Introduction
Colon cancer (CC) is the fifth most common cancer
worldwide, with an annual worldwide incidence of approximately
1,148,500 cases and 576,800 deaths (1). However, CC prognosis has improved in
recent years owing to advances in chemotherapy and the development
of various anticancer drugs. Currently, CC up to stage III has a
relatively good prognosis if treated with appropriate anticancer
agents (2,3).
The American Society of Clinical Oncology (ASCO),
European Society for Medical Oncology (ESMO), National
Comprehensive Cancer Network (NCCN), and Japanese Society for
Cancer of the Colon and Rectum (JSCCR) guidelines identify various
high-risk factors for recurrence in patients with stage II CC.
Postoperative adjuvant chemotherapy is recommended for patients
with pathological stage (pStage) II CC with the identified risk
factors and for those with pStage III CC (4–8). T4
has been reported to be the most powerful prognostic factor among
the risk factors for stage II CC (9). In contrast, patients with pathological
T1-3N1 (pT1-3N1) CC have a relatively low risk of recurrence.
Previous studies have discussed the possibility of shortening the
duration of chemotherapy with oxaliplatin for patients with
low-risk stage III CC (10,11) based on data demonstrating that
shortening the administration duration does not worsen prognosis
but rather decreases adverse events. However, certain patients
receiving adjuvant chemotherapy have poor prognoses, and the
International Duration Evaluation of Adjuvant Therapy (IDEA)
Collaboration has identified the following study limitations.
First, the study included an integrated analysis of six studies;
however, subgroup analyses were performed without adjusting for
multiplicity. Second, no standardized follow-up procedures were
performed in the six trials, including intervals for imaging and
laboratory assessments. Third, the study did not examine recurrence
in the low-risk group based on clinicopathological background
factors. Certain patients in the low-risk group may have a higher
probability of recurrence. Therefore, shortening the duration of
adjuvant chemotherapy based on the pStage remains controversial
(10,12,13).
We aimed to elucidate the risk factors for CC in a
group with a low recurrence risk and investigate the effect of a
shorter duration of oxaliplatin treatment. We believe the results
of this analysis will contribute to a better prognosis by
identifying the patients for whom postoperative adjuvant
chemotherapy duration should be shortened.
Materials and methods
Patients and data collection
This study included 396 patients who underwent
surgery for CC at the Kyoto Prefectural University of Medicine
between January 2008 and December 2020 and at the Japanese Red
Cross Kyoto Daiichi Hospital between January 2013 and December
2020. The inclusion criteria were as follows: patients (a)
pathologically diagnosed with colon adenocarcinoma, (b) with pT4N0
and pStage III CC (14), (c)
undergoing curative resection for CC, and (d) who received adjuvant
chemotherapy (at any time). Patients who underwent emergency
primary tumor resection were excluded. In the present study, CC was
defined as colon cancer from the cecum to the sigmoid colon or
rectal sigmoid colon cancer. Appendiceal and rectal cancers were
excluded from this study.
The present study was a retrospective analysis of
de-identified data. The requirement for written informed consent
from individual participants was waived owing to the retrospective
design, in accordance with the standards of the Kyoto Prefectural
University of Medicine Institutional Medical Ethics Review
Committee. The present study was approved by the ethics committee
of Kyoto Prefectural University of Medicine (approval no.
ERB-C-1178, 1178-1, 1178-2, 1178-3). We submitted the notification
required by the Ministry of Health, Labor, and Welfare to the
Japanese Red Cross Kyoto Daiichi Hospital and obtained permission
to access the database through proper procedures. After creating
the correspondence table, all information identifying individual
patients was excluded, and only the information to be used for
analysis was received from Kyoto Daiichi Red Cross Hospital. The
correspondence table is maintained at Kyoto Daiichi Red Cross
Hospital. The dates when the databases were accessed for data
collection and data collection started were April 1, 2023 for Kyoto
Prefectural University of Medicine and July 29, 2024 for the
Japanese Red Cross Kyoto Daiichi Hospital.
Measurement of tumor markers
Preoperative tumor markers utilized in the analysis
were measured during the first outpatient visit (approximately 1
month preoperatively), and postoperative tumor markers were
measured during the first postoperative visit (approximately 1
month postoperatively).
Surgical procedure, follow-up, and
diagnosis of recurrence
Primary tumor resection and lymph node dissection
were performed according to the JSCCR Guidelines for the Treatment
of Colorectal Cancer (8). Resected
specimens were assessed by pathologists based on the
tumor-node-metastasis (TNM) (8th edition) staging system by the
Union for International Cancer Control (UICC) (14). After primary tumor resection for CC,
patients were followed up at regular intervals by determining serum
carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9
levels every 3 months; computed tomography of the chest, abdomen,
and pelvis every 6 months; and colonoscopy at 1 and 3 years
postoperatively, according to the JSCCR guidelines (8). The first follow-up was performed 1
month after primary tumor resection, and patients were subsequently
followed up every 3 months for up to 3 years. All patients were
followed up until their death or at least 3 years after primary
tumor resection. After primary tumor resection, adjuvant
chemotherapy regimens were recommended based on the JSCCR
guidelines, unless the patient's performance status was unsuitable
for the recommended chemotherapy regimens or if the patient
declined chemotherapy.
Statistical analysis
Data are presented as medians (ranges) and
percentages. Prognostic curves were generated using the
Kaplan-Meier method, and the log-rank test was performed to
evaluate intergroup differences. Significant parameters in the
univariate analyses were further assessed using multivariate Cox
models. Preoperative and postoperative high CEA levels were
considered as separate two types of multivariate Cox models because
of confounding. In one model (model #1), preoperative CEA level was
incorporated as an explanatory variable. In the other model (model
#2), postoperative CEA level was incorporated as an explanatory
variable. Hazard ratios (HRs) and 95% confidence intervals (CIs)
were calculated using Cox proportional hazard models. The
statistical significance of the differences was set at P<0.05,
as derived from two-tailed tests. Statistical analyses were
performed using the JMP software version 10 (JMP, Cary, NC,
USA).
