Introduction
Accounting for ~10 million cancer deaths recorded in
2020, cancer is a major cause of death worldwide (1). Among these patients, ~90% die from
secondary cancer rather than primary cancer (2). Cancer most frequently metastasises to
the lymph nodes, lungs, liver and bones (3,4).
Secondary bone cancers are commonly observed in advanced stages of
breast (76–100%) and prostate cancer (76–100%), as well as in lung
(26–50%) and kidney cancer (26–50%) (5). Skeletal metastases affect the bone
remodelling process by stimulating bone formation
(osteoblastic/osteosclerotic phenotype), enhancing bone resorption
(osteolytic phenotype) or increasing both bone synthesis and
degradation (mixed phenotype). For instance, the majority of
skeletal metastases in patients with prostate cancer are considered
osteosclerotic (6,7), whereas most breast cancer bone
metastases are osteolytic (8).
Osseous metastases can have a serious impact on the skeleton,
ultimately affecting the patient's quality of life (QOL) (9). Once metastatic bone disease has
formed, the median overall survival is 6 months (9). However, the life expectancy of a
patient can range from 2.8 to 57 months, depending on the primary
cancer (9,10). In most cases, there is no cure for
bone metastasis, but the progression of the disease can be delayed.
Therefore, improving the understanding of the metastatic cascade
may help to advance treatment strategies and improve the QOL of
patients.
Bone sialoprotein (BSP) is part of the small
integrin-binding ligand N-linked glycoprotein (SIBLING) family of
proteins (11). It is an acidic,
glycosylated phosphoprotein almost exclusively expressed in
mineralised tissues (12). Bone
modelling and remodelling is mediated by BSP (13) and BSP is secreted into the
non-mineralised extracellular matrix (ECM) when the osteoid is
formed and the bone tissue is mineralised (14,15).
To date, several functions of BSP have been described, such as the
binding and potential induction of hydroxyapatite nucleation
(16,17), and the stimulation of osteoclast
differentiation (18). A previous
review has described the role of BSP in bone development and
turnover in detail (13). However,
BSP is also involved in cancerous growth and metastasis. Cancer
cell proliferation (19), migration
(20), invasion (21), tumour cell evasion of immune system
surveillance (22) and angiogenesis
(23) are all stimulated by BSP.
This strongly indicates that BSP plays a crucial role in driving
cancer progression. BSP also regulates cancer cell adhesion
(24,25) and the adhesion of cancer cells to
BSP appears to be mediated by the binding of the
arginine-glycine-aspartic acid (RGD) sequence of BSP to cell
surface integrin receptors (26).
Integrins are a large family of cell adhesion
molecules (to date the 18 α- and eight β-integrin subunits have
been described in humans) and they recognise various integrin
ligands, for example, fibronectin (FN), vitronectin (VN) and BSP
(27,28). Integrin αvβ3 (VN receptor) on
osteoclasts, osteosarcoma and osteoblast-like cells can bind to the
RGD motif of BSP to induce cell attachment (28,29).
This sequence is highly conserved among the SIBLING protein family
(11), indicating an essential
regulatory function. The RGD cell attachment domain of BSP is
located at the C-terminus (30) and
is flanked by tyrosine residues, which can be post-translationally
modified, for instance by sulfation (31). The C-terminal tyrosine-rich region
in BSP may drive integrin-mediated cell attachment, independent of
the RGD sequence (29,31). Glycosylation modification (N-linked
and O-linked glycosylation) of BSP potentially alters the activity
of the protein as well as inhibits cell attachment (32). BSP can also bind to
glycosaminoglycans, such as heparin, in an ionic-dependent manner,
to increase the cell-binding activity of the RGD motif to the
transmembrane integrin receptors (33). The heparin-binding sequence of BSP
itself potentially requires the presence of the RGD domain to
sufficiently induce cell adhesion (34).
The RGD motif of BSP was reported to bind to αvβ5 on
human breast cancer cells (SKBR3) to stimulate cell attachment
(35). At present, it is still not
clearly defined which integrin receptors are involved in
BSP-mediated cancer cell attachment, as findings from different
studies seem contradictory. Sung et al (26) reported that the adhesion of the
human melanoma cancer cell line MDA-MB-435 to BSP was
αvβ5-dependent, whereas Byzova et al (35) showed that BSP-adhesion of MDA-MB-435
was αvβ3-mediated. In addition, MDA-MB-435 cells moderately to
strongly express αvβ3 and αvβ5 integrin receptors (26). Furthermore, whether BSP can bind to
other RGD-binding integrins on cancer cells, such as αvβ6, to
induce cell adhesion remains unclear. Cells may also employ
different integrin receptors in adhesion and chemotaxis (26). In addition, most studies have
focused on osteosarcoma, breast cancer or melanoma, whereas little
is known about the relation between BSP and the adhesion of
prostate and lung cancer cells. The human prostate adenocarcinoma
cell line PC-3 and the human lung cancer cell line NCI-H460 express
both αvβ3 and αvβ5 integrin receptors, although the expression
levels vary from weak to very strong (36–40).
Integrins promote tumour progression and metastasis
by regulating tumour cell survival, proliferation, migration and
invasion (41). Expression of the
integrins αvβ3 and αvβ5 in tumour cells is associated with cancer
progression (41). Interestingly,
elevated BSP expression in the sera and in tumour sections of
cancer patients is correlated with disease progression and poor
survival as well (42–44). Furthermore, BSP has been identified
as a potential risk factor for bone metastasis development in
breast and lung cancer patients (45,46).
Given that αvβ3 and BSP are co-expressed in prostate cancer cell
lines (47) and primary breast
invasive ductal carcinoma samples (21), the binding of BSP to integrins may
contribute to tumour progression by connecting cancer cells to the
ECM and activating downstream signalling pathways.
Signalling pathways that are stimulated upon
BSP-integrin binding or BSP treatment include, for instance, focal
adhesion kinase (FAK), extracellular signal-regulated kinase (ERK),
phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and
AP-1 signalling (20,25). These signalling pathways promote
tumour growth (48) and metastasis
(49,50). BSP can not only bind to integrins
but can also bind to and activate pro matrix metalloproteinase
(MMP)-2 (51). MMPs play an
important role in tumour metastasis by degrading the ECM and
basement membrane to promote cancer cell migration and invasion
(52). Karadag et al
(53) demonstrated enhanced cancer
cell invasiveness when BSP formed a trimolecular complex with αvβ3
and matrix metalloproteinase (MMP)-2. Cancer cell invasion was
RGD-dependent as the replacement of the RGD motif of BSP with the
motif KAE (lysine-alanine-glutamic acid) inhibited cancer cell
invasiveness (53). This highlights
the importance of the RGD sequence in cancer cell chemotaxis.
Collectively, the current literature suggests that
the interactions of BSP with αvβ3-integrins play a central role in
driving cancer cell chemotaxis and promoting the development of
skeletal metastases in malignant cells. BSP-integrin-induced cancer
cell adhesion and motility seem to be RGD-dependent. However, the
role of BSP/αvβ5-integrin binding in cancer cell adhesion and
tumour progression remains unclear. Because the BSP-integrin
interactions could be essential for the progression of cancer and
metastasis, the present study aimed to elucidate whether BSP
enhances the adhesion of cancer cells by binding its RGD sequence
to the integrin receptors αvβ3 and αvβ5. Adhesion of breast,
prostate and lung cancer cell lines was investigated using a cell
adhesion assay. We hypothesised that breast adenocarcinoma,
prostate adenocarcinoma and large-cell lung cancer cells adhere to
BSP and that this attachment of cancer cells to BSP is mediated by
the RGD sequence of BSP and either the αvβ3-integrin receptors
and/or the αvβ5-integrin receptors.
Materials and methods
Reagents and antibodies
Human plasma FN was purchased from Merck Millipore.
Human BSP (huBSP2) was provided by Immundiagnostik AG. Human VN was
purchased from Sartorius (Göttingen, Germany). Phosphate-buffered
saline (PBS) was purchased from Sigma-Aldrich Chemie GmbH
(Taufkirchen, Germany). FN-derived RGD peptide
glycine-arginine-glycine-aspartic acid-serine-proline (GRGDSP) was
also purchased from Sigma-Aldrich (Merck KGaA). Isotype control,
anti-mouse IgG1 (catalogue no. 02-6100) was purchased from Gibco
(Thermo Fisher Scientific, Inc.). Anti-integrin αvβ3 mouse
monoclonal antibody, (clone LM609; catalogue no. MAB1976Z) and
anti-integrin αvβ5 mouse monoclonal antibody (clone P1F6; catalogue
no. MAB1961Z) were obtained from Millipore (Merck KGaA).
AlamarBlue™ cell viability reagent (catalogue no. DAL1100) was
purchased from Invitrogen (Thermo Fisher Scientific, Inc.).
