Introduction
Lung cancer is one of the most prevalent types of
malignant tumors worldwide and is characterized by a high degree of
malignancy and poor prognosis. Despite the advancements in
understanding risk factors, development mechanisms and treatment
modalities, lung cancer remains a leading cause of cancer-related
mortality worldwide (1). The
prognosis for lung cancer is relatively good as the 5-year survival
rate for early-stage lung cancer (stage IA) is up to 90%, whereas
for advanced-stage lung cancer (stage IVB) the 5-year survival rate
is <10% (2). A total of ~83% of
lung cancer cases are classed as non-small cell lung cancer (NSCLC)
(3). The early symptoms of NSCLC
are not easily detectable, which contributes to its high mortality
rate (the mortality in China was 37 per 100,000 population in 2014)
(4,5). Systemic therapy, including
chemotherapy, targeted therapy or immunotherapy combined with local
radiotherapy, is the primary treatment for patients with inoperable
advanced NSCLC. However, patients with advanced NSCLC who develop
drug resistance after targeted therapy or experience disease
progression following chemotherapy show poor response rates to
subsequent chemotherapy regimens, with a median survival time of no
longer than 10 months (6).
Consequently, further research is essential to develop effective
treatment strategies for advanced NSCLC. Currently, targeted
therapy is the first-line treatment for advanced NSCLC with
positive driver genes. Second-line treatments include pemetrexed,
docetaxel and programmed cell death 1 (PD-1)/programmed
death-ligand 1 (PD-L1) inhibitors. However, options for third-line
and beyond are limited, particularly for patients with squamous
cell carcinoma who do not respond to PD-1/PD-L1 inhibitors
(2). In recent years,
antiangiogenic drugs have emerged as an viable treatment option.
VEGF and VEGFR are crucial factors in angiogenesis, which makes
them effective targets for anticancer therapies (7,8).
Anlotinib, an innovative oral multitarget receptor tyrosine kinase
inhibitor, strongly inhibits multiple targets, such as VEGFR,
platelet-derived growth factor receptors, fibroblast growth factor
receptors and c-Kit. This inhibition can suppress tumor
angiogenesis, cell proliferation, migration and invasion in various
types of tumor cells, including lung tumor cells. (9–11).
Anlotinib effectively suppresses tumor angiogenesis and
proliferation signaling pathways, exhibiting broad-spectrum
inhibitory effects on tumor angiogenesis and growth (12–14).
The ALTER-0303 trial, a randomized, double-blind multicenter phase
3 clinical trial, reported that anlotinib was superior to placebo
in the third-line treatment of patients with advanced NSCLC, while
also highlighting its manageable toxicities and side effects
(7). Based on these findings, the
China Food and Drug Administration approved anlotinib as a
third-line treatment for refractory advanced NSCLC on May 8, 2018
(15). According to the 2022
Chinese Society of Clinical Oncology (CSCO) guidelines, anlotinib
has been accorded level I recommendations (class 1 evidence) as
third-line therapy for stage IV NSCLC without driver genes
(16).
S-1 is a combination of tegafur, gimeracil and oxo
(17) that generates fluorouracil
in both plasma and tumor tissues, exerting antitumor effects.
Previous studies have reported the efficacy and tolerability of S-1
in advanced NSCLC, which led to its approval for NSCLC treatment in
2004 in Japan (18,19). Additionally, combining
antiangiogenic drugs with chemotherapy has shown promise for lung
cancer treatment (20). Clinical
trials have reported that adding anti-angiogenic agents to
conventional chemotherapy significantly improves progression-free
survival (PFS) and overall survival (OS) rates in patients with
advanced NSCLC (21–23).
A number of clinical trials have reported that
adding anti-angiogenic drugs to conventional chemotherapy can
significantly improve the PFS and OS of patients with advanced
NSCLC (21–23). Novel antiangiogenic agents combined
with chemotherapy are expected to further enhance survival outcomes
for these patients. Both anlotinib and S-1 have demonstrated good
responses in advanced NSCLC, with previous studies indicating
favorable efficacy and safety outcomes when they are combined for
the treatment of advanced NSCLC. However, systematic reviews of
this combination therapy are lacking. Therefore, the present study
aimed to systematically evaluate whether third-line or later-line
treatment with anlotinib combined with S-1 for advanced NSCLC is
more effective than anlotinib monotherapy. The findings of the
present study could provide valuable insights into the clinical
application of this combined regimen for the future treatment of
NSCLC.
Materials and methods
Literature retrieval
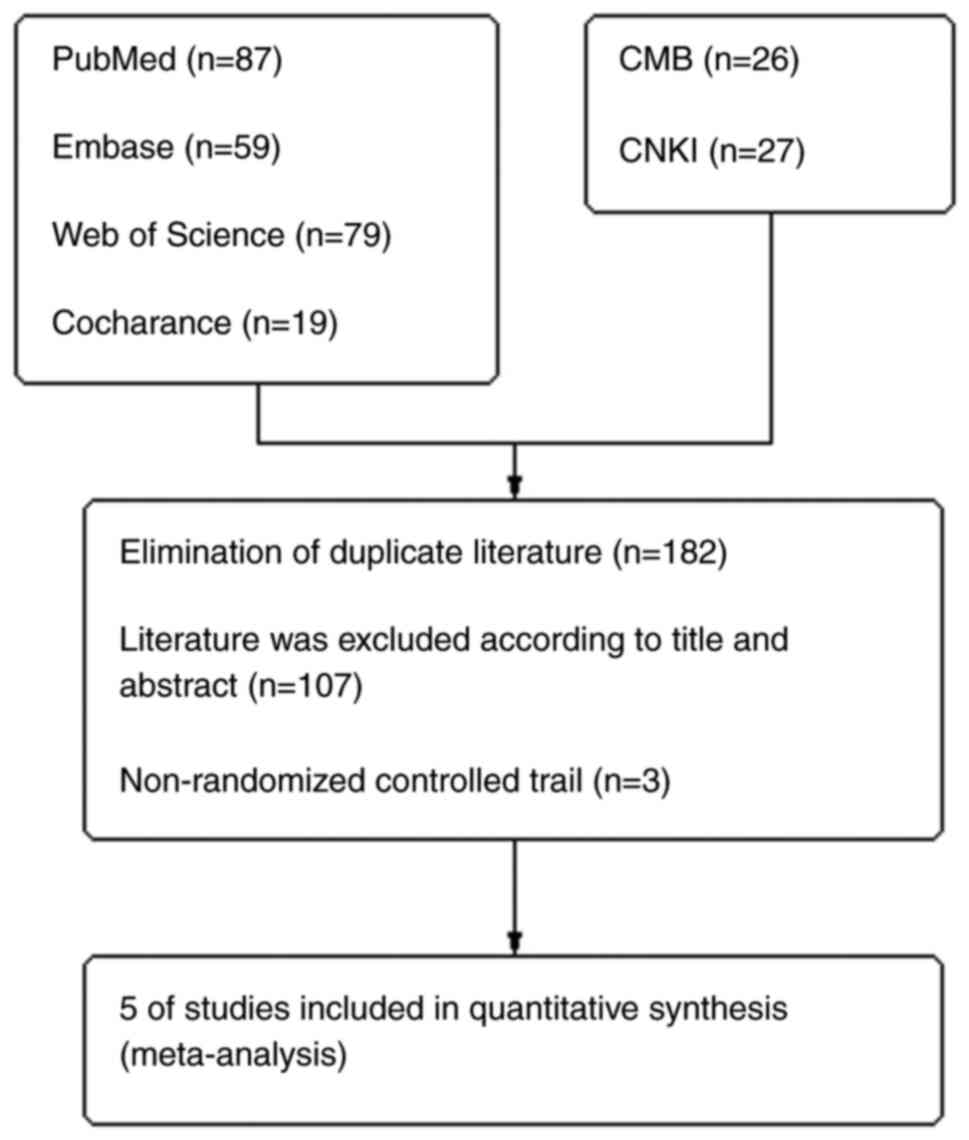
A systematic evaluation and meta-analysis was
conducted following the Preferred Reporting Items for Systematic
Reviews and Meta-analyses (PRISMA) statement and the PRISMA
extension statement for network meta-analysis (24) (Fig.
