Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of cancer and the third leading cause of cancer-related
deaths worldwide (1). Current
systemic therapies for HCC include immune checkpoint inhibitors,
angiogenesis inhibitors and tyrosine kinase inhibitors. However,
the response rates to these treatments are limited, and there is a
high risk of HCC recurrence (2).
Hepatitis B virus (HBV) infection is the primary risk factor for
HCC development, accounting for ~50% of HCC cases worldwide and
~85% of cases in China. HBV-DNA integration is detected in >80%
of HBV-related HCC tumor cells (3).
Therefore, targeting the HBV-related surface antigen is seen as a
promising strategy in the fight against HCC. Clinical trials
exploring T-cell immunotherapy targeting HBV-specific antigens are
underway.
T-cell receptor-engineered T cells (TCR-Ts) are
autologous T cells modified to express a specific TCR that
recognizes tumor-associated antigens presented by human leukocyte
antigen molecules on cancer cells. These T cells are genetically
engineered using viral vectors to enhance specificity and affinity
for the target antigen. Upon reinfusion into the patient, the
engineered TCRs guide T cells to recognize and eliminate tumor
cells by triggering cytotoxic responses, including cytokine release
and direct lysis (4). Similar to
chimeric antigen receptor T-cell (CAR-T) therapy, which is another
type of T-cell immunotherapy that mainly targets B cell lineages
and is used to treat leukemia and lymphomas, TCR-T therapies can
also be complicated by the potentially life-threatening adverse
event of cytokine release syndrome (CRS). TCR-T therapy is being
explored in clinical trials involving melanoma, synovial sarcoma,
hepatocellular carcinoma, mesothelioma and various solid tumors
(4). The incidence of CRS in these
TCR-T clinical trials ranges from 10–50% (5). While the precise mechanism remains to
be elucidated, CRS manifests clinically as a severe systemic
inflammatory response resulting in hypotension, hypoxia and
multi-organ dysfunction (6). In
severe cases of CRS, these systemic inflammatory responses can be
fatal, with a reported mortality rate as high as 10% (7).
Apart from high-quality supportive care, tocilizumab
and glucocorticoids serve as first-line treatments for CRS.
However, for severe cases that are unresponsive to repeated
tocilizumab and pulse glucocorticoids, limited second-line
therapies are available. Plamsa exchange (PE) is a extracorporeal
blood purification technique in which the patient's plasma is
separated from the blood cells and discarded, while a replacement
fluid, commonly fresh frozen plasma, is used to replenish the
plasma. PE not only eliminates all inflammatory mediators and
circulating damaged molecules, but also replenishes essential
plasma components depleted by the disease process. Case reports and
cohort studies have shown promising outcomes in using PE to manage
severe CRS following chimeric antigen receptor (CAR)-T therapy
(8–10). However, the efficacy of PE in the
treatment of TCR-T-related CRS has not yet been reported.
In the present study, the case of a patient with HCC
who underwent PE to effectively treat severe CRS following TCR-T
therapy for metastatic HBV-related HCC is reported. To the best of
our knowledge, this is the first reported case of PE being used to
manage CRS following TCR-T therapy for solid tumors.
Case report
The present study reports the case of a 35-year-old
male who presented to Peking Union Medical College Hospital
(Beijing, China) in September 2023 with HBV-related metastatic HCC
after failing previous multiline treatments. The patient was
diagnosed with metastatic HCC at 1 year prior to the presentation
to Peking Union Medical College Hospital, based on a biopsy of
liver nodules and elevated serum α-fetoprotein levels. A positron
emission tomography scan had demonstrated multiple metastatic
lesions in both lungs and lymph nodes at an external hospital.
Following diagnosis, the patient had undergone microwave ablation
of liver nodules, followed by 3 months of immunotherapy with 200 mg
sintilimab every 3 weeks and 0.4 g sorafenib twice a day,
accompanied by transarterial chemoembolization (TACE), additional
microwave ablation of liver lesions and microwave ablation of lung
metastases. Systemic therapy was then transitioned to 200 mg
toripalimab every 3 weeks and 0.2 g donafenib twice a day for 4
months, during which time, the lung metastases increased in size
and number. The patient subsequently received another TACE and
computed tomography (CT)-guided microwave ablation of the left lung
metastases. Despite 1 month of Sintilimab 200 mg and bevacizumab
700 mg every 3 weeks, the lung lesions progressed (Video S1 and S2). The patient was then enrolled in a
clinical trial of hepatitis B virus surface antigen-specific
(HBsAg)-specific TCR-engineered autologous T cells for HBV-related
HCC (trial registration no. CTR20222173; August 2022) (11). The patient had a medical history of
HBV infection in 2003 and had been taking 5 mg entecavir orally
once a day since then, with normal liver enzyme levels. The HBV DNA
count and HBsAg levels prior to TCR-T therapy were <20 IU/ml
(normal range, <20 IU/ml) and >250 IU/ml (normal range,
<0.05 IU/ml), respectively.