Cell culture
The human breast carcinoma cell line MDA-MB-231
[DSMZ ACC 732 (RRID:CVCL 0062)] was purchased from Leibniz
Institute DSMZ-German Collection of Microorganisms and Cell
Cultures. Established in 1972, this cell line originated from the
pleural effusion of a woman who had undergone chemotherapy for
breast cancer. MDA-MB-231 cells were cultured in Roswell Park
Memorial Institute (RPMI) 1640 medium (Life Technologies, Ltd.),
supplemented with 10% heat-inactivated fetal calf serum (FCS;
Biochrom GmbH), 1% penicillin-streptomycin (P/S; Sigma-Aldrich;
Merck KGaA), 2 mM L-Alanyl-L-Glutamine (Sigma-Aldrich; Merck KGaA),
5 ml Minimum Essential Medium non-essential amino acids (MEM NEAA),
100X (Gibco; Thermo Fisher Scientific, Inc.) and 1 mM sodium
pyruvate (Gibco; Thermo Fisher Scientific, Inc.). The human
prostate cancer cell line PC-3 (RRID:CVCL 0035) was kindly provided
by Dr. Eva Jüngel and was grown in Iscove's Basal Medium (Biochrom
GmbH), supplemented with 10% heat-inactivated FCS and 1% P/S. The
human large-cell lung carcinoma cell line NCI-H460 [H460] [ATCC
HTB-177 (RRID:CVCL 0459)] was grown in RPMI 1640 medium,
supplemented with 10% heat-inactivated FCS and 1% P/S.
Authentication of cells was conducted using short tandem repeat
profiling. Cells were confirmed to be mycoplasma-free. All cell
lines were grown in a humidified environment at 37°C and 5%
CO2. Medium was renewed twice a week.
Cell adhesion assay
The adhesion assay protocol by Oliveira-Ferrer et
al (54) was modified and used
in the present study, and the adhesion assay was carried out once
in quintuples. Briefly, 24-well suspension culture plates
(Cellstar®; Greiner Bio-One GmbH) were pre-coated (250
µl/well) with PBS, 10 µg/ml human plasma FN and 3 µg/ml huBSP2
diluted in PBS. The concentration of BSP was selected based on
preliminary, unpublished data from the authors' laboratory. The
plates were incubated overnight at room temperature. Cultured cell
lines were washed with PBS, detached from the flask using Accutase
(Sigma-Aldrich; Merck KGaA), centrifuged (5 min at 353 × g and room
temperature) and diluted to 2.4×105 cells/ml in reduced
growth medium (GM) with 2.5% FCS. After removing the supernatant
from the pre-coated suspension culture plates, cells were added to
the wells (6×104 cells/250 µl/well) and were allowed to
adhere for 2 h at 37°C and 5% CO2. The supernatant
containing the unattached cells was then removed and wells were
washed once with PBS (500 µl/well). AlamarBlue™ cell viability
reagent was diluted 1:10 in reduced GM according to the
manufacturer's instructions and added to the plate (500 µl/well).
Blank wells with no coating solution and no cells were also
included. Fluorescence was read at 540 nm excitation wavelength and
590 nm emission wavelength with a fluorescence multi-well reader
(GloMax® Multi+ Detection System; Promega Corporation)
after 4 h (37°C, 5% CO2) using the
alamarBlue® assay.
The alamarBlue® assay is a common method
to study cell viability and cytotoxicity (55). AlamarBlue™ cell viability reagent
(catalogue no. DAL1100) contains resazurin and upon accepting
electrons, the initial blue-coloured and non-fluorescent
resazurin-based solution changes to the pink-coloured and
fluorescent resorufin solution (56). The level of fluorescence is directly
proportional to the number of viable cells.
The alamarBlue® assay was carried out in
quadruplicate (100 µl/well) on a 96-well microplate (Greiner
Bio-One GmbH) to measure cell viability (metabolic activity). Cell
viability was used to quantify the number of adherent cells.
Dose-response curve of BSP and
adherent cells
A BSP dose-response curve (concentration-dependent
adhesion assay) was established for each cancer cell line. In
short, huBSP2 was serially diluted to various concentrations (0 to
≤10 µg/ml) in PBS and coated in triplicate (250 µl/well) on 24-well
suspension culture plates overnight at room temperature. The
ensuing protocol steps were identical to the previous
experiment.
The BSP concentration-dependent adhesion assay was
carried out three times in total and the results were pooled
following completion of the experiments. The concentration of BSP
needed to induce 80% of maximal cell adhesion (EC80) was
calculated for each cell line. In short, EC80 values
were obtained by plotting the dose response data (BSP
concentrations against mean fluorescence readings) using a
sigmoidal curve with a variable slope. The EC80 of
huBSP2 was used in the subsequent RGD-binding and integrin-binding
assays.
RGD-binding assay
To investigate the mechanistic link behind the cell
adhesion of human cancer cell lines to BSP and hence the ECM, an
RGD-binding assay was performed by Stachurska et al
(57) with the following
modification. 24-well suspension culture plates were pre-coated
with 10 µg/ml human VN in sextuplicate (250 µl/well) and the
EC80 of huBSP2 (15–18 wells, 250 µl/well). The
EC80 of huBSP2 was derived from the dose-response curve
of BSP and adherent cells. Plates were incubated overnight at room
temperature. The next day, cultured cells were diluted to
2.4×105 cells/ml in reduced GM with 2.5% FCS and cell
suspensions were incubated for 30 min (37°C and 5% CO2)
with FN-derived RGD peptide GRGDSP at various concentrations (0 to
≤120 µM). In the intervening time, the supernatant of the plate
coating solution was removed and the 24-well plate was incubated
with 3% bovine serum albumin (BSA; PAA Laboratories GmbH) in PBS
(250 µl/well) for 30 min at RT to block non-specific binding sites.
After removal of the blocking solution, wells were washed twice
with PBS (500 µl/well) and the incubated cell suspension was added
to the plate (6×104 cells/250 µl/well). Following a 2-h
incubation period (37°C, 5% CO2), the supernatant was
discarded and wells were washed again with PBS (500 µl/well).
AlamarBlue™ cell viability reagent (1:10 in reduced GM) was added
to the wells (500 µl/well). A blank was also pipetted. The
fluorescence signal was read after 4 h (37%, 5% CO2) to
measure cell viability. Adherent cells were also imaged with
phase-contrast microscopy to evaluate the degree of cell spreading.
The RGD-binding assay was performed in triplicate in three
independent experiments.
Integrin-binding assay
The contribution of the cell surface integrin
receptors αvβ3 and αvβ5 in the cell adhesive activities of BSP was
determined by performing a receptor inhibition assay. The
experiment was carried out as described in the RGD-binding assay
with some modifications. 10 µg/ml human VN and the EC80
of huBSP2 were coated onto a 24-well suspension culture plate at
250 µl per well, in sextuplicate each. Following incubation
overnight at room temperature, the supernatant of the coated plate
was removed and replaced with 3% BSA blocking solution (250
µl/well) for 30 min at room temperature. Meanwhile, cell
suspensions of cultured cell lines (2.4×105 cells/ml in
reduced GM with 2.5% FCS) were incubated with 10 µg/ml
anti-integrin αvβ3 mouse monoclonal antibody, 10 µg/ml
anti-integrin αvβ5 mouse monoclonal antibody or 10 µg/ml isotype
control in duplicate (250 µl/well) for 60 min (37°C, 5%
CO2). The blocking solution was removed and the plate
was washed twice with PBS (500 µl/well). Cells were then added to
the coated culture plate (6×104 cells/250 µl/well) and
incubated for 2 h (37°C, 5% CO2). The supernatant
containing the non-adherent cells was discarded and the plate was
washed once with PBS (500 µl/well). PBS was removed through vacuum
suction and 500 µl of alamarBlue™ cell viability reagent (1:10 in
2.5% FCS GM) was added into each well. A blank was also included.
The attached cells were quantified by measuring the cell viability
after 4 h (37°C, 5% CO2) using the alamarBlue™ assay.
Two independent integrin-binding experiments were carried out in
duplicate.
Statistical analysis
The cell viability assay was used as an indirect
measurement of cell adhesion. The fluorescence signals were
corrected by subtracting the mean fluorescence value of the blank
(i.e. auto-fluorescence of alamarBlue™ cell viability reagent) from
all individual sample readings. The individual sample readings of
each group were averaged for quantitative analysis of cell
adhesion. Percentages were calculated relative to the negative
control as follows: % control=[(control-treated)/control] ×100.
Values are presented as mean ± standard deviation.