1).
As of January 10, 2024, two investigators
independently conducted comprehensive literature searches using the
following databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/landing?status=grey), Web of
Science (https://www.webofscience.com/wos/woscc/basic-search),
Cochrane Library (https://www.wiley.com/en-cn/professionals), CMB
(https://www.sinomed.ac.cn/index.jsp)
and China National Knowledge Infrastructure (https://www.cnki.net/). The search items included
‘lung cancer’, ‘anlotinib’ and ‘S-1’. Additionally, original
references, reviews and internal medicine clinical trial data were
searched. Relevant studies were included regardless of publication
status, language or publication year.
Inclusion and exclusion criteria
The following inclusion criteria were used for the
present study: i) Patients clinically diagnosed with advanced
NSCLC; ii) randomized controlled clinical studies evaluating the
combination of anlotinib and S-1 in the treatment of advanced
NSCLC; and iii) treatment regimen is third-line or later-line. The
following exclusion criteria were used for the present study: i)
Studies with missing efficacy and adverse reaction data; ii)
studies based on duplicate patient samples; iii) case studies,
reviews, abstracts and conference reports; and iv) non-clinical
randomized controlled trials.
Data extraction and quality
assessment
A total of two researchers independently screened
the literature, extracted data based on the inclusion and exclusion
criteria and crosschecked the information. In cases of
disagreement, a third researcher made the final judgment. The main
content of the extracted information included: i) Title of the
included study, author, source of literature and publication date;
ii) number and age of patients, intervention measures and methods
and treatment duration in the experimental and control groups; iii)
study types and factors related to the risk of bias assessment; and
iv) outcome indicators such as objective response rate (ORR),
disease control rate (DCR), PFS, OS and adverse reactions.
The Cochrane Handbook for Systematic Reviews of
Interventions (version 5.1.0) was used to evaluate the included
studies. It includes the following contents: i) Random sequence
production; ii) allocation hiding; iii) blinding the subjects and
investigators; iv) blinding outcome assessors; v) incomplete data;
vi) selective result reporting; and vii) other biases (25,26).
Statistical analysis
The meta-analysis was conducted using RevMan
(version 5.3) software (Cochrane Collaboration). Odds ratios (OR)
and 95% confidence intervals (Cis) were used for binary categorical
variables. For continuous outcome measures, mean differences (MD)
or standardized MD (SMD) and 95% CI were calculated. The
χ2 test was used to determine heterogeneity among the
included studies. Due to the clinical heterogeneity of the studies,
a random-effects model was used for the meta-analysis. The
I2 statistic assessment was used to estimate the
curative effect of heterogeneity in the results of the
meta-analysis. According to the statistical standards, each ending
heterogeneity could be divided into the following categories: i)
Not important (I2, 0–40%); ii) medium (I2,
30–60%); iii) significant (I2, 50–90%); or iv)
equivalent (I2, 75–100%). If there were overlapping
values, it was necessary to observe the I2 values: i)
the magnitude and direction of the effect; and ii) the strength of
evidence of heterogeneity (e.g., P-values obtained from
χ2 tests, or CIs for I2) (27). If heterogeneity was too large to be
resolved, descriptive analyses were performed. Further, publication
bias was estimated using funnel plots, Begg's test and Egger's test
(28).
Results
Literature search results and basic
features of included studies
A total of five randomized controlled trials were
included. The basic characteristics of the included studies were
presented in Table I (29–33).
 | Table I.Basic characteristics of the included
studies. |
