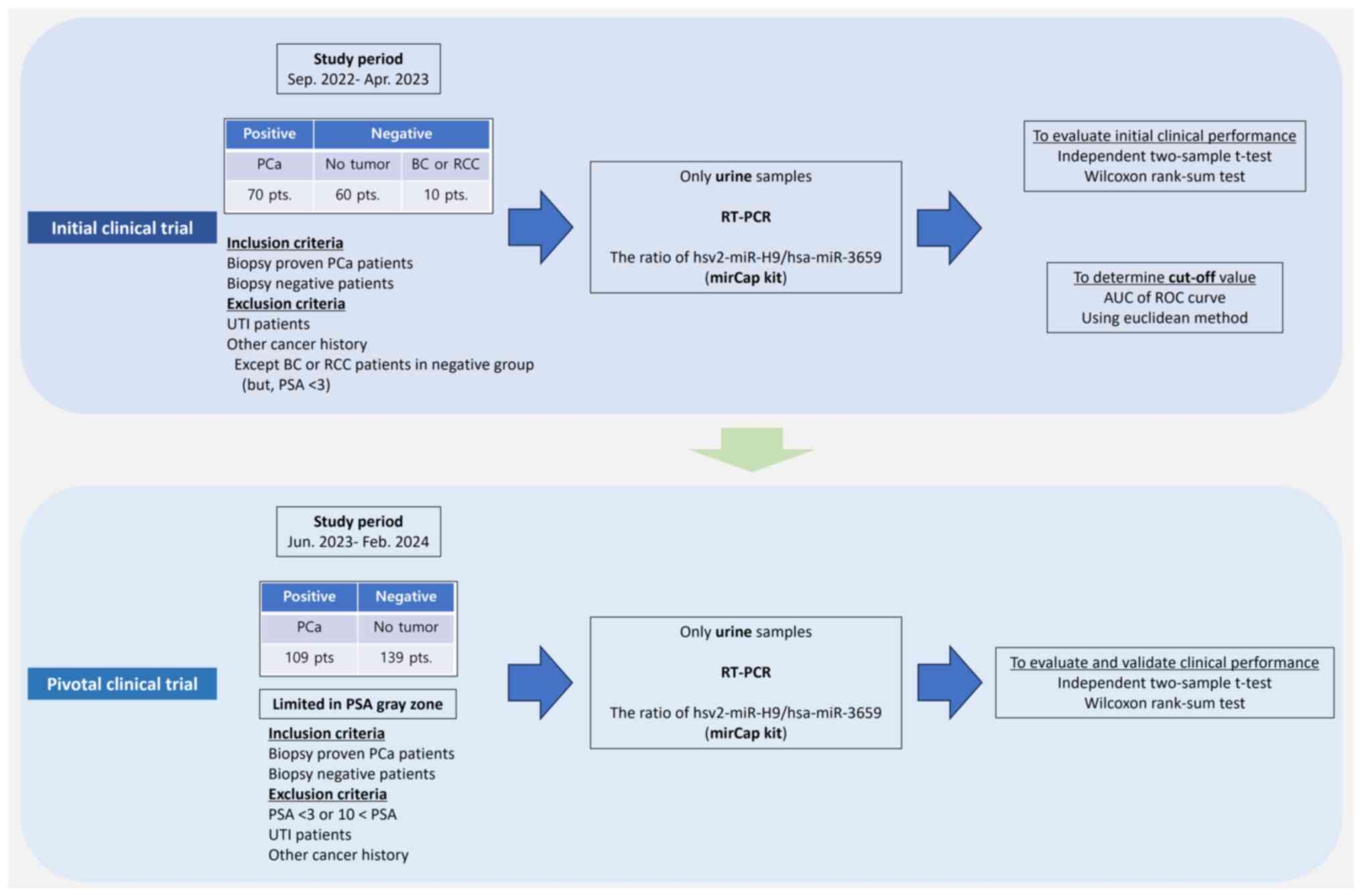
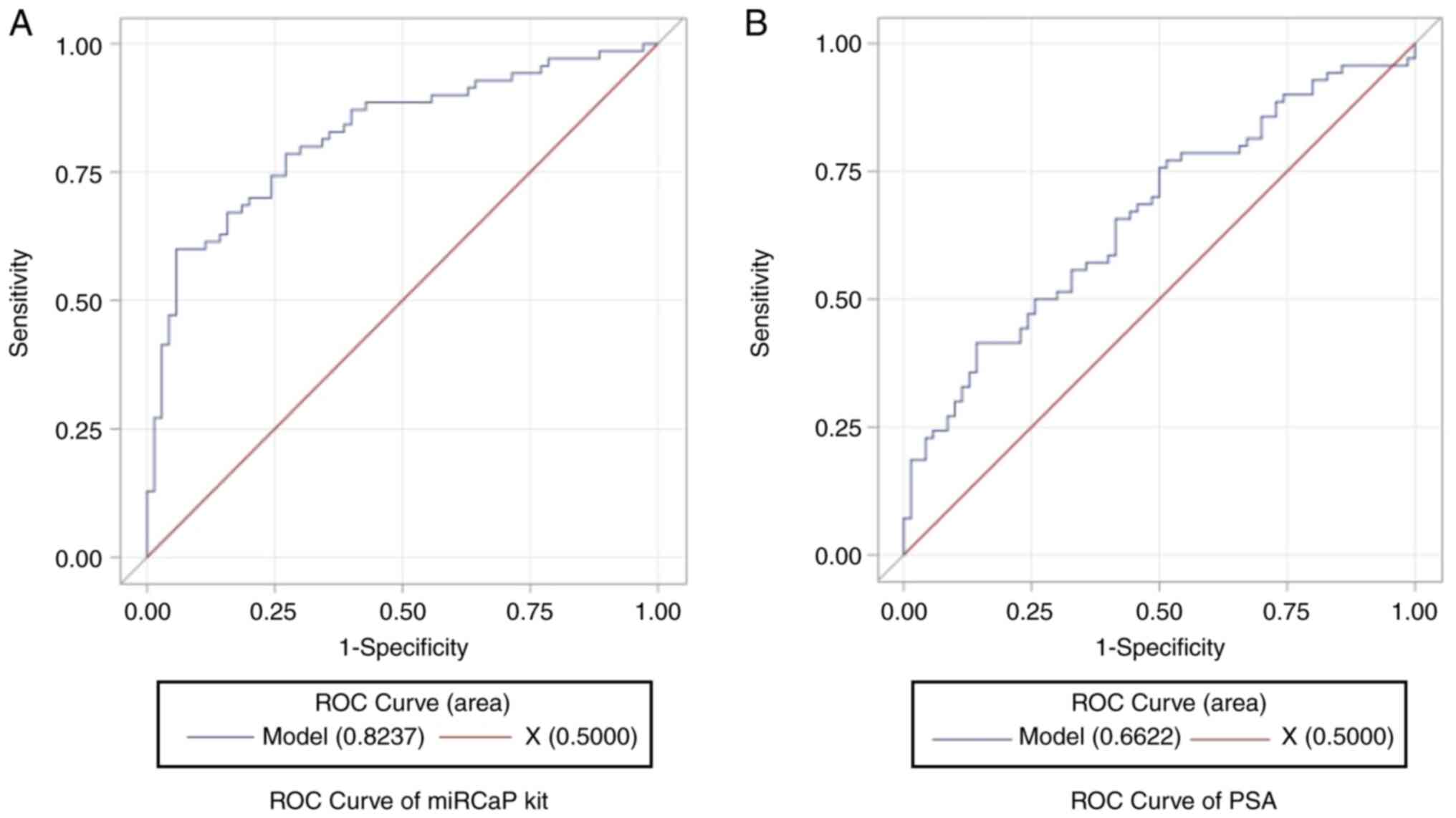
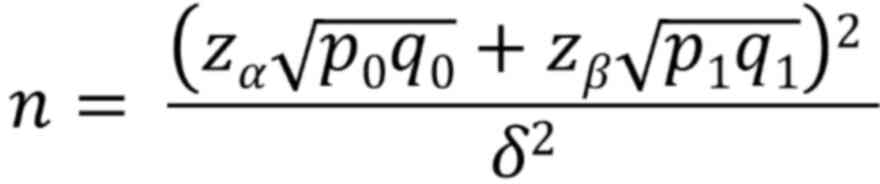|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Obirieze AC, Moten A, Allen D and Ahaghotu
CA: African-American men with low-risk prostate cancer: Modern
treatment and outcome trends. J Racial Ethn Health Disparities.
2:295–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gann PH, Hennekens CH and Stampfer MJ: A
prospective evaluation of plasma prostate-specific antigen for
detection of prostatic cancer. JAMA. 273:289–294. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shahab AA, Soebadi DM, Djatisoesanto W,
Hardjowijoto S, Soetojo S and Hakim L: Prostate-specific antigen
and prostate-specific antigen density cutoff points among
Indonesian population suspected for prostate cancer. Prostate Int.
