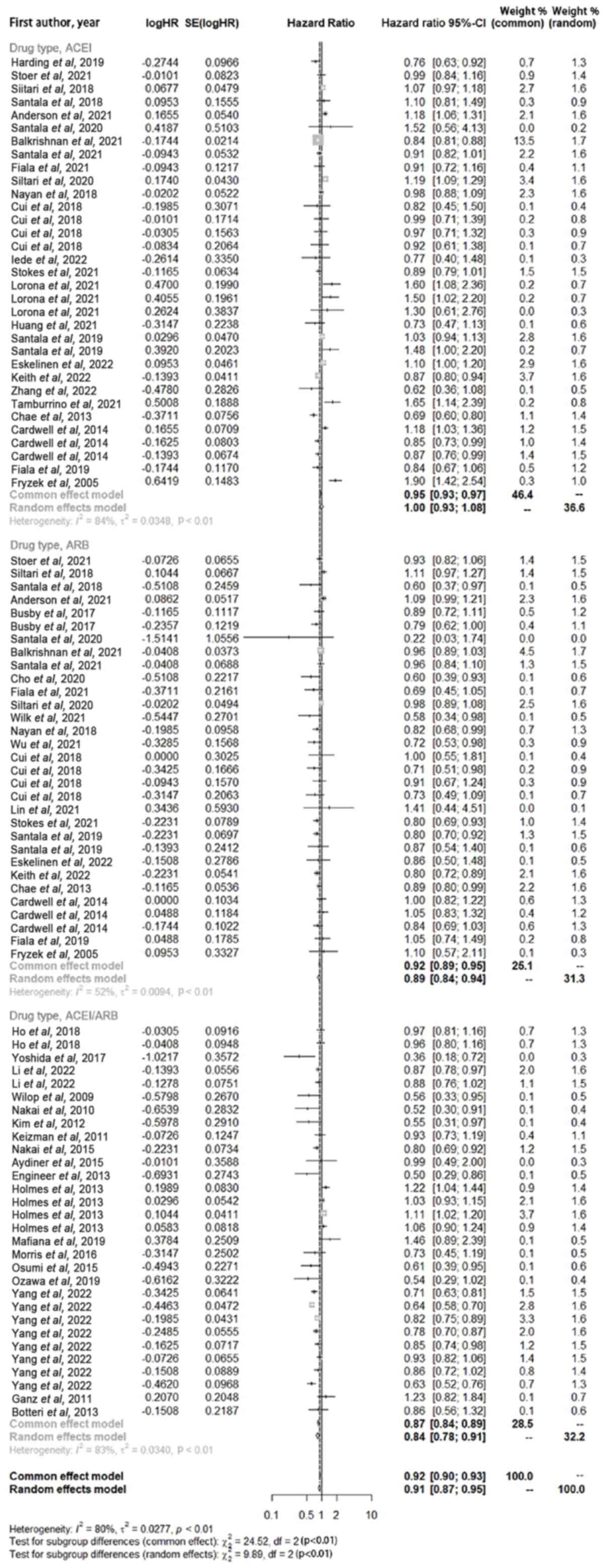
combination (Fig. 4). The use of
ACEI drugs alone did not lead to an significant extension of the
overall survival period of cancer patients (HR, 1.00; 95% CI,
0.93–1.08; P<0.01), while the use of ARB drugs alone (HR, 0.89;
95% CI, 0.84–0.94; P<0.01) or the combined application of
ACEI/ARB drugs (HR, 0.84; 95% CI, 0.78–0.91; P<0.01)
significantly improved the survival period of tumor patients
compared with patients with hypertension and cancer who did not use
ARB drugs or who were not taking ACEI/ARB drugs.
Impact of ACEI and ARB use on the OS
of patients with cancer according to study type
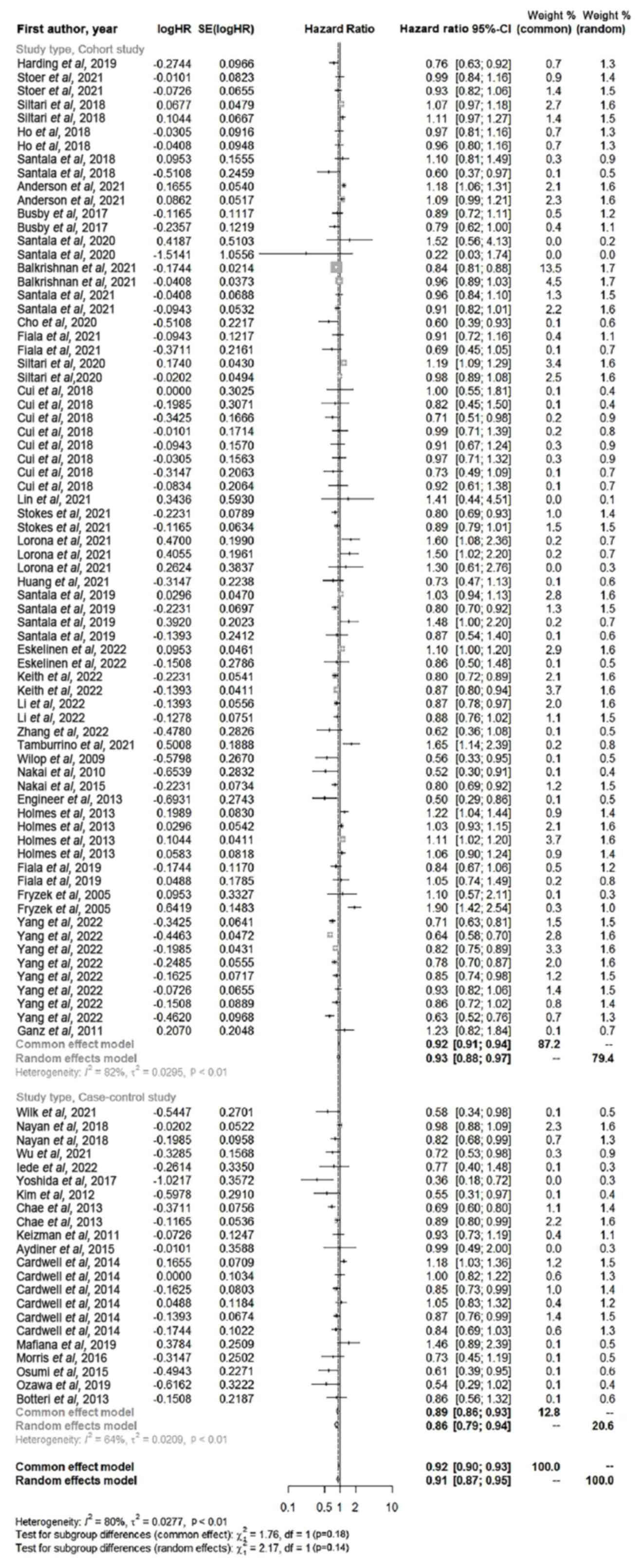
Subgroup analysis was performed on the included
studies according to study type (Fig.
