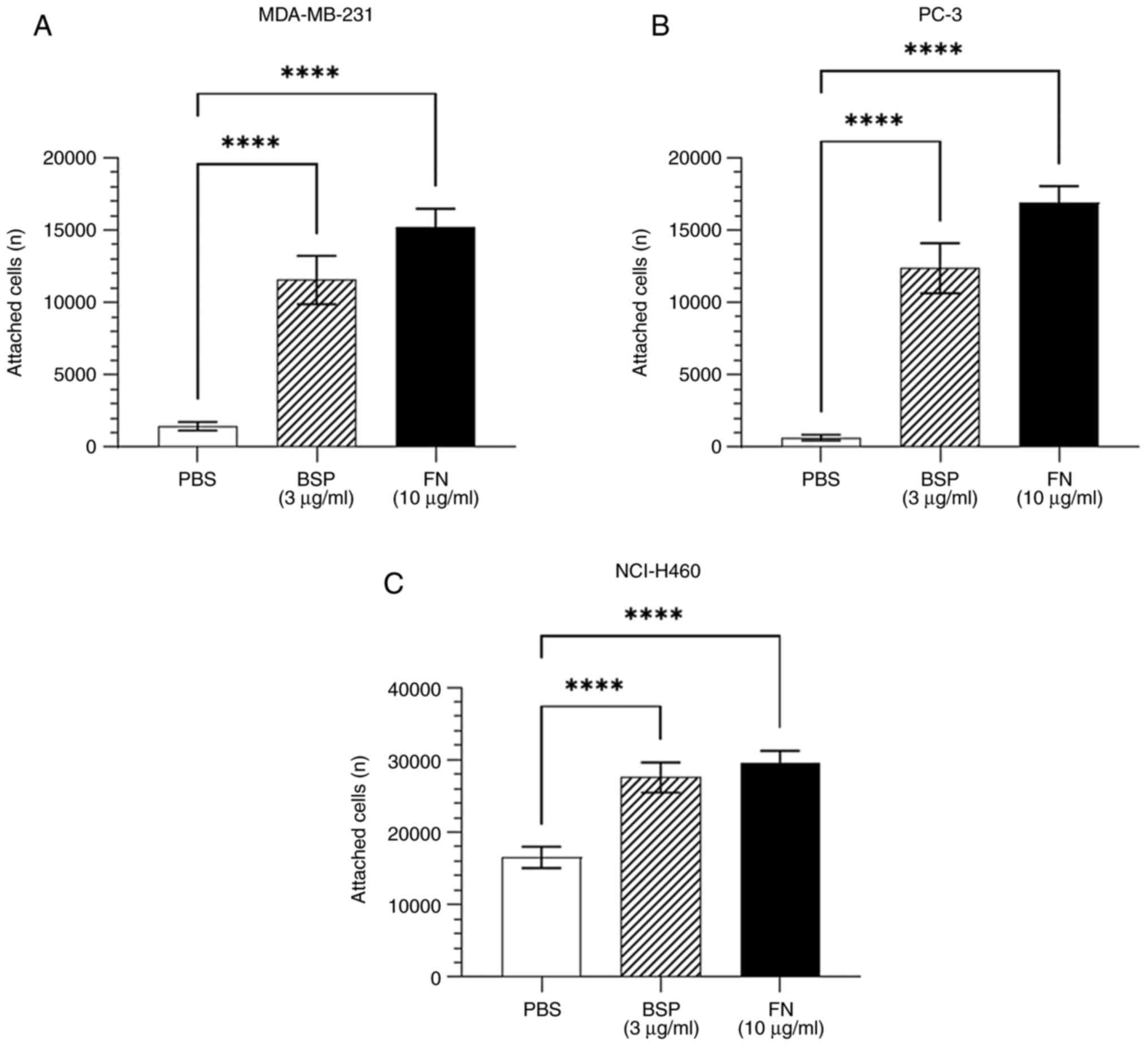
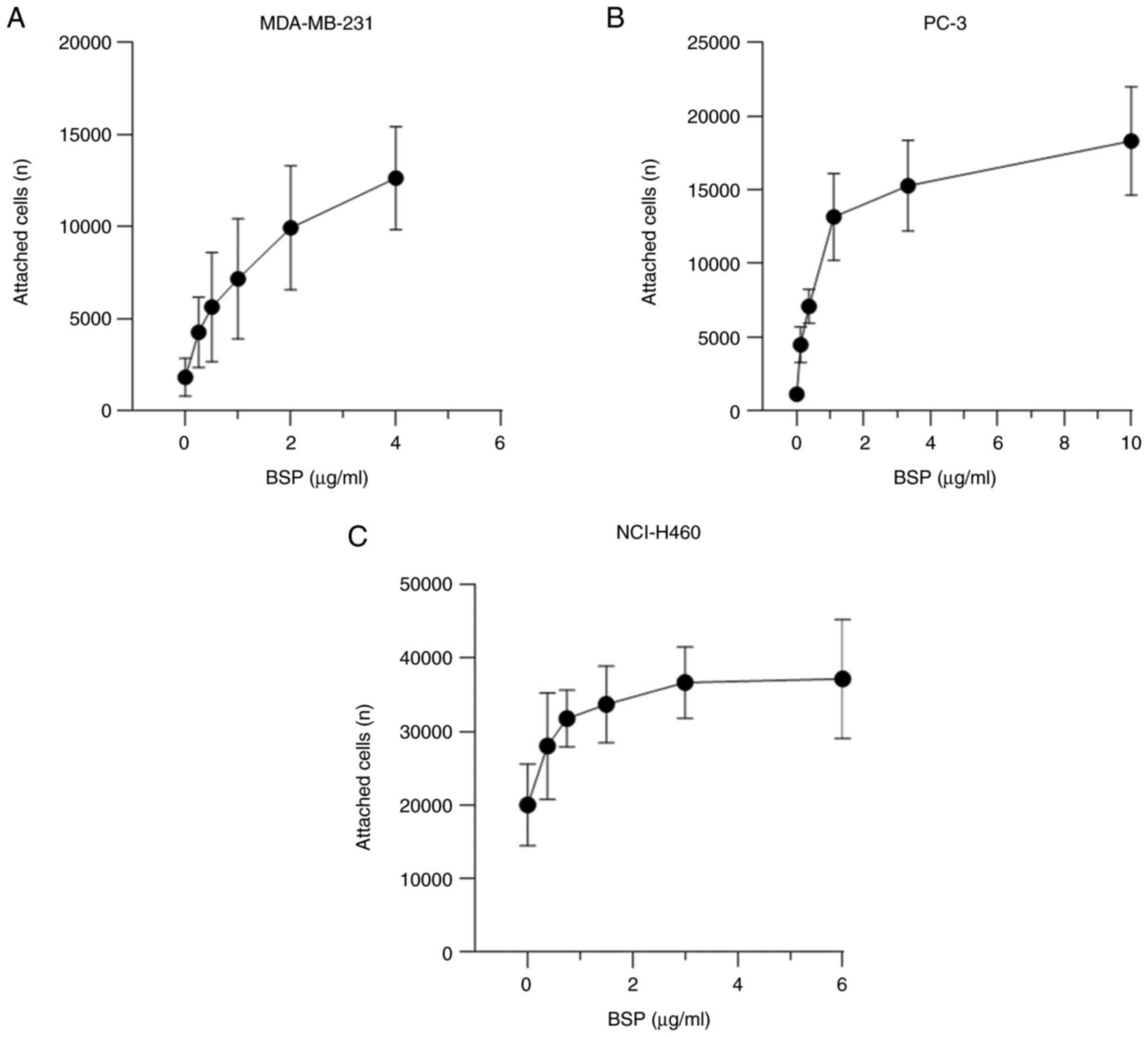
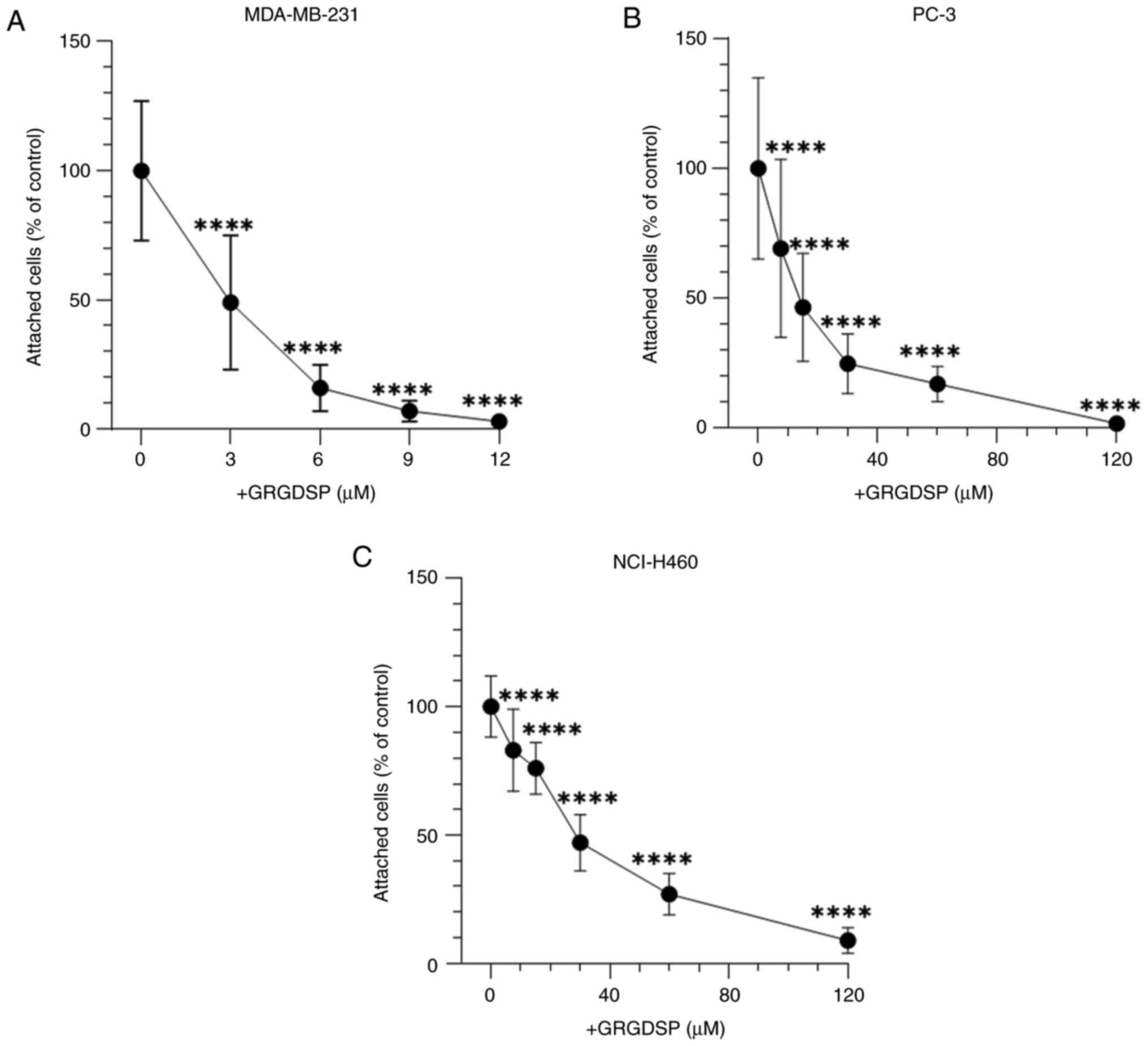
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Pineros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. 5:335882021.
|
|
2
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Disibio G and French SW: Metastatic
patterns of cancers: Results from a large autopsy study. Arch
Pathol Lab Med. 132:931–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riihimäki M, Thomsen H, Sundquist K,
Sundquist J and Hemminki K: Clinical landscape of cancer
metastases. Cancer Med. 7:5534–5542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Budczies J, von Winterfeld M, Klauschen F,
Bockmayr M, Lennerz JK, Denkert C, Wolf T, Warth A, Dietel M,
Anagnostopoulos I, et al: The landscape of metastatic progression
patterns across major human cancers. Oncotarget. 6:570–583. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uprimny C, Svirydenka A, Fritz J, Kroiss
AS, Nilica B, Decristoforo C, Haubner R, von Guggenberg E, Buxbaum
S, Horninger W and Virgolini IJ: Comparison of
[68Ga]Ga-PSMA-11 PET/CT with [18F]NaF PET/CT
in the evaluation of bone metastases in metastatic prostate cancer
patients prior to radionuclide therapy. Eur J Nucl Med Mol Imaging.