Results
Clinicopathological characteristics
and survival analyses of all patients
The clinicopathological characteristics of 396
patients are presented in Table I.
The median age of the patients was 68 years, with 213 male (53.8%)
and 183 female (46.2%) patients. Most patients had advanced T
(T3/T4) stage CC (345/396, 87.1%) and positive lymph nodes
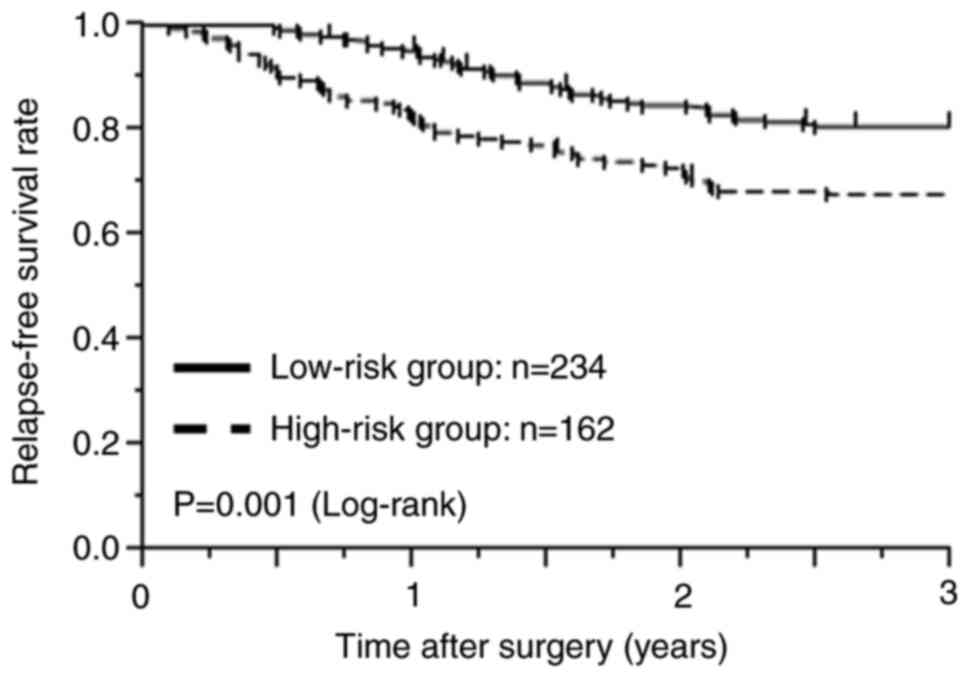
(363/396, 91.7%) with their primary tumors. Overall, 234 (59.1%)
were classified into the low-risk group, including those with pT4N0
or low-risk pStage III (T1-3N1) CC, whereas 162 (40.9%) were in the
high-risk group, including those with high-risk pStage III (T4N1 or
any T, N2) CC. In total, 92 patients completed 6 months of adjuvant
chemotherapy with oxaliplatin. The median follow-up period after
colectomy was 36 months (average, 34.5 months; range, 3.6–36
months). The 3-year recurrence-free survival (RFS) rate of the
low-risk group, for which the duration of adjuvant chemotherapy may
be shortened, was significantly better than that of the high-risk
group, for which conventional adjuvant chemotherapy is recommended
(low-risk group: 80.8% vs. high-risk group: 67.8%, P=0.001;
Fig. 1).
 | Table I.Clinicopathological characteristics
of pT4N0 or pStage III patients with adjuvant chemotherapy. |
Table I.
Clinicopathological characteristics
of pT4N0 or pStage III patients with adjuvant chemotherapy.
| Variables | All patients
(n=396) |
|---|
| Age, years |
|
|
Median | 68 |
|
Range | 21-87 |
| Sex, n |
|
|
Male | 213 |
|
Female | 183 |
| CEAa, n |
|
| <5
ng/ml | 224 |
| ≥5
ng/ml | 172 |
| CA19-9a, n |
|
| <37
U/ml | 346 |
| ≥37
U/ml | 50 |
| Obstruction, n |
|
|
Absence | 339 |
|