One-way ANOVA, followed by Dunnett's multiple comparison test where
appropriate, was performed using GraphPad Prism 9 software for
Windows (GraphPad Software, Inc.; Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
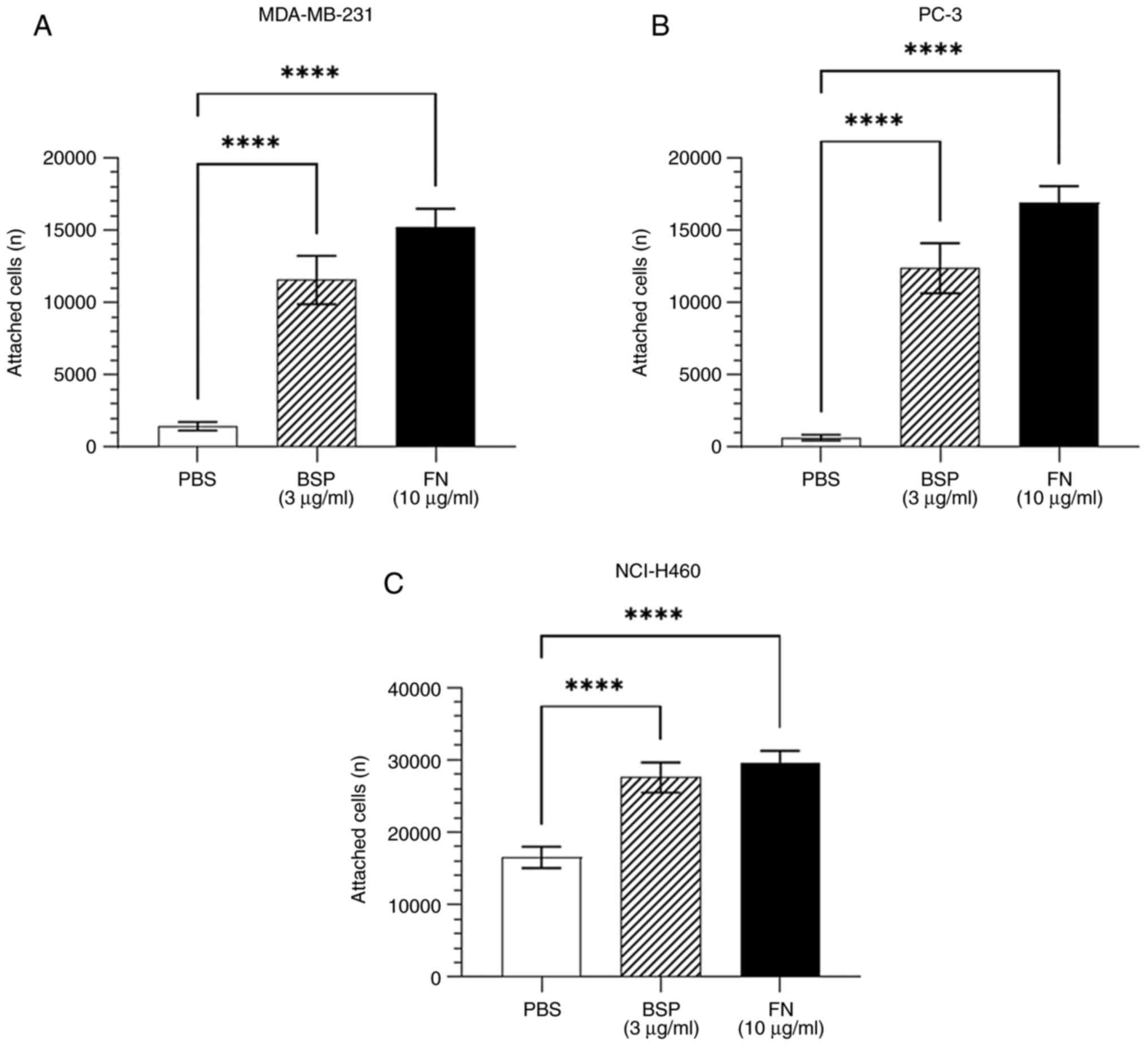
Cancer cells adhere to BSP in
vitro
Adhesion assays were performed to determine whether
human cancer cells adhere to BSP. As shown in Fig. 1, BSP significantly supported the
attachment of breast adenocarcinoma MDA-MB-231, prostate
adenocarcinoma PC-3 and large-cell lung cancer NCI-H460 cell lines
compared with the negative control (PBS).
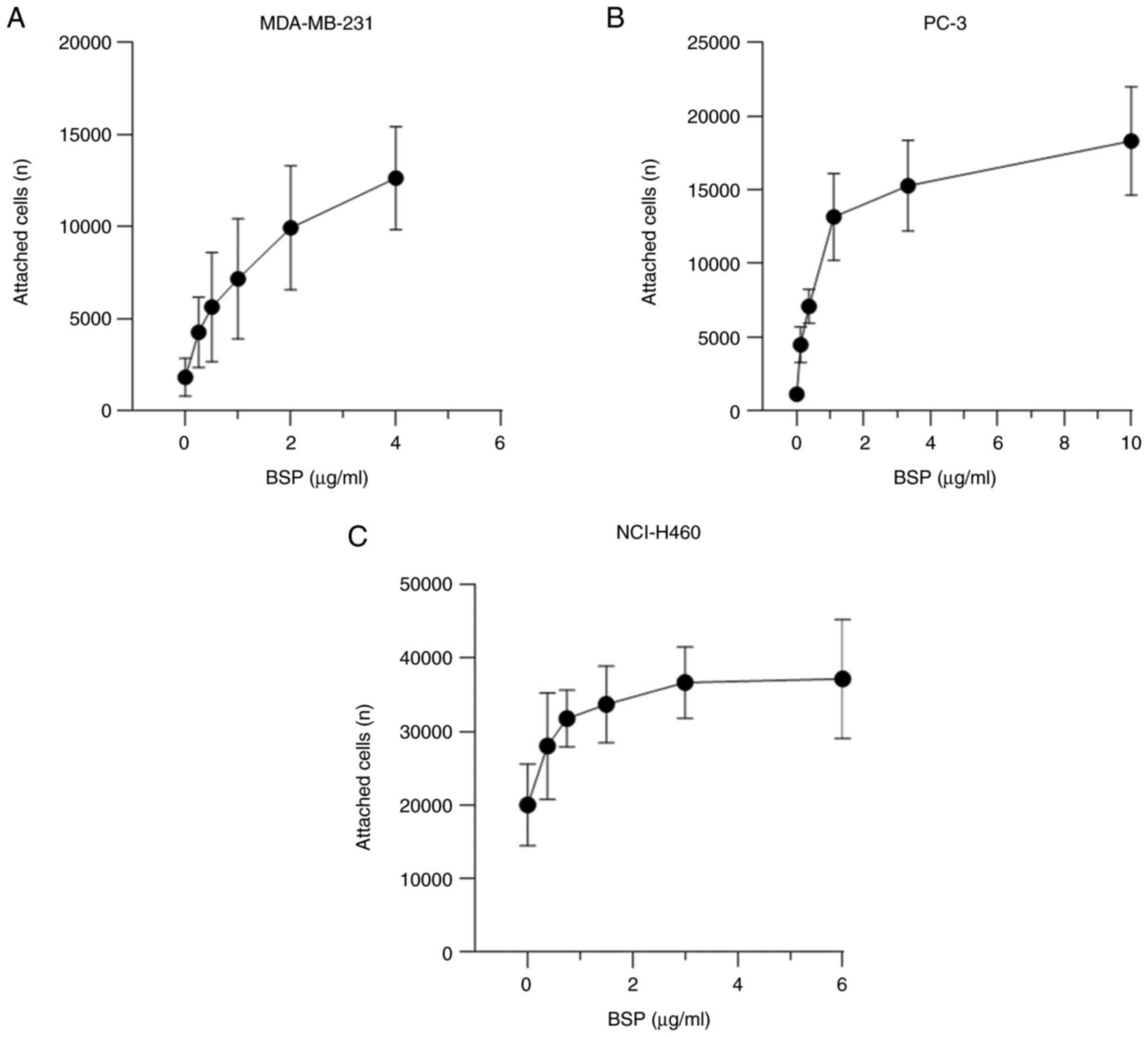
Cancer cell adhesion to BSP is
dose-dependent
The current findings demonstrate a dose-dependent
stimulation of cell attachment to BSP (Fig. 2). The dose-response curve of BSP and
adherent breast adenocarcinoma MDA-MB-231 cells was constructed
using the following huBSP2 concentrations: 4, 2, 1, 0.5, 0.25 and 0
µg/ml. huBSP2 was serially diluted to 10, 3.33, 1.11, 0.37, 0.123
and 0 µg/ml for PC-3, while it was serially diluted to 6, 3, 1.5,
0.75, 0.375 and 0 µg/ml for NCI-H460. The amount of adherent cancer
cells increased with increasing protein concentrations. The
concentration of BSP that led to EC80 was 1.209 µg/ml
for MDA-MB-231, 3.5 µg/ml for PC-3 and 1.403 µg/ml for
NCI-H460.