5). Among the 34 cohort studies, the use of ACEIs or ARBs,
alone or in combination, significantly increased the OS of patients
with cancer (HR, 0.92; 95% CI, 0.88–0.97; P<0.01) compared with
those who were not treated. The use of ACEIs or ARBs, alone or in
combination, significantly increased the OS of patients with cancer
among the 14 case-control studies (HR, 0.86; 95% CI, 0.79–0.94;
P<0.01).
Sensitivity and publication bias
analyses
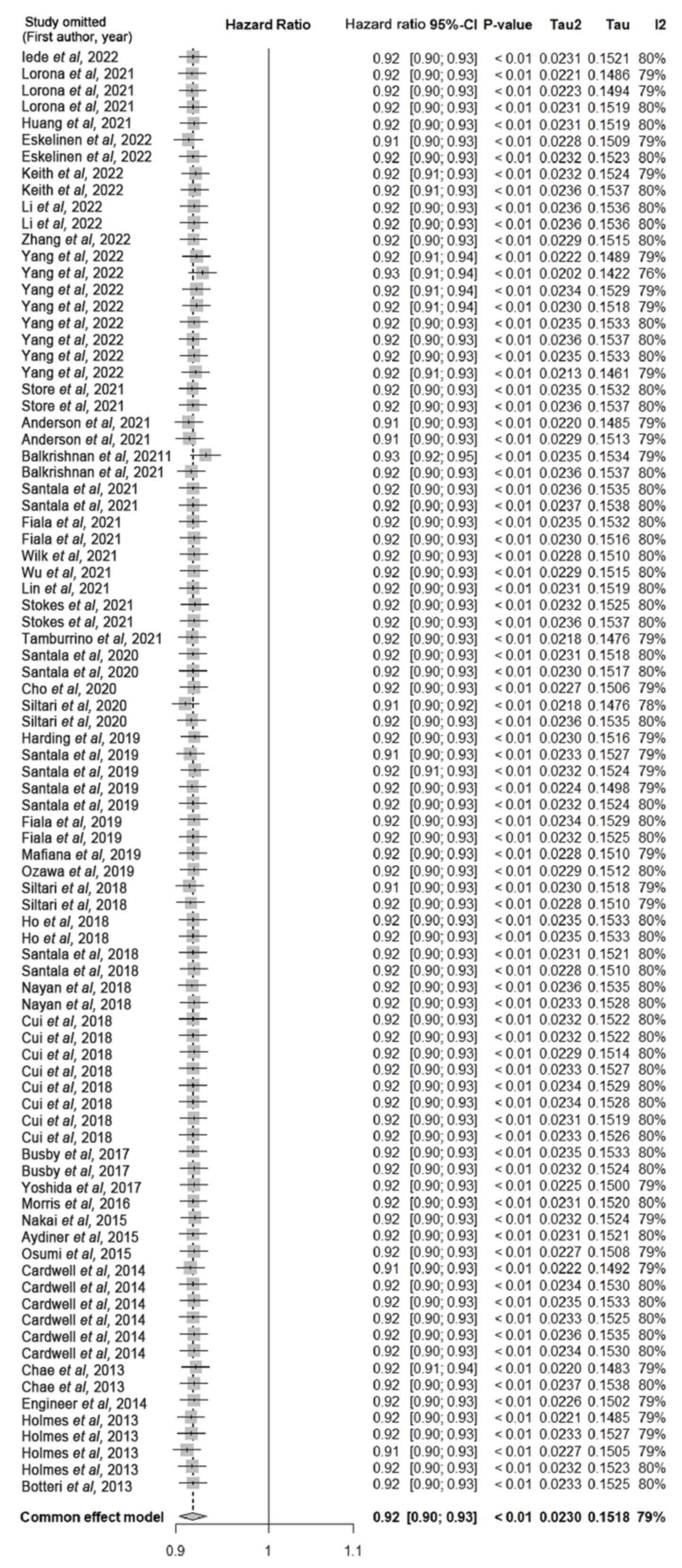
Sensitivity analysis was performed through the
individual elimination of each included study from the merged
studies (Fig. 6). The results of
this analysis indicated no significant change in the combined
effect size.
