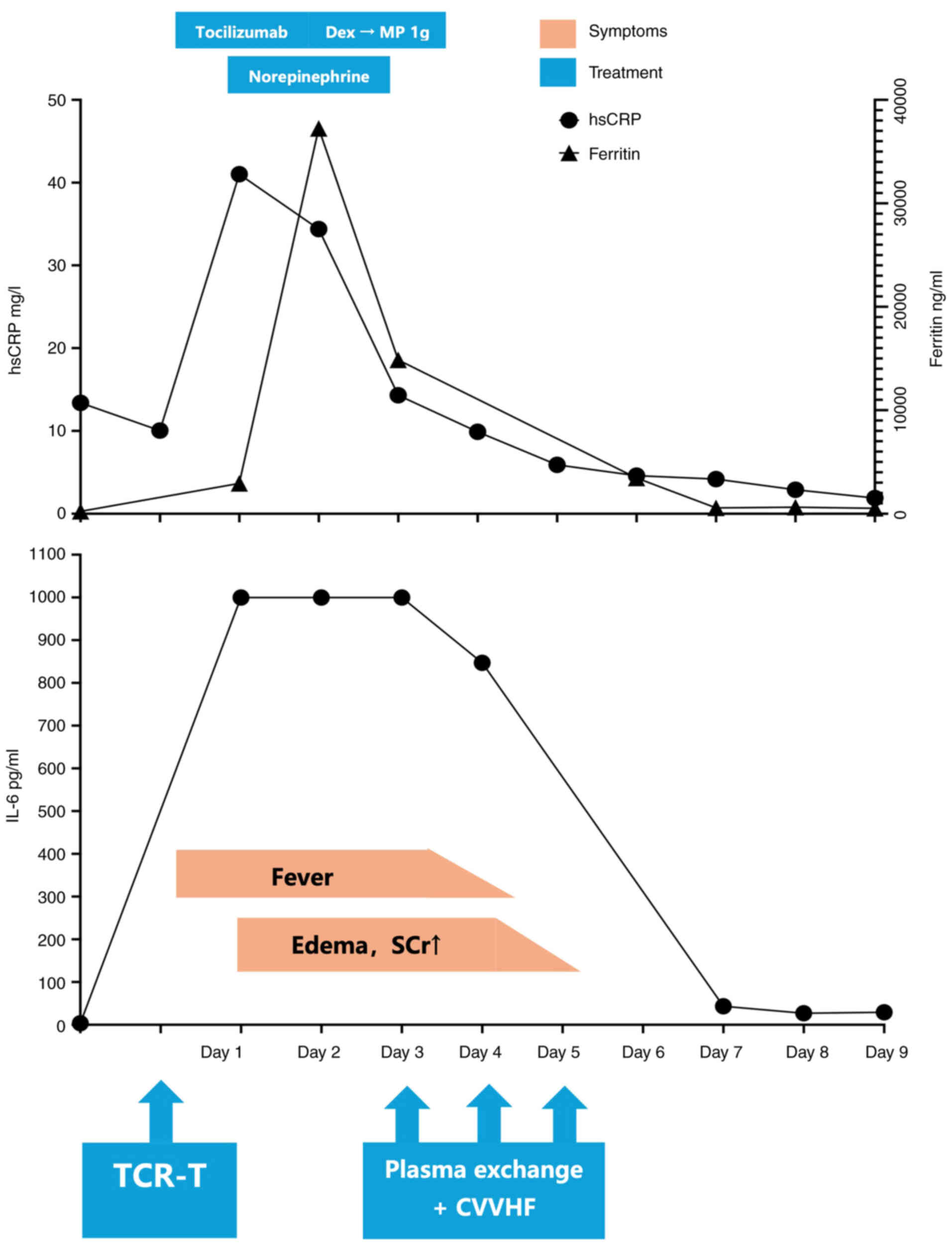
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA A Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar
|
|
2
|
Fan Y, Xue H and Zheng H: Systemic therapy
for hepatocellular carcinoma: Current updates and outlook. J
Hepatocell Carcinoma. 9:233–263. 2022. View Article : Google Scholar
|
|
3
|
Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu
X, Xia C, Yang Z, Li H, Wei W, et al: Liver cancer incidence and
mortality in China: Temporal trends and projections to 2030. Chin J
Cancer Res. 30:571–579. 2018. View Article : Google Scholar
|
|
4
|
Tsimberidou AM, Van Morris K, Vo HH, Eck
S, Lin YF, Rivas JM and Andersson BS: T-cell receptor-based
therapy: An innovative therapeutic approach for solid tumors. J
Hematol Oncol. 14:1022021. View Article : Google Scholar
|
|
5
|
Baulu E, Gardet C, Chuvin N and Depil S:
TCR-engineered T cell therapy in solid tumors: State of the art and
perspectives. Sci Adv. 9:eadf37002024. View Article : Google Scholar
|
|
6
|
Cobb DA and Lee DW: Cytokine release
syndrome biology and management. Cancer J. 27:1192021. View Article : Google Scholar
|
|
7
|
Liu LL, Skribek M, Harmenberg U and
Gerling M: Systemic inflammatory syndromes as life-threatening side
effects of immune checkpoint inhibitors: Case report and systematic
review of the literature. J Immunother Cancer. 11:e0058412024.