Upon presentation in September 2023, no icterus or
sclera were observed on the skin. Pulmonary auscultation
demonstrated no rales. The patient's abdomen was soft and
non-tender, and shifting dullness was negative. As part of the
clinical trial treatment, peripheral blood mononuclear cells were
harvested in advance to construct engineered T cells. SCG101, an
autologous TCR-T designed to target specific epitopes of HBsAg, was
used for the investigational cell therapy. SCG101 was engineered
for high affinity and avidity toward intracellular antigens
presented by major histocompatibility complex (MHC) on solid tumors
(12). The engineered and expanded
TCR-Ts were infused back into the patient 3 days after
lymphodepletion with cyclophosphamide (900 mg on days 1–3) and
fludarabine (45 mg on days 1–3). Prior to infusion, the vital signs
were stable, with a blood pressure (BP) of 109/65 mmHg, a pulse
rate of 61 bpm and a temperature of 36.4°C (normal range of vital
signs: systolic BP, 90–120 mmHg; diastolic BP, 60–80 mmHg; pulse,
60–100 bpm; temperature, 36.5–37.3°C).
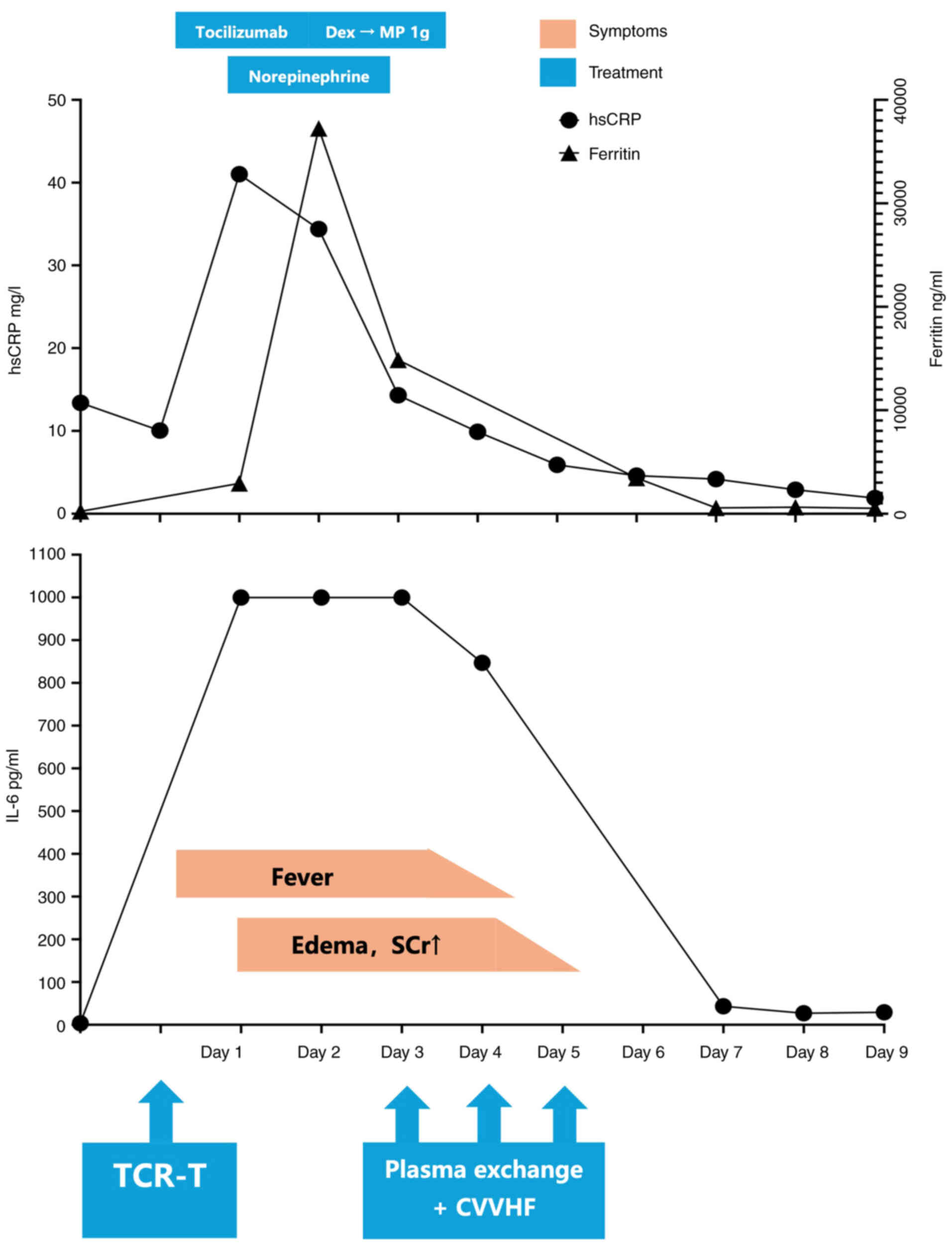
Prior to infusion, the patient was preconditioned
with 12.5 mg promethazine, 650 mg paracetamol and 2 g ceftazidime
once. A total of six bags, each containing 1.13×109
TCR-T cells, were infused. At 30 min post-infusion, the patient
experienced a fever, soreness and chills, with a temperature of
38°C and a BP of 92/54 mmHg. Therefore, CRS was suspected.
Aggressive hydration was initiated and tocilizumab at a dose of 560
mg was administered. Despite treatment, the fever persisted into
the next day and the BP remained at <90/60 mmHg, which led to
the diagnosis of grade 3 CRS. A norepinephrine infusion was
initiated and two additional doses of tocilizumab were
administered. The patient was also treated with dexamethasone (10
mg, every 6 h). Despite the aforementioned treatments, the patient