45:1873–1883. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roudier MP, Corey E, True LD, Hiagno CS,
Ott SM and Vessell RL: Histological, immunophenotypic and
histomorphometric characterization of prostate cancer bone
metastases. Cancer Treat Res. 118:311–339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hansen JA, Naghavi-Behzad M, Gerke O, Baun
C, Falch K, Duvnjak S, Alavi A, Hoilund-Carlsen PF and Hildebrandt
MG: Diagnosis of bone metastases in breast cancer: Lesion-based
sensitivity of dual-time-point FDG-PET/CT compared to low-dose CT
and bone scintigraphy. PLoS One. 16:e02600662021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phanphaisarn A, Patumanond J, Settakorn J,
Chaiyawat P, Klangjorhor J and Pruksakorn D: Prevalence and
survival patterns of patients with bone metastasis from common
cancers in Thailand. Asian Pac J Cancer Prev. 17:4335–4340.
2016.PubMed/NCBI
|
|
10
|
Huang JF, Shen JF, Li X, Rengan R,
Silvestris N, Wang M, Derosa L, Zheng XQ, Belli A, Zhang XL, et al:
Incidence of patients with bone metastases at diagnosis of solid
tumors in adults: A large population-based study. Ann Transl Med.
8:4822020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bellahcene A, Castronovo V, Ogbureke KU,
Fisher LW and Fedarko NS: Small integrin-binding ligand N-linked
glycoproteins (SIBLINGs): Multifunctional proteins in cancer. Nat
Rev Cancer. 8:212–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Staines KA, MacRae VE and Farquharson C:
The importance of the SIBLING family of proteins on skeletal
mineralisation and bone remodelling. J Endocrinol. 214:241–255.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bouleftour W, Juignet L, Bouet G, Granito
RN, Vanden-Bossche A, Laroche N, Aubin JE, Lafage-Proust MH, Vico L
and Malaval L: The role of the SIBLING, bone sialoprotein in
skeletal biology-contribution of mouse experimental genetics.
Matrix Biol. 52–54. 60–77. 2016.
|
|
14
|
Bianco P, Fisher LW, Young MF, Termine JD
and Robey PG: Expression of bone sialoprotein (BSP) in developing
human tissues. Calcif Tissue Int. 49:421–426. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Midura RJ, Midura SB, Su X and Gorski JP:
Separation of newly formed bone from older compact bone reveals
clear compositional differences in bone matrix. Bone. 49:1365–1374.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baht GS, Hunter GK and Goldberg HA: Bone
sialoprotein-collagen interaction promotes hydroxyapatite
nucleation. Matrix Biol. 27:600–608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldberg HA, Warner KJ, Li MC and Hunter
GK: Binding of bone sialoprotein, osteopontin and synthetic
polypeptides to hydroxyapatite. Connect Tissue Res. 42:25–37. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malaval L, Wade-Gueye NM, Boudiffa M, Fei
J, Zirngibl R, Chen F, Laroche N, Roux JP, Burt-Pichat B, Duboeuf
F, et al: Bone sialoprotein plays a functional role in bone
formation and osteoclastogenesis. J Exp Med. 205:1145–1153. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kovacheva M, Zepp M, Berger SM and Berger
MR: Sustained conditional knockdown reveals intracellular bone
sialoprotein as essential for breast cancer skeletal metastasis.
Oncotarget. 5:5510–5522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen WC, Chang AC, Tsai HC, Liu PI, Huang
CL, Guo JH, Liu CL, Liu JF, Thuong LH and Tang CH: Bone
sialoprotein promotes lung cancer osteolytic bone metastasis via
MMP14-dependent mechanisms. Biochem Pharmacol. 211:1155402023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Song L, Li J, Wang Y, Yang C, Kou
X, Xiao B, Zhang W, Li L, Liu S and Wang J: Bone
sialoprotein-alphavbeta3 integrin axis promotes breast cancer
metastasis to the bone. Cancer Sci. 110:3157–3172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fedarko NS, Fohr B, Robey PG, Young MF and
Fisher LW: Factor H binding to bone sialoprotein and osteopontin
enables tumor cell evasion of complement-mediated attack. J Biol
Chem. 275:16666–16672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kriegel A, Langendorf E, Kottmann V,
Kammerer PW, Armbruster FP, Wiesmann-Imilowski N, Baranowski A,
Gercek E, Drees P, Rommens PM and Ritz U: Bone sialoprotein
immobilized in collagen type I enhances angiogenesis in vitro and
in ovo. Polymers (Basel). 15:10072023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu B, Xu M, Guo Z, Liu J, Chu X and Jiang
H: Interleukin-8 promotes prostate cancer bone metastasis through
upregulation of bone sialoprotein. Oncol Lett. 17:4607–4613.
