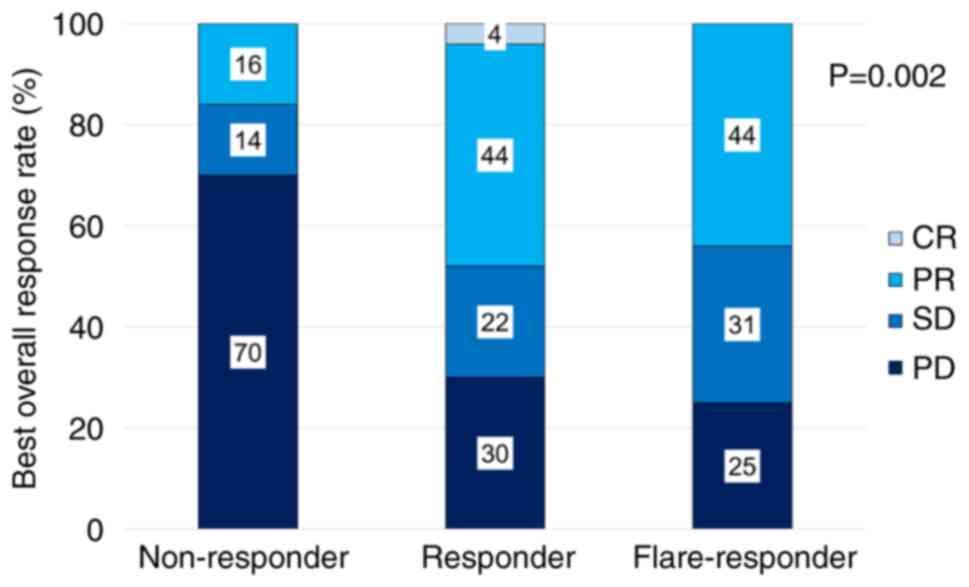
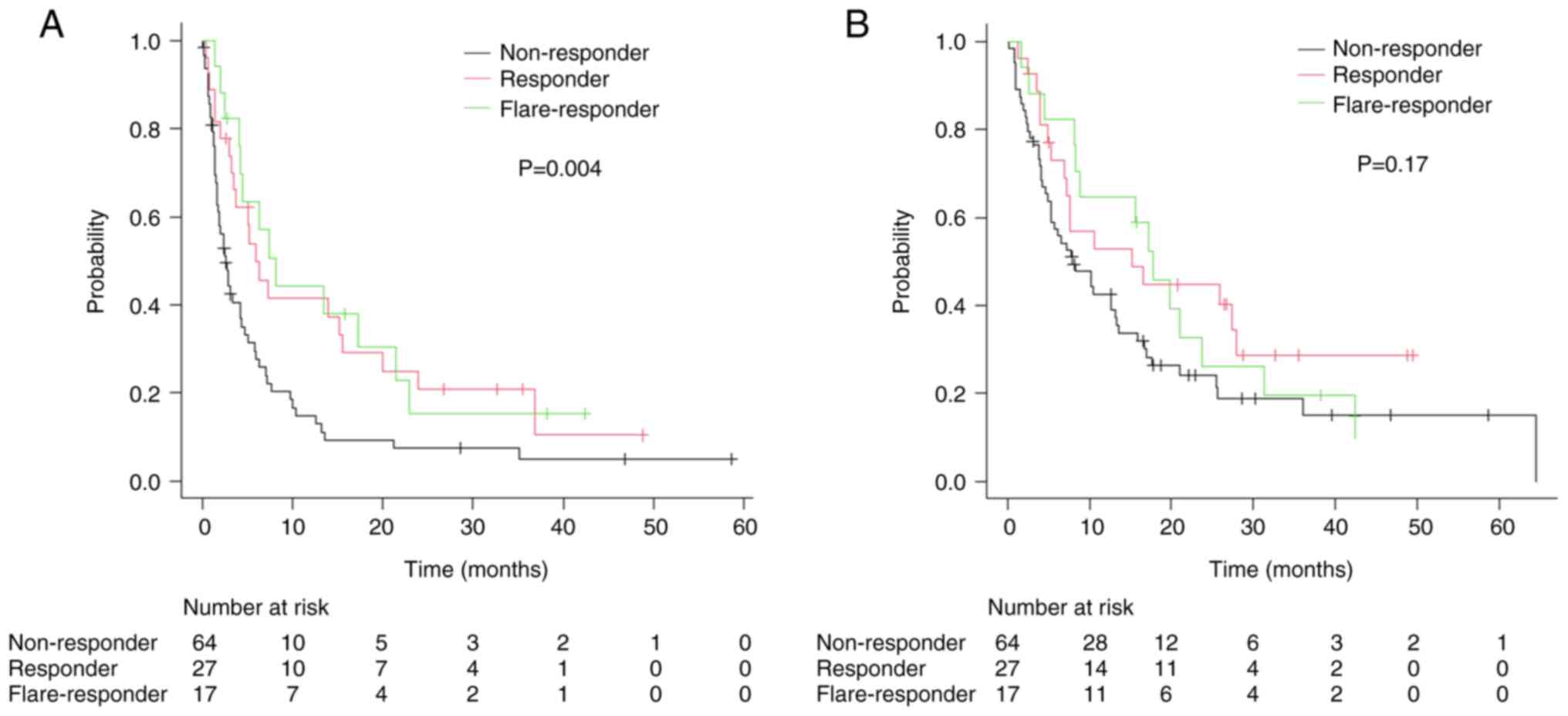
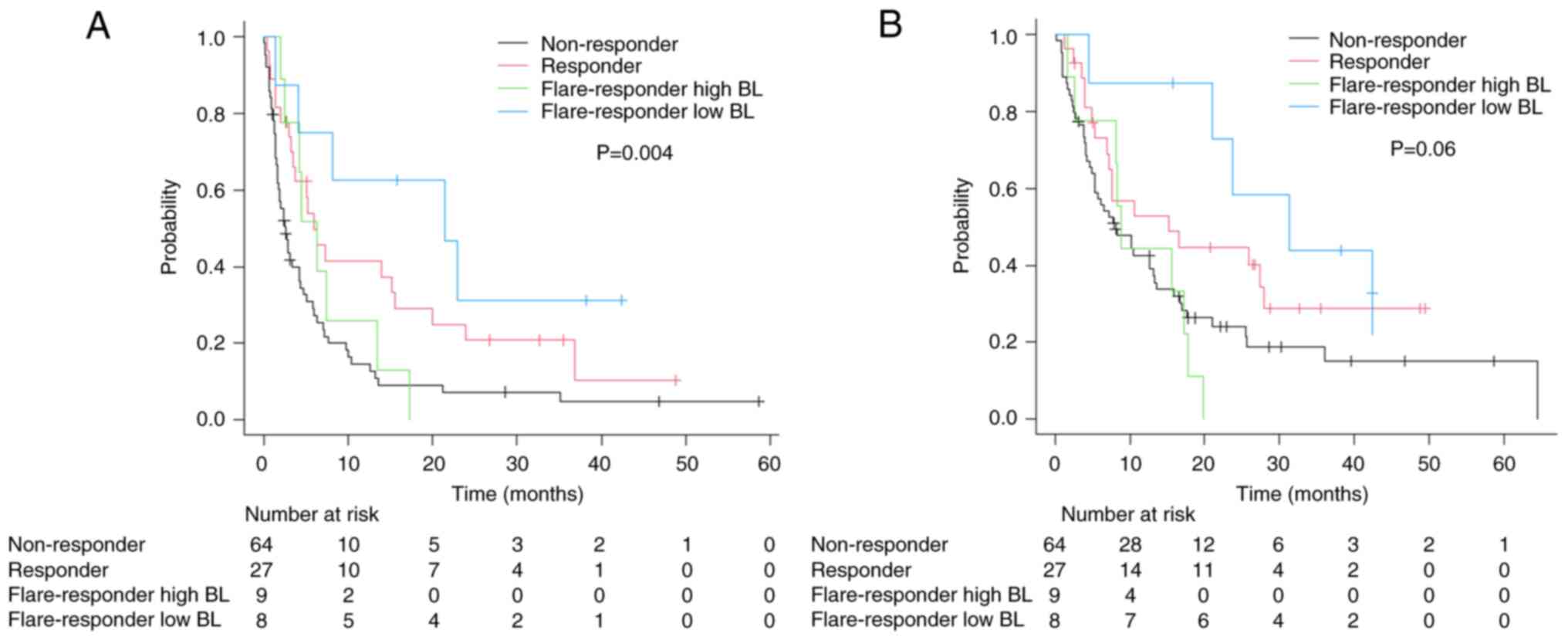
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Flaig TW, Spiess PE, Abern M, Agarwal N,
Bangs R, Buyyounouski MK, Chan K, Chang SS, Chang P, Friedlander T,
et al: NCCN guidelines® insights: Bladder cancer,
version 3.2024. J Natl Compr Canc Netw. 22:216–225. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones RJ, Crabb SJ, Linch M, Birtle AJ,
McGrane J, Enting D, Stevenson R, Liu K, Kularatne B and Hussain
SA: Systemic anticancer therapy for urothelial carcinoma: UK
oncologists' perspective. Br J Cancer. 130:897–907. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taguchi S, Kawai T, Nakagawa T, Miyakawa
J, Kishitani K, Sugimoto K, Nakamura Y, Kamei J, Obinata D,
Yamaguchi K, et al: Improved survival in real-world patients with
advanced urothelial carcinoma: A multicenter propensity
score-matched cohort study comparing a period before the