Presence | 57 |
| Lymph node
dissection, n |
|
|
≥12 | 333 |
|
<12 | 63 |
| pT, n |
|
|
pT1 | 27 |
|
pT2 | 24 |
|
pT3 | 210 |
|
pT4 | 135 |
| pN, n |
|
|
Absence | 33 |
|
Presence | 363 |
| Venous invasion,
n |
|
|
Absence | 146 |
|
Presence | 250 |
| Lymphatic invasion,
n |
|
|
Absence | 101 |
|
Presence | 295 |
| Histopathological
type, n |
|
|
Differentiated | 349 |
|
Undifferentiated | 47 |
Clinicopathological characteristics
and survival analyses of patients in the low-risk group
We examined the prognostic factors of 234 patients
in the low-risk group, including those with pT4N0 and low-risk
stage III CC. The details of adjuvant chemotherapy are summarized
in Table II. Of the 234 patients,
113 (48.3%) received an oxaliplatin-based regimen, and 46 (19.7%)
completed 6 months of oxaliplatin treatment. The median follow-up
period after colectomy was 36 months (average, 34.9 months; range,
3.6–36 months). We classified the patients into two groups
according to various clinicopathological background factors and
conducted univariate and multivariate analyses for the 3-year RFS,
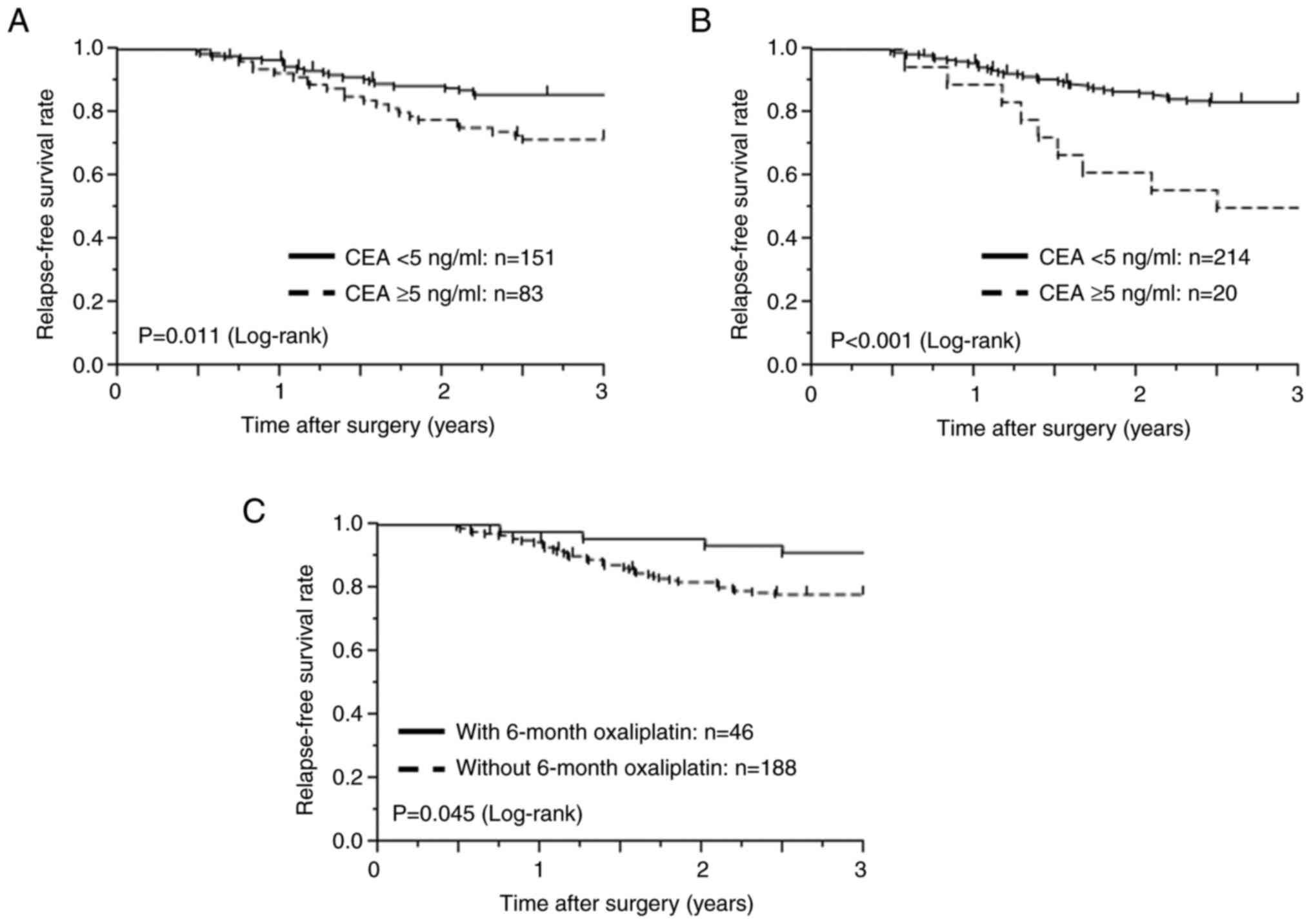
as shown in Table III. The
univariate analysis demonstrated that patients with a high
preoperative serum CEA level (≥5 ng/ml), a high postoperative serum
CEA level, and those who did not receive the 6-month treatment with
oxaliplatin had poor 3-year RFS. The survival curves are depicted
in Fig. 2. The 3-year RFS rate in
patients with high preoperative CEA levels was 71.6%, significantly
lower than that in patients with low preoperative CEA levels
(85.8%; P=0.011, Fig. 2A),
and postoperative CEA was also significantly different on
univariate analysis (P<0.001, Fig.