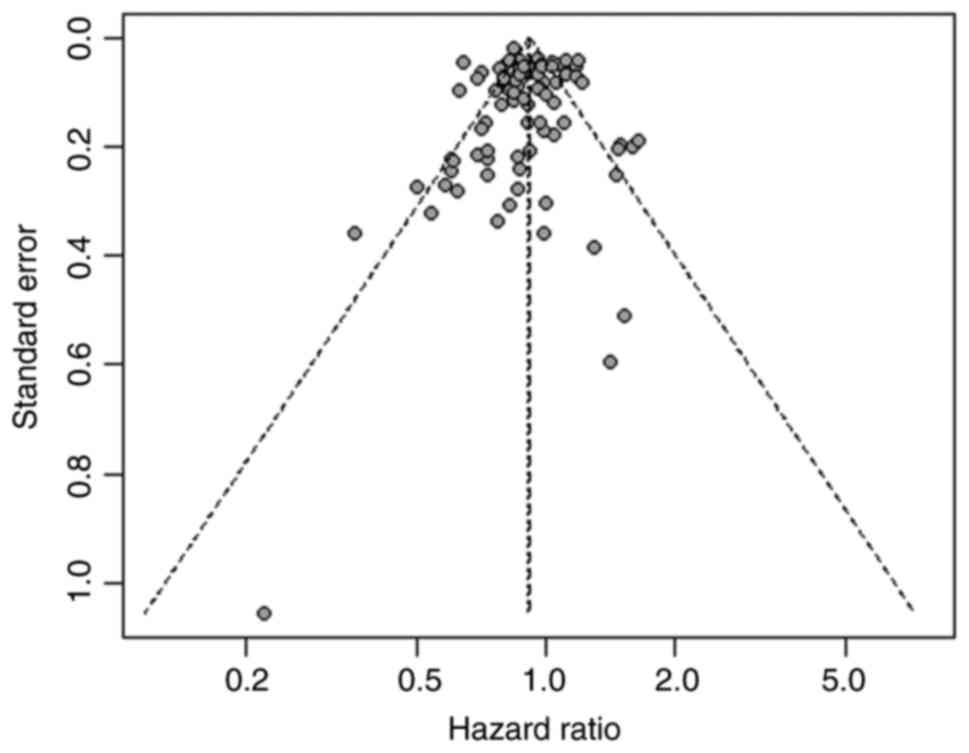
Egger's regression test was used to conduct a
publication bias analysis of the 48 articles that explored the
correlation between neutrophil-lymphocyte ratio and the OS and no
significant publication bias was demonstrated (Fig. 7; P=0.321).
Discussion
Hypertension is a common and frequently occurring
disease (64), and in clinical
practice, the prognosis of a number of patients with cancer with
concurrent hypertension is subject to potential effects of the
antihypertensive drugs, such as ACEIs and ARBs (65). However, the impact of ACEIs and ARBs
on prognosis is currently unclear. The present meta-analysis
included 48 studies, which involved the data of 923,134 patients
and demonstrated that patients who used ACEIs and ARBs had a
significantly increased OS compared with patients with cancer who
did not use these drugs. The increase in OS was significant in
patients with ovarian, pancreatic, prostate, hepatocellular, lung,
esophageal, gastric, colon, nasopharyngeal, head and neck,
gallbladder and rectal cancers. However, the OS of patients with
breast tumors and urothelial carcinoma, was significantly decreased
with the use of ACEIs and ARBs compared with the OS of patients who
did not use them. No significant differences in overall survival
were observed among patients with renal tumors, regardless of
whether they were treated with ACEI or ARB drugs. In terms of the
specific drugs used and the method of administration, the use of
ACEIs alone did not significantly change the OS of patients with
cancer; however, the use of ARBs alone and the combined use of
ACEIs and ARBs significantly increased the OS of patients.
The effects of antihypertensive drugs on the
development and progression of tumors is a topic of notable
importance. Previous studies have shown that Ang II and AT1 are
upregulated in a number of types of cancer tissues and that RAAS
disorders are closely associated with hypertension (66). Therefore, the present study aimed to
investigate whether the administration of RAAS-inhibiting
antihypertensive drugs in hypertensive patients with cancer affects
their prognosis. In comparison with a number of previous studies on
the effect of RAAS inhibitors on the prognosis of patients with
cancer (21,32), the present study incorporated data
from a large number of types of cancer, which included ovarian,
pancreatic, prostate, hepatocellular carcinoma, lung, esophageal
gastric, colon, nasopharyngeal, head and neck, gallbladder cancer,
rectal, renal, urothelial carcinoma and breast cancers. The impact
of ACEI and ARB use on the OS of hypertensive patients with various
types of cancer was systematically analyzed. The present study
evaluated the survival rate following the diagnosis of cancer,
collecting information on the use of medication in patients with
cancer, which may provide a better reflection of the impact of
hypertension treatment drugs on cancer risk and prognosis. In 2019,
Cui et al (25) utilized a
time-dependent Cox regression model to examine the association
between common antihypertensive drugs and the OS in breast,
colorectal, lung and gastric cancers, and used data from 2 large
prospective cohort studies in Shanghai, China. By contrast, the
present study included sample information on patients with
different types of cancer from the United States, Japan, Finland,
China, England, Canada, the Czech Republic, Italy, Denmark,
Germany, South Korea, North Korea, Norway, Oman and Poland. This
approach was used to minimize the analytical errors that could
result from different races of the patients included in the
studies. In contrast to the study conducted by Mc Menamin et
al (67), which assessed the
impact of RAAS inhibitors on overall survival in patients with
pancreatic cancer, lung cancer, renal cell carcinoma, breast
cancer, colorectal cancer, prostate cancer, and multiple myeloma by
assessing 10 relevant studies, the present study included 48
relevant studies and used a larger sample to conduct a more
comprehensive analysis of overall survival in patients with
pancreatic cancer, lung cancer, renal cell carcinoma, breast
cancer, colorectal cancer, prostate cancer, and multiple myeloma
who had used ACEI/ARB drugs, making the results more generalizable.
In contrast to a number of studies on that evaluated the impact of
antihypertensive drugs on the prognosis of cancer patients
(68,69), in terms of drug type, the present
study investigated RAAS inhibitors, which have a well-established
mechanism of action in tumor tissues. However, ACEIs and ARBs,
which both act as inhibitors in the various steps of the RAAS
cascade reaction, may not have the same effect on tumors. By
analyzing the impact of using ACEI/ARB drugs alone or in
combination on the overall survival of cancer patients, it was
found that inhibiting different steps of the RAAS cascade may have
different effects on the overall survival of cancer patients of
different types, these results may help to guide future
research.
The RAAS is an endocrine pathway that participates
in the regulation of cardiovascular and neuroendocrine functions
and is closely associated with the pathogenesis of hypertension
(70). In the RAAS, the
angiotensin-converting enzyme (ACE) is a key enzyme that primarily
converts Ang I into Ang II. Ang II binds to the AT1 and AT2
receptors, playing a role in various physiological pathways,
including vasoconstriction, aldosterone and vasopressin release,
sodium and water retention, and sympathetic activation (71,72).