introduction of pembrolizumab (2003-2011) and a more recent period
(2016-2020). Int J Urol. 29:1462–1469. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balar AV, Castellano DE, Grivas P, Vaughn
DJ, Powles T, Vuky J, Fradet Y, Lee JL, Fong L, Vogelzang NJ, et
al: Efficacy and safety of pembrolizumab in metastatic urothelial
carcinoma: Results from KEYNOTE-045 and KEYNOTE-052 after up to 5
years of follow-up. Ann Oncol. 34:289–299. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bellmunt J, de Wit R, Fradet Y, Climent
MA, Petrylak DP, Lee JL, Fong L, Necchi A, Sternberg CN, O'Donnell
PH, et al: Putative biomarkers of clinical benefit with
pembrolizumab in advanced urothelial cancer: Results from the
KEYNOTE-045 and KEYNOTE-052 landmark trials. Clin Cancer Res.
28:2050–2060. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park HS, Park JY and Yu R: Relationship of
obesity and visceral adiposity with serum concentrations of CRP,
TNF-alpha and IL-6. Diabetes Res Clin Pract. 69:29–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burger PM, Koudstaal S, Mosterd A, Fiolet
ATL, Teraa M, van der Meer MG, Cramer MJ, Visseren FLJ, Ridker PM
and Dorresteijn JAN; UCC-SMART study group, : C-reactive protein
and risk of incident heart failure in patients with cardiovascular
disease. J Am Coll Cardiol. 82:414–426. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu M, Ma Z, Zhang X, Hang D, Yin R, Feng
J, Xu L and Shen H: C-reactive protein and cancer risk: A
pan-cancer study of prospective cohort and Mendelian randomization
analysis. BMC Med. 20:3012022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernandes JV, Cobucci RN, Jatobá CA,
Fernandes TA, de Azevedo JW and de Araújo JM: The role of the
mediators of inflammation in cancer development. Pathol Oncol Res.
21:527–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mosca M, Nigro MC, Pagani R, De Giglio A
and Di Federico A: Neutrophil-to-lymphocyte ratio (NLR) in NSCLC,
gastrointestinal, and other solid tumors: Immunotherapy and beyond.