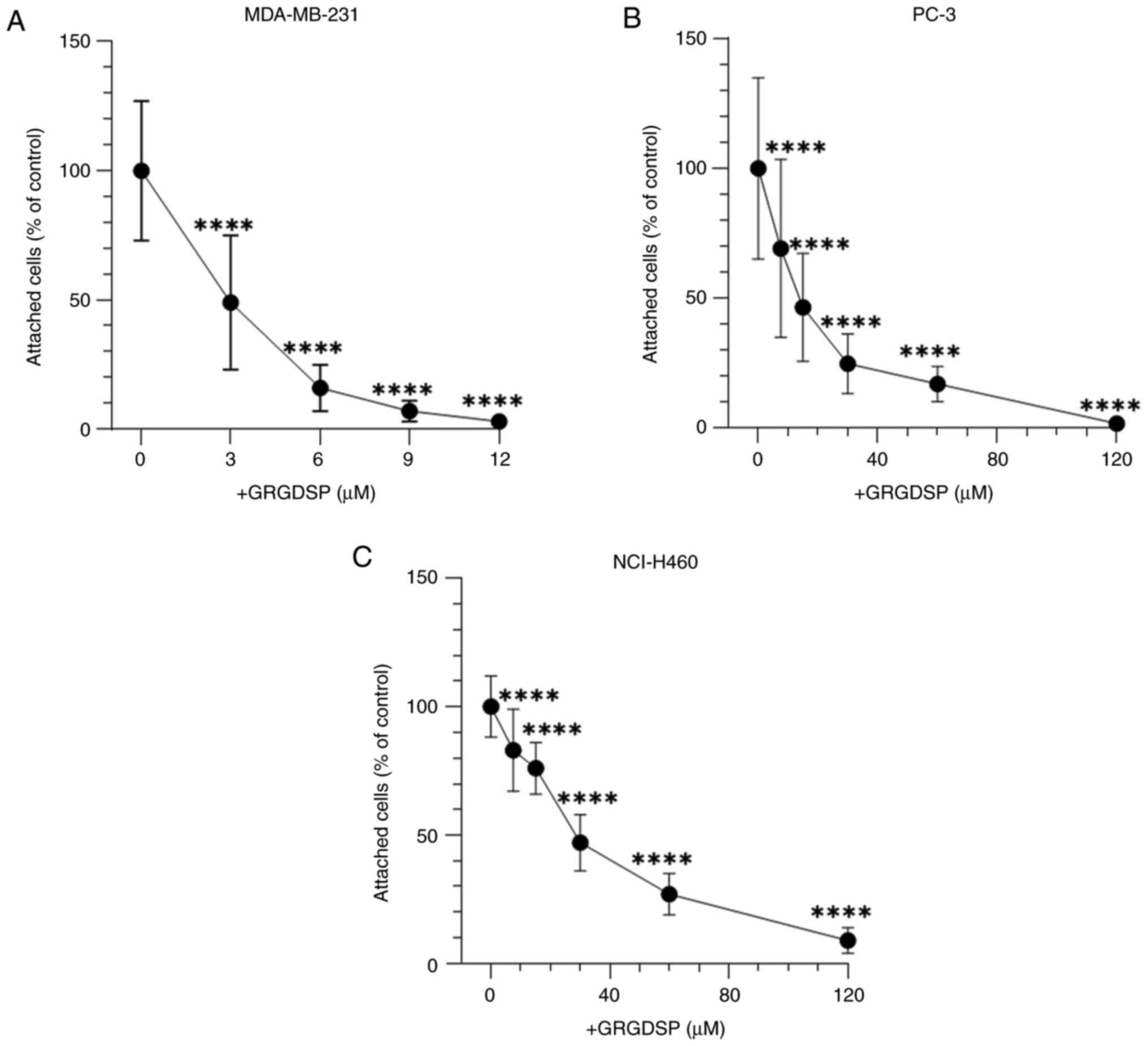
RGD sequence strongly regulates the
adhesion of cancer cells to BSP
The involvement of the RGD sequence in BSP-mediated
cancer cell adhesion was examined. GRGDSP significantly inhibited
the attachment of all cancer cell lines to BSP in a
concentration-dependent manner (Fig.
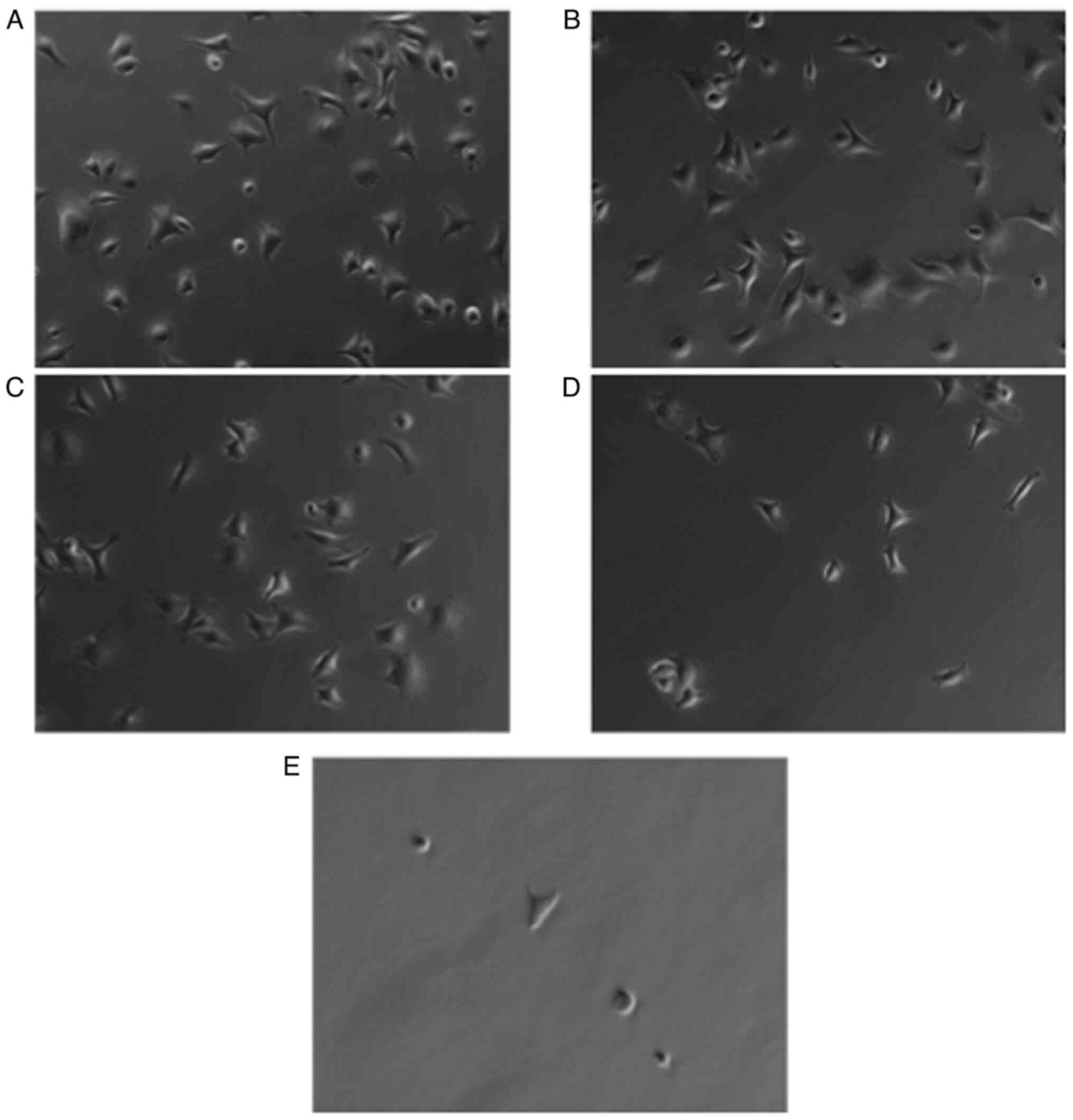
3). Cell shape of cancer cells was elongated in the absence and
at low concentrations of GRGDSP peptide (Fig. 4A-C). With elevated levels of
RGD-containing peptide, cells became more round-shaped and detached
from BSP-coated plates (Fig. 4D and
E).
The exogenously added RGD peptide had a limited
effect on the level of breast adenocarcinoma MDA-MB-231 and
large-cell lung cancer cell NCI-H460 adhesion to VN (data not
shown). Cell adhesion to VN decreased by 7.6% for MDA-MB-231 at 12
µM RGD-containing peptide compared with the prior incubation with 0
µM GRGDSP [55723±5560 (fluorescence 560/590 nm) vs. 60311±8150] and
by 6.3% for NCI-H460 at 120 µM RGD motif-containing peptide
(39549±5697 vs. 42217±6853). Exogenous RGD peptide could inhibit
prostate carcinoma cell attachment, though the inhibition achieved
with GRGDSP was not complete (data not shown). 120 µM GRGDSP
strongly inhibited the attachment of PC-3 to VN by 62.8% with
respect to 0 µM GRGDSP (13226±7038 vs. 35575±14194).