Previous studies have reported that some tumor cells express renin
and Ang II receptors, and the activation or deactivation of these
receptors plays distinct physiological roles in the development of
cancer through various signaling pathways (73). The activation of AT1 receptor and
PRR receptor signaling leads to the activation of MAPK,
PI3K/AKT/MTOR, NF-κB and JAK/STAT signaling pathways, as well as an
increase in VEGF, TGFβ1, EGFR, and fibronectin, ultimately leading
to cell proliferation, angiogenesis, fibrosis, tumor invasion and
metastasis (74). These pathways
are inhibited by the AT2 receptor and angiotensin-(1–7)-mediated Mas signaling. Therefore, the
AT1 receptor is considered to serve a role in the promotion of
tumorigenesis (7,66). By contrast, the AT2 receptor has a
direct antiproliferative effect (75,76)
and Ang (1–7) directly inhibits angiogenesis and cell
proliferation (77). ACEIs inhibit
ACE, which thereby prevents the conversion of Ang I into Ang II and
indirectly inhibits the binding of Ang II to AT1 and AT2. By
contrast, ARBs directly block the binding of Ang II to AT1. Both
ACEIs and ARBs may exert their effects by directly or indirectly
inhibiting the signaling pathways of AT1 and AT2 receptors, which
leads to the inhibition of tumor cell growth and formation of
peripheral vessels (78).
A previous analysis of various types of cancer
demonstrated that patients with breast cancer had a decreased
survival period after the use of ACEIs or ARBs (32). However, a cohort analysis including
1,435 cases of breast cancer, 1,511 cases of colorectal cancer and
1,184 cases of prostate cancer demonstrated that in all patients,
the use of ACEIs or ARBs did not increase the cancer-specific risk
of death; therefore, ACEIs and ARBs drugs were considered to be
safe for patients diagnosed with breast, colorectal and prostate
cancer (21). A previous
meta-analysis suggested that ARBs have antiproliferative effects on
breast cancer (79). There are a
number of molecular types of breast cancer, and the specific type
is determined from the expression levels of indicators such as
estrogen and progesterone receptors, HER2 and Ki-67 through IHC;
the clinical features, degree of malignancy, treatment and
prognosis vary among the different molecular types of breast cancer
(80). It could be suggested that
the differential results reported on the prognosis and OS of
hypertensive patients with breast cancer after treatment with ACEI
and ARB analogs may be because endocrine therapy is preferred in
patients with high levels of estrogen and progesterone receptors in
the molecular typing of breast cancer (81). In the present study, analysis of
ACEIs and ARBs use, alone or in combination, on the OS of patients
with cancer, ACEIs alone had no significant effect on the survival
of these patients, whereas the use of ARBs or ACEI and ARB in
combination increased patient survival. The mechanism of action of
ACEIs against hypertension is to inhibit the ACE and
bradykinin-degrading enzymes, reduce the conversion of Ang I to Ang
II, and through vasodilatory effects, slow down the degradation of
bradykinin through and promote the release of prostaglandins, which
together leads to vasodilatation and blood pressure reduction
(82). However, it has been
reported that kinins are not only involved in blood pressure
regulation, but also serve a role in the regulation of
physiological functions of the cardiovascular system, kidneys and
nervous system. Kinins are closely related to the occurrence of
diseases such as heart disease, kidney disease, inflammatory
reactions and cancer (83).
Previous studies reported that bradykinin mediates the migration
and invasion of various human cancer cells (84,85).
Hsin-Shan Yu et al found that bradykinin induced VEGF
expression and promoted angiogenesis in human prostate cancer
through activation of the B2 receptor and the Akt, mTOR, and NF-k
AP-1 signaling pathways. Bradykinin promotes gastric cancer cell
proliferation, migration, invasion and tumor growth through the ERK
signaling pathway (86). The
mechanism of action of ARBs against hypertension, by contrast, is
to selectively block the binding of Ang II to AT1, which leads to a
dose-dependent reduction in peripheral vascular resistance and a
decrease in blood pressure (87).
This could potentially be due to the previous studies on breast or
gynecological cancers on the impact of hormones and hormone
therapies where the status of estrogen receptors were unclear,
which may have impacted the subsequent analysis. Analysis of the
impact of the use of ACEIs and ARBs alone or in combination on the
OS of patients with cancer in the present study demonstrated that
the use of ACEIs alone did not significantly affect the OS of these
patients. ACEIs block Ang II production by suppressing ACE and
indirectly inhibiting Ang II binding to AT1 and AT2 (88). ARBs selectively block the binding of
Ang II to AT1, the AT1 receptor is upregulated in cancer tissues
and promotes cell proliferation and angiogenesis (89). Previous studies have reported that
AT1 receptor antagonists can significantly slow the progression of
tumors, and in the maintenance of blood pressure, water and
electrolyte homeostasis, AT1 and AT2 receptors antagonize each
other to maintain a regulatory balance (89). The present analysis demonstrated no
significant impact of the use of ACEIs alone on the OS of patients
with cancer, whereas the use of ARBs alone significantly the OS of
these patients. Therefore, determining the specific roles of AT1
and AT2 receptors in tumors could potentially increase the
understanding of the increased OS of patients with cancer who used
ARBs alone or in combination with an ARB or ACEI. Hence, conducting
further investigation into the involvement of the RAAS in local
tumors by selectively inhibiting AT1 and AT2 receptors in
hypertensive cancer patients through experimental studies may yield
deeper insights into this mechanism.
The impact of antihypertensive drugs on the
prognosis of cancer patients may be influenced by multiple factors,
with a key constraint being heterogeneity, as well as factors such
as race, lifestyle, geographical environment, underlying diseases,
comorbidities, health status and therapeutic methods.
Simultaneously, specific types of bias should be considered, for
example, in a previous study where hypertension history was
collected through self-report, there may be a few cases of recall
bias among patients; the data collected in this way will be
partially biased (90).