2019.PubMed/NCBI
|
|
25
|
Gordon JA, Sodek J, Hunter GK and Goldberg
HA: Bone sialoprotein stimulates focal adhesion-related signaling
pathways: Role in migration and survival of breast and prostate
cancer cells. J Cell Biochem. 107:1118–1128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sung V, Stubbs JT III, Fisher L, Aaron AD
and Thompson EW: Bone sialoprotein supports breast cancer cell
adhesion proliferation and migration through differential usage of
the alpha(v)beta3 and alpha(v)beta5 integrins. J Cell Physiol.
176:482–494. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bachmann M, Kukkurainen S, Hytönen VP and
Wehrle-Haller B: Cell adhesion by integrins. Physiol Rev.
99:1655–1699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oldberg A, Franzen A, Heinegård D,
Pierschbacher M and Ruoslahti E: Identification of a bone
sialoprotein receptor in osteo-sarcoma cells. J Biol Chem.
263:19433–19436. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rapuano BE and MacDonald DE:
Structure-activity relationship of human bone sialoprotein
peptides. Eur J Oral Sci. 121:600–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ganss B, Kim RH and Sodek J: Bone
sialoprotein. Crit Rev Oral Biol Med. 10:79–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stubbs JT III, Mintz KP, Eanes ED, Torchia
DA and Fisher LW: Characterization of native and recombinant bone
sialoprotein: Delineation of the mineral-binding and cell adhesion
domains and structural analysis of the RGD domain. J Bone Miner
Res. 12:1210–1222. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujisawa R, Nodasaka Y and Kuboki Y:
Further characterization of interaction between bone sialoprotein
(BSP) and collagen. Calcif Tissue Int. 56:140–144. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang Y and Kiick KL:
Heparin-functionalized polymeric biomaterials in tissue engineering
and drug delivery applications. Acta Biomater. 10:1588–1600. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rezania A and Healy KE: Biomimetic peptide
surfaces that regulate adhesion, spreading, cytoskeletal
organization, and mineralization of the matrix deposited by
osteoblast-like cells. Biotechnol Prog. 15:19–32. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Byzova TV, Kim W, Midura RJ and Plow EF:
Activation of integrin alpha(V)beta(3) regulates cell adhesion and
migration to bone sialoprotein. Exp Cell Res. 254:299–308. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albert JM, Cao C, Geng L, Leavitt L,
Hallahan DE and Lu B: Integrin alpha v beta 3 antagonist
Cilengitide enhances efficacy of radiotherapy in endothelial cell
and non-small-cell lung cancer models. Int J Radiat Oncol Biol
Phys. 65:1536–1543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He X, Hao Y, Long W, Song N, Fan S and
Meng A: Exploration of peptide T7 and its derivative as integrin
αvβ3-targeted imaging agents. Onco Targets Ther. 8:1483–1491.
2015.PubMed/NCBI
|
|
38
|
Sutherland M, Gordon A, Shnyder SD,
Patterson LH and Sheldrake HM: RGD-binding integrins in prostate
cancer: Expression patterns and therapeutic prospects against bone
metastasis. Cancers (Basel). 4:1106–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takayama K, Ueno H, Pei XH, Nakanishi Y,
Yatsunami J and Hara N: The levels of integrin alpha v beta 5 may
predict the susceptibility to adenovirus-mediated gene transfer in
human lung cancer cells. Gene Ther. 5:361–368. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu X, Zhang R, Liu F, Ping J, Wen X, Wang
H, Wang K, Sun X, Zou H, Shen B and Wu L: 19F MRI in
orthotopic cancer model via intratracheal administration of
ανβ3-targeted perfluorocarbon nanoparticles.