2B). The 3-year RFS rate in patients who did not receive the
6-month treatment with oxaliplatin was 78.1%, significantly lower
than that in patients who received the 6-month treatment with
oxaliplatin (91.3%; P=0.045, Fig. 2C). The multivariate Cox model #1
demonstrated that a high preoperative CEA level (HR, 2.120; 95% CI,
1.171–3.858; P=0.013) and incomplete 6-month adjuvant
chemotherapy with oxaliplatin (HR, 2.737; 95% CI, 1.103–9.118;
P=0.028) were independent risk factors for poor RFS. In
multivariate Cox model #2, a high postoperative CEA (HR, 3.456; 95%
CI, 1.557–6.897; P=0.004) and incomplete 6-month adjuvant
chemotherapy with oxaliplatin (HR, 2.592; 95% CI, 1.043–8.643;
P=0.039) were identified as independent risk factors for
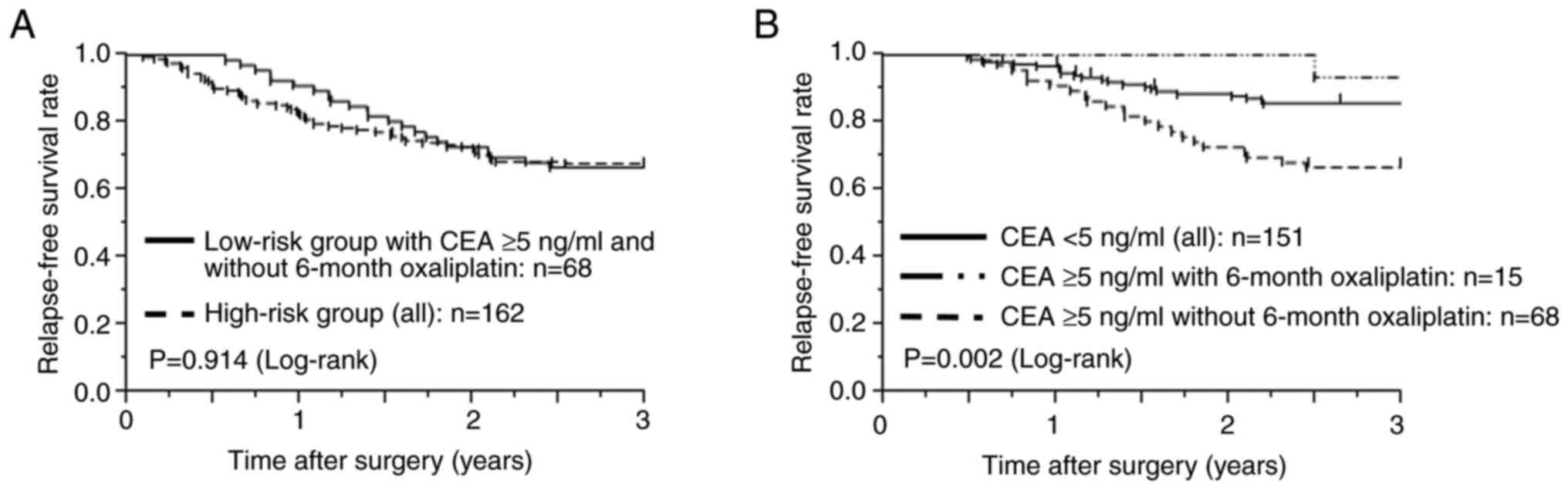
poor RFS. Further analyses were performed to investigate the effect
of high preoperative CEA levels on prognosis. The 3-year RFS rate
of the 68 patients in the low-risk group who had abnormal
preoperative CEA levels and did not complete the 6-month adjuvant
treatment with oxaliplatin was similar to that of all patients in
the high-risk group (3-year RFS rates: 66.7% vs. 67.8%,
P=0.914, Fig. 3A). However,
among patients in the low-risk group, the 3-year RFS of those with
high preoperative CEA levels who received the 6-month treatment
with oxaliplatin (93.3%) was similar to that of all patients with
low preoperative CEA levels (85.8%, P=0.402) and tended to
be better than that of patients with high preoperative CEA levels
who did not receive the 6-month treatment with oxaliplatin (66.7%,
P=0.044) (Fig. 3B).
 | Table II.Summary of adjuvant chemotherapy in
pT4N0 or pT1-3N1 patients. |
Table II.
Summary of adjuvant chemotherapy in
pT4N0 or pT1-3N1 patients.
| Chemotherapy
regimen | All patients, n (%)
(n=234) | T4N0 patients, n
(%) (n=33) | T1-2N1 patients, n
(%) (n=49) | T3N1 patients, n
(%) (n=152) |
|---|
| UFT | 79 (33.8) | 15 (45.5) | 14 (28.6) | 50 (32.9) |
| S-1 | 6 (2.6) | 1 (3.0) | 1 (2.0) | 4 (2.6) |
| Capecitabine | 36 (15.4) | 5 (15.2) | 7 (14.3) | 24 (15.8) |
| CAPOX | 99 (42.3) | 10 (30.3) | 24 (49.0) | 65 (42.8) |
| FOLFOX | 13 (5.6) | 2 (6.1) | 2 (4.1) | 9 (5.9) |
| SOX | 1 (0.4) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Usage of
oxaliplatin | 113 (48.3) | 12 (36.4) | 27 (55.1) | 74 (48.7) |
| Completeness of
6-month treatment with oxaliplatin | 46 (19.7) | 1 (3.0) | 12 (24.5) | 33 (21.7) |
 | Table III.Univariate and multivariate survival
analyses of RFS in pT4N0 or pT1-3N1 patients with adjuvant
chemotherapy. |
Table III.
Univariate and multivariate survival
analyses of RFS in pT4N0 or pT1-3N1 patients with adjuvant
chemotherapy.