Additionally, the potential effects of changes in the therapeutic
regimen for hypertension on the analysis results should be
considered. For example, in a previous study, a number of patients
started using thiazide diuretics or calcium channel blockers due to
poor blood pressure control or the subsequent development of other
diseases (91). However, the effect
of other antihypertensive drugs on the previous stages of cancer is
currently unclear. Furthermore, the present study demonstrated that
the inclusion and exclusion criteria for single tumor studies
varied among populations. A study by Wilk et al (58) with stringent exclusion criteria
included 93 patients with metastatic castration-resistant prostate
cancer who had received docetaxel and androgen deprivation therapy
and had developed metastases, all of whom had a clear pathological
diagnosis and radiological evidence of metastasis, and had received
docetaxel prior to the start of ABI to evaluate the impact of prior
chronic diseases and concomitant medications on the abiraterone
acetate treatment process in this patient cohort. It was reported
that the use of ACEI/ARB drugs may prolong the survival of these
patients; however, the aforementioned study requires further
support through prospective studies. Another previous study
investigated the relationship between antihypertensive drugs and
prostate cancer prognosis through a survey of 8,253 patients with
prostate cancer and reported that the use of RAS inhibitors, ACEIs
and AT receptor blockers were associated with improved survival
rates in patients with prostate cancer (52). Although the aforementioned study had
a large sample size, the inclusion and exclusion criteria were not
set for the study population's age, disease stage or treatment
received. Therefore, the aforementioned study may only represent
the overall prognosis trend of patients with prostate cancer who
use antihypertensive drugs to a certain extent and cannot
accurately reflect the impact of antihypertensive drugs use on the
prognosis of certain specific groups of prostate cancer patients
(52). The present meta-analysis is
a preliminary study of the prognostic impact of ACEIs and ARBs in
hypertensive patients with cancer; therefore, the inclusion and
exclusion criteria were set broadly. With an increase in the number
of randomized controlled trials and clinical studies on the use of
ACEIs and ARBs in hypertensive patients with different types of
cancers, meta-analyses for a single type of tumors could be
performed with more stringent exclusion criteria to obtain accurate
study conclusions in the future. For instance, to further
investigate the impact of ACEI/ARB drugs on the overall survival of
a specific subtype of breast cancer patients with comorbid
hypertension undergoing endocrine therapy, establishing inclusion
criteria for individuals diagnosed with this subtype of breast
cancer and receiving endocrine therapy. Subsequently, it would be
possible to prospectively assess the prognosis of this cohort to
yield more valuable research findings.
ACEIs and ARBs may increase the survival of
hypertensive patients with cancers and the specific mechanism
underlying this effect may be associated with the promotion of cell
proliferation and angiogenesis by AT1 (73). In the future, further clinical and
biological research could potentially improve the understanding of
the mechanisms underlying the anticancer effects of ACEIs and ARBs,
demonstrate the potential of ACEIs and ARBs in adjunctive cancer
therapy, identify the patient populations that benefit the most
from these treatments and provide novel treatment options to
improve the prognosis of hypertensive patients with cancer.
Acknowledgments
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YX was responsible for the study methodology,
software use, data curation, manuscript writing, review and
editing. YX and XC contributed to study conceptualization. XC
contributed to formal analysis of the data. WL participated in data
investigation. WL and XC curated the data. YX, XC and WL performed
data validation. WZ and XL contributed significantly to the
conceptualization and design of the study, and supervised the
research activities, and validated the integrity of all primary
data. WZ and XL confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang MC and Lloyd-Jones DM: Cardiovascular
risk assessment in hypertensive patients. Am J Hypertens.
34:569–577. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Małyszko J, Małyszko M, Kozlowski L,
Kozlowska K and Małyszko J: Hypertension in malignancy-an
underappreciated problem. Oncotarget. 9:20855–20871. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raimondi S, Botteri E, Munzone E, Cipolla
C, Rotmensz N, DeCensi A and Gandini S: Use of beta-blockers and
angiotensin-converting enzyme inhibitors and angiotensin receptor
blockers and breast cancer survival: Systematic review and
meta-analysis. Int J Cancer. 139:212–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng Y, Xie Y, Wang M, Xu P, Wei B, Li N,
Wu Y, Yang S, Zhou L, Hao Q, et al: Effects of antihypertensive
drugs use on risk and prognosis of colorectal cancer: A
meta-analysis of 37 observational studies. Front Pharmacol.
12:6706572022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allen AM: Role of angiotensin in the
rostral ventrolateral medulla in the development and maintenance of
hypertension. Curr Opin Pharmacol. 11:117–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dinh DT, Frauman AG, Johnston CI and
Fabiani ME: Angiotensin receptors: Distribution, signalling and
function. Clin Sci (Lond). 100:481–492. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arendse LB, Danser AHJ, Poglitsch M, Touyz
RM, Burnett JC Jr, Llorens-Cortes C, Ehlers MR and Sturrock ED:
Novel therapeutic approaches targeting the renin-angiotensin system
and associated peptides in hypertension and heart failure.
Pharmacol Rev. 71:539–570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Escobar E, Rodríguez-Reyna TS, Arrieta O
and Sotelo J: Angiotensin II and cell proliferation and
angiogenesis regulator: Biologic and therapeutic implications in
cancer. Curr Vasc Pharmacol. 2:385–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ram CV: Antihypertensive drugs: An
overview. Am J Cardiovasc Drugs. 2:77–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neo JH, Malcontenti-Wilson C, Muralidharan
V and Christophi C: Effect of ACE inhibitors and angiotensin II
receptor antagonists in a mouse model of colorectal cancer liver
metastases. J Gastroenterol Hepatol. 22:577–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfeffer MA, Swedberg K, Granger CB, Held
P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S and
Pocock S; CHARM Investigators Committees, : Effects of candesartan
on mortality and morbidity in patients with chronic heart failure:
The CHARM-Overall programme. Lancet. 362:759–766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tamburrino D, Guarneri G, Partelli S,
Crippa S, Falconi M and Capurso G: Does chronic consumption of
angiotensin-converting enzyme inhibitors affect survival after
surgical resection of pancreatic ductal adenocarcinoma? Dig Liver
Dis. 53:1065–1067. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgins JPT and Green S: Cochrane handbook
for systematic reviews of interventions. Version 5.1.0 [Updated
March 2011]. The Cochrane Collaboration. 2011.Available from.
www.handbook.cochrane.org
|
|
15
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. PLoS Med.
6:e10000972009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality if nonrandomized studies in
meta-analyses. Available from URL:. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm[cited
2009 Oct 19].
|
|
17
|
Anderson JL, Knowlton KU, Muhlestein JB,
Bair TL, Le VT and Horne BD: Evaluation of treatment with
angiotensin converting enzyme inhibitors and the risk of lung
cancer: ERACER-an observational cohort study. J Cardiovasc
Pharmacol Ther. 26:321–327. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aydiner A, Ciftci R and Sen F:
Renin-angiotensin system blockers may prolong survival of
metastatic non-small cell lung cancer patients receiving erlotinib.