Biomolecules. 13:18032023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, Fletcher CD, O'Reilly DSJ, Foulis AK, Horgan PG and McMillan
DC: A comparison of inflammation-based prognostic scores in
patients with cancer. A glasgow inflammation outcome study. Eur J
Cancer. 47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han CL, Meng GX, Ding ZN, Dong ZR, Chen
ZQ, Hong JG, Yan LJ, Liu H, Tian BW, Yang LS, et al: The predictive
potential of the baseline C-reactive protein levels for the
efficiency of immune checkpoint inhibitors in cancer patients: A
systematic review and meta-analysis. Front Immunol. 13:8277882022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishihara H, Takagi T, Kondo T, Fukuda H,
Tachibana H, Yoshida K, Iizuka J, Okumi M, Ishida H and Tanabe K:
Predictive impact of an early change in serum C-reactive protein
levels in nivolumab therapy for metastatic renal cell carcinoma.
Urol Oncol. 38:526–532. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kijima T, Yamamoto H, Saito K, Kusuhara S,
Yoshida S, Yokoyama M, Matsuoka Y, Numao N, Sakai Y, Matsubara N,
et al: Early C-reactive protein kinetics predict survival of
patients with advanced urothelial cancer treated with
pembrolizumab. Cancer Immunol Immunother. 70:657–665. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujiwara M, Yuasa T, Urasaki T, Komai Y,
Fujiwara R, Numao N, Yamamoto S and Yonese J: Effectiveness and
safety profile of pembrolizumab for metastatic urothelial cancer: A
retrospective single-center analysis in Japan. Cancer Rep 4. Cancer
Rep (Hoboken). 4:e13982021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto Y, Yatsuda J, Shimokawa M, Fuji
N, Aoki A, Sakano S, Yamamoto M, Suga A, Tei Y, Yoshihiro S, et al:
Prognostic value of pre-treatment risk stratification and
post-treatment neutrophil/lymphocyte ratio change for pembrolizumab
in patients with advanced urothelial carcinoma. Int J Clin Oncol.
26:169–177. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ozawa Y, Amano Y, Kanata K, Hasegwa H,
Matsui T, Kakutani T, Koyauchi T, Tanahashi M, Niwa H, Yokomura K
and Suda T: Impact of early inflammatory cytokine elevation after
commencement of PD-1 inhibitors to predict efficacy in patients
with non-small cell lung cancer. Med Oncol. 36:332019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukuda S, Saito K, Yasuda Y, Kijima T,
Yoshida S, Yokoyama M, Ishioka J, Matsuoka Y, Kageyama Y and Fujii
Y: Impact of C-reactive protein flare-response on oncological
outcomes in patients with metastatic renal cell carcinoma treated
with nivolumab. J Immunother Cancer. 9:e0015642021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tomisaki I, Harada M, Tokutsu K, Minato A,
Nagata Y, Kimuro R, Matsumoto M and Fujimoto N: Impact of
C-reactive protein flare response in patients with advanced
urothelial carcinoma who received pembrolizumab. In Vivo.
35:3563–3568. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klümper N, Sikic D, Saal J, Büttner T,
Goldschmidt F, Jarczyk J, Becker P, Zeuschner P, Weinke M,
Kalogirou C, et al: C-reactive protein flare predicts response to
anti-PD-(L)1 immune checkpoint blockade in metastatic urothelial
carcinoma. Eur J Cancer. 167:13–22. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yasuoka S, Yuasa T, Nishimura N, Ogawa M,
Komai Y, Numao N, Yamamoto S, Kondo Y and Yonese J: Initial
experience of pembrolizumab therapy in japanese patients with
metastatic urothelial cancer. Anticancer Res. 39:3887–3892. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagasaka H, Yamamoto S, Suzuki A, Usui K,
Terao H, Nakaigawa N and Kishida T: C-reactive protein is a
prognostic factor for survival in metastatic upper tract urothelial
carcinoma patients receiving pembrolizumab. In Vivo. 38:1823–1828.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tamura D, Jinnouchi N, Abe M, Ikarashi D,
Matsuura T, Kato R, Maekawa S, Kato Y, Kanehira M, Trakata R and
Obara W: Prognostic outcomes and safety in patients treated with
pembrolizumab for advanced urothelial carcinoma: Experience in
real-world clinical practice. Int J Clin Oncol. 25:899–905. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoeh B, Garcia CC, Banek S, Klümper N, Cox
A, Ellinger J, Schmucker P, Hahn O, Mattigk A, Zengerling F, et al:
Early CRP kinetics to predict long-term efficacy of first-line
immune-checkpoint inhibition combination therapies in metastatic
renal cell carcinoma: An updated multicentre real-world experience
applying different CRP kinetics definitions. Clin Transl
Immunology. 12:e14712023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klümper N, Saal J, Berner F,
Lichtensteiger C, Wyss N, Heine A, Bauernfeind FG, Ellinger J,
Brossart P, Diem S, et al: C reactive protein flare predicts
response to checkpoint inhibitor treatment in non-small cell lung
cancer. J Immunother Cancer. 10:e0040242022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saal J, Bald T, Eckstein M, Ritter M,
Brossart P, Ellinger J, Hölzel M and Klümper N: Early C-reactive
protein kinetics predicts immunotherapy response in non-small cell
lung cancer in the phase III OAK trial. JNCI Cancer Spectr.