developed peripheral edema, ascites and pleural effusion. Urine
output declined and the serum creatinine levels rose from 86 to 155
µmol/l (normal range, 59–104 µmol/l). Due to hemodynamic
instability and impending multiorgan failure, the patient was
admitted to the intensive care unit (ICU) on the second day after
infusion.
In addition to tocilizumab, pulse methylprednisolone
and broad-spectrum antibiotics, daily PE was initiated on the
second day until the fifth day after infusion. A total of three
sessions were administered with a treatment volume of 3,000 ml per
session. Fresh frozen plasma was used as the replacement fluid. Due
to hyperkalemia, continuous veno-venous hemofiltration (CVVHF) was
also initiated on the third day after infusion. Post-TCR-T therapy,
the patient exhibited notably elevated interleukin-6 (IL-6),
high-sensitive C-reactive protein (hsCRP) and ferritin levels.
Notably, the levels of IL-6 rose to >1,000 pg/ml, while the
normal range should be <5 pg/ml, and even in critically ill
COVID-19 patients, the median IL-6 level is only 21 pg/ml (13). Following PE, the IL-6, hsCRP and
ferritin levels were all notably decreased (Fig. 1), with the resolution of the
patient's hypotension and fever. By the fifth day post-infusion,
the patient no longer required vasopressors and the urine output
was improved. The patient was discharged from the ICU and continued
to receive 2 g ceftazidime twice a day for an additional week prior
to discharge from the hospital.
After discharge, the patient was followed up in the
outpatient clinic on a monthly basis. At 3 months after TCR-T
infusion, the patient's renal and liver functions had returned to
within normal ranges (Table I).
Both metastatic and primary liver tumors were stable in size, thus
indicating that the patient remained in a state of stable disease
(Video S3 and S4).
 | Table I.Changes in biochemistry and
coagulation parameters with treatment. |
Table I.