Medicine (Baltimore). 94:e8872015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balkrishnan R, Desai RP, Narayan A,
Camacho FT, Flausino LE and Chammas R: Associations between
initiating antihypertensive regimens on stage I–III colorectal
cancer outcomes: A medicare SEER cohort analysis. Cancer Med.
10:5347–5357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Botteri E, Munzone E, Rotmensz N, Cipolla
C, De Giorgi V, Santillo B, Zanelotti A, Adamoli L, Colleoni M,
Viale G, et al: Therapeutic effect of β-blockers in triple-negative
breast cancer postmenopausal women. Breast Cancer Res Treat.
140:567–575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Busby J, McMenamin Ú, Spence A, Johnston
BT, Hughes C and Cardwell CR: Angiotensin receptor blocker use and
gastro-oesophageal cancer survival: A population-based cohort
study. Aliment Pharmacol Ther. 47:279–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cardwell CR, Mc Menamin ÚC, Hicks BM,
Hughes C, Cantwell MM and Murray LJ: Drugs affecting the
renin-angiotensin system and survival from cancer: A population
based study of breast, colorectal and prostate cancer patient
cohorts. BMC Med. 12:282014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chae YK, Brown EN, Lei X, Melhem-Bertrandt
A, Giordano SH, Litton JK, Hortobagyi GN, Gonzalez-Angulo AM and
Chavez-Macgregor M: Use of ACE inhibitors and angiotensin receptor
blockers and primary breast cancer outcomes. J Cancer. 4:549–556.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho MA, Jeong SY, Sohn I, Kim MS, Kang JH,
Paik ES, Lee YY and Choi CH: Impact of angiotensin receptor
blockers, beta blockers, calcium channel blockers and thiazide
diuretics on survival of ovarian cancer patients. Cancer Res Treat.
52:645–654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui Y, Wen W, Zheng T, Li H, Gao YT, Cai
H, You M, Gao J, Yang G, Zheng W, et al: Use of antihypertensive
medications and survival rates for breast, colorectal, lung, or
stomach cancer. Am J Epidemiol. 188:1512–1528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Engineer DR, Burney BO, Hayes TG and
Garcia JM: Exposure to ACEI/ARB and β-blockers is associated with
improved survival and decreased tumor progression and
hospitalizations in patients with advanced colon cancer. Transl
Oncol. 6:539–545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eskelinen T, Veitonmäki T, Kotsar A,
Tammela TLJ, Pöyhönen A and Murtola TJ: Improved renal cancer
prognosis among users of drugs targeting renin-angiotensin system.
Cancer Causes Control. 33:313–320. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fiala O, Ostasov P, Sorejs O, Liska V,
Buchler T, Poprach A and Finek J: Incidental use of beta-blockers
is associated with outcome of metastatic colorectal cancer patients
treated with bevacizumab-based therapy: A single-institution
retrospective analysis of 514 patients. Cancers (Basel).
11:18562019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fiala O, Ostašov P, Rozsypalová A, Hora M,
Šorejs O, Šustr J, Bendová B, Trávníček I, Filipovský J, Fínek J
and Büchler T: Impact of concomitant cardiovascular medication on
survival of metastatic renal cell carcinoma patients treated with
sunitinib or pazopanib in the first line. Target Oncol. 16:643–652.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fryzek JP, Poulsen AH, Johnsen SP,
McLaughlin JK, Sørensen HT and Friis S: A cohort study of
antihypertensive treatments and risk of renal cell cancer. Br J
Cancer. 92:1302–1306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ganz PA, Habel LA, Weltzien EK, Caan BJ
and Cole SW: Examining the influence of beta blockers and ACE
inhibitors on the risk for breast cancer recurrence: Results from
the LACE cohort. Breast Cancer Res Treat. 129:549–556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harding BN, Delaney JA, Urban RR and Weiss
NS: Post-diagnosis use of antihypertensive medications and the risk
of death from ovarian cancer. Gynecol Oncol. 154:426–431. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holmes S, Griffith EJ, Musto G and Minuk
GY: Antihypertensive medications and survival in patients with
cancer: A population-based retrospective cohort study. Cancer
Epidemiol. 37:881–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang T, Townsend MK, Dood RL, Sood AK and
Tworoger SS: Antihypertensive medication use and ovarian cancer
survival. Gynecol Oncol. 163:342–347. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Keith SW, Maio V, Arafat HA, Alcusky M,
Karagiannis T, Rabinowitz C, Lavu H and Louis DZ: Angiotensin
blockade therapy and survival in pancreatic cancer: A population
study. BMC Cancer. 22:1502022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Keizman D, Huang P, Eisenberger MA, Pili
R, Kim JJ, Antonarakis ES, Hammers H and Carducci MA: Angiotensin
system inhibitors and outcome of sunitinib treatment in patients
with metastatic renal cell carcinoma: A retrospective examination.
Eur J Cancer. 47:1955–1961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim ST, Park KH, Oh SC, Seo JH, Kim JS,
Shin SW and Kim YH: How does inhibition of the renin-angiotensin
system affect the prognosis of advanced gastric cancer patients
receiving platinum-based chemotherapy? Oncology. 83:354–360. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iede K, Yamada T, Ueda M, Tsuda Y,
Nakashima S, Ohta K, Tanida T, Matsuyama J, Ikenaga M and Tominaga
S: Do antihypertensive drugs really have antitumor effects?
Baseline differences in hypertensive and non-hypertensive patients
with advanced pancreatic cancer. Medicine (Baltimore).
101:e295322022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li PC, Huang RY, Yang YC, Hsieh KP and
Yang YH: Prognostic impact of angiotensin-converting enzyme
inhibitors and angiotensin receptors blockers in esophageal or
gastric cancer patients with hypertension-a real-world study. BMC
Cancer. 22:4302022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin YT, Wang HC, Tsai MH, Su YY, Yang MY
and Chien CY: Angiotensin II receptor blockers valsartan and
losartan improve survival rate clinically and suppress tumor growth
via apoptosis related to PI3K/AKT signaling in nasopharyngeal
carcinoma. Cancer. 127:1606–1619. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lorona NC, Cook LS, Tang MTC, Hill DA,
Wiggins CL and Li CI: Antihypertensive medications and risks of
recurrence and mortality in luminal, triple-negative, and
HER2-overexpressing breast cancer. Cancer Causes Control.
