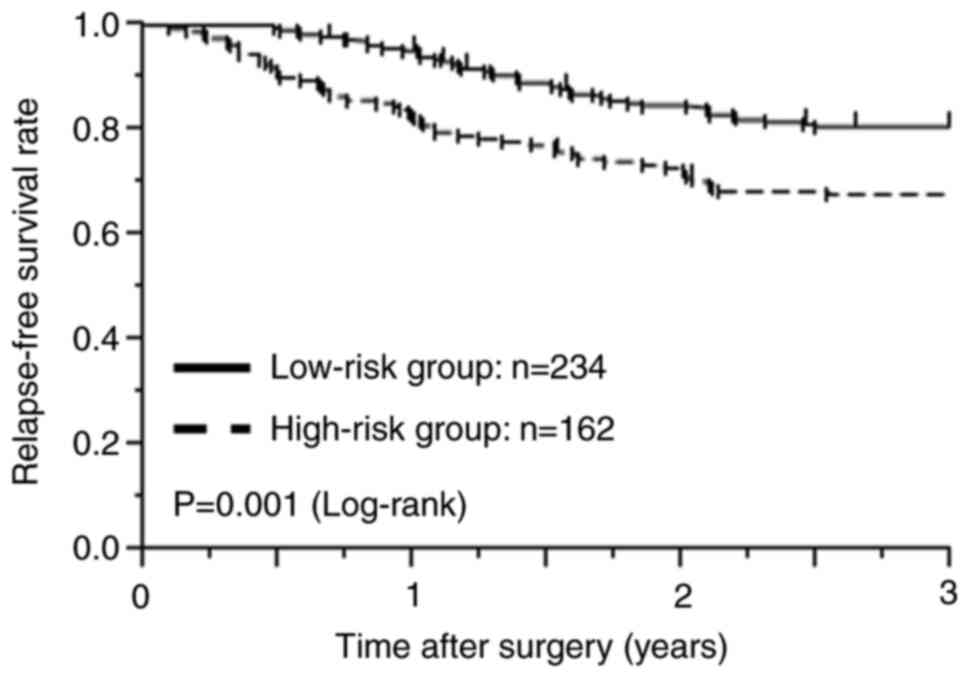
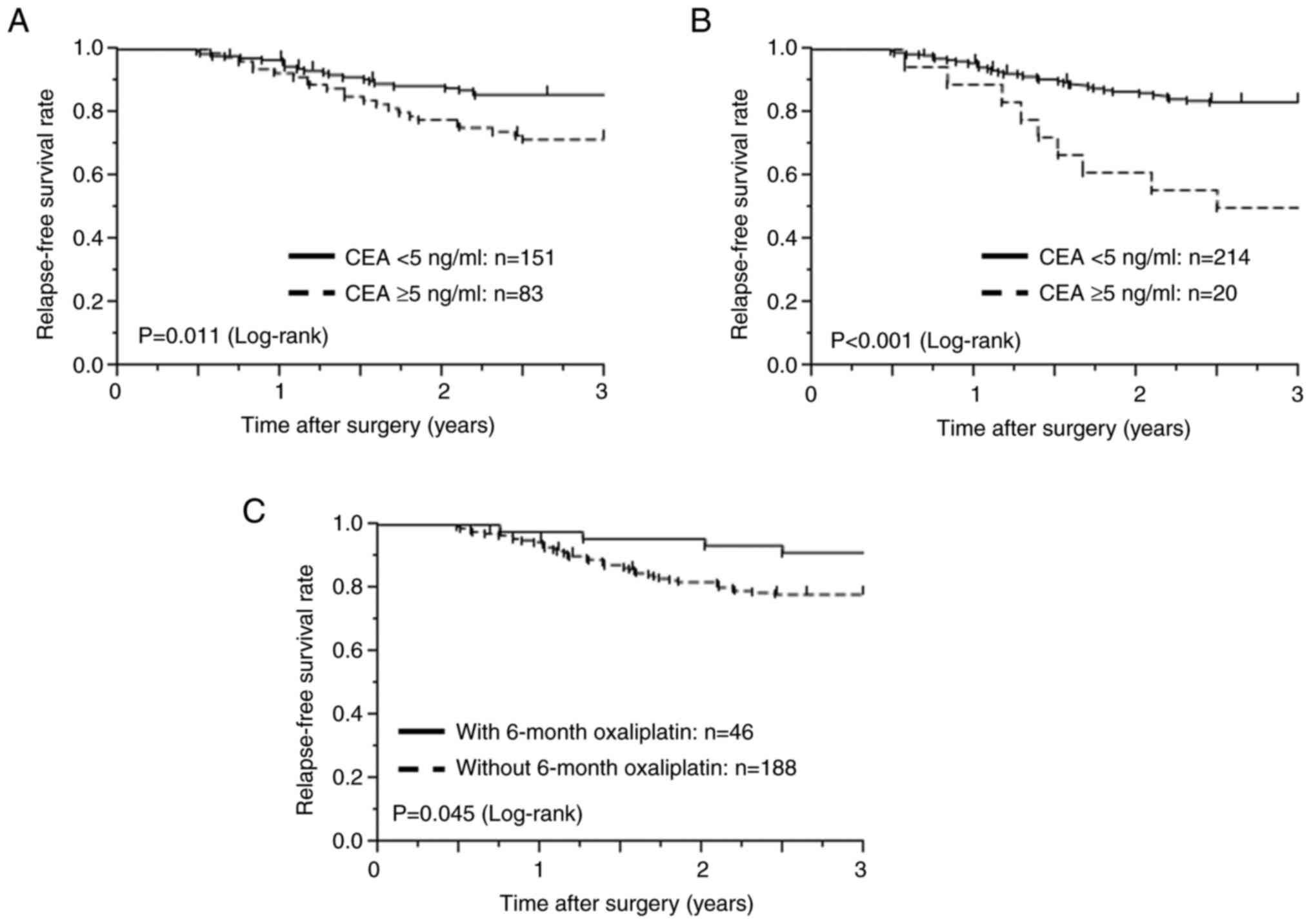
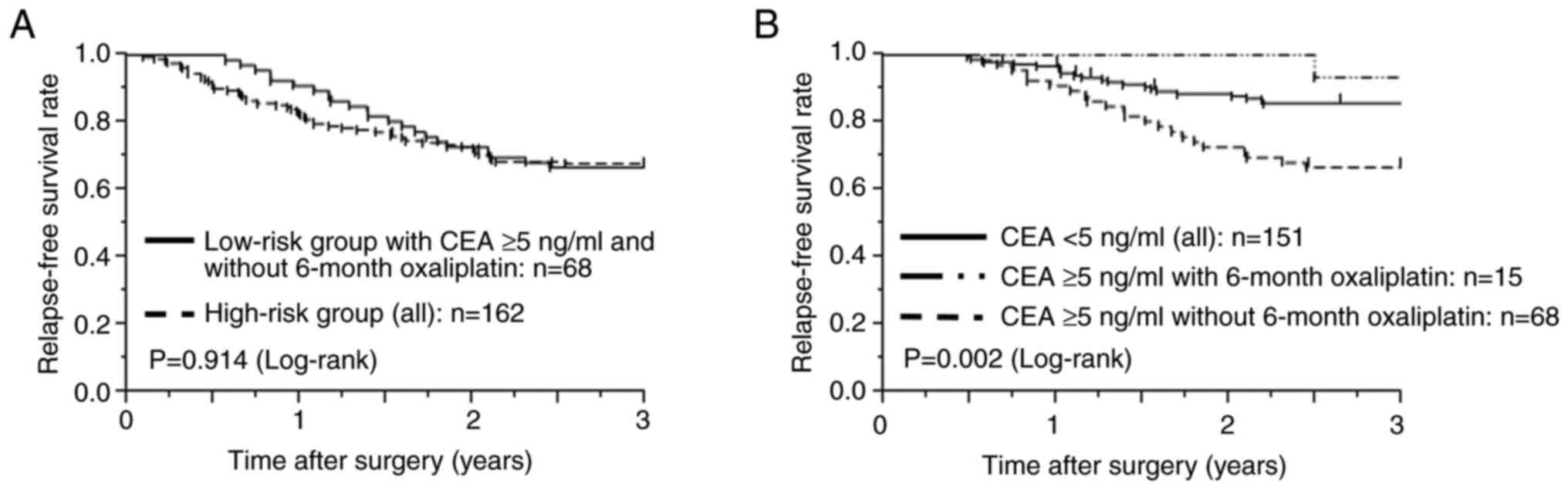
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society: Colorectal cancer
facts and figures 2023-2025, . American Cancer Society; Atlanta,
GA: 2023
|
|
3
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benson AB III, Schrag D, Somerfield MR,
Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J,
McAllister P, Van Cutsem E, et al: American Society of Clinical
Oncology recommendations on adjuvant chemotherapy for stage II
colon cancer. J Clin Oncol. 22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baxter NN, Kennedy EB, Bergsland E, Berlin
J, George TJ, Gill S, Gold PJ, Hantel A, Jones L, Lieu C, et al:
Adjuvant therapy for stage II colon cancer: ASCO guideline update.