Table I.
Basic characteristics of the included
studies.
| First author,
year | Patient group | Number of cases,
n | Mean age,
years | Male/female, n |
Adeno-carcinoma/squamous cell carcinoma,
n | Treatment
options | Median overall
survival, months | Median
progression-free survival, months | Outcomes
measured | (Refs.) |
|---|
| Xie et
al, | Experimental | 40 | 63.1 | 36/4 | 0/40 | Anlotinib 12
mg/d | 8.07 | 3.87 | PFS, OS, OOR, | (29) |
| 2020 |
|
|
|
|
| qd D1-14 + S-1 |
|
| DCR,
gastrointestinal |
|
|
|
|
|
|
|
| 25 mg bid
D1-14 |
|
| reactions,
hypertension |
|
|
|
|
|
|
|
|
|
|
| and fatigue |
|
|
| Control | 30 | 61.7 | 24/6 | 0/30 | Anlotinib 12
mg/d | 6.17 | 3.00 |
|
|
|
|
|
|
|
|
| qd D1-14 |
|
|
|
|
| Liu, | Experimental | 36 | 55.32 | 22/14 | 13/23 | Anlotinib 10
mg/d | 10.59 | 6.34 | PFS, OS, OOR,
DCR, | (30) |
| 2021 |
|
|
|
|
| qd D1-14 + S-1 |
|
| gastrointestinal
reactions, |
|
|
|
|
|
|
|
| 40 mg bid
D1-14 |
|
| hypertension,
fatigue, |
|
|
|
|
|
|
|
|
|
|
| bone marrow
suppressison, |
|
|
|
|
|
|
|
|
|
|
| hand-foot
dysfunction |
|
|
|
|
|
|
|
|
|
|
| syndrome and
proteinuria |
|
|
| Control | 36 | 55.21 | 21/15 | 24/12 | Anlotinib 10
mg/d | 8.23 | 4.83 |
|
|
|
|
|
|
|
|
| qd D1-14 |
|
|
|
|
| Kong, | Experimental | 30 | 59 | 18/12 | 24/6 | Anlotinib 12
mg/d | 8.1 | 5.2 | PFS, OS ORR,
DCR, | (31) |
| 2021 |
|
|
|
|
| qd D1-14 + S-1 |
|
| gastrointestinal
reactions, |
|
|
|
|
|
|
|
| 40–60 mg qd
D1-14 |
|
| hypertension,
fatigue, |
|
|
|
|
|
|
|
|
|
|
| bone marrow
suppression, |
|
|
|
|
|
|
|
|
|
|
| abnormal liver and
kidney |
|
|
|
|
|
|
|
|
|
|
| function,
hand-foot |
|
|
|
|
|
|
|
|
|
|
| dysfunction
syndrome and |
|
|
|
|
|
|
|
|
|
|
| proteinuria |
|
|
| Control | 30 | 63 | 14/16 | 23/7 | Anlotinib 12
mg/d | 3.7 | 6.7 |
|
|
|
|
|
|
|
|
| qd D1-14 |
|
|
|
|
| Zhan et
al, | Experimental | 22 | 61.71 | 15/7 | 22/10 | Anlotinib 12
mg/d | N/A | N/A | ORR, DCR,
gastrointestinal | (32) |
| 2021 |
|
|
|
|
| qd D1-14 + S-1 |
|
| reactions,
hypertension, |
|
|
|
|
|
|
|
| 40–60 mg qd
D1-14 |
|
| fatigue, bone
marrow |
|
|
|
|
|
|
|
|
|
|
| suppression,
abnormal liver |
|
|
|
|
|
|
|
|
|
|
| and kidney
function, hand- |
|
|
|
|
|
|
|
|
|
|
| foot dysfunction
syndrome |
|
|
|
|
|
|
|
|
|
|
| and
proteinuria |
|
|
| Control | 23 | 61.71 | 16/7 | 12/11 | Anlotinib 12
mg/d | N/A | N/A |
|
|
|
|
|
|
|
|
| qd D1-14 |
|
|
|
|
| Wang et
al, | Experimental | 35 | 60.41 | 18/17 | 18/17 | Anlotinib 8–12
mg/ | N/A | 11.8 | ORR, DCR,
gastrointestinal | (33) |
| 2022 |
|
|
|
|
| d qd D1-14 +
S-1 |
|
| reactions,
hypertension, |
|
|
|
|
|
|
|
| 40–60 mg qd
D1-14 |
|
| fatigue, bone
marrow |
|
|
|
|
|
|
|
|
|
|
| suppression,
abnormal liver |
|
|
|
|
|
|
|
|
|
|
| and kidney
function, hand- |
|
|
|
|
|
|
|
|
|
|
| foot dysfunction
syndrome |
|
|
|
|
|
|
|
|
|
|
| and
proteinuria |
|
|
| Control | 35 | 59.82 | 22/13 | 23/12 | Anlotinib 8–12
mg/ | N/A | 10.2 |
|
|
|
|
|
|
|
|
| d qd D1-14 |
|
|
|
|
Literature quality evaluation
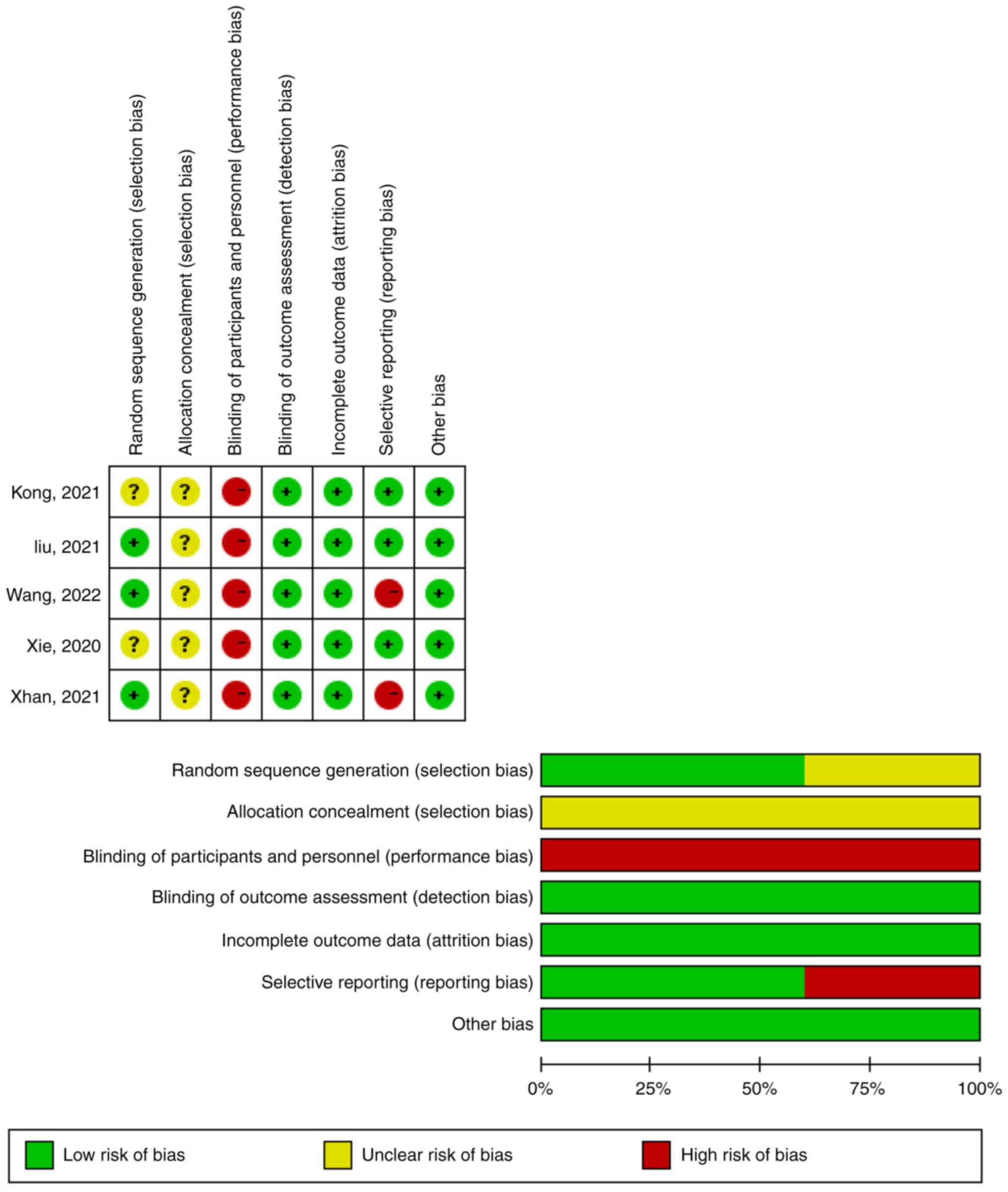
In two articles, the investigators did not describe
whether random methods were used in the sequence generation
process, so whether there was a selection bias caused by
inappropriate methods of generating random sequences was unknown
(Fig. 2). Therefore, the text
selection bias was not clear in these two cases (29,31).
In addition, five articles did not clarify whether researchers
understood the distribution, which could lead to selective bias
(29–33). The subjects of the five articles may
have received different medications within their groups, therefore,
there was a risk of implementation bias across all studies
(29–33). Furthermore, two studies did not
report the expected long-term results, namely OS and PFS,
therefore, reporting bias was suspected (32,33).
Meta-analysis results
Drug efficacy
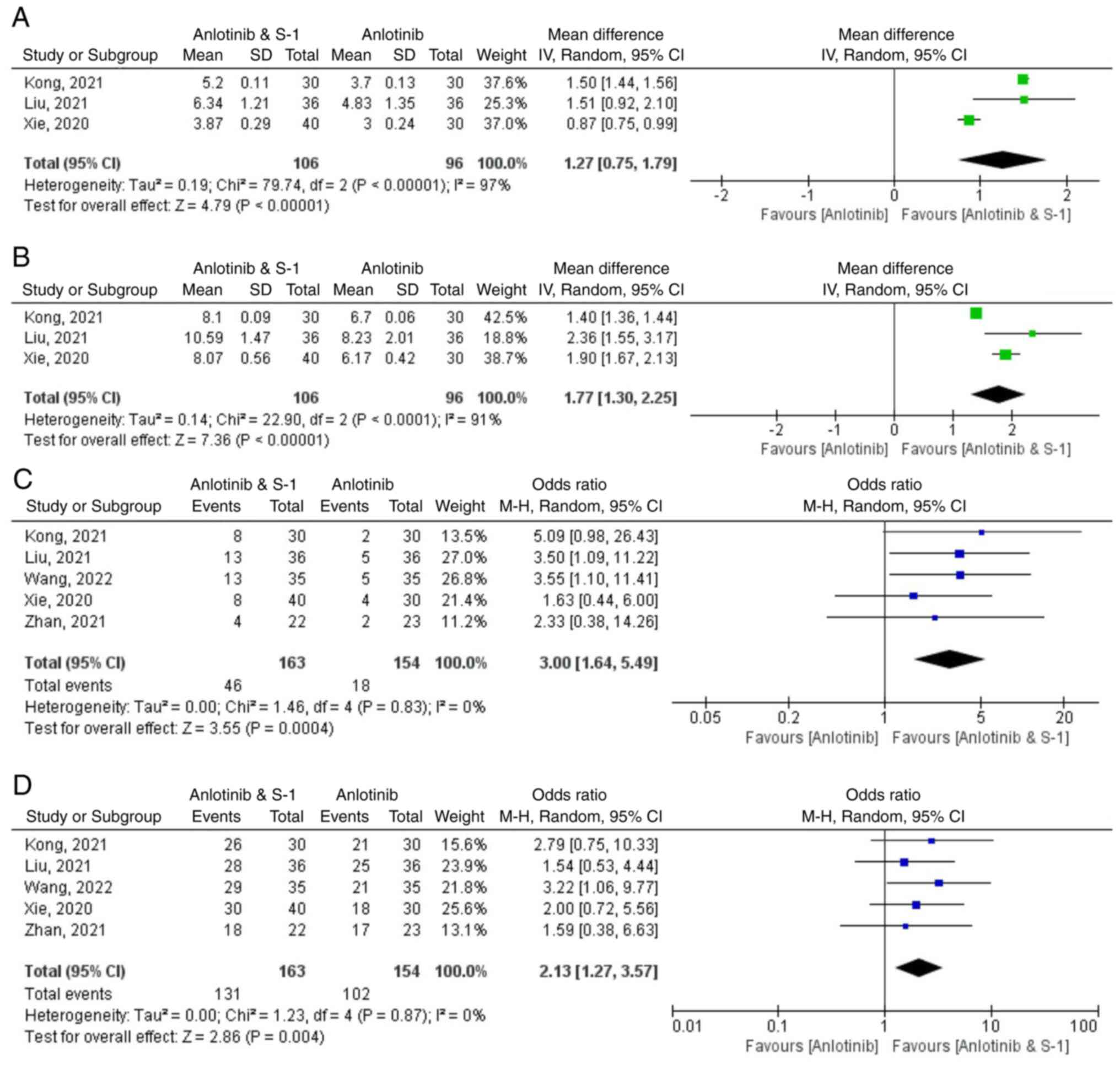
Long-term evaluations of drug efficacy included OS
and PFS, whereas short-term evaluations focused on ORR and DCR
(Fig. 3).