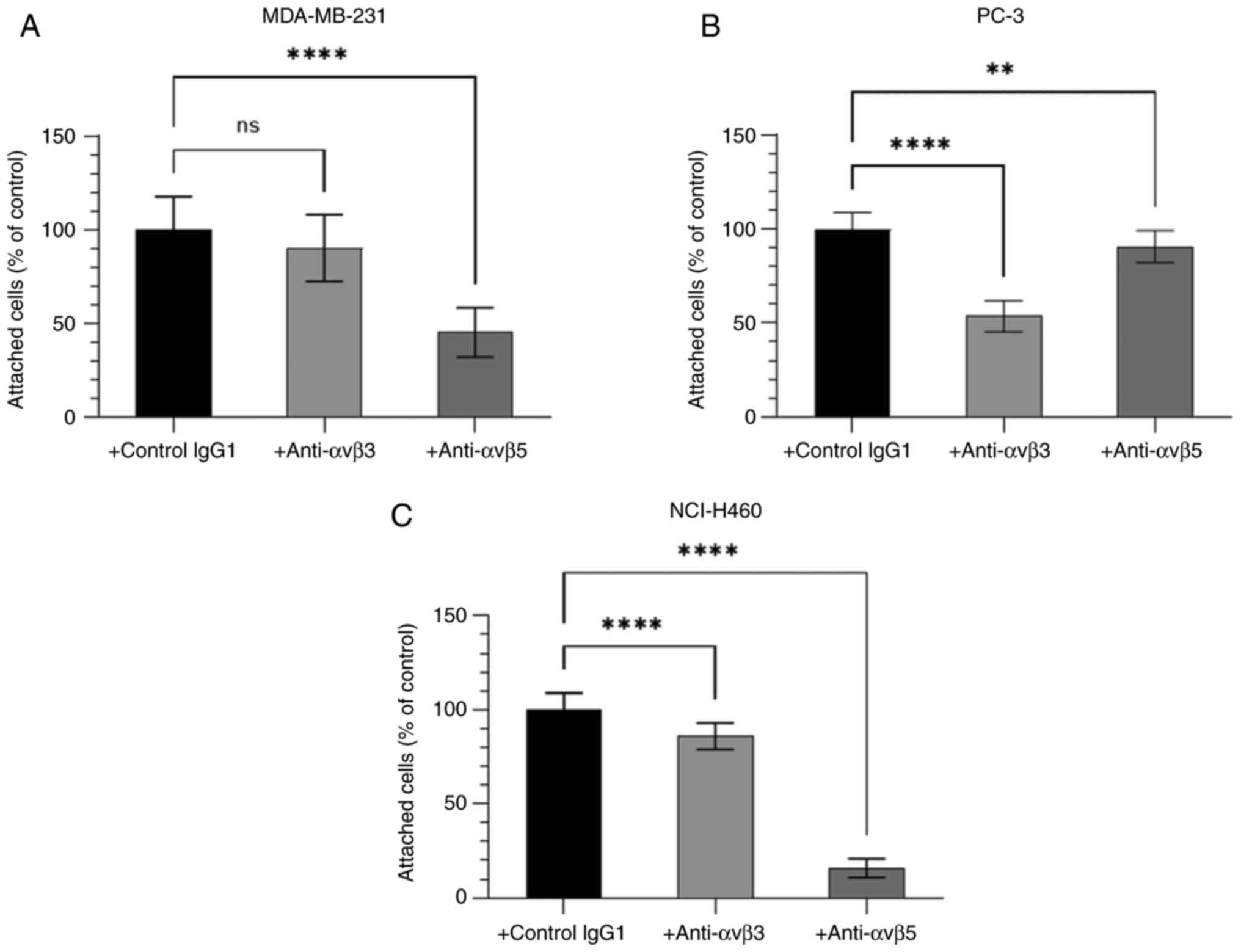
Binding of cancer cells to BSP is
integrin-mediated
Since the RGD domain can bind to integrin receptors,
the function of the integrin receptors αvβ3 and αvβ5 in cancer cell
adhesion to BSP was further characterised. The adhesion of human
cancer cell lines MDA-MB-231, PC-3 and NCI-H460 to BSP was
dependent upon the αvβ3-integrin receptors and the αvβ5-integrin
receptors (Fig. 5). The degree of
contribution of integrins αvβ3 and αvβ5 to BSP-induced cancer cell
adhesion differed among cell lines.
Discussion
Several studies have shown that BSP is involved in
multiple steps in tumour progression, including cancer cell
attachment (35). Cancer cell
adhesion to the extracellular matrix of bone is a critical step in
the metastatic cascade. Previous research suggests that the RGD
sequence of BSP can bind to αvβ3 and αvβ5 integrins on cancer cells
to induce cancer cell attachment (26,35).
Elucidating the role of BSP in cancer cell attachment will help to
understand the development of bone metastases, improve precision
medicine and ultimately advance current cancer treatment
strategies.
The present authors performed several in
vitro adhesion assays to identify the mechanism behind the
homing of human breast, prostate and lung cancer cells to the bone.
We hypothesised that cancer cell lines adhere to BSP and that the
adhesion is regulated by the RGD-binding domain of BSP and the
αvβ3-integrin and/or αvβ5-integrin receptors. To the best of our
knowledge, the present study is the first to demonstrate that
large-cell lung carcinoma NCI-H460 cells adhere to BSP. The current
results also showed that BSP promotes the cell attachment of
prostate adenocarcinoma PC-3 and breast adenocarcinoma MDA-MB-231
cells, which is in agreement with previous studies (25,26).
We did not investigate downstream signalling pathways in
BSP-induced cancer cell adhesion. However, previous studies showed
that BSP is able to stimulate several signalling pathways involved
in cancerous growth and spread (20,25).
Gordon et al (25) observed
BSP-mediated activation of FAK-ERK after adherence of PC-3 and
MDA-MB-231 cells to rat BSP for one hour. Importantly, an intact
RGD-integrin binding sequence of BSP is required for FAK and MAPK
activation, as MDA-MB-231 cells infected with mutated BSP (BSP-KAE)
exhibit lower FAK and ERK phosphorylation (25). Similar to BSP-induced adhesion of
PC-3 and MDA-MB-231 cells, adherence of NCI-H460 cells to BSP
potentially activates RGD-dependent FAK and ERK signalling. The
attachment of all three cancer cell lines to BSP was extensively
mediated by the RGD motif. This was previously observed in murine
cementoblasts (58), murine
osteoblast-like cells (29), human
bone fibroblasts (59) and human
skin fibroblasts (59) as well. By
contrast, van der Pluijm et al (60) reported no effect of adding
commercially available (non-BSP derived) GRGDS peptide at
concentrations up to 300 µM on the attachment of the human breast
cancer cell line MDA-MB-231 to ECM of human bone cells.
Nonetheless, cyclic synthetic BSP peptides containing the EPRGDNYR
sequence were strong inhibitors of breast cancer cell adhesion to
human ECM at concentrations of only 2 µM (60). The authors argued that the adhesion
of MDA-MB-231 cells to the ECM of bone is not solely regulated by
the RGD sequence of BSP. In the present study, FN-derived
RGD-peptide GRGDSP was effective, almost completely blocking (97%)
breast adenocarcinoma adhesion to BSP. Mintz et al (59) also reported that 0.4 mM synthetic
GRGDS peptide completely inhibited the attachment of human bone
cells and human skin fibroblasts to rat BSP. The contribution of
tyrosine-rich repeat to BSP cell attachment activity was likely
negligible as attachment of breast cancer, prostate adenocarcinoma
and NSCLC cells was greatly diminished with GRGDSP (31). Cell attachment independent of
tyrosine residues was previously described in osteopontin (OPN),
another member of the SIBLING family of proteins, as well (61).
The present study showed for the first time that the
adhesion of the PC-3 cell line to BSP is mainly αvβ3-dependent,
whereas the adhesion of the NCI-H460 cell line to BSP is
essentially αvβ5-mediated. BSP-induced breast cancer adhesion
mainly used αvβ5 receptors. The data also suggests that large-cell
lung carcinoma cells partially use the αvβ3 integrin receptors to
regulate BSP-induced cell adhesion, whereas prostate cancer cells
use, to a small, but significant degree, the αvβ5 integrin
receptors to regulate BSP-induced cell adhesion. Colon
adenocarcinoma cells also use the integrins αvβ3 and αvβ5 to adhere
to OPN (61). Depending on the
cancer cell line, the integrin heterodimers αvβ3 and αvβ5 may
preferentially bind to BSP. NCI-H460 cell adhesion to BSP was
greatly diminished in the presence of αvβ5-integrin antibody.
However, neither the αvβ3- nor the αvβ5-integrin antibody was able
to completely inhibit BSP-induced adhesion of the NSCLC, prostate
adenocarcinoma and breast adenocarcinoma cells. Stimulation of
cancer cells with an exogenous agonist, such as phorbol
12-myristate 13-acetate (PMA) or adenosine diphosphate (ADP), after
antibody incubation, could potentially increase the binding of the
integrin receptors to BSP (62,63).
However, the results of the present study showed that integrin
activation was not required for the adhesion of cancer cells to
BSP. Future studies should pre-treat cells with anti-αvβ3 and
anti-αvβ5 integrin antibodies, followed by stimulation with 200 nM
or 200 ng/ml PMA or various concentrations of ADP (2–2,000 µM) to
examine the effect on breast adenocarcinoma, prostate
adenocarcinoma and large-cell lung cancer cell attachment to BSP.
BSP may also adjust the expression of integrins in cancer cells to
regulate cancer cell adhesion. BSP-infected MDA-MB-231 and PC-3
cells display elevated levels of the integrin subunits αv, β3 and
β5, leading to greater focal adhesion formation in relation to
non-infected cells or cells infected with mutated BSP (BSP-KAE)
(25). The expression of BSP by a
cancer cell may increase the cell's potential to metastasise.