J Clin Oncol. 40:892–910. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Argilés G, Tabernero J, Labianca R,
Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P,
Yoshino T, Taieb J, et al: Localised colon cancer: ESMO Clinical
Practice Guidelines for diagnosis, treatment and Follow-up. Ann
Oncol. 31:1291–1305. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology. NCCN;
Plymouth Meeting, PA: 2023
|
|
8
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Babcock BD, Aljehani MA, Jabo B, Choi AH,
Morgan JW, Selleck MJ, Luca F, Raskin E, Reeves ME, Garberoglio CA,
et al: High-risk stage II colon cancer: Not all risks are created
equal. Ann Surg Oncol. 25:1980–1985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grothey A, Sobrero AF, Shields AF, Yoshino
T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, et al:
Duration of adjuvant chemotherapy for stage III colon cancer. N
Engl J Med. 378:1177–1188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
André T, Meyerhardt J, Iveson T, Sobrero
A, Yoshino T, Souglakos I, Grothey A, Niedzwiecki D, Saunders M,
Labianca R, et al: Effect of duration of adjuvant chemotherapy for
patients with stage III colon cancer (IDEA collaboration): Final
results from a prospective, pooled analysis of six randomised,
phase 3 trials. Lancet Oncol. 21:1620–1629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshino T, Yamanaka T, Kotaka M, Manaka D,
Eto T, Hasegawa J, Takagane A, Nakamura M, Kato T, Munemoto Y, et
al: LBA24-efficacy of 3 versus 6 months of oxaliplatin-based
adjuvant chemotherapy for stage III colon cancer (CC): Results from
phase III ACHIEVE trial as part of the International Duration
Evaluation of Adjuvant therapy (IDEA) Collaboration. Ann Oncol.
28:v6142017. View Article : Google Scholar
|
|
13
|
Kotaka M, Yamanaka T, Yoshino T, Manaka D,
Eto T, Hasegawa J, Takagane A, Nakamura M, Kato T, Munemoto Y, et
al: Safety data from the phase III Japanese ACHIEVE trial: Part of
an international, prospective, planned pooled analysis of six phase
III trials comparing 3 versus 6 months of Oxaliplatin-based
adjuvant chemotherapy for stage III colon cancer. ESMO Open.
3:e0003542018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumors. (8th edition).
2017.
|
|
15
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmoll HJ, Cartwright T, Tabernero J,
Nowacki MP, Figer A, Maroun J, Price T, Lim R, Van Cutsem E, Park
YS, et al: Phase III trial of capecitabine plus oxaliplatin as
adjuvant therapy for stage III colon cancer: A planned safety
analysis in 1,864 patients. J Clin Oncol. 25:102–109. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haller DG, Tabernero J, Maroun J, de Braud
F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K and
Schmoll HJ: Capecitabine plus oxaliplatin compared with
fluorouracil and folinic acid as adjuvant therapy for stage III
colon cancer. J Clin Oncol. 29:1465–1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
André T, de Gramont A, Vernerey D,
Chibaudel B, Bonnetain F, Tijeras-Raballand A, Scriva A, Hickish T,
Tabernero J, Van Laethem JL, et al: Adjuvant fluorouracil,
leucovorin, and oxaliplatin in stage II to III colon cancer:
Updated 10-year survival and outcomes according to BRAF mutation
and mismatch repair status of the MOSAIC study. J Clin Oncol.