|
|
| Univariate
analysis | Multivariate
analysis (model #1) | Multivariate
analysis (model #2) |
|---|
|
|
|
|
|
|
|---|
| Variables | All patients, n
(n=234) | 3-year RFS, % | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
<65 | 87 | 81.2 | 0.885 |
|
|
|
|
|
≥65 | 147 | 80.5 |
|
|
|
|
|
| Sex |
|
|
|
|
|
|
|
|
Male | 135 | 80.9 | 0.950 |
|
|
|
|
|
Female | 99 | 80.6 |
|
|
|
|
|
| CEAa, ng/ml |
|
|
|
|
|
|
|
|
<5 | 151 | 85.8 | 0.011c | 1 (reference) |
|
|
|
| ≥5 | 83 | 71.6 |
| 2.120
(1.171–3.858) | 0.013c | NA | NA |
| CEAb, ng/ml |
|
|
|
|
|
|
|
|
<5 | 214 | 83.4 |
<0.001c |
|
| 1 (reference) |
|
| ≥5 | 20 | 50.0 |
| NA | NA | 3.456
(1.557–6.897) | 0.004c |
| CA19-9a, U/ml |
|
|
|
|
|
|
|
|
<37 | 216 | 82.0 | 0.124 |
|
|
|
|
|
≥37 | 18 | 66.7 |
|
|
|
|
|
| CA19-9b, U/ml |
|
|
|
|
|
|
|
|
<37 | 230 | 80.9 | 0.695 |
|
|
|
|
|
≥37 | 4 | 75.0 |
|
|
|
|
|
| Obstruction |
|
|
|
|
|
|
|
|
Absence | 202 | 82.3 | 0.158 |
|
|
|
|
|
Presence | 32 | 70.3 |
|
|
|
|
|
| Lymph node
dissection |
|
|
|
|
|
|
|
|
≥12 | 191 | 90.2 | 0.123 |
|
|
|
|
|
<12 | 43 | 78.8 |
|
|
|
|
|
| Venous
invasion |
|
|
|
|
|
|
|
|
Absence | 101 | 80.6 | 0.997 |
|
|
|
|
|
Presence | 133 | 80.9 |
|
|
|
|
|
| Lymphatic
invasion |
|
|
|
|
|
|
|
|
Absence | 75 | 85.3 | 0.226 |
|
|
|
|
|
Presence | 159 | 78.5 |
|
|
|
|
|
| Histopathological
type |
|
|
|
|
|
|
|
|
Differentiated | 208 | 79.3 | 0.152 |
|
|
|
|
|
Undifferentiated | 26 | 92.3 |
|
|
|
|
|
| Completeness of
6-month treatment with oxaliplatin |
|
|
|
|
|
|
|
|
Yes | 46 | 91.3 | 0.045c | 1 (reference) |
| 1 (reference) |
|
| No | 188 | 78.1 |
| 2.737
(1.103–9.118) | 0.028c | 2.592
(1.043–8.643) | 0.039c |
Discussion
In the present study, we investigated the
relationship between various clinicopathological factors and
recurrence in patients with T4N0 and Stage III CC who received
adjuvant chemotherapy. We performed multivariate Cox model analysis
for patients in the low-risk group (T4N0 and low-risk stage III CC)
and identified two factors, high preoperative serum CEA levels and
incomplete 6-month adjuvant chemotherapy with oxaliplatin, as
independent risk factors for poor RFS. Furthermore, patients in the
low-risk group with high CEA levels and without the 6-month
oxaliplatin had a poor prognosis, similar to that of patients in
the high-risk group. However, the prognosis of patients in the
low-risk group with high CEA levels improved with a 6-month
adjuvant treatment with oxaliplatin to a similar level to that of
all patients with low CEA levels in the low-risk group. This study
identified patients for whom chemotherapy duration should not be
shortened by determining the prognostic factors in those with T4N0
and low-risk stage III CC.
In recent years, the concepts of high-risk stage II,
low-risk stage III, and high-risk stage III CC have been
established for CC, and distinctions between treatment strategies
according to the risk of recurrence have been discussed (10,15–19). A
previous study demonstrated that pT4 has high prognostic importance
in stage II–III CC and that pT4 stage II CC has a worse prognosis
than does low-risk (pT1-2N1-2) stage III CC (20). Another previous study revealed worse
5-year disease-free survival (DFS) rates in patients with T4N0
tumors than in those with T1-2N1 tumors (5-year DFS: 73.6 vs.
88.0%) (21). Staging paradoxes
have been reported in our institution, particularly in pT4N0 and
pT1-3N1 CC (22). Therefore,
whether pT4N0 and pT1-3N1 can be considered equal-risk groups
remains unclear. However, in this study, patients with pT4 CC,
representing the high-risk stage II group, and those with pT1-3N1
CC, representing the low-risk stage III group, were classified
together as a group in which adjuvant therapy duration could be
shortened to 3 months, because pT4N0 and pT1-3N1 have a lower risk
of recurrence than do other high-risk stage III CCs.
Numerous reports have indicated that high-risk stage
II and low-risk stage III CC can be treated with a shorter duration
of adjuvant chemotherapy, and such cases are often encountered in
clinical practice (10,11,23).
The JSCCR guidelines recommend 6 months of adjuvant chemotherapy
for high-risk stage II, low-risk stage III, and high-risk stage III
CC; however, 3 months of adjuvant chemotherapy is also an option if
capecitabine and oxaliplatin (CAPOX) are used (8). In contrast, ASCO and ESMO state that
postoperative adjuvant chemotherapy should be administered for 6
months for high-risk stage III CC, whereas either 6 or 3 months of
adjuvant chemotherapy may be offered for high-risk stage II and
low-risk stage III CC (4–6,24). The
NCCN suggests adjuvant treatment options of 3 months of CAPOX or
3–6 months of fluorouracil, leucovorin, and oxaliplatin (FOLFOX)
for high-risk stage II and low-risk stage III CC (7). Minor differences exist between the
guidelines; nevertheless, the worldwide trend is to shorten the
duration of postoperative adjuvant chemotherapy with oxaliplatin
from 6 to 3 months in patients with high-risk stage II and low-risk
stage III CC. In addition, the ACHIEVE-2, open-label, multicenter,
randomized phase III trial, demonstrated that the shortened therapy
duration did not affect the 3-year disease-free survival rate,
suggesting that a 3-month course of CAPOX can be an effective
treatment option (25).
However, some reports have shown that chemotherapy
duration should not be shortened, suggesting that a shorter CAPOX
duration is significantly associated with worse survival (26,27).
The IDEA study did not examine the low-risk recurrence group based
on clinicopathological background factors in detail; therefore,
some patients in the low-risk recurrence group may have had a high
probability of recurrence (10). We
agree that the low-risk group can generally be treated with CAPOX
for a shorter duration and support the ACHIEVE-2 results and the
IDEA study results; however, considering the controversial reports
in the literature, we believe that certain high-risk cases may
exist within the low-risk group. This is the reason for the
discrepancy between the ACHIEVE-2 results and the results of the
present study, and we believe that most of the low-risk group may
shorten postoperative adjuvant chemotherapy, but some among the
low-risk group should not shorten chemotherapy. Therefore, in the
present study, we investigated the prognosis of a low-risk group
based on various clinicopathological background factors, focusing
on the factors used to determine high-risk stage II CC.