7:pkad0272023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin Q, Kou X, Zheng Y, Zhou F, Zhang X and
Liu H: Early C-reactive protein kinetics predict response to immune
checkpoint blockade in unresectable hepatocellular carcinoma. J
Hepatocell Carcinoma. 10:2009–2019. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haas M, Lein A, Fuereder T, Schnoell J,
Brkic FF, Liu DT, Kadletz-Wanke L, Heiduschka G and Jank BJ: Early
on-treatment C-reactive protein and its kinetics predict survival
and response in recurrent and/or metastatic head and neck cancer
patients receiving first-line pembrolizumab. Invest New Drugs.
41:727–736. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakayama T, Saito K, Kumagai J, Nakajima
Y, Kijima T, Yoshida S, Kihara K and Fujii Y: Higher serum
C-reactive protein level represents the immunosuppressive tumor
microenvironment in patients with clear cell renal cell carcinoma.
Clin Genitourin Cancer. 16:e1151–e1158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshida T, Ichikawa J, Giuroiu I, Laino
AS, Hao Y, Krogsgaard M, Vassallo M, Woods DM, Stephen Hodi F and
Weber J: C reactive protein impairs adaptive immunity in immune
cells of patients with melanoma. J Immunother Cancer.
8:e0002342020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hussaini S, Chehade R, Boldt RG, Raphael
J, Blanchette P, Maleki Vareki S and Fernandes R: Association
between immune-related side effects and efficacy and benefit of
immune checkpoint inhibitors-a systematic review and meta-analysis.
Cancer Treat Rev. 92:1021342021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abolhassani AR, Schuler G, Kirchberger MC
and Heinzerling L: C-reactive protein as an early marker of
immune-related adverse events. J Cancer Res Clin Oncol.
145:2625–2631. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsieh MC, Sung MT, Chiang PH, Huang CH,
Tang Y and Su YL: The prognostic impact of histopathological
variants in patients with advanced urothelial carcinoma. PLoS One.
10:e01292682015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Minato A, Murooka K, Okumura Y, Takaba T,
Higashijima K, Nagata Y, Tomisaki I, Harada K and Fujimoto N:
Efficacy of platinum-based chemotherapy in patients with metastatic
urothelial carcinoma with variant histology. In Vivo. 38:873–880.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kobayashi M, Narita S, Matsui Y, Kanda S,
Hidaka Y, Abe H, Tsuzuki T, Ito K, Kojima T, Kato M, et al: Impact
of histological variants on outcomes in patients with urothelial
carcinoma treated with pembrolizumab: A propensity score matching
analysis. BJU Int. 130:226–234. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Minato A, Furubayashi N, Harada M, Negishi
T, Sakamoto N, Song Y, Hori Y, Tomoda T, Tamura S, Kuroiwa K, et
al: Efficacy of pembrolizumab in patients with variant urothelial
carcinoma: A multicenter retrospective study. Clin Genitourin
Cancer. 20:499.e1–499.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|