Future studies should treat or infect cells with BSP prior to cell
seeding.
As mentioned earlier, cells use integrin receptors
in adhesion and chemotaxis differently, which could provide another
possible explanation as to why the adhesion of cells to BSP was not
completely blocked by integrin antibodies. Sung et al
(26) observed that the attachment
of MDA-MB-231 cells to BSP was αvβ5-dependent, whereas the
αvβ3-integrin receptors mediated the migration of MDA-MB-231 cells
to BSP-derived RGD peptides. The results in the present study
suggested that integrins αvβ3 and αvβ5 differentially mediate cell
adhesion depending on the cell type in which they are expressed.
Sung et al (26) did not
examine additional integrin receptors in the blocking experiments.
Likewise, the current study only looked at the αvβ3- and the
αvβ5-integrin receptors and did not quantify the expression levels
of these receptors on the cell surface. However, previous research
indicated that the breast adenocarcinoma cell line MDA-MB-231 and
the prostate adenocarcinoma cell line PC-3 strongly express αvβ5
integrin receptors (26,38,64).
Less is known about αvβ5 integrin receptors in the NSCLC cell line
NCI-H460. One study reported weak expression levels of αvβ5 in
NCI-H460 cells (39), while αvβ3
integrin receptor expression varies within and between cancer cell
lines, ranging from low to moderate expression in the breast cancer
cell line MDA-MB-231 (26,64) and from moderate to very high
expression in the cancer cell lines NCI-H460 and PC-3 (36–38,40).
It is therefore plausible that prior incubation with anti-integrin
αvβ5 antibody led to a greater reduction in the number of attached
MDA-MB-231 cells to BSP compared with the αvβ3 antibody incubation.
By contrast, the similar expression levels of integrin αvβ5 and
αvβ3 in PC-3 cannot explain the findings of the present
investigation. The results seen in NCI-H460 should be interpreted
with caution given the limited knowledge of αvβ5 integrin
expression. Whilst integrin αvβ3 is expressed at a significantly
greater level on NCI-H460 in comparison with integrin αvβ5,
integrin αvβ3 may not be the main integrin receptor driving NSCLC
cell attachment to BSP. This would also support the notion of the
differential employment of integrins in cell adhesion and
chemotaxis. Flow cytometric evaluation of αvβ5 integrin receptor
expression on NCI-H460 cells will improve the current understanding
of integrin-mediated large-cell lung cancer adhesion to BSP.
Other RGD-binding integrin receptors could have been
involved in the cell adhesion assays. The breast adenocarcinoma
cell line MDA-MB-231 expresses, apart from the αvβ5 and αvβ3
integrin receptors, also the αvβ6 RGD-binding integrin receptor
(64) and the RGD-binding integrin
subunit β1 (65). The prostate
cancer cell line PC-3 expresses multiple RGD-binding integrin
receptors including the αvβ5, αvβ3, αvβ6, α5β1 and αvβ1 receptors
(38). NCI-H460 cells express the
RGD-recognising integrin receptor α5β1 and the RGD-binding integrin
subunits α5, αv, β1 and β6 (66).
Cell-specific integrin receptor expression may also explain why
different RGD peptide concentrations were needed to strongly or
completely inhibit the attachment of the three cancer cell lines to
BSP. The GRGDSP peptide potentially bound to integrin receptors on
MDA-MB-231 cells at a higher affinity in contrast to PC-3 and
NCI-H460 cells (67,68). Further tests will elucidate whether
other integrin receptors, such as α5β1, αvβ1 or αvβ6, mediate
BSP-induced cancer cell adhesion. Among these, αvβ6 is a promising
candidate as the receptor can recognise the RGD sequence of OPN
(61).
Additional BSP sequences could have regulated
BSP-induced cancer cell attachment. The present study did not
investigate the role of the heparin-binding sequence of human BSP,
leucine-histidine-arginine-arginine-valine-lysine-isoleucine
(LHRRVKI), in cancer cell adhesion. Other studies demonstrated that
the heparin-binding sequence of rat BSP,
phenylalanine-histidine-arginine-arginine-isoleucine-lysine-alanine
(FHRRIKA), plays a role in BSP-mediated cell adhesion. For
instance, Rezania and Healy (34)
showed that less rat osteoblast-like cells attach to homogenously
coated FHRRIKA surfaces when compared with surfaces coated with
both RGD and FHRRIKA. Furthermore, no focal contact formation by
bone cells is observed on homogenous FHRRIKA-coated surfaces with
respect to mimetic peptide surfaces (RGD and FHRRIKA) (34). Further in vitro cell adhesion
assays should pre-treat cell suspensions with an LHRRVKI-containing
peptide at various concentrations to determine the involvement of
the heparin-binding domain in human cancer cell adhesion to BSP.
Co-incubation of cells with an LHRRVKI-containing peptide and
FN-derived GRGDSP peptide will provide additional information on
the potential interplay between the two protein sequences in
BSP-induced cancer cell adhesion.
A major limitation in the current study is the use
of the alamarBlue® assay to determine cell adherence.
The assay is commonly used to quantify cytotoxicity and cell
viability. Alternatively, adherent cells may be fixed and stained
with crystal violet (CV) (69).
Dying cells lose their ability to adhere. CV dye stains the DNA and
proteins of cells (70). The number
of attached cells can be counted by eluting the CV dye and reading
the absorbance with a spectrophotometer (69). However, previous studies also solely
used the alamarBlue® assay to quantify cell adhesion
(71–73). Furthermore, the
alamarBlue® assay was previously confirmed as a suitable
method to measure the migration and invasion of choriocarcinoma
cells (74). Therefore, we believe
that using only the alamarBlue® assay to evaluate cell
adhesion did not negatively impact our results or conclusions.
The present results have important implications for
drug development. Knockdown of BSP or the use of BSP inhibitors may
be a more feasible and effective approach for cancer therapy than
integrin-targeted therapeutics due to several reasons, that is,
integrins sharing the same subunits and the complexity of the
integrin signalling cascade. To date, only seven integrin
inhibitors have been approved by the U.S. Food and Drug
Administration. None of the current drugs on the market target the
integrins αvβ3 or αvβ5 or are used in cancer therapy (75), emphasising the need for new cancer
drugs to block integrin-ECM interactions and tumour progression.
Previous studies on animal models showed that silencing of BSP in
human breast cancer and human lung cancer cells inhibits the
development of bone metastases (20,76,77),
possibly owing to decreased expression levels of αvβ3 (77) and MMP-14 (20). Silencing of BSP may also suppress
tissue remodelling by MMPs and inhibit cancer cell invasion into
surrounding tissue, as intact BSP is able to stimulate the activity
of proMMP-2 (51). In vitro,
treatment of cancer cells with BSP enhances the binding of cancer
cells to MMP-2 (53) and increases
the mRNA expression of MMPs MMP-14, MMP-2 and MMP-9 (20,25),
leading to enhanced cancer cell migration and invasion (20). Interfering with BSP expression may
suppress the formation of skeletal metastases in vivo, in
part, by inhibiting MMP-dependent cancer cell chemotaxis. Thus,
future cancer drug development may focus on BSP-targeted
therapeutics.
In conclusion, the present study demonstrated that
the RGD sequence is essential to BSP-mediated adhesion of
adenocarcinoma, prostate adenocarcinoma and NSCLC cells. Cancer
cell attachment to BSP occurs through the binding of αvβ3 and αvβ5
integrins. The RGD-integrin interaction serves as a mechanistic
link for the homing of cancer cells to bone. Targeting this
cell-ECM adhesion with antibodies or RGD-containing peptides may
provide a new approach to the prevention and treatment of skeletal
metastases.
Acknowledgements
The authors would like to thank Dr Eva Jüngel
(Department of Urology and Pediatric Urology, University Medical
Center Mainz, Mainz, Germany) for providing the PC-3 cell line.
This research article is part of the doctoral thesis of VK.
Funding
Immundiagnostik AG (Bensheim, Germany) provided financial
support.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
UR and FC devised the project, and FPA, EG and PD
contributed to the conception and design of the study. UR, FC and
AB administered the project. VK, EK, GB, AB and FC designed
experiments. VK, EK and GB performed experiments. UR, PD, EG and
FPA provided resources. UR, VK and AB analysed data. VK designed
the figures. VK wrote the manuscript with support from UR. AB, FC,
FPA, PD and EG performed critical revisions of the intellectual
content of the manuscript. UR and VK confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
FPA is the CEO of Immundiagnostik AG and FC is an
employee of Immundiagnostik AG. AB was an employee of
Immundiagnostik AG at the time of the study. Immundiagnostik AG
supplied the human bone sialoprotein and provided funding. The
other authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
BSP
|
bone sialoprotein
|
|
ECM
|
extracellular matrix
|
|
GRGDSP
|
glycine-arginine-glycine-aspartic
acid-serine-proline
|
|
RGD
|
arginine-glycine-aspartic acid
|
|
SIBLING
|
small integrin-binding ligand N-linked
glycoprotein
|
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Pineros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. 5:335882021.
|
|
2
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Disibio G and French SW: Metastatic
patterns of cancers: Results from a large autopsy study. Arch
Pathol Lab Med. 132:931–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riihimäki M, Thomsen H, Sundquist K,
Sundquist J and Hemminki K: Clinical landscape of cancer
metastases. Cancer Med. 7:5534–5542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Budczies J, von Winterfeld M, Klauschen F,
Bockmayr M, Lennerz JK, Denkert C, Wolf T, Warth A, Dietel M,
Anagnostopoulos I, et al: The landscape of metastatic progression
patterns across major human cancers. Oncotarget. 6:570–583. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uprimny C, Svirydenka A, Fritz J, Kroiss
AS, Nilica B, Decristoforo C, Haubner R, von Guggenberg E, Buxbaum
S, Horninger W and Virgolini IJ: Comparison of
[68Ga]Ga-PSMA-11 PET/CT with [18F]NaF PET/CT
in the evaluation of bone metastases in metastatic prostate cancer
patients prior to radionuclide therapy. Eur J Nucl Med Mol Imaging.