Median PFS (mPFS)
mPFS was reported in three studies included
(29–31), which encompassed a total of 202
patients (n=106 anlotinib plus S-1 group). There was considerable
heterogeneity among the studies (P<0.00001; I2=97%).
The results indicated that PFS in the anlotinib combined with the
S-1 group was significantly longer compared with that of the
control group Weighted Mean Difference (WMD)=1.27; 95% CI 0.75,
1.79; P<0.00001). The mPFS of the anlotinib combined with S-1
group was 3.87–6.34 months, which was 1.27 months longer compared
with that of anlotinib alone group. (Fig. 3A). After excluding the study
published by Xie et al (29), which contributed to the
heterogeneity (WMD=1.50; 95% CI 1.44, 1.56; P<0.00001;
I2=0%), the heterogeneity of PFS decreased.
mOS
The mOS was reported in three of the included
studies (29–31), which comprised a total of 202
patients, with 106 patients in the anlotinib plus S-1 group. There
was significant heterogeneity among these studies (P<0.00001;
I2=91%). These results demonstrated that PFS in the
anlotinib combined with the S-1 group was significantly longer than
that in the control group (WMD=1.77; 95% CI 1.30, 2.25;
P<0.00001). The mOS of anlotinib combined with S-1 group was
8.07–10.59 months, which was 1.77 months longer compared with that
of the anlotinib alone group (Fig.
3B). After excluding the Kong study (31), which contributed to the
heterogeneity (WMD=1.96; 95% CI 1.66, 2.26; P<0.00001;
I2=12%), the heterogeneity of OS decreased.
ORR
ORR was reported in five of the included studies
(29–33), which encompassed a total of 317
patients, with 163 patients in the anlotinib plus S-1 group.
Homogeneity among the studies was not statistically significant.
(P=0.83; I2=0%). These results indicated that the ORR of
the anlotinib combined with the S-1 group was significantly higher
compared with that in the control group (RR=3.00; 95% CI 1.64,
5.49; P=0.004) (Fig. 3C).
DCR
Further, five studies reported the DCR (29–33),
which totaled 317 patients, with 163 in the anlotinib plus S-1
group. Homogeneity was not statistically significant among the
studies (P=0.87; I2=0%). These results indicated that
the DCR of the anlotinib combined with the S-1 group was
significantly higher compared with that in the control group
(RR=2.13; 95% CI 1.27, 3.57; P=0.004) (Fig. 3D).
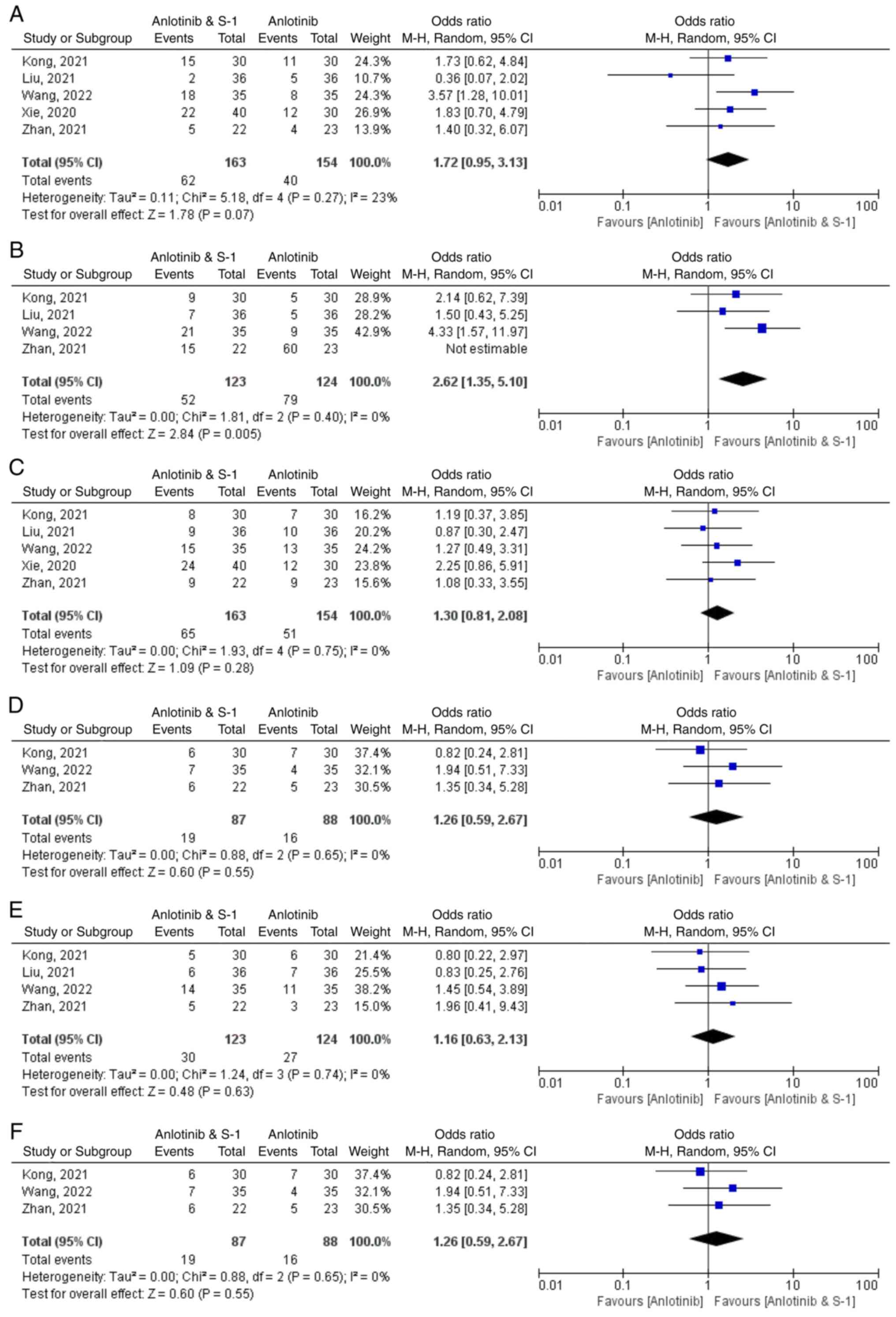
Adverse reactions
Adverse drug reactions were evaluated across various
categories, which included gastrointestinal adverse reactions, bone
marrow suppression, hypertension, fatigue, proteinuria, hepatic and
renal insufficiency and functional hand-foot syndrome (Fig. 4).