Changes in biochemistry and
coagulation parameters with treatment.
| Parameter | Baseline (before PBMC
harvesting and lymphodepletion) | Before TCR-T
infusion, (after PBMC harvesting and lymphodepletion) | Day 2 after TCR-T
infusion (before PE) | Day 6 after TCR-T
infusion (after PE) | 3-months after TCR-T
infusion | Normal range |
|---|
| Alanine transaminase,
U/l | 38 | 22 | 3,084a | 477a | 26 | 9-50 |
| Aspartate
aminotransferase, U/l | 28 | 34 | 4,153a | 231a | 29 | 15-40 |
| Total bilirubin,
µmol/l | 6.1 | 15.3 | 45.1a | 44.0a | 8.3 | 5.1–22.2 |
| Direct bilirubin,
µmol/l | 2.2 | 4.8 | 30.7 | 20.5 | 3.4 | <6.8 |
| Albumin, g/l | 48 | 46 | 25b | 36 | 49 | 35-52 |
| Serum creatinine,
µmol/l | 61 | 53 | 155a | 94 | 56 | 59-104 |
| Urea, mmol/l | 5.32 | 3.52 | 8.12a | 11.80a | 5.36 | 2.78–7.14 |
| Potassium,
mmol/l | 4.4 | 4.1 | 6.5 | 3.6 | 4.0 | 3.5–5.5 |
| Phosphate,
mmol/l | 1.06 | 1.12 | NA | 1.02 | 1.26 | 0.81–1.45 |
| Fibrinogen, g/l | 4.16 | 4.28 | 0.78 | 1.32 | 2.22 | 1.80–3.50 |
| White blood cells, n
(×109/l) | 6.01 | 1.20b | 0.52b | 2.31b | 3.63 | 3.50–9.50 |
| Lymphocytes, n
(×109/l) | 1.26 | 0.04b | 0.04b | 0.34b | 1.10 | 0.80–4.00 |
| Platelets, n
(×109/l) | 162 | 103 | 73b | 45b | 107 | 100-350 |
| Hemoglobin, g/l | 158 | 144 | 133 | 83b | 136 | 120-160 |
| Hepatitis B virus
DNA, IU/ml | <20 | <20 | <20 | <20 | <20 | 20 |
| Hepatitis B surface
antigen, IU/ml | 799.52a | 952.75a | 819.07a | 6.41a | 3.74a | <0.05 |
| α-fetoprotein,
ng/ml | 4.7 | 17.4 | NA | NA | 1.5 | ≤20.0 |
Discussion
TCR-T therapy is a promising form of immunotherapy
for solid tumors. With ongoing clinical trials, novel targets and
engineered T cells are emerging to bolster the treatment of various
types of cancer. However, the mortality rate for severe cases of
CRS remains as high as 10% (7) and
treatment options are limited. The present study reports a case of
TCR-T-related CRS successfully managed with PE as a salvage therapy
to tocilizumab and glucosteroids, which suggested that PE could be
a potential strategy for the treatment of refractory CRS.
The symptoms of CRS, such as hypotension, hypoxia
and capillary leaking, are associated with the supraphysiological
levels of inflammatory cytokines due to the overstimulation of
immune effector cells. Consequently, treatment of CRS primarily
revolves around anti-inflammatory and anti-cytokine therapies, such
as tocilizumab and glucocorticoids (14). Even as such, deaths have been
reported for grade 3 and 4 CRS (15), thus limiting the application of
T-cell immunotherapies, including TCR-T therapy.
Emerging evidence has suggested that blood
purification techniques, including hemofiltration, immunoadsorption
and PE, can mitigate systemic inflammatory reactions in
inflammatory syndromes, such as sepsis (16), COVID-19-related cytokine storms
(17) and hemophagocytic
lymphohistiocytosis (HLH) (10),
with some successful treatment outcomes reported in case studies.
These techniques may be applied to CRS as well (9).
Hemofiltration effectively clears small molecules,
such as ILs, via a method of convection. However, hemofiltration is
unable to remove molecules larger than albumin (>66.5 kDa).
Immunoadsorption can remove toxic substances or inflammatory
cytokines by binding them with solvent or adsorptive materials in
the extracorporeal circuit (16).
Bottari et al (18) reported
a case where hemoadsorption using a Cytosorb column and continuous
renal replacement therapy were employed to manage grade 4 CRS
associated with secondary HLH after CAR-T therapy for acute
lymphoblastic leukemia. Both hemofiltration and adsorption have the
disadvantage of being unable to replace plasma components (16).