45:1873–1883. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roudier MP, Corey E, True LD, Hiagno CS,
Ott SM and Vessell RL: Histological, immunophenotypic and
histomorphometric characterization of prostate cancer bone
metastases. Cancer Treat Res. 118:311–339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hansen JA, Naghavi-Behzad M, Gerke O, Baun
C, Falch K, Duvnjak S, Alavi A, Hoilund-Carlsen PF and Hildebrandt
MG: Diagnosis of bone metastases in breast cancer: Lesion-based
sensitivity of dual-time-point FDG-PET/CT compared to low-dose CT
and bone scintigraphy. PLoS One. 16:e02600662021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phanphaisarn A, Patumanond J, Settakorn J,
Chaiyawat P, Klangjorhor J and Pruksakorn D: Prevalence and
survival patterns of patients with bone metastasis from common
cancers in Thailand. Asian Pac J Cancer Prev. 17:4335–4340.
2016.PubMed/NCBI
|
|
10
|
Huang JF, Shen JF, Li X, Rengan R,
Silvestris N, Wang M, Derosa L, Zheng XQ, Belli A, Zhang XL, et al:
Incidence of patients with bone metastases at diagnosis of solid
tumors in adults: A large population-based study. Ann Transl Med.
8:4822020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bellahcene A, Castronovo V, Ogbureke KU,
Fisher LW and Fedarko NS: Small integrin-binding ligand N-linked
glycoproteins (SIBLINGs): Multifunctional proteins in cancer. Nat
Rev Cancer. 8:212–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Staines KA, MacRae VE and Farquharson C:
The importance of the SIBLING family of proteins on skeletal
mineralisation and bone remodelling. J Endocrinol. 214:241–255.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bouleftour W, Juignet L, Bouet G, Granito
RN, Vanden-Bossche A, Laroche N, Aubin JE, Lafage-Proust MH, Vico L
and Malaval L: The role of the SIBLING, bone sialoprotein in
skeletal biology-contribution of mouse experimental genetics.
Matrix Biol. 52–54. 60–77. 2016.
|
|
14
|
Bianco P, Fisher LW, Young MF, Termine JD
and Robey PG: Expression of bone sialoprotein (BSP) in developing
human tissues. Calcif Tissue Int. 49:421–426. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Midura RJ, Midura SB, Su X and Gorski JP:
Separation of newly formed bone from older compact bone reveals
clear compositional differences in bone matrix. Bone. 49:1365–1374.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baht GS, Hunter GK and Goldberg HA: Bone
sialoprotein-collagen interaction promotes hydroxyapatite
nucleation. Matrix Biol. 27:600–608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldberg HA, Warner KJ, Li MC and Hunter
GK: Binding of bone sialoprotein, osteopontin and synthetic
polypeptides to hydroxyapatite. Connect Tissue Res. 42:25–37. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malaval L, Wade-Gueye NM, Boudiffa M, Fei
J, Zirngibl R, Chen F, Laroche N, Roux JP, Burt-Pichat B, Duboeuf
F, et al: Bone sialoprotein plays a functional role in bone
formation and osteoclastogenesis. J Exp Med. 205:1145–1153. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kovacheva M, Zepp M, Berger SM and Berger
MR: Sustained conditional knockdown reveals intracellular bone
sialoprotein as essential for breast cancer skeletal metastasis.
Oncotarget. 5:5510–5522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen WC, Chang AC, Tsai HC, Liu PI, Huang
CL, Guo JH, Liu CL, Liu JF, Thuong LH and Tang CH: Bone
sialoprotein promotes lung cancer osteolytic bone metastasis via
MMP14-dependent mechanisms. Biochem Pharmacol. 211:1155402023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Song L, Li J, Wang Y, Yang C, Kou
X, Xiao B, Zhang W, Li L, Liu S and Wang J: Bone
sialoprotein-alphavbeta3 integrin axis promotes breast cancer
metastasis to the bone. Cancer Sci. 110:3157–3172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fedarko NS, Fohr B, Robey PG, Young MF and
Fisher LW: Factor H binding to bone sialoprotein and osteopontin
enables tumor cell evasion of complement-mediated attack. J Biol
Chem. 275:16666–16672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kriegel A, Langendorf E, Kottmann V,
Kammerer PW, Armbruster FP, Wiesmann-Imilowski N, Baranowski A,
Gercek E, Drees P, Rommens PM and Ritz U: Bone sialoprotein
immobilized in collagen type I enhances angiogenesis in vitro and
in ovo. Polymers (Basel). 15:10072023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu B, Xu M, Guo Z, Liu J, Chu X and Jiang
H: Interleukin-8 promotes prostate cancer bone metastasis through
upregulation of bone sialoprotein. Oncol Lett. 17:4607–4613.
2019.PubMed/NCBI
|
|
25
|
Gordon JA, Sodek J, Hunter GK and Goldberg
HA: Bone sialoprotein stimulates focal adhesion-related signaling
pathways: Role in migration and survival of breast and prostate
cancer cells. J Cell Biochem. 107:1118–1128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sung V, Stubbs JT III, Fisher L, Aaron AD
and Thompson EW: Bone sialoprotein supports breast cancer cell
adhesion proliferation and migration through differential usage of
the alpha(v)beta3 and alpha(v)beta5 integrins. J Cell Physiol.
176:482–494. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bachmann M, Kukkurainen S, Hytönen VP and
Wehrle-Haller B: Cell adhesion by integrins. Physiol Rev.
99:1655–1699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oldberg A, Franzen A, Heinegård D,
Pierschbacher M and Ruoslahti E: Identification of a bone
sialoprotein receptor in osteo-sarcoma cells. J Biol Chem.
263:19433–19436. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rapuano BE and MacDonald DE:
Structure-activity relationship of human bone sialoprotein
peptides. Eur J Oral Sci. 121:600–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ganss B, Kim RH and Sodek J: Bone
sialoprotein. Crit Rev Oral Biol Med. 10:79–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stubbs JT III, Mintz KP, Eanes ED, Torchia
DA and Fisher LW: Characterization of native and recombinant bone
sialoprotein: Delineation of the mineral-binding and cell adhesion
domains and structural analysis of the RGD domain. J Bone Miner
Res. 12:1210–1222. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujisawa R, Nodasaka Y and Kuboki Y:
Further characterization of interaction between bone sialoprotein
(BSP) and collagen. Calcif Tissue Int. 56:140–144. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang Y and Kiick KL:
Heparin-functionalized polymeric biomaterials in tissue engineering
and drug delivery applications. Acta Biomater. 10:1588–1600. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rezania A and Healy KE: Biomimetic peptide
surfaces that regulate adhesion, spreading, cytoskeletal
organization, and mineralization of the matrix deposited by
osteoblast-like cells. Biotechnol Prog. 15:19–32. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Byzova TV, Kim W, Midura RJ and Plow EF:
Activation of integrin alpha(V)beta(3) regulates cell adhesion and
migration to bone sialoprotein. Exp Cell Res. 254:299–308. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albert JM, Cao C, Geng L, Leavitt L,
Hallahan DE and Lu B: Integrin alpha v beta 3 antagonist
Cilengitide enhances efficacy of radiotherapy in endothelial cell
and non-small-cell lung cancer models. Int J Radiat Oncol Biol
Phys. 65:1536–1543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He X, Hao Y, Long W, Song N, Fan S and
Meng A: Exploration of peptide T7 and its derivative as integrin
αvβ3-targeted imaging agents. Onco Targets Ther. 8:1483–1491.
2015.PubMed/NCBI
|
|
38
|
Sutherland M, Gordon A, Shnyder SD,
Patterson LH and Sheldrake HM: RGD-binding integrins in prostate
cancer: Expression patterns and therapeutic prospects against bone
metastasis. Cancers (Basel). 4:1106–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takayama K, Ueno H, Pei XH, Nakanishi Y,
Yatsunami J and Hara N: The levels of integrin alpha v beta 5 may
predict the susceptibility to adenovirus-mediated gene transfer in
human lung cancer cells. Gene Ther. 5:361–368. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu X, Zhang R, Liu F, Ping J, Wen X, Wang
H, Wang K, Sun X, Zou H, Shen B and Wu L: 19F MRI in
orthotopic cancer model via intratracheal administration of
ανβ3-targeted perfluorocarbon nanoparticles.