32:1375–1384. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mafiana RN, Al-Kindi MS, Mafiana N, Al
Lawati AS and Al Moundhri M: Impact of metabolic syndrome diagnosis
and its treatment on survival of colorectal cancer patients. J
Cancer Epidemiol. 2019:65274572019.PubMed/NCBI
|
|
43
|
Ho CM, Lee CH, Lee MC, Zhang JF, Wang JY,
Hu RH and Lee PH: Comparative effectiveness of
angiotensin-converting enzyme inhibitors and angiotensin II
receptor blockers in chemoprevention of hepatocellular carcinoma: A
nationwide high-risk cohort study. BMC Cancer. 18:4012018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morris ZS, Saha S, Magnuson WJ, Morris BA,
Borkenhagen JF, Ching A, Hirose G, McMurry V, Francis DM, Harari
PM, et al: Increased tumor response to neoadjuvant therapy among
rectal cancer patients taking angiotensin-converting enzyme
inhibitors or angiotensin receptor blockers. Cancer. 122:2487–2495.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nakai Y, Isayama H, Ijichi H, Sasaki T,
Sasahira N, Hirano K, Kogure H, Kawakubo K, Yagioka H, Yashima Y,
et al: Inhibition of renin-angiotensin system affects prognosis of
advanced pancreatic cancer receiving gemcitabine. Br J Cancer.
103:1644–1648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakai Y, Isayama H, Sasaki T, Takahara N,
Saito K, Ishigaki K, Hamada T, Mizuno S, Miyabayashi K, Yamamoto K,
et al: The inhibition of renin-angiotensin system in advanced
pancreatic cancer: An exploratory analysis in 349 patients. J
Cancer Res Clin Oncol. 141:933–939. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nayan M, Juurlink DN, Austin PC, Macdonald
EM, Finelli A, Kulkarni GS and Hamilton RJ: Canadian Drug Safety
and Effectiveness Research Network (CDSERN): Medication use and
kidney cancer survival: A population-based study. Int J Cancer.
142:1776–1785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Osumi H, Matsusaka S, Wakatsuki T, Suenaga
M, Shinozaki E and Mizunuma N: Angiotensin II type-1 receptor
blockers enhance the effects of bevacizumab-based chemotherapy in
metastatic colorectal cancer patients. Mol Clin Oncol. 3:1295–1300.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ozawa T, Hashiguchi Y, Yagi T, Fukushima
Y, Shimada R, Hayama T, Tsuchiya T, Nozawa K, Iinuma H, Ishihara S
and Matsuda K: Angiotensin I-converting enzyme
inhibitors/angiotensin II receptor blockers may reduce tumor
recurrence in left-sided and early colorectal cancers. Int J
Colorectal Dis. 34:1731–1739. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Santala EEE, Kotsar A, Veitonmäki T,
Tammela TLJ and Murtola TJ: Risk of urothelial cancer death among
people using antihypertensive drugs-a cohort study from Finland.
Scand J Urol. 53:185–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Santala EEE, Murto MO, Artama M, Pukkala
E, Visvanathan K and Murtola TJ: Angiotensin receptor blockers
associated with improved breast cancer survival-a nationwide cohort
study from Finland. Cancer Epidemiol Biomarkers Prev. 29:2376–2382.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Santala EE, Rannikko A and Murtola TJ:
Antihypertensive drugs and prostate cancer survival after radical
prostatectomy in Finland-A nationwide cohort study. Int J Cancer.
144:440–447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Siltari A, Murtola TJ, Talala K, Taari K,
Tammela TLJ and Auvinen A: Antihypertensive drug use and prostate
cancer-specific mortality in Finnish men. PLoS One.
15:e02342692020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Santala EEE, Artama M, Pukkala E,
Visvanathan K, Staff S and Murtola TJ: Antihypertensive drug use
and the risk of ovarian cancer death among Finnish ovarian cancer
patients-a nationwide cohort study. Cancers (Basel). 13:20872021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Siltari A, Murtola TJ, Talala K, Taari K,
Tammela TLJ and Auvinen A: Antihypertensive drugs and prostate
cancer risk in a Finnish population-based cohort. Scand J Urol.
52:321–327. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Støer NC, Bouche G, Pantziarka P, Sloan
EK, Andreassen BK and Botteri E: Use of non-cancer drugs and
survival among patients with pancreatic adenocarcinoma: A
nationwide registry-based study in Norway. Acta Oncol.
60:1146–1153. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Stokes WA, Molina E, McDermott JD, Morgan
RL, Bickett T, Fakhoury KR, Amini A and Karam SD: Survival impact
of angiotensin-converting enzyme inhibitors and angiotensin II
receptor antagonists in head and neck cancer. Head Neck.
43:3255–3275. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wilk M, Waśko-Grabowska A, Skoneczna I and
Szmit S: Angiotensin system inhibitors may improve outcomes of
patients with castration-resistant prostate cancer during
abiraterone acetate treatment-a cardio-oncology study. Front Oncol.
11:6647412021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wilop S, von Hobe S, Crysandt M, Esser A,
Osieka R and Jost E: Impact of angiotensin I converting enzyme
inhibitors and angiotensin II type 1 receptor blockers on survival
in patients with advanced non-small-cell lung cancer undergoing
first-line platinum-based chemotherapy. J Cancer Res Clin Oncol.
135:1429–1435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu CN, Wu SC, Chen WC, Yang YH, Chin JC,
Chien CY, Fang FM, Li SH, Luo SD and Chiu TJ: Angiotensin II
receptor blockers and oral squamous cell carcinoma survival: A
propensity-score-matched cohort study. PLoS One. 16:e02607722021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yoshida T, Kinoshita H, Fukui K, Matsuzaki
T, Yoshida K, Mishima T, Yanishi M, Komai Y, Sugi M, Inoue T, et
al: Prognostic impact of renin-angiotensin inhibitors in patients
with bladder cancer undergoing radical cystectomy. Ann Surg Oncol.