i) Gastrointestinal adverse reactions. All
five studies reported gastrointestinal adverse reactions (29–33),
with a total of 317 patients which included 163 in the anlotinib
plus S-1 group. Analysis of the studies demonstrated that there was
not significant homogeneity among the studies (P=0.27;
I2=23%). These results indicated that the anlotinib
combined with S-1 group had significantly more gastrointestinal
adverse reactions compared with the control group (RR=1.72; 95% CI
0.95, 3.13; P=0.07) (Fig. 4A).
ii) Bone marrow suppression. Myelosuppression
was reported in four studies (30–33),
encompassing a total of 247 patients, with 123 in the anlotinib
plus S-1 group. Homogeneity among the studies was not statistically
significant (P=0.40; I2=0%). Myelosuppression was
significantly higher in the anlotinib combined with the S-1 group
compared with the control group (RR=2.62; 95% CI 1.35, 5.10;
P=0.005) (Fig. 4B).
iii) Hypertension. Hypertension was reported
in 5 studies (29–33), which involved 317 patients, with 163
patients in the anlotinib plus S-1 group. Homogeneity among the
studies was not statistically significant (P=0.75;
I2=0%). These results demonstrated a higher incidence of
hypertension in the anlotinib plus S-1 group compared with the
control group (RR=1.30; 95% CI 0.81, 2.08; P=0.28), although this
difference was not statistically significant (Fig. 4C).
iv) Fatigue. Fatigue was reported in 5
included studies (29–33), in a total of 317 patients, including
163 patients in the anlotinib plus S-1 group. Homogeneity among the
studies was not statistically significant (P=0.79;
I2=0%). These results indicated that the incidence of
fatigue in the anlotinib combined with S-1 group was significantly
higher compared with the control group (RR=1.66; 95% CI 1.04, 2.66;
P=0.03) (Fig 4D).
v) Proteinuria. Proteinuria was reported in 4
of the included studies (30–33),
which involved 247 patients, with 123 patients in the anlotinib
plus S-1 group. Homogeneity was not statistically significant among
the studies (P=0.74; I2=0%). These results indicated
that the incidence of proteinuria in the anlotinib plus S-1 group
was higher compared with that in the control group (RR=1.16; 95% CI
0.63, 2.13; P=0.63); however, this difference was not statistically
significant (Fig. 4E).
vi) Hepatic and renal insufficiency. Hepatic
and renal dysfunctions were reported in 3 of the included studies
(29–33), which involved 175 patients,
including 87 in the anlotinib plus S-1 group. Homogeneity was not
statistically significant among the studies (P=0.65;
I2=0%). These results indicated that the incidence of
hepatic and renal insufficiency in the anlotinib plus S-1 group was
higher compared with that in the control group (RR=1.26; 95% CI
0.59, 2.67; P=0.55); however, this difference was not statistically
significant (Fig. 4F).
vii) Functional hand-foot syndrome.
Functional hand-foot syndrome was reported in four of the included
studies (30–33), which involved 247 cases, with 123 in
the anlotinib plus S-1 group. Homogeneity among the studies was not
statistically significant (P=0.99; I2=0%). These results
demonstrated that the incidence of functional hand-foot syndrome in
the anlotinib plus S-1 group was higher compared with that in the
control group (RR=1.43; 95% CI 0.78, 2.63; P=0.25); however, the
difference was not statistically significant (Fig. 4G).
Discussion
Lung cancer is a significant public health concern
due to its high morbidity, mortality and recurrence rates (34). Despite advancements in treatment
options over the past four decades, treatment strategies for
advanced NSCLC, such as third-line treatments, have remained
elusive. Currently, anlotinib and single-agent chemotherapy, such
as platinum, pemetrexed, docetaxel and paclitaxel, are the standard
recommendations for patients with NSCLC for whom third-line or
later-line treatment was not successful in China. However, these
patients often derive limited benefits from single-agent
chemotherapy (2,3). For instance, the mPFS of the
third-line treatment with platinum, pemetrexed, docetaxel and
paclitaxel intravenous chemotherapy ranges from 0.7–1.4 months and
mOS from 4.0–6.3 months. Moreover, after multiline treatment,
patients are generally in poor condition and often cannot tolerate
the toxic and side effects of the above intravenous chemotherapy
drugs (35). Given these
challenges, there is a pressing clinical need for novel and
effective antitumor therapies. Anlotinib and S-1 represent
promising oral antineoplastic drugs that are convenient for daily
use. The present study conducted a comprehensive systematic review
and meta-analysis to evaluate the efficacy and safety profile of
combining anlotinib with S-1 compared to anlotinib alone in
patients with advanced NSCLC. To the best of our knowledge, the
present study was the first meta-analysis to compare anlotinib
combined with S-1 vs. anlotinib alone in patients with advanced
non-small cell lung cancer using the PubMed, Embase, Web of
Science, Cochrane Library, CMB and CNKI databases. Therefore, the
results detailed in the present study were innovative and
novel.
In previous years, the exploration of antiangiogenic
drugs in combination with chemotherapy has shown considerable
progress in lung cancer treatment. A number of clinical trials have
demonstrated that integrating antiangiogenic drugs with
conventional chemotherapy can reduce drug resistance and
significantly improve treatment efficacy (21–23,36,37).
The present study did not retrieve data from the included
literature on the cost-effectiveness of combination therapy with
anlotinib compared with other therapies, such as single-agent
chemotherapy or anlotinib single-agent targeted therapy. Therefore,
the present study discussed the therapeutic difference between
efficacy and adverse reactions caused by anlotinib combined with
S-1 and anlotinib alone.
The findings from the present study highlighted
significant improvements in OS, PFS, ORR and DCR in patients
treated with anlotinib combined with the S-1 group, compared with
those treated with anlotinib alone. Specifically, the combination
therapy extended mPFS and mOS by 1.27 months and 1.77 months,
respectively, when compared with the anlotinib alone group. These
results highlighted the promising potential of this regimen in the
treatment of patients with advanced NSCLC. Compared with
single-agent chemotherapy, the mOS and mPFS achieved with anlotinib
combined with the S-1 group showed a notable increase. These
results indicated that the combination of anlotinib and S-1 yielded
favorable clinical outcomes compared with the single agent
chemotherapy. In China, anlotinib, in combination with
chemotherapy, has been studied for the treatment of advanced NSCLC.
A retrospective study compared the efficacy of anlotinib with
chemotherapy compared with that of chemotherapy alone. These
results showed that the DCR of the combination group was
significantly higher than that of the chemotherapy alone group (78%
vs. 51%, respectively) and the mPFS of the combination group was
1.5 months longer than that of the chemotherapy alone group (5.0
months vs. 3.5 months) (38). These
results suggest that anlotinib combined with chemotherapy can
improve clinical efficacy. In another retrospective study by Meng
et al (39), anlotinib
combined with chemotherapy was compared with bevacizumab combined
with paclitaxel and carboplatin for the treatment of advanced lung
adenocarcinoma. The results showed that the ORR and DCR of
anlotinib combined with chemotherapy and bevacizumab combined with
chemotherapy were significantly better compared with those of
chemotherapy alone and that the ORR and DCR of the anlotinib group
were higher compared with those of the bevacizumab group.