PE is a blood purification technique in which the
patient's plasma is separated from the blood cells and discarded,
while a replacement fluid, commonly fresh frozen plasma, is used to
replenish the plasma. PE not only eliminates all inflammatory
mediators and circulating damaged molecules, but also replenishes
essential plasma components depleted by the disease process
(19). In CRS, multiple cytokines
and activated immune cell products, including IL-6, IL-10 and INF-γ
can contribute to the disease process (14). In the later stages of CRS,
endothelial damage and tumor cell products further aggravate the
clinical severity (20). Since CRS
is multifactorial and the specific causative molecule is unclear,
in our opinion, PE would be the preferred blood purification
modality. The use of PE to address refractory CRS in CAR-T therapy
has been reported by a number of studies. In 2019, Xiao et
al (8) reported successful PE
in a case of treatment-resistant CRS following CAR-T therapy for
acute lymphoblastic leukemia. A recent study of a retrospective
cohort reported the use of PE in 17 refractory cases of CRS after
CAR-T therapy (9). In these cases,
it was verified that PE could effectively mitigate CRS symptoms and
reduce the serum levels of inflammatory mediators. Nonetheless, the
use of TCR-T for the treatment of solid tumors is still under
investigation and, to the best of our knowledge, there are
currently no reports on the efficacy of PE for treating
TCR-T-induced CRS.
Markedly increased ferritin levels in CRS could
suggest the co-existence of HLH (6), although the distinction between the
two is unclear without further diagnostic tests. Nonetheless, in
the present study, PE therapy normalized the ferritin levels
without necessitating additional immunosuppressive therapy, thus
suggesting that an HLH-like presentation could represent a stage of
CRS.
Whether PE interferes with the efficacy of T-cell
therapy is another potential point of debate. PE primarily removes
plasma and does not significantly affect infused effector T cell
levels. However, T-cell therapies can induce the release of various
cytokines and soluble factors that play a role in the antitumor
response. PE can remove these cytokines and factors, potentially
dampening the overall immune response against cancer cells. There
is limited specific research directly addressing the interaction
between PE and T-cell immunotherapy. In the present case study, the
cancer remained in a stable disease state at the 3-month follow-up
and we speculate that PE did not interfere with the effect of TCR-T
therapy.
In summary, the present case study demonstrated the
successful use of PE in managing CRS associated with TCR-T therapy.
As TCR-T therapy expands across different types of solid tumors,
more CRS cases are likely to arise. Nevertheless, tocilizumab and
glucosteroids remain the first-line therapy for such cases.
However, further research is needed to determine PE indications,
optimal dosage and its potential combination with CVVHF and other
blood purification modalities.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was partially supported by a grant from the
National High-Level Hospital Clinical Research Funding (grant no.
2022-PUMCH-B-020).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ designed the study, analyzed patient data and
wrote the manuscript. YW designed the study, advised on patient
treatment and edited the manuscript. XZ and YW confirm the
authenticity of all the raw data. SZ, HW and JX helped with the PE
process, data collection and clinical management of the patient. KZ
advised on patient treatment. YQ advised on patient treatment,
helped with the PE process and obtained funding for the
publication. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Peking Union Medical College Hospital (Beijing, China;
approval no. I-23PJ1746).
Patient consent for publication
Written consent was obtained from the patient for
academic use of their medical record.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA A Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar
|
|
2
|
Fan Y, Xue H and Zheng H: Systemic therapy
for hepatocellular carcinoma: Current updates and outlook. J
Hepatocell Carcinoma. 9:233–263. 2022. View Article : Google Scholar
|
|
3
|
Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu
X, Xia C, Yang Z, Li H, Wei W, et al: Liver cancer incidence and
mortality in China: Temporal trends and projections to 2030. Chin J
Cancer Res. 30:571–579. 2018. View Article : Google Scholar
|
|
4
|
Tsimberidou AM, Van Morris K, Vo HH, Eck
S, Lin YF, Rivas JM and Andersson BS: T-cell receptor-based
therapy: An innovative therapeutic approach for solid tumors. J
Hematol Oncol. 14:1022021. View Article : Google Scholar
|
|
5
|
Baulu E, Gardet C, Chuvin N and Depil S:
TCR-engineered T cell therapy in solid tumors: State of the art and
perspectives. Sci Adv. 9:eadf37002024. View Article : Google Scholar
|
|
6
|
Cobb DA and Lee DW: Cytokine release
syndrome biology and management. Cancer J. 27:1192021. View Article : Google Scholar
|
|
7
|
Liu LL, Skribek M, Harmenberg U and
Gerling M: Systemic inflammatory syndromes as life-threatening side
effects of immune checkpoint inhibitors: Case report and systematic
review of the literature. J Immunother Cancer. 11:e0058412024.