24:823–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang Y, Song M, Chan AT, Meyerhardt JA,
Willett WC and Giovannucci EL: Long-term use of antihypertensive
medications and hypertension and colorectal cancer risk mortality:
A prospective cohort study. Br J Cancer. 127:1974–1982. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang A, Wu H, Lau ESH, Shi M, Fan B, Kong
APS, Ma RCW, Luk AOY, Chan JCN and Chow E: Effects of RAS
inhibitors on all-site cancers and mortality in the Hong Kong
diabetes surveillance database (2002–2019). EBioMedicine.
83:1042192022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Israili ZH, Hernández-Hernández R and
Valasco M: The future of antihypertensive treatment. Am J Ther.
14:121–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li XY, Sun JF and Hu SQ: The
renin-angiotensin system blockers as adjunctive therapy for cancer:
A meta-analysis of survival outcome. Eur Rev Med Pharmacol Sci.
21:1375–1383. 2017.PubMed/NCBI
|
|
66
|
Deshayes F and Nahmias C: Angiotensin
receptors: A new role in cancer? Trends Endocrinol Metab.
16:293–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mc Menamin ÚC, Murray LJ, Cantwell MM and
Hughes CM: Angiotensin-converting enzyme inhibitors and angiotensin
receptor blockers in cancer progression and survival: A systematic
review. Cancer Causes Control. 23:221–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li J, Lam ASM, Yau STY, Yiu KKL and Tsoi
KKF: Antihypertensive treatments and risks of lung cancer: A large
population-based cohort study in Hong Kong. BMC Cancer.
21:12022021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mehrdad SA, Mirzavi F, Seyedi SMR and
Asoodeh A: The effect of angiotensin receptor blockers and
angiotensin-converting enzyme inhibitors on progression of gastric
cancer: Systematic review and meta-analysis. Anticancer Drugs.
33:983–988. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Te Riet L, van Esch JH, Roks AJ, van den
Meiracker AH and Danser AH: Hypertension:
renin-angiotensin-aldosterone system alterations. Circ Res.
116:960–975. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Timmermans PB, Wong PC, Chiu AT, Herblin
WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA and Smith RD:
Angiotensin II receptors and angiotensin II receptor antagonists.
Pharmacol Rev. 45:205–251. 1993.PubMed/NCBI
|
|
72
|
Burnier M: Angiotensin II type 1 receptor
blockers. Circulation. 103:904–912. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hassani B, Attar Z and Firouzabadi N: The
renin-angiotensin-aldosterone system (RAAS) signaling pathways and
cancer: Foes versus allies. Cancer Cell Int. 23:2542023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Imai N, Hashimoto T, Kihara M, Yoshida S,
Kawana I, Yazawa T, Kitamura H and Umemura S: Roles for host and
tumor angiotensin II type 1 receptor in tumor growth and
tumor-associated angiogenesis. Lab Invest. 87:189–198. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Judd SJ, Alderman J, Bowden J and
Michailov L: Evidence against the involvement of opiate neurons in
mediating the effect of clomiphene citrate on
gonadotropin-releasing hormone neurons. Fertil Steril. 47:574–578.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pei N, Mao Y, Wan P, Chen X, Li A, Chen H,
Li J, Wan R, Zhang Y, Du H, et al: Angiotensin II type 2 receptor
promotes apoptosis and inhibits angiogenesis in bladder cancer. J
Exp Clin Cancer Res. 36:772017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu J, Fan J, Wu F, Huang Q, Guo M, Lv Z,
Han J, Duan L, Hu G, Chen L, et al: The ACE2/angiotensin-(1–7)/Mas
receptor axis: Pleiotropic roles in cancer. Front Physiol.
8:2762017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ferreira AJ and Santos RA: Cardiovascular
actions of angiotensin-(1–7). Braz J Med Biol Res. 38:499–507.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Rhodes DR, Ateeq B, Cao Q, Tomlins SA,
Mehra R, Laxman B, Kalyana-Sundaram S, Lonigro RJ, Helgeson BE,
Bhojani MS, et al: AGTR1 overexpression defines a subset of breast
cancer and confers sensitivity to losartan, an AGTR1 antagonist.
Proc Natl Acad Sci USA. 106:10284–10289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Weigelt B, Geyer FC and Reis-Filho JS:
Histological types of breast cancer: How special are they? Mol
Oncol. 4:192–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cushman DW and Ondetti MA: History of the
design of captopril and related inhibitors of angiotensin
converting enzyme. Hypertension. 17:589–592. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Burch RM, Farmer SG and Steranka LR:
Bradykinin receptor antagonists. Med Res Rev. 10:237–269. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Montana V and Sontheimer H: Bradykinin
promotes the chemotactic invasion of primary brain tumors. J
Neurosci. 31:4858–4867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cheng CY, Kuo CT, Lin CC, Hsieh HL and
Yang CM: IL-1beta induces expression of matrix metalloproteinase-9
and cell migration via a c-Src-dependent, growth factor receptor
transactivation in A549 cells. Br J Pharmacol. 160:1595–1610. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yu HS, Lin TH and Tang CH: Involvement of
intercellular adhesion molecule-1 up-regulation in bradykinin
promotes cell motility in human prostate cancers. Int J Mol Sci.
14:13329–13345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hill RD and Vaidya PN: Angiotensin II
receptor blockers (ARB). StatPearls [Internet]. StatPearls
Publishing; Treasure Island, FL: 2024
|
|
88
|
Piepho RW: Overview of the
angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm.
57 (Suppl 1):S3–S7. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Colin M, Delaitre C, Foulquier S and
Dupuis F: The AT1/AT2 receptor equilibrium is
a cornerstone of the regulation of the renin angiotensin system
beyond the cardiovascular system. Molecules. 28:54812023.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kidoguchi S, Sugano N, Yokoo T, Kaneko H,
Akazawa H, Mukai M, Node K, Yano Y and Nishiyama A:
Antihypertensive drugs and cancer risk. Am J Hypertens. 35:767–783.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tsioufis C and Thomopoulos C: Combination
drug treatment in hypertension. Pharmacol Res. 125:266–271. 2017.
View Article : Google Scholar : PubMed/NCBI
|