In the present study, a high preoperative CEA level
was identified as a poor prognostic factor by analyzing the data of
patients with high-risk stage II and low-risk stage III CC, which
are considered to have a relatively low risk of recurrence among
the stages for which chemotherapy is recommended. Multiple previous
reports have suggested that a high preoperative CEA level is a poor
prognostic factor for CC (28–30).
The findings of this study are consistent with those of previous
reports, even when the patient populations are restricted to those
with high-risk stage II and low-risk stage III CC. Moreover,
preoperative serum CA19-9 may be a prognostic factor for high-risk
stage II and low-risk stage III CC, as some reports demonstrated
that preoperative serum CA19-9 was an additional prognostic factor
for CC (31,32). However, the number of patients with
CA19-9-positivity was small in the present study and did not reach
statistical significance; therefore, CA19-9 was not considered a
prognostic factor in this study. We aim to examine the prognostic
effect of CA19-9 in future studies with the accumulation of
additional cases. In addition, some researchers have claimed that a
postoperative, rather than preoperative, high CEA level is an
independent poor prognostic factor (33,34).
In our study, high postoperative CEA level was also an independent
prognostic factor (HR, 3.456; 95% CI, 1.557–6.897; P=0.004), as
shown in Fig. 2B and Table III. Therefore, we suggest that the
duration of chemotherapy with oxaliplatin should not be shortened
in patients with high postoperative CEA levels. However, it has
long been known that serum CEA levels generally decrease after
curative resection in CC because CEA is produced by cancer cells,
and thus the number of patients with high postoperative CEA levels
is small and sensitivity is low in this study (35,36);
therefore, high postoperative CEA levels may be unsuitable as a
marker to identify patients for whom adjuvant chemotherapy duration
should not be shortened because of the small number of patients
with high postoperative CEA values and low sensitivity. We
recommend that preoperative high CEA level be utilized as a
biomarker for 6 months of oxaliplatin treatment.
Notably, the 3-year RFS rate of patients with high
CEA levels in the low-risk group was similar to that of all
patients in the high-risk group. Interestingly, this study showed
that among patients in the low-risk group, the prognosis of
patients with high CEA levels improved with a 6-month adjuvant
treatment with oxaliplatin to a level similar to that of all
patients with low CEA levels. These results suggest that the
duration of adjuvant chemotherapy with oxaliplatin should not be
shortened in patients in the low-risk group with preoperative CEA
levels >5 ng/ml. To the best of our knowledge, this is the first
report to examine the risk factors for recurrence in patients with
high-risk stage II and low-risk stage III CC using available data
in general clinical practice.
The present study has several limitations. First, a
large number of patients in this study were treated with a regimen
that did not include oxaliplatin. The JSCCR guidelines did not
strongly recommend oxaliplatin-based regimens, and recommended
fluoride pyrimidines alone and oxaliplatin-based regimens equally
until the 2016 edition; the 2019 guidelines strongly recommend
oxaliplatin-based regimens for stage III CC. In addition, no
recommendation for postoperative adjuvant chemotherapy for patients
with high-risk stage II CC was proposed until 2019 (8,37).
Therefore, multiple patients in this cohort were treated with
regimens that did not include oxaliplatin owing to different
treatment strategies initiated during different years (38,39).
We analyzed a relatively large cohort from two centers to overcome
this limitation; however, our results require validation in a
larger prospective patient cohort from multiple institutions.
Second, in the low-risk group, the 3-year RFS rate of patients with
high CEA levels treated with an oxaliplatin-based regimen for 6
months was comparable to that of patients with low CEA levels;
however, whether this relationship was non-inferior remains
unclear. Third, we did not consider cases with positive perineural
invasion (40,41), tumor budding (42,43),
or poor nutritional indices (44,45),
which have recently attracted attention as prognostic factors owing
to their associations with a poor prognosis. The usual duration of
chemotherapy should be considered for low-risk groups with
recurrence or poor prognostic factors.
Despite these limitations, this study challenges the
current argument that it is acceptable to shorten the duration of
adjuvant chemotherapy for all patients with T4N0 and low-risk stage
III CC. Furthermore, this study identified the prognostic factors
for this patient population. Further prospective studies with
larger sample sizes are necessary to identify patients for whom
adjuvant chemotherapy duration can be appropriately shortened.
In conclusion, this study demonstrated that patients
with high preoperative serum CEA levels had poor prognoses among
those with T4N0 and low-risk stage III CC, suggesting that the
duration of adjuvant chemotherapy should not be uniformly
shortened.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HIn and HS contributed to the conception and design
of the study. All authors discussed the patient's treatment plan at
a preoperative conference. HIn, HS, JK, KN, TA, YK and EO performed
the surgeries. HIn, HS, JK, KN, TA, TO, YY, HK, RM, AS, HIk, TK and
HF collected the clinical samples, acquired data and assessed the
clinical data. HIn and HS contributed to the analysis and
interpretation of data, and writing of the manuscript. HIn, HS, YK,
TA, KN, JK, TO, YY, HK, RM, AS, HIk, TK, HF and EO discussed and
revised the analysis and interpretation of data. In addition, YK
and EO made critical revisions. HIn, HS and YK confirmed the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures involving human participants were
performed in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. The present retrospective study was approved by the
Medical Ethics Review Committee of the Kyoto Prefectural University
of Medicine (approval nos. ERB-C-1178, 1178-1, 1178-2, 1178-3;
Kyoto, Japan). It was determined to be a retrospective analysis of
de-identified data, and the requirement for obtaining written
informed consent from the participants was waived, in accordance
with the standards of the Kyoto Prefectural University of Medicine
Institutional Medical Ethics Review Committee. The notification
required by the Ministry of Health, Labor and Welfare was submitted
to the Japanese Red Cross Kyoto Daiichi Hospital (Kyoto, Japan) and
permission was obtained to access the database through proper
procedures.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society: Colorectal cancer
facts and figures 2023-2025, . American Cancer Society; Atlanta,
GA: 2023
|
|
3
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benson AB III, Schrag D, Somerfield MR,
Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J,
McAllister P, Van Cutsem E, et al: American Society of Clinical
Oncology recommendations on adjuvant chemotherapy for stage II
colon cancer. J Clin Oncol. 22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baxter NN, Kennedy EB, Bergsland E, Berlin
J, George TJ, Gill S, Gold PJ, Hantel A, Jones L, Lieu C, et al:
Adjuvant therapy for stage II colon cancer: ASCO guideline update.