33:4176–4187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gunderson LL, Jessup JM, Sargent DJ,
Greene FL and Stewart AK: Revised TN categorization for colon
cancer based on national survival outcomes data. J Clin Oncol.
28:264–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim MJ, Jeong SY, Choi SJ, Ryoo SB, Park
JW, Park KJ, Oh JH, Kang SB, Park HC, Heo SC and Park JG: Survival
paradox between stage IIB/C (T4N0) and stage IIIA (T1-2N1) colon
cancer. Ann Surg Oncol. 22:505–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimizu H, Kuriu Y, Arita T, Kiuchi J,
Yamamoto Y, Konishi H, Morimura R, Shiozaki A, Ikoma H, Kubota T,
et al: Staging paradox and discrepancy in adjuvant chemotherapy in
patients with T4N0, T1-2N1, and T3N1 colon cancer. World J Surg.
45:1561–1568. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Souglakos J, Boukovinas I, Kakolyris S,
Xynogalos S, Ziras N, Athanasiadis A, Androulakis N, Christopoulou
A, Vaslamatzis M, Ardavanis A, et al: Three-versus six-month
adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon
cancer patients: The efficacy results of Hellenic Oncology Research
Group (HORG) participation to the International Duration Evaluation
of Adjuvant Chemotherapy (IDEA) project. Ann Oncol. 30:1304–1310.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lieu C, Kennedy EB, Bergsland E, Berlin J,
George TJ, Gill S, Gold PJ, Hantel A, Jones L, Mahmoud N, et al:
Duration of oxaliplatin-containing adjuvant therapy for stage III
colon cancer: ASCO clinical practice guideline. J Clin Oncol.
37:1436–1447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamazaki K, Yamanaka T, Shiozawa M, Manaka
D, Kotaka M, Gamoh M, Shiomi A, Makiyama A, Munemoto Y, Rikiyama T,
et al: Oxaliplatin-based adjuvant chemotherapy duration (3 versus 6
months) for high-risk stage II colon cancer: The randomized phase
III ACHIEVE-2 trial. Ann Oncol. 32:77–84. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu IS, Pereira AAL, Lee M, Korphaisarn K,
Marshall J, Segelov E, O'Callaghan C, Lim HJ, Kopetz S and Loree
JM: Medical oncologists' perspectives on how the results of the
IDEA Collaboration impact the adjuvant treatment of stage III colon
cancer. Oncologist. 25:229–234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boyne DJ, Cheung WY, Hilsden RJ, Sajobi
TT, Batra A, Friedenreich CM and Brenner DR: Association of a
shortened duration of adjuvant chemotherapy with overall survival
among individuals with stage III colon cancer. JAMA Netw Open.
4:e2135872021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baqar AR, Wilkins S, Staples M, Angus Lee
CH, Oliva K and McMurrick P: The role of preoperative CEA in the
management of colorectal cancer: A cohort study from two cancer
centres. Int J Surg. 64:10–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takagawa R, Fujii S, Ohta M, Nagano Y,
Kunisaki C, Yamagishi S, Osada S, Ichikawa Y and Shimada H:
Preoperative serum carcinoembryonic antigen level as a predictive
factor of recurrence after curative resection of colorectal cancer.
Ann Surg Oncol. 15:3433–3439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wiratkapun S, Kraemer M, Seow-Choen F, Ho
YH and Eu KW: High preoperative serum carcinoembryonic antigen
predicts metastatic recurrence in potentially curative colonic
cancer: Results of a five-year study. Dis Colon Rectum. 44:231–235.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang R, Xu B, Sun M, Pang X, Wang X, Zhu
J, Lian J and Lu H: Dynamic monitoring of serum CEA and CA19-9
predicts the prognosis of postoperative stage II colon cancer. Eur
J Surg Oncol. 49:1071382023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ushigome M, Shimada H, Kaneko T, Miura Y,
Nagashima Y, Suzuki T, Kagami S, Kurihara A and Funahashi K:
Preoperative CRP(-)/CEA(-)/CA19-9(-)/non-T4 in stage III colorectal
cancer is favorable risk for recurrence. J Anus Rectum Colon.