Nanomedicine (Lond). 13:2551–2562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He JJ, Zhi K and Liu GF: Predictive value
of serum bone sialoprotein in patients with bone metastasis of
non-small cell lung cancer. Onkologie. 34:584–588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Papotti M, Kalebic T, Volante M, Chiusa L,
Bacillo E, Cappia S, Lausi P, Novello S, Borasio P and Scagliotti
GV: Bone sialoprotein is predictive of bone metastases in
resectable non-small-cell lung cancer: A retrospective case-control
study. J Clin Oncol. 24:4818–4824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Righi L, Bollito E, Ceppi P, Mirabelli D,
Tavaglione V, Chiusa L, Porpiglia F, Brunelli M, Martignoni G,
Terrone C and Papotti M: Prognostic role of bone sialoprotein in
clear cell renal carcinoma. Anticancer Res. 33:2679–2687.
2013.PubMed/NCBI
|
|
45
|
Niu Y, Lin Y, Pang H, Shen W, Liu L and
Zhang H: Risk factors for bone metastasis in patients with primary
lung cancer: A systematic review. Medicine (Baltimore).
98:e140842019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pulido C, Vendrell I, Ferreira AR,
Casimiro S, Mansinho A, Alho I and Costa L: Bone metastasis risk
factors in breast cancer. Ecancermedicalscience. 11:7152017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu M, Jiang H, Wang H, Liu J, Liu B and
Guo Z: SB225002 inhibits prostate cancer invasion and attenuates
the expression of BSP, OPN and MMP-2. Oncol Rep. 40:726–736.
2018.PubMed/NCBI
|
|
48
|
Wang J, Guo X, Xie C and Jiang J: KIF15
promotes pancreatic cancer proliferation via the MEK-ERK signalling
pathway. Br J Cancer. 117:245–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dou P, Zhang D, Cheng Z, Zhou G and Zhang
L: PKIB promotes cell proliferation and the invasion-metastasis
cascade through the PI3K/Akt pathway in NSCLC cells. Exp Biol Med
(Maywood). 241:1911–1918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu HJ, Hao M, Yeo SK and Guan JL: FAK
signaling in cancer-associated fibroblasts promotes breast cancer
cell migration and metastasis by exosomal miRNAs-mediated
intercellular communication. Oncogene. 39:2539–2549. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fedarko NS, Jain A, Karadag A and Fisher
LW: Three small integrin binding ligand N-linked glycoproteins
(SIBLINGs) bind and activate specific matrix metalloproteinases.
FASEB J. 18:734–736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Karadag A, Ogbureke KU, Fedarko NS and
Fisher LW: Bone sialoprotein, matrix metalloproteinase 2, and
alpha(v)beta3 integrin in osteotropic cancer cell invasion. J Natl
Cancer Inst. 96:956–965. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Oliveira-Ferrer L, Rößler K, Haustein V,
Schröder C, Wicklein D, Maltseva D, Khaustova N, Samatov T,
Tonevitsky A, Mahner S, et al: c-FOS suppresses ovarian cancer
progression by changing adhesion. Br J Cancer. 110:753–763. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rampersad SN: Multiple applications of
Alamar Blue as an indicator of metabolic function and cellular
health in cell viability bioassays. Sensors (Basel).
12:12347–12360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mommsen TP and Moon TW: Biochemistry and
molecular biology of fishes. Environ Toxicol. 6:51–56. 2005.
|
|
57
|
Stachurska A, Elbanowski J and
Kowalczyńska HM: Role of α5β1 and αvβ3 integrins in relation to
adhesion and spreading dynamics of prostate cancer cells
interacting with fibronectin under in vitro conditions. Cell Biol
Int. 36:883–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nagasaki K, Chavez MB, Nagasaki A, Taylor
JM, Tan MH, Ma M, Ralston E, Thew ME, Kim DG, Somerman MJ and
Foster BL: The bone sialoprotein RGD domain modulates and maintains
periodontal development. J Dent Res. 101:1238–1247. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mintz KP, Grzesik WJ, Midura RJ, Robey PG,
Termine JD and Fisher LW: Purification and fragmentation of
nondenatured bone sialoprotein: Evidence for a cryptic,
RGD-resistant cell attachment domain. J Bone Miner Res. 8:985–995.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
van der Pluijm G, Vloedgraven HJ, Ivanov
B, Robey FA, Grzesik WJ, Robey PG, Papapoulos SE and Lowik CW: Bone
sialoprotein peptides are potent inhibitors of breast cancer cell
adhesion to bone. Cancer Res. 56:1948–1955. 1996.PubMed/NCBI
|
|
61
|
Yokosaki Y, Tanaka K, Higashikawa F,
Yamashita K and Eboshida A: Distinct structural requirements for
binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5,
alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol.