Collectively, the aforementioned studies suggest that anlotinib
combination therapy offers enhanced clinical benefits compared with
chemotherapy, immunotherapy or targeted therapy alone.
Previous studies have reported the pharmacological
mechanisms underlying the combined administration of anlotinib,
with studies showing the effect of anlotinib on tumor blood vessels
(40–43). Tumor blood vessels are often
characterized by pericyte shedding, abnormal basement membranes and
increased vascular leakage, which leads to inadequate blood
perfusion, increased interstitial fluid pressure, difficulty in
drug delivery and persistent hypoxia (40). Anlotinib functions by inhibiting
tumor angiogenesis, thereby reducing nutrient supply to tumors.
Moreover, it promotes a process known as tumor vascular matrix
reprogramming. This mechanism involves enhancing the integrity of
tight junctions in tumor endothelial cells, which increases
pericyte coverage around blood vessels and facilitates vascular
normalization to improve blood vessel morphology and enhance tumor
blood perfusion. This ultimately leads to the restoration of normal
tumor interstitial fluid pressure and an increased distribution of
chemotherapy and other drugs in the tumor tissue for a more
effective antitumor effect. Therefore, anlotinib can improve the
antitumor effects of chemotherapy drugs, targeted drugs or immune
checkpoint inhibitors and enhance tumor tissue distribution of
drugs (41–43). Previous studies reported that
anlotinib combined with S-1 was superior when compared with
anlotinib alone in the treatment of advanced NSCLC, which suggests
that anlotinib improves the distribution of anti-tumor drugs in
tumor tissues and enhances the anti-tumor effect (29–33).
The advantages of combination therapy with anlotinib still require
further elucidation through additional clinical studies. The
efficacy of anlotinib combined with other antineoplastic drugs and
whether the adverse reactions of the combination drugs are
controllable warrants further study. Additional studies are
required to determine any efficacy advantages and potential adverse
reactions caused by anlotinib combined with other antineoplastic
drugs, such as chemotherapy drugs or immune checkpoint inhibitors,
compared with other antineoplastic drugs alone. Furthermore, the
specific use and medication management of the combination drugs in
clinical practice (such as drug dosage selection, management of
adverse drug reactions, formulation of treatment cycles, etc.)
still need a large number of clinical trials and practice to
accumulate experience in the treatment of advanced tumors with
anlotinib, in order to form robust evidence-based medical
guidelines to inform clinical practice.
A previous study by Kong (31) reported that the efficacy of
anlotinib combined with S-1 was not significantly influenced by the
previous lines of treatment received by patients, possibly because
the mechanism of action of this combination is entirely independent
of previous treatments. Furthermore, patients who have received
prior antiangiogenic therapy or EGFR-TKI treatment can still derive
benefits from continuing with anlotinib, which can effectively
sustain treatment outcomes for patients with advanced NSCLC.
Traditionally, an increase in the number of chemotherapy drugs used
can lead to tumor cell drug resistance. Long-term exposure to
chemotherapy may result in both functional and structural
cross-resistance to different drugs, thus reducing the
effectiveness of subsequent treatments (44). Xie et al (29) reported that by improving comorbidity
management, patients' performance status scores may improve even
after multiple lines of treatment failure, which could enable
patients to receive systemic treatment again. These studies
highlight the potential advantages of combining anlotinib and S-1
in clinical practice.
Data on adverse effects from a study of antirotinib
combined with chemotherapy vs. chemotherapy (docetaxel,
gemcitabine, vinorelbine or pemetrexel) in advanced NSCLC suggest
that AEs is significantly more common in the combined treatment
group compared with the chemotherapy alone group. The incidence of
adverse events, such high blood pressure, hand, foot and skin
reactions and hypothyroidism was significantly higher in the joint
treatment group compared with the chemotherapy alone group.
According to the CTCAE 5.0 classification of adverse events
(divided into grade I, II, III and IV) (45), in the joint group, adverse reactions
of grade III and grade IV are mainly bone marrow suppression and
gastrointestinal reaction, but there were no statistically
significant difference between the joint group and the anlotinib
group (31,38). In general, most toxicity was limited
to grades I or II and was both well-tolerated and controlled
(38). The present systematic
review of the adverse effects of anlotinib combined with S-1 vs.
anlotinib alone highlighted that the anlotinib group experienced
fewer gastrointestinal adverse reactions, bone marrow suppression,
hypertension, fatigue, proteinuria, liver and kidney dysfunction
and hand and foot dysfunction compared with the anlotinib combined
with S-1 group. The differences between the bone marrow suppression
and fatigue results of the two groups were statistically
significant and demonstrated heterogeneity. S-1 is a cytotoxic
chemotherapeutic agent and previous studies have reported that
adverse reactions to S-1 mainly manifest in the blood and digestive
systems. Adverse reactions observed with the combination therapy of
anlotinib and S-1 include bone marrow suppression, anorexia and
fatigue and were mostly grade I and II reactions, with a low
incidence of grade III reactions (46,47).
This suggested that compared with anlotinib monotherapy, the
incidence of adverse events is significantly increased in the
combined therapy group compared with the chemotherapy alone group,
primarily due to S-1 chemotherapy-related reactions involving the
blood and digestive systems. No deaths were caused by adverse
reactions in the anlotinib combined with S-1 group. This indicates
that combination therapy did not significantly increase the
incidence of adverse reactions. Most adverse reactions could be
treated through drug intervention, with a few patients
discontinuing treatment due to adverse reactions. For the majority
of advanced NSCLC patients who are suitable for three-line
treatment, the biggest challenge in completing treatment lies in
the need for long-term systemic therapy, which may lead to repeated
hospitalizations and intolerable drug side effects. Anlotinib and
S-1 are both oral capsule preparations. The combination of
anlotinib and S-1 can significantly improve patient convenience by
allowing follow-up in outpatient clinics, reducing the need for
repeated hospitalizations and enabling advanced cancer patients to
reintegrate into society and family life, thereby enhancing their
overall quality of life. Furthermore, oral anticancer drugs
generally have lower severity and incidence of adverse reactions
compared with intravenous antineoplastic drugs. For example, a
study on the efficacy and adverse reactions of oral S-1 and
intravenous fluorouracil (5-FU) in the chemotherapy of rectal
cancer showed that S-1 can serve a similar clinical effect with
5-FU in the chemotherapy of advanced rectal cancer, as both S-1 and
5-FU are fluorouracil chemotherapeutic drugs. The two drugs cause
few adverse reactions and patients exhibit good tolerance,
therefore, both these drugs have value for clinical applications
(48). The heterogeneity of mPFS
was mainly derived from the study reported by Xie et al
(29). The population included in
this study included all patients with EGFR mutation-negative
advanced squamous cell lung cancer at baseline, whereas the
populations included in the other two studies included a mix of
patients with EGFR mutation-positive status, lung adenocarcinoma
and lung squamous cell carcinoma (30,31).
Subgroup analyses of EGFR mutation status and lung cancer
pathological types were not performed because detailed information
was not available. The heterogeneity of mOS was mainly derived from
Kong's study (31) and may be
related to differences in regional economic development in China.
Economic development is closely related to the survival rates of
patients with cancer (49). The
aforementioned study was conducted in Liaoning Province, China,
whereas the other two studies were conducted in the Guangdong
Province, China. As one of the most developed provinces in China,
the Guangdong Province has a higher level of economic development
than most other regions in China, including Liaoning Province
(50), which may explain a number
of differences observed between the Kong study and those of the
other two studies.