J Clin Oncol. 40:892–910. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Argilés G, Tabernero J, Labianca R,
Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P,
Yoshino T, Taieb J, et al: Localised colon cancer: ESMO Clinical
Practice Guidelines for diagnosis, treatment and Follow-up. Ann
Oncol. 31:1291–1305. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology. NCCN;
Plymouth Meeting, PA: 2023
|
|
8
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Babcock BD, Aljehani MA, Jabo B, Choi AH,
Morgan JW, Selleck MJ, Luca F, Raskin E, Reeves ME, Garberoglio CA,
et al: High-risk stage II colon cancer: Not all risks are created
equal. Ann Surg Oncol. 25:1980–1985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grothey A, Sobrero AF, Shields AF, Yoshino
T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, et al:
Duration of adjuvant chemotherapy for stage III colon cancer. N
Engl J Med. 378:1177–1188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
André T, Meyerhardt J, Iveson T, Sobrero
A, Yoshino T, Souglakos I, Grothey A, Niedzwiecki D, Saunders M,
Labianca R, et al: Effect of duration of adjuvant chemotherapy for
patients with stage III colon cancer (IDEA collaboration): Final
results from a prospective, pooled analysis of six randomised,
phase 3 trials. Lancet Oncol. 21:1620–1629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshino T, Yamanaka T, Kotaka M, Manaka D,
Eto T, Hasegawa J, Takagane A, Nakamura M, Kato T, Munemoto Y, et
al: LBA24-efficacy of 3 versus 6 months of oxaliplatin-based
adjuvant chemotherapy for stage III colon cancer (CC): Results from
phase III ACHIEVE trial as part of the International Duration
Evaluation of Adjuvant therapy (IDEA) Collaboration. Ann Oncol.
28:v6142017. View Article : Google Scholar
|
|
13
|
Kotaka M, Yamanaka T, Yoshino T, Manaka D,
Eto T, Hasegawa J, Takagane A, Nakamura M, Kato T, Munemoto Y, et
al: Safety data from the phase III Japanese ACHIEVE trial: Part of
an international, prospective, planned pooled analysis of six phase
III trials comparing 3 versus 6 months of Oxaliplatin-based
adjuvant chemotherapy for stage III colon cancer. ESMO Open.
3:e0003542018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumors. (8th edition).
2017.
|
|
15
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmoll HJ, Cartwright T, Tabernero J,
Nowacki MP, Figer A, Maroun J, Price T, Lim R, Van Cutsem E, Park
YS, et al: Phase III trial of capecitabine plus oxaliplatin as
adjuvant therapy for stage III colon cancer: A planned safety
analysis in 1,864 patients. J Clin Oncol. 25:102–109. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haller DG, Tabernero J, Maroun J, de Braud
F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K and
Schmoll HJ: Capecitabine plus oxaliplatin compared with
fluorouracil and folinic acid as adjuvant therapy for stage III
colon cancer. J Clin Oncol. 29:1465–1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
André T, de Gramont A, Vernerey D,
Chibaudel B, Bonnetain F, Tijeras-Raballand A, Scriva A, Hickish T,
Tabernero J, Van Laethem JL, et al: Adjuvant fluorouracil,
leucovorin, and oxaliplatin in stage II to III colon cancer:
Updated 10-year survival and outcomes according to BRAF mutation
and mismatch repair status of the MOSAIC study. J Clin Oncol.
33:4176–4187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gunderson LL, Jessup JM, Sargent DJ,
Greene FL and Stewart AK: Revised TN categorization for colon
cancer based on national survival outcomes data. J Clin Oncol.
28:264–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim MJ, Jeong SY, Choi SJ, Ryoo SB, Park
JW, Park KJ, Oh JH, Kang SB, Park HC, Heo SC and Park JG: Survival
paradox between stage IIB/C (T4N0) and stage IIIA (T1-2N1) colon
cancer. Ann Surg Oncol. 22:505–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimizu H, Kuriu Y, Arita T, Kiuchi J,
Yamamoto Y, Konishi H, Morimura R, Shiozaki A, Ikoma H, Kubota T,
et al: Staging paradox and discrepancy in adjuvant chemotherapy in
patients with T4N0, T1-2N1, and T3N1 colon cancer. World J Surg.
45:1561–1568. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Souglakos J, Boukovinas I, Kakolyris S,
Xynogalos S, Ziras N, Athanasiadis A, Androulakis N, Christopoulou
A, Vaslamatzis M, Ardavanis A, et al: Three-versus six-month
adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon
cancer patients: The efficacy results of Hellenic Oncology Research
Group (HORG) participation to the International Duration Evaluation
of Adjuvant Chemotherapy (IDEA) project. Ann Oncol. 30:1304–1310.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lieu C, Kennedy EB, Bergsland E, Berlin J,
George TJ, Gill S, Gold PJ, Hantel A, Jones L, Mahmoud N, et al:
Duration of oxaliplatin-containing adjuvant therapy for stage III
colon cancer: ASCO clinical practice guideline. J Clin Oncol.