24:418–427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Khawaja AA, Pericleous C, Ripoll VM,
Porter JC and Giles IP: Autoimmune rheumatic disease IgG has
differential effects upon neutrophil integrin activation that is
modulated by the endothelium. Sci Rep. 9:12832019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee SH, Sud N, Lee N, Subramaniyam S and
Chung CY: Regulation of integrin α6 recycling by
calcium-independent phospholipase A2 (iPLA2) to promote microglia
chemotaxis on laminin. J Biol Chem. 291:23645–23653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Taherian A, Li X, Liu Y and Haas TA:
Differences in integrin expression and signaling within human
breast cancer cells. BMC Cancer. 11:2932011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ziperstein MJ, Guzman A and Kaufman LJ:
Breast cancer cell line aggregate morphology does not predict
invasive capacity. PLoS One. 10:e01395232015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gaud G, Iochmann S, Guillon-Munos A,
Brillet B, Petiot S, Seigneuret F, Touzé A, Heuzé-Vourc'h N, Courty
Y, Lerondel S, et al: TFPI-2 silencing increases tumour progression
and promotes metalloproteinase 1 and 3 induction through
tumour-stromal cell interactions. J Cell Mol Med. 15:196–208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kapp TG, Rechenmacher F, Neubauer S,
Maltsev OV, Cavalcanti-Adam EA, Zarka R, Reuning U, Notni J, Wester
HJ, Mas-Moruno C, et al: A comprehensive evaluation of the activity
and selectivity profile of ligands for RGD-binding integrins. Sci
Rep. 7:398052017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhou P, Feng F, Song Y, Li J, Li Q, Xu Z,
Shi J, Qin L, He F, Li H, et al: Novel RGD-containing peptides
exhibited improved abilities to integrin receptor binding and
cultures of human induced pluripotent stem cells. Mater Design.
219:1107622022. View Article : Google Scholar
|
|
69
|
Kariya Y, Kanno M, Matsumoto-Morita K,
Konno M, Yamaguchi Y and Hashimoto Y: Osteopontin O-glycosylation
contributes to its phosphorylation and cell-adhesion properties.
Biochem J. 463:93–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Feoktistova M, Geserick P and Leverkus M:
Crystal violet assay for determining viability of cultured cells.
Cold Spring Harb Protoc 2016: pdb prot087379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Castelletto V, Gouveia RM, Connon CJ,
Hamley IW, Seitsonen J, Nykänen A and Ruokolainen J: Alanine-rich
amphiphilic peptide containing the RGD cell adhesion motif: A
coating material for human fibroblast attachment and culture.
Biomater Sci. 2:362–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Silva JC, Moura CS, Alves N, Cabral JMS
and Ferreira FC: Effects of different fibre alignments and
bioactive coatings on mesenchymal stem/stromal cell adhesion and
proliferation in poly (ε-caprolactone) scaffolds towards cartilage
repair. Procedia Manuf. 12:132–140. 2017. View Article : Google Scholar
|
|
73
|
Uto K, Mano SS, Aoyagi T and Ebara M:
Substrate fluidity regulates cell adhesion and morphology on
poly(ε-caprolactone)-based materials. ACS Biomater Sci Eng.
2:446–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Al-Nasiry S, Geusens N, Hanssens M, Luyten
C and Pijnenborg R: The use of Alamar blue assay for quantitative
analysis of viability, migration and invasion of choriocarcinoma
cells. Hum Reprod. 22:1304–1309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Slack RJ, Macdonald SJF, Roper JA, Jenkins
RG and Hatley RJD: Emerging therapeutic opportunities for integrin
inhibitors. Nat Rev Drug Discov. 21:60–78. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Reufsteck C, Lifshitz-Shovali R, Zepp M,
Bäuerle T, Kübler D, Golomb G and Berger MR: Silencing of skeletal
metastasis-associated genes impairs migration of breast cancer
cells and reduces osteolytic bone lesions. Clin Exp Metastasis.
29:441–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang J, Wang L, Xia B, Yang C, Lai H and
Chen X: BSP gene silencing inhibits migration, invasion, and bone
metastasis of MDA-MB-231BO human breast cancer cells. PLoS One.
8:e629362013. View Article : Google Scholar : PubMed/NCBI
|