Nanomedicine (Lond). 13:2551–2562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He JJ, Zhi K and Liu GF: Predictive value
of serum bone sialoprotein in patients with bone metastasis of
non-small cell lung cancer. Onkologie. 34:584–588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Papotti M, Kalebic T, Volante M, Chiusa L,
Bacillo E, Cappia S, Lausi P, Novello S, Borasio P and Scagliotti
GV: Bone sialoprotein is predictive of bone metastases in
resectable non-small-cell lung cancer: A retrospective case-control
study. J Clin Oncol. 24:4818–4824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Righi L, Bollito E, Ceppi P, Mirabelli D,
Tavaglione V, Chiusa L, Porpiglia F, Brunelli M, Martignoni G,
Terrone C and Papotti M: Prognostic role of bone sialoprotein in
clear cell renal carcinoma. Anticancer Res. 33:2679–2687.
2013.PubMed/NCBI
|
|
45
|
Niu Y, Lin Y, Pang H, Shen W, Liu L and
Zhang H: Risk factors for bone metastasis in patients with primary
lung cancer: A systematic review. Medicine (Baltimore).
98:e140842019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pulido C, Vendrell I, Ferreira AR,
Casimiro S, Mansinho A, Alho I and Costa L: Bone metastasis risk
factors in breast cancer. Ecancermedicalscience. 11:7152017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu M, Jiang H, Wang H, Liu J, Liu B and
Guo Z: SB225002 inhibits prostate cancer invasion and attenuates
the expression of BSP, OPN and MMP-2. Oncol Rep. 40:726–736.
2018.PubMed/NCBI
|
|
48
|
Wang J, Guo X, Xie C and Jiang J: KIF15
promotes pancreatic cancer proliferation via the MEK-ERK signalling
pathway. Br J Cancer. 117:245–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dou P, Zhang D, Cheng Z, Zhou G and Zhang
L: PKIB promotes cell proliferation and the invasion-metastasis
cascade through the PI3K/Akt pathway in NSCLC cells. Exp Biol Med
(Maywood). 241:1911–1918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu HJ, Hao M, Yeo SK and Guan JL: FAK
signaling in cancer-associated fibroblasts promotes breast cancer
cell migration and metastasis by exosomal miRNAs-mediated
intercellular communication. Oncogene. 39:2539–2549. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fedarko NS, Jain A, Karadag A and Fisher
LW: Three small integrin binding ligand N-linked glycoproteins
(SIBLINGs) bind and activate specific matrix metalloproteinases.
FASEB J. 18:734–736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Karadag A, Ogbureke KU, Fedarko NS and
Fisher LW: Bone sialoprotein, matrix metalloproteinase 2, and
alpha(v)beta3 integrin in osteotropic cancer cell invasion. J Natl
Cancer Inst. 96:956–965. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Oliveira-Ferrer L, Rößler K, Haustein V,
Schröder C, Wicklein D, Maltseva D, Khaustova N, Samatov T,
Tonevitsky A, Mahner S, et al: c-FOS suppresses ovarian cancer
progression by changing adhesion. Br J Cancer. 110:753–763. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rampersad SN: Multiple applications of
Alamar Blue as an indicator of metabolic function and cellular
health in cell viability bioassays. Sensors (Basel).
12:12347–12360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mommsen TP and Moon TW: Biochemistry and
molecular biology of fishes. Environ Toxicol. 6:51–56. 2005.
|
|
57
|
Stachurska A, Elbanowski J and
Kowalczyńska HM: Role of α5β1 and αvβ3 integrins in relation to
adhesion and spreading dynamics of prostate cancer cells
interacting with fibronectin under in vitro conditions. Cell Biol
Int. 36:883–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nagasaki K, Chavez MB, Nagasaki A, Taylor
JM, Tan MH, Ma M, Ralston E, Thew ME, Kim DG, Somerman MJ and
Foster BL: The bone sialoprotein RGD domain modulates and maintains
periodontal development. J Dent Res. 101:1238–1247. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mintz KP, Grzesik WJ, Midura RJ, Robey PG,
Termine JD and Fisher LW: Purification and fragmentation of
nondenatured bone sialoprotein: Evidence for a cryptic,
RGD-resistant cell attachment domain. J Bone Miner Res. 8:985–995.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
van der Pluijm G, Vloedgraven HJ, Ivanov
B, Robey FA, Grzesik WJ, Robey PG, Papapoulos SE and Lowik CW: Bone
sialoprotein peptides are potent inhibitors of breast cancer cell
adhesion to bone. Cancer Res. 56:1948–1955. 1996.PubMed/NCBI
|
|
61
|
Yokosaki Y, Tanaka K, Higashikawa F,
Yamashita K and Eboshida A: Distinct structural requirements for
binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5,
alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol.
24:418–427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Khawaja AA, Pericleous C, Ripoll VM,
Porter JC and Giles IP: Autoimmune rheumatic disease IgG has
differential effects upon neutrophil integrin activation that is
modulated by the endothelium. Sci Rep. 9:12832019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee SH, Sud N, Lee N, Subramaniyam S and
Chung CY: Regulation of integrin α6 recycling by
calcium-independent phospholipase A2 (iPLA2) to promote microglia
chemotaxis on laminin. J Biol Chem. 291:23645–23653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Taherian A, Li X, Liu Y and Haas TA:
Differences in integrin expression and signaling within human
breast cancer cells. BMC Cancer. 11:2932011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ziperstein MJ, Guzman A and Kaufman LJ:
Breast cancer cell line aggregate morphology does not predict
invasive capacity. PLoS One. 10:e01395232015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gaud G, Iochmann S, Guillon-Munos A,
Brillet B, Petiot S, Seigneuret F, Touzé A, Heuzé-Vourc'h N, Courty
Y, Lerondel S, et al: TFPI-2 silencing increases tumour progression
and promotes metalloproteinase 1 and 3 induction through
tumour-stromal cell interactions. J Cell Mol Med. 15:196–208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kapp TG, Rechenmacher F, Neubauer S,
Maltsev OV, Cavalcanti-Adam EA, Zarka R, Reuning U, Notni J, Wester
HJ, Mas-Moruno C, et al: A comprehensive evaluation of the activity
and selectivity profile of ligands for RGD-binding integrins. Sci
Rep. 7:398052017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhou P, Feng F, Song Y, Li J, Li Q, Xu Z,
Shi J, Qin L, He F, Li H, et al: Novel RGD-containing peptides
exhibited improved abilities to integrin receptor binding and
cultures of human induced pluripotent stem cells. Mater Design.
219:1107622022. View Article : Google Scholar
|
|
69
|
Kariya Y, Kanno M, Matsumoto-Morita K,
Konno M, Yamaguchi Y and Hashimoto Y: Osteopontin O-glycosylation
contributes to its phosphorylation and cell-adhesion properties.
Biochem J. 463:93–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Feoktistova M, Geserick P and Leverkus M:
Crystal violet assay for determining viability of cultured cells.
Cold Spring Harb Protoc 2016: pdb prot087379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Castelletto V, Gouveia RM, Connon CJ,
Hamley IW, Seitsonen J, Nykänen A and Ruokolainen J: Alanine-rich
amphiphilic peptide containing the RGD cell adhesion motif: A
coating material for human fibroblast attachment and culture.
Biomater Sci. 2:362–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Silva JC, Moura CS, Alves N, Cabral JMS
and Ferreira FC: Effects of different fibre alignments and
bioactive coatings on mesenchymal stem/stromal cell adhesion and
proliferation in poly (ε-caprolactone) scaffolds towards cartilage
repair. Procedia Manuf. 12:132–140. 2017. View Article : Google Scholar
|
|
73
|
Uto K, Mano SS, Aoyagi T and Ebara M:
Substrate fluidity regulates cell adhesion and morphology on
poly(ε-caprolactone)-based materials. ACS Biomater Sci Eng.
2:446–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Al-Nasiry S, Geusens N, Hanssens M, Luyten
C and Pijnenborg R: The use of Alamar blue assay for quantitative
analysis of viability, migration and invasion of choriocarcinoma
cells. Hum Reprod. 22:1304–1309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Slack RJ, Macdonald SJF, Roper JA, Jenkins
RG and Hatley RJD: Emerging therapeutic opportunities for integrin
inhibitors. Nat Rev Drug Discov. 21:60–78. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Reufsteck C, Lifshitz-Shovali R, Zepp M,
Bäuerle T, Kübler D, Golomb G and Berger MR: Silencing of skeletal
metastasis-associated genes impairs migration of breast cancer
cells and reduces osteolytic bone lesions. Clin Exp Metastasis.
29:441–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang J, Wang L, Xia B, Yang C, Lai H and
Chen X: BSP gene silencing inhibits migration, invasion, and bone
metastasis of MDA-MB-231BO human breast cancer cells. PLoS One.
8:e629362013. View Article : Google Scholar : PubMed/NCBI
|