37:1436–1447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamazaki K, Yamanaka T, Shiozawa M, Manaka
D, Kotaka M, Gamoh M, Shiomi A, Makiyama A, Munemoto Y, Rikiyama T,
et al: Oxaliplatin-based adjuvant chemotherapy duration (3 versus 6
months) for high-risk stage II colon cancer: The randomized phase
III ACHIEVE-2 trial. Ann Oncol. 32:77–84. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu IS, Pereira AAL, Lee M, Korphaisarn K,
Marshall J, Segelov E, O'Callaghan C, Lim HJ, Kopetz S and Loree
JM: Medical oncologists' perspectives on how the results of the
IDEA Collaboration impact the adjuvant treatment of stage III colon
cancer. Oncologist. 25:229–234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boyne DJ, Cheung WY, Hilsden RJ, Sajobi
TT, Batra A, Friedenreich CM and Brenner DR: Association of a
shortened duration of adjuvant chemotherapy with overall survival
among individuals with stage III colon cancer. JAMA Netw Open.
4:e2135872021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baqar AR, Wilkins S, Staples M, Angus Lee
CH, Oliva K and McMurrick P: The role of preoperative CEA in the
management of colorectal cancer: A cohort study from two cancer
centres. Int J Surg. 64:10–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takagawa R, Fujii S, Ohta M, Nagano Y,
Kunisaki C, Yamagishi S, Osada S, Ichikawa Y and Shimada H:
Preoperative serum carcinoembryonic antigen level as a predictive
factor of recurrence after curative resection of colorectal cancer.
Ann Surg Oncol. 15:3433–3439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wiratkapun S, Kraemer M, Seow-Choen F, Ho
YH and Eu KW: High preoperative serum carcinoembryonic antigen
predicts metastatic recurrence in potentially curative colonic
cancer: Results of a five-year study. Dis Colon Rectum. 44:231–235.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang R, Xu B, Sun M, Pang X, Wang X, Zhu
J, Lian J and Lu H: Dynamic monitoring of serum CEA and CA19-9
predicts the prognosis of postoperative stage II colon cancer. Eur
J Surg Oncol. 49:1071382023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ushigome M, Shimada H, Kaneko T, Miura Y,
Nagashima Y, Suzuki T, Kagami S, Kurihara A and Funahashi K:
Preoperative CRP(-)/CEA(-)/CA19-9(-)/non-T4 in stage III colorectal
cancer is favorable risk for recurrence. J Anus Rectum Colon.
6:264–273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sonoda H, Yamada T, Matsuda A, Ohta R,
Shinji S, Yokoyama Y, Takahashi G, Iwai T, Takeda K, Ueda K, et al:
Elevated serum carcinoembryonic antigen level after curative
surgery is a prognostic biomarker of stage II-III colorectal
cancer. Eur J Surg Oncol. 47:2880–2887. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pu H, Yang W, Liu M, Pang X, Chen Y and
Xiong Q: Elevated postoperative carcinoembryonic antigen guides
adjuvant chemotherapy for stage II colon cancer: A multicentre
cohort retrospective study. Sci Rep. 14:68892024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mach JP, Jaeger P, Bertholet MM,
Ruegsegger CH, Loosli RM and Pettavel J: Detection of recurrence of
large-bowel carcinoma by radioimmunoassay of circulating
carcinoembryonic antigen (C.E.A.). Lancet. 2:535–540. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung TD, Yoo JH, Lee MJ, Park HK, Shin JH,
An MS, Ha TK, Kim KH, Bae KB, Kim TH, et al: Prognostic
significance of the decreased rate of perioperative serum
carcinoembryonic antigen level in the patients with colon cancer
after a curative resection. Ann Coloproctol. 29:115–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Watanabe T, Muro K, Ajioka Y, Hashiguchi
Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S,
et al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2016 for the treatment of colorectal cancer. Int J Clin
Oncol. 23:1–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimada Y, Hamaguchi T, Mizusawa J, Saito
N, Kanemitsu Y, Takiguchi N, Ohue M, Kato T, Takii Y, Sato T, et
al: Randomised phase III trial of adjuvant chemotherapy with oral
uracil and tegafur plus leucovorin versus intravenous fluorouracil
and levofolinate in patients with stage III colorectal cancer who
have undergone Japanese D2/D3 lymph node dissection: Final results
of JCOG0205. Eur J Cancer. 50:2231–2240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida M, Ishiguro M, Ikejiri K,
Mochizuki I, Nakamoto Y, Kinugasa Y, Takagane A, Endo T, Shinozaki
H, Takii Y, et al: S-1 as adjuvant chemotherapy for stage III colon
cancer: A randomized phase III study (ACTS-CC trial). Ann Oncol.
25:1743–1749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Q, Liu T, Liu P, Luo J, Zhang N, Lu K,
Ju H, Zhu Y, Wu W, Zhang L, et al: Perineural and lymphovascular
invasion predicts for poor prognosis in locally advanced rectal
cancer after neoadjuvant chemoradiotherapy and surgery. J Cancer.
10:2243–2249. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qin L, Heng Y, Deng S, Gu J, Mao F, Xue Y,
Jiang Z, Wang J, Cheng D, Wu K, et al: Perineural invasion affects
prognosis of patients undergoing colorectal cancer surgery: A
propensity score matching analysis. BMC Cancer. 23:4522023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lugli A, Karamitopoulou E and Zlobec I:
Tumour budding: A promising parameter in colorectal cancer. Br J
Cancer. 106:1713–1717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rogers AC, Winter DC, Heeney A, Gibbons D,
Lugli A, Puppa G and Sheahan K: Systematic review and Meta-analysis
of the impact of tumour budding in colorectal cancer. Br J Cancer.
115:831–840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie H, Wei L, Yuan G, Liu M, Tang S and
Gan J: Prognostic value of prognostic nutritional index in patients
with colorectal cancer undergoing surgical treatment. Front Nutr.
9:7944892022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mao Y and Lan J: Prognostic value of the
geriatric nutritional index in colorectal cancer patients
undergoing surgical intervention: A systematic review and
meta-analysis. Front Oncol. 12:10664172022. View Article : Google Scholar : PubMed/NCBI
|