The present study had some limitations. There were
five studies included in this paper, of which three were
prospective studies and two were retrospective studies. The sample
size of the included studies was not calculated according to the
response rate to anlotinib in the treatment of advanced
non-small-cell lung cancer. According to the ORR (anlotinib
combined with S-1 was designed to be ~30%) as the primary endpoint,
the effective rate of anlotinib monotherapy was 9%, the two-sided α
was 0.05 and the weight β was 80%. According to the 1:1 enrollment
design, 52 patients were required for each group, so a
meta-analysis comprised of five studies was required to include at
least 520 patients. However, the actual sample size of the five
studies was 317. Therefore, the sample size was small and it could
be suggested that the subjects of the present study were not
sufficiently representative and that there were some limitations in
external validity. Systematic reviews based on small sample
randomized controlled trials (RCTs) may lead to a greater risk of
publication bias (51,52). Moreover, retrospective studies
cannot guarantee that conditions other than intervention measures
are the same, which may cause bias. A considerable part of the
information in the present study was obtained from studies with an
uncertain risk of bias. In addition, the included studies provided
baseline data including patient demographics and NSCLC staging but
did not analyze treatment outcomes related to patient demographics
and NSCLC staging baseline data, which prevented the present study
from conducting group analysis. As patient sex, age and condition
(such as lung cancer classification, whether there is brain
metastasis and gene mutation) were not explored in the included
articles with respect to anlotinib S-1 treatment, the present study
lacked access to certain data and was unable to consider other
patient characteristics, such as sex, age or previous lung disease.
In future research, there is a requirement to expand sample size,
reduce bias and design a prospective multicenter RCT.
In summary, the present study suggested that
combining anlotinib with S-1 may offer superior efficacy compared
with anlotinib alone as a third- or later-line treatment for
advanced NSCLC. In terms of adverse reactions, the combination
therapy was generally well-tolerated. The present meta-analysis
demonstrated that anlotinib combined with S-1 was superior to the
anlotinib monotherapy recommended by the CSCO guidelines for NSCLC,
which may provide clinicians with new ideas for the treatment of
patients with advanced NSCLC, who have failed multiple lines of
treatment. After the efficacy and adverse reactions of the dual
drug combination and the single drug combination were analyzed, it
could be suggested that further clinical trials with a larger
sample size are required to promote the standardization and
recommendation of the dual drug combination in the treatment of
advanced NSCLC.
Acknowledgements
Not applicable.
Funding
This work was supported by the State Key Laboratory of
Ultrasound in Medicine and Engineering (grant nos. 2020KFKT015 and
2020KFKT018).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HX and YL drafted the manuscript, HX, YL, WT and XY
participated in the data review and collection for the study. XD
conceived the study and reviewed and edited the manuscript. HX and
XD confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CSCO
|
Chinese Society of Clinical
Oncology
|
|
DCR
|
disease control rate
|
|
mOS
|
median overall survival
|
|
PFS
|
progression-free survival
|
|
mPFS
|
median PFS
|
|
NSCLC
|
non-small cell lung cancer
|
|
ORR
|
objective response rate
|
|
PRISMA
|
Preferred Reporting Items for
Systematic Reviews and Meta-Analyses
|
|
RCT
|
randomized controlled trial
|
References
|
1
|
Toumazis I, Bastani M, Han SS and
Plevritis SK: Risk-based lung cancer screening: A systematic
review. Lung Cancer. 147:154–186. 2020. View Article : Google Scholar
|
|
2
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar
|
|
3
|
Wang M, Herbst RS and Boshoff C: Toward
personalized treatment approaches for non-small-cell lung cancer.
Nat Med. 27:1345–1356. 2021. View Article : Google Scholar
|
|
4
|
Osarogiagbon RU, Veronesi G, Fang W, Ekman
S, Suda K, Aerts JG and Donington J: Early-stage NSCLC: Advances in
thoracic oncology 2018. J Thorac Oncol. 14:968–978. 2019.
View Article : Google Scholar
|
|
5
|
Zhou C: Lung cancer molecular epidemiology
in China: Recent trends. Transl Lung Cancer Res. 3:270–279.
2014.
|
|
6
|
Lima ABC, Macedo LT and Sasse AD: Addition
of bevacizumab to chemotherapy in advanced non-small cell lung
cancer: A systematic review and meta-analysis. PLoS One.
6:e226812011. View Article : Google Scholar
|
|
7
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang
Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a
third-line or further treatment on overall survival of patients
with advanced non-small cell lung cancer: The ALTER 0303 phase 3
randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018.
View Article : Google Scholar
|
|
8
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar
|
|
9
|
Jiang T, Su C, Li X, Zhao C, Zhou F, Ren
S, Zhou C and Zhang J: EGFR TKIs plus WBRT demonstrated no survival
benefit other than that of TKIs alone in patients with NSCLC and
EGFR mutation and brain metastases. J Thorac Oncol. 11:1718–1728.
2016. View Article : Google Scholar
|
|
10
|
Lee JH, Chen HY, Hsu FM, Chen JS, Liao WY,
Shih JY, Yu CJ, Chen KY, Tsai TH and Yang JC: Cranial irradiation
for patients with epidermal growth factor receptor (EGFR) mutant
lung cancer who have brain metastases in the era of a new
generation of EGFR inhibitors. Oncologist. 24:e1417–e1425. 2019.
View Article : Google Scholar
|
|
11
|
Wang C, Lu X, Zhou Z, Wang J, Hui Z, Liang
J, Feng Q, Chen D, Xiao Z, Lv J, et al: The efficacy of upfront
intracranial radiation with TKI compared to TKI alone in the NSCLC
patients harboring EGFR mutation and brain metastases. J Cancer.
10:1985–1990. 2019. View Article : Google Scholar
|
|
12
|
Gao Y, Liu P and Shi R: Anlotinib as a
molecular targeted therapy for tumors. Oncol Lett. 20:1001–1014.
2020. View Article : Google Scholar
|
|
13
|
Mross K, Frost A, Steinbild S, Hedbom S,
Büchert M, Fasol U, Unger C, Krätzschmar J, Heinig R, Boix O and
Christensen O: A phase I dose-escalation study of regorafenib (BAY
73–4506), an inhibitor of oncogenic, angiogenic, and stromal
kinases, in patients with advanced solid tumors. Clin Cancer Res.
18:2658–2667. 2012. View Article : Google Scholar
|
|
14
|
Strumberg D, Richly H, Hilger RA,
Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D,
Haase CG, et al: Phase I clinical and pharmacokinetic study of the
Novel Raf kinase and vascular endothelial growth factor receptor
inhibitor BAY 43–9006 in patients with advanced refractory solid
tumors. J Clin Oncol. 23:965–972. 2005. View Article : Google Scholar
|
|
15
|
Chen XZ: Anlotinib for refractory advanced
non-small cell lung cancer in China. JAMA Oncol. 5:116–117. 2019.
View Article : Google Scholar
|
|
16
|
Zhong R, Wang Y, Han B, et al:
Interpretation of the clinical diagnosis and treatment guidelines
for lung cancer (2022 edition) of the Chinese medical association.
Chin J Thorac Cardiovasc Surg. 29:1402–1406. 2022.
|
|
17
|
Chhetri P, Giri A, Shakya S, Shakya S,
Sapkota B and Pramod KC: Current development of anti-cancer drug
S-1. J Clin Diagn Res. 10:XE01–XE05. 2016.
|
|
18
|
Ma PQ: Research progress of new anti-tumor
drug Tegafur potassium. Chin J Med Guide. 499–502. 2007.
|
|
19
|
Cheng XW, Leng WH and Mu CL: Efficacy and
safety of S-1 maintenance therapy in advanced non-small-cell lung
cancer patients. World J Clin Cases. 8:5172–5179. 2020. View Article : Google Scholar
|
|
20
|
Kawahara M: Efficacy of S-1 in non-small
cell lung cancer. Expert Opin Pharmacother. 15:1927–1942. 2014.