6:264–273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sonoda H, Yamada T, Matsuda A, Ohta R,
Shinji S, Yokoyama Y, Takahashi G, Iwai T, Takeda K, Ueda K, et al:
Elevated serum carcinoembryonic antigen level after curative
surgery is a prognostic biomarker of stage II-III colorectal
cancer. Eur J Surg Oncol. 47:2880–2887. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pu H, Yang W, Liu M, Pang X, Chen Y and
Xiong Q: Elevated postoperative carcinoembryonic antigen guides
adjuvant chemotherapy for stage II colon cancer: A multicentre
cohort retrospective study. Sci Rep. 14:68892024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mach JP, Jaeger P, Bertholet MM,
Ruegsegger CH, Loosli RM and Pettavel J: Detection of recurrence of
large-bowel carcinoma by radioimmunoassay of circulating
carcinoembryonic antigen (C.E.A.). Lancet. 2:535–540. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung TD, Yoo JH, Lee MJ, Park HK, Shin JH,
An MS, Ha TK, Kim KH, Bae KB, Kim TH, et al: Prognostic
significance of the decreased rate of perioperative serum
carcinoembryonic antigen level in the patients with colon cancer
after a curative resection. Ann Coloproctol. 29:115–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Watanabe T, Muro K, Ajioka Y, Hashiguchi
Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S,
et al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2016 for the treatment of colorectal cancer. Int J Clin
Oncol. 23:1–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimada Y, Hamaguchi T, Mizusawa J, Saito
N, Kanemitsu Y, Takiguchi N, Ohue M, Kato T, Takii Y, Sato T, et
al: Randomised phase III trial of adjuvant chemotherapy with oral
uracil and tegafur plus leucovorin versus intravenous fluorouracil
and levofolinate in patients with stage III colorectal cancer who
have undergone Japanese D2/D3 lymph node dissection: Final results
of JCOG0205. Eur J Cancer. 50:2231–2240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida M, Ishiguro M, Ikejiri K,
Mochizuki I, Nakamoto Y, Kinugasa Y, Takagane A, Endo T, Shinozaki
H, Takii Y, et al: S-1 as adjuvant chemotherapy for stage III colon
cancer: A randomized phase III study (ACTS-CC trial). Ann Oncol.
25:1743–1749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Q, Liu T, Liu P, Luo J, Zhang N, Lu K,
Ju H, Zhu Y, Wu W, Zhang L, et al: Perineural and lymphovascular
invasion predicts for poor prognosis in locally advanced rectal
cancer after neoadjuvant chemoradiotherapy and surgery. J Cancer.
10:2243–2249. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qin L, Heng Y, Deng S, Gu J, Mao F, Xue Y,
Jiang Z, Wang J, Cheng D, Wu K, et al: Perineural invasion affects
prognosis of patients undergoing colorectal cancer surgery: A
propensity score matching analysis. BMC Cancer. 23:4522023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lugli A, Karamitopoulou E and Zlobec I:
Tumour budding: A promising parameter in colorectal cancer. Br J
Cancer. 106:1713–1717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rogers AC, Winter DC, Heeney A, Gibbons D,
Lugli A, Puppa G and Sheahan K: Systematic review and Meta-analysis
of the impact of tumour budding in colorectal cancer. Br J Cancer.
115:831–840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie H, Wei L, Yuan G, Liu M, Tang S and
Gan J: Prognostic value of prognostic nutritional index in patients
with colorectal cancer undergoing surgical treatment. Front Nutr.
9:7944892022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mao Y and Lan J: Prognostic value of the
geriatric nutritional index in colorectal cancer patients
undergoing surgical intervention: A systematic review and
meta-analysis. Front Oncol. 12:10664172022. View Article : Google Scholar : PubMed/NCBI
|