View Article : Google Scholar
|
|
8
|
Xiao X, He X, Li Q, Zhang H, Meng J, Jiang
Y, Deng Q and Zhao M: Plasma exchange can be an alternative
therapeutic modality for severe cytokine release syndrome after
chimeric antigen receptor-T cell infusion: A case report. Clin
Cancer Res. 25:29–34. 2019. View Article : Google Scholar
|
|
9
|
Pu Y, Zhao Y, Qi Y, Liu Y, Zhang M, Xiao
X, Lyu H, Meng J, Zhu H, Xu K, et al: Multi-centers experience
using therapeutic plasma exchange for
corticosteroid/tocilizumab-refractory cytokine release syndrome
following CAR-T therapy. Int Immunopharmacol. 130:1117612024.
View Article : Google Scholar
|
|
10
|
Pandey PK, Kaul E, Agarwal N and Goel S:
Effectiveness of therapeutic plasma exchange in a critically ill
child with secondary hemophagocytic lymphohistiocytosis. Asian J
Transfus Sci. 13:145–147. 2019. View Article : Google Scholar
|
|
11
|
Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin
J, Wu J, Li Y, Shi L, Fu JL, et al: Immunotherapy of HBV-related
advanced hepatocellular carcinoma with short-term HBV-specific TCR
expressed T cells: Results of dose escalation, phase I trial.
Hepatol Int. 15:1402–1412. 2021. View Article : Google Scholar
|
|
12
|
Wan X, Wu W, Liu Y, Du S, Li W, Quan D,
Wang X, Protzer U, Zhou Y and Qu X: 691 First-in-human trial of
novel HBsAg-specific TCR T cell therapy (SCG101) in patients with
HBV-related hepatocellular carcinoma. Regular and Young
Investigator Award Abstracts. BMJ Publishing Group Ltd; pp.
ppA783–A783. 2023, View Article : Google Scholar
|
|
13
|
Zhang J, Hao Y, Ou W, Ming F, Liang G,
Qian Y, Cai Q, Dong S, Hu S, Wang W and Wei S: Serum interleukin-6
is an indicator for severity in 901 patients with SARS-CoV-2
infection: A cohort study. J Transl Med. 18:4062020. View Article : Google Scholar
|
|
14
|
Shimabukuro-Vornhagen A, Gödel P, Subklewe
M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B and von
Bergwelt-Baildon MS: Cytokine release syndrome. J Immunother
Cancer. 6:562018. View Article : Google Scholar
|
|
15
|
Neelapu SS, Tummala S, Kebriaei P, Wierda
W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et
al: Chimeric antigen receptor T-cell therapy-assessment and
management of toxicities. Nat Rev Clin Oncol. 15:47–62. 2018.
View Article : Google Scholar
|
|
16
|
Jarczak D, Kluge S and Nierhaus A: Septic
Hyperinflammation-is there a role for extracorporeal blood
purification techniques? Int J Mol Sci. 25:31202024. View Article : Google Scholar
|
|
17
|
Fonseca-González G, Alamilla-Sánchez M,
García-Macas V, Herrera-Acevedo J, Villalobos-Brito M, Tapia-Rangel
E, Maldonado-Tapia D, López-Mendoza M, Cano-Cervantes JH,
Orozco-Vázquez J, et al: Impact of plasmapheresis on severe
COVID-19. Sci Rep. 13:1632023. View Article : Google Scholar
|
|
18
|
Bottari G, Merli P, Guzzo I, Stoppa F,
Ruggeri A, Di Nardo M, Del Bufalo F, Galaverna F, Corrado C and
Locatelli F: Multimodal therapeutic approach of cytokine release
syndrome developing in a child given chimeric antigen
receptor-modified T cell infusion. Crit Care Explor. 2:e00712020.
View Article : Google Scholar
|
|
19
|
Bauer PR, Ostermann M, Russell L, Robba C,
David S, Ferreyro BL, Cid J, Castro P, Juffermans NP, Montini L, et
al: Plasma exchange in the intensive care unit: A narrative review.
Intensive Care Med. 48:1382–1396. 2022. View Article : Google Scholar
|
|
20
|
Morris EC, Neelapu SS, Giavridis T and
Sadelain M: Cytokine release syndrome and associated neurotoxicity
in cancer immunotherapy. Nat Rev Immunol. 22:85–96. 2022.
View Article : Google Scholar
|