View Article : Google Scholar
|
|
21
|
Li Y, Yi Y, Lin A, Luo P and Zhang J: A
comparison of the efficacy of antiangiogenic agents combined with
chemotherapy for the treatment of non-small cell lung cancer: A
network meta-analysis. Cancer Cell Int. 20:5482020. View Article : Google Scholar
|
|
22
|
Garon EB, Ciuleanu TE, Arrieta O, Prabhash
K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel
J, et al: Ramucirumab plus docetaxel versus placebo plus docetaxel
for second-line treatment of stage IV non-small-cell lung cancer
after disease progression on platinum-based therapy (REVEL): A
multicentre, double-blind, randomised phase 3 trial. Lancet.
384:665–673. 2014. View Article : Google Scholar
|
|
23
|
Horn L and Sandler A: Chemotherapy and
antiangiogenic agents in non-small-cell lung cancer. Clin Lung
Cancer. 8 (Suppl 2):S68–S73. 2007. View Article : Google Scholar
|
|
24
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. Int J Surg.
8:336–341. 2010. View Article : Google Scholar
|
|
25
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar
|
|
26
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the Cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10:ED0001422019.
|
|
27
|
Higgins JPT, Thomas J, Chandler J,
Cumpston M, Li T, Page MJ and Welch VA: Cochrane handbook for
systematic reviews of interventions. 2nd edition. Chichester (UK):
John Wiley & Sons Ltd; 2019, View Article : Google Scholar
|
|
28
|
Wang D, Zhai JX, Mou ZY, Zong HX, Zhao XD,
Wang XY and Gu P: Discussing on the research of heterogeneity in
meta-analysis. Chin J Evid-Based Med. 9:1115–1118. 2009.
|
|
29
|
Xie XH, Wang F, Lin XQ, Qin YY, Xie ZH,
Zhang JX, Ouyang M and Zhou CZ: Anlotinib plus S-1 for patients
with EGFR mutation-negative advanced squamous cell lung cancer with
ps scores of 2–3 after progression of second-line or later-line
treatment. Cancer Manag Res. 12:12709–12714. 2020. View Article : Google Scholar
|
|
30
|
Liu KL: Efficacy analysis of antirotinib
combined with S-1 in the treatment of advanced non-small cell lung
cancer. Chin J Mod Drug Appl. 15:162–164. 2021.
|
|
31
|
Kong DH: Efficacy and safety of anlotinib
combined with tegafur in the treatment of third-line and later
advanced non-small cell lung cancer. China Med Univ; 2021
|
|
32
|
Zhan LF, Yang QL and Li YY: Effects of
combination therapy with anlotinib and S-1 in thirdly-line
treatment of advanced non-small cell lung cancer. Guide China Med.
19:95–96+99. 2021.
|
|
33
|
Wang Y, Li XZ, Jang SL, et al: Clinical
efficacy of anlotinib combined with S-1 of advanced non-small cell
lung cancer after second-line treatment. Anhui Med J. 43:36–40.
2022.
|
|
34
|
Wu F, Wang L and Zhou C: Lung cancer in
China: Current and prospect. Curr Opin Oncol. 33:40–46. 2021.
View Article : Google Scholar
|
|
35
|
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang
Z, Zhao F, Ahmad R and Zhao J: Anlotinib: A novel multi-targeting
tyrosine kinase inhibitor in clinical development. J Hematol Oncol.
11:1202018. View Article : Google Scholar
|
|
36
|
Wang L, Dong X, Ren Y, Luo J, Liu P, Su D
and Yang X: Targeting EHMT2 reverses EGFR-TKI resistance in NSCLC
by epigenetically regulating the PTEN/AKT signaling pathway. Cell
Death Dis. 9:1292018. View Article : Google Scholar
|
|
37
|
Jiang W, Sun W, Li W, Gao J, Wang H, Zhou
W, Liang J, Aa L and Wang L: Real-world treatment pattern and
comprehensive comparative effectiveness of Endostar plus different
chemotherapy in advanced patients with non-small cell lung cancer.
Sci Rep. 12:108412022. View Article : Google Scholar
|
|
38
|
Wang HY, Chu JF, Zhao Y, Tang H, Wang LL,
Zhou MQ, Yan Z, Liu YY and Yao ZH: A trial of the safety and
efficacy of chemotherapy plus anlotinib vs chemotherapy alone as
second- or third-line salvage treatment for advanced non-small cell
lung cancer. Cancer Manag Res. 12:3827–3834. 2020. View Article : Google Scholar
|
|
39
|
Meng L, Zeng Q, Meng Q, et al: Clinical
effect of antirotinib and bevacizumab combined with paclitaxel plus
carboplatin in the treatment of advanced lung adenocarcinoma. Chin
Med. 14:1164–1168. 2019.
|
|
40
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar
|
|
41
|
Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu
P, Wang L, Xia Y, Qiao Y, Sun W, et al: Autophagy inhibition
potentiates the anti-angiogenic property of multikinase inhibitor
anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung
cancer cells. J Exp Clin Cancer Res. 38:712019. View Article : Google Scholar
|
|
42
|
Xu Q, Wang J, Sun Y, Lin Y, Liu J, Zhuo Y,
Huang Z, Huang S, Chen Y, Chen L, et al: Efficacy and safety of
sintilimab plus anlotinib for PD-L1-positive recurrent or
metastatic cervical cancer: A multicenter, single-arm, prospective
phase II trial. J Clin Oncol. 40:1795–1805. 2022. View Article : Google Scholar
|
|
43
|
Su Y, Luo B, Lu Y, Wang D, Yan J, Zheng J,
Xiao J, Wang Y, Xue Z, Yin J, et al: Anlotinib induces a T
cell-inflamed tumor microenvironment by facilitating vessel
normalization and enhances the efficacy of PD-1 checkpoint blockade
in neuroblastoma. Clin Cancer Res. 28:793–809. 2022. View Article : Google Scholar
|
|
44
|
Dallavalle S, Dobričić V, Lazzarato L,
Gazzano E, Machuqueiro M, Pajeva I, Tsakovska I, Zidar N and
Fruttero R: Improvement of conventional anti-cancer drugs as new
tools against multidrug resistant tumors. Drug Resist Updat.
50:1006822020. View Article : Google Scholar
|
|
45
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar
|
|
46
|
Lin H, Xie Q, Zhong AH, et al: Efficacy
and safety of S-1 in postoperative adjuvant chemotherapy for
non-small cell lung cancer: A meta-analysis. Chin Foreign Med Res.
18:1–6. 2020.
|
|
47
|
Chen J, Wang J, Wu X, Che X, Zou Y, Weng
M, Miao Q and Zheng Q: Meta-analysis for the efficacy of S-1-based
regimens as the first-line treatment in Asian chemotherapy-naive
patients with advanced non-small-cell lung cancer. Future Oncol.
13:2195–2207. 2017. View Article : Google Scholar
|
|
48
|
Li Y, Ji Y and Peng X: Efficacy and
tolerability of S-1 and 5-FU on advanced rectal cancer
chemotherapy. J Colorectal Anal Surg (China). 23:194–197. 2017.
|
|
49
|
Exarchakou A, Rachet B, Belot A, Maringe C
and Coleman MP: Impact of national cancer policies on cancer
survival trends and socioeconomic inequalities in England,
1996–2013: Population based study. BMJ. 360:K7642018. View Article : Google Scholar
|
|
50
|
Wang JD: Guangdong has ranked first in GDP
for 32 consecutive years. Insight China. 38–39. 2021.
|
|
51
|
Begg CB and Berlin JA: Publication bias
and dissemination of clinical research. J Natl Cancer Inst.
81:107–115. 1989. View Article : Google Scholar
|
|
52
|
Ioannidis JPA: Why most published research
findings are false. PLoS Med. 2:e1242005. View Article : Google Scholar
|