View Article : Google Scholar
|
|
8
|
Xiao X, He X, Li Q, Zhang H, Meng J, Jiang
Y, Deng Q and Zhao M: Plasma exchange can be an alternative
therapeutic modality for severe cytokine release syndrome after
chimeric antigen receptor-T cell infusion: A case report. Clin
Cancer Res. 25:29–34. 2019. View Article : Google Scholar
|
|
9
|
Pu Y, Zhao Y, Qi Y, Liu Y, Zhang M, Xiao
X, Lyu H, Meng J, Zhu H, Xu K, et al: Multi-centers experience
using therapeutic plasma exchange for
corticosteroid/tocilizumab-refractory cytokine release syndrome
following CAR-T therapy. Int Immunopharmacol. 130:1117612024.
View Article : Google Scholar
|
|
10
|
Pandey PK, Kaul E, Agarwal N and Goel S:
Effectiveness of therapeutic plasma exchange in a critically ill
child with secondary hemophagocytic lymphohistiocytosis. Asian J
Transfus Sci. 13:145–147. 2019. View Article : Google Scholar
|
|
11
|
Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin
J, Wu J, Li Y, Shi L, Fu JL, et al: Immunotherapy of HBV-related
advanced hepatocellular carcinoma with short-term HBV-specific TCR
expressed T cells: Results of dose escalation, phase I trial.
Hepatol Int. 15:1402–1412. 2021. View Article : Google Scholar
|
|
12
|
Wan X, Wu W, Liu Y, Du S, Li W, Quan D,
Wang X, Protzer U, Zhou Y and Qu X: 691 First-in-human trial of
novel HBsAg-specific TCR T cell therapy (SCG101) in patients with
HBV-related hepatocellular carcinoma. Regular and Young
Investigator Award Abstracts. BMJ Publishing Group Ltd; pp.
ppA783–A783. 2023, View Article : Google Scholar
|
|
13
|
Zhang J, Hao Y, Ou W, Ming F, Liang G,
Qian Y, Cai Q, Dong S, Hu S, Wang W and Wei S: Serum interleukin-6
is an indicator for severity in 901 patients with SARS-CoV-2
infection: A cohort study. J Transl Med. 18:4062020. View Article : Google Scholar
|
|
14
|
Shimabukuro-Vornhagen A, Gödel P, Subklewe
M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B and von
Bergwelt-Baildon MS: Cytokine release syndrome. J Immunother
Cancer. 6:562018. View Article : Google Scholar
|
|
15
|
Neelapu SS, Tummala S, Kebriaei P, Wierda
W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et
al: Chimeric antigen receptor T-cell therapy-assessment and
management of toxicities. Nat Rev Clin Oncol. 15:47–62. 2018.
View Article : Google Scholar
|
|
16
|
Jarczak D, Kluge S and Nierhaus A: Septic
Hyperinflammation-is there a role for extracorporeal blood
purification techniques? Int J Mol Sci. 25:31202024. View Article : Google Scholar
|
|
17
|
Fonseca-González G, Alamilla-Sánchez M,
García-Macas V, Herrera-Acevedo J, Villalobos-Brito M, Tapia-Rangel
E, Maldonado-Tapia D, López-Mendoza M, Cano-Cervantes JH,
Orozco-Vázquez J, et al: Impact of plasmapheresis on severe
COVID-19. Sci Rep. 13:1632023. View Article : Google Scholar
|
|
18
|
Bottari G, Merli P, Guzzo I, Stoppa F,
Ruggeri A, Di Nardo M, Del Bufalo F, Galaverna F, Corrado C and
Locatelli F: Multimodal therapeutic approach of cytokine release
syndrome developing in a child given chimeric antigen
receptor-modified T cell infusion. Crit Care Explor. 2:e00712020.
View Article : Google Scholar
|
|
19
|
Bauer PR, Ostermann M, Russell L, Robba C,
David S, Ferreyro BL, Cid J, Castro P, Juffermans NP, Montini L, et
al: Plasma exchange in the intensive care unit: A narrative review.
Intensive Care Med. 48:1382–1396. 2022. View Article : Google Scholar
|
|
20
|
Morris EC, Neelapu SS, Giavridis T and
Sadelain M: Cytokine release syndrome and associated neurotoxicity
in cancer immunotherapy. Nat Rev Immunol. 22:85–96. 2022.
View Article : Google Scholar
|