1:23–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stamey TA, Yang N, Hay AR, McNeal JE,
Freiha FS and Redwine E: Prostate-specific antigen as a serum
marker for adenocarcinoma of the prostate. N Engl J Med.
317:909–916. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayashi M, Kijima T, Yashi M and Kamai
T: Prostate-specific antigen kinetics contributes to decision
making for biopsy referral: The predictive implication for PSA
retest in patients with elevated PSA levels. Prostate Int.
11:27–33. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson S and Crawford ED: Screening for
prostate cancer: Current recommendations. Urol Clin North Am.
31:219–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim WT and Kim WJ: MicroRNAs in prostate
cancer. Prostate Int. 1:3–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Liu S, Zhou Z, Yan H and Xiao J: A
herpes simplex virus type 2-encoded microRNA promotes tumor cell
metastasis by targeting suppressor of cytokine signaling 2 in lung
cancer. Tumor Biol. 39:10104283177016332017.PubMed/NCBI
|
|
10
|
Choi J, Kwon SM, Moon SU, Yoon S, Shah M,
Lee BG, Yang J, Park YN, Wang HJ and Woo HG: TPRG1-AS1 induces
RBM24 expression and inhibits liver cancer progression by sponging
miR-4691-5p and miR-3659. Liver Int. 41:2788–2800. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yun SJ, Jeong P, Kang HW, Kim YH, Kim EA,
Yan C, Choi YK, Kim D, Kim JM, Kim SK, et al: Urinary MicroRNAs of
prostate cancer: Virus-Encoded hsv1-miRH18 and hsv2-miR-H9-5p could
be valuable diagnostic markers. Int Neurourol J. 19:74–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Byun YJ, Kang HW, Piao X, Zheng CM, Moon
SK, Choi YH, Kim WT, Lee SC, Yun SJ and Kim WJ: Expression of
hsv1-miR-H18 and hsv2-miR-H9 as a field defect marker for detecting
prostate cancer. Prostate Int. 10:1–6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang HW, Byun YJ, Kim K, Moon SM, Kim K,
Piao XM, Zheng CM, Moon SK, Choi YH, Kim WT, et al: Urinary
hsv2-miR-H9 to hsa-miR-3659 ratio is an effective marker for
discriminating prostate cancer from benign prostate hyperplasia in
patients within the prostate-specific antigen grey zone. Invest
Clin Urol. 63:238–244. 2022. View Article : Google Scholar
|
|
14
|
Byun YJ, Piao XM, Jeong P, Kang HW, Seo
SP, Moon SK, Lee JY, Choi YH, Lee HY, Kim WT, et al: Urinary
microRNA-1913 to microRNA-3659 expression ratio as a non-invasive
diagnostic biomarker for prostate cancer. Invest Clin Urol.
62:340–348. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Borley N and Feneley MR: Prostate cacner:
Diagnosis and staging. Asian J Androl. 11:74–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yun SJ, Jeong P, Kim W, Kim TH, Lee YS,
Song PH, Choi YH, Kim IY, Moon SK and Kim WJ: Cell-free microRNAs
in urine as diagnostic and prognostic biomarkers of bladder cancer.
Int J Oncol. 41:1871–1878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim WT, Jeong P, Yan C, Kim YH, Lee IS,
Kang HW, Kim YJ, Lee SC, Kim SJ, Kim YT, et al: UBE2C cell-free RNA
in urine can discriminate between bladder cancer and hematuria.
Oncotarget. 7:58193–58202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piao XM, Jeong P, Kim YH, Byun YJ, Xu Y,
Kang HW, Ha YS, Kim WT, Lee JY, Woo SH, et al: Urinary cell-free
microRNA biomarker could discriminate bladder cancer from benign
hematuria. Int J Cancer. 144:380–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho JS: Optimal prostate-specific antigen
cutoff value in Korean. Korean J Urol Oncol. 5:1–5. 2007.(In
Korean).
|
|
20
|
Oesterling JE, Jacobsen SJ and Cooner WH:
The use of age-specific reference ranges for serum prostate
specific antigen in men 60 years old or older. J Urol.
153:1160–1163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee R, Localio AR, Armstrong K, Malkowicz
SB and Schwartz JS; Free PSA Study Group, : A meta-analysis of the
performance characteristics of the free prostate-specific antigen
test. Urology. 67:762–768. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang P, Du W, Xie K, Deng X, Fu J, Chen H
and Yang W: Transition zone PSA density improves the prostate
cancer detection rate both in PSA 4.0–10.0 and 10.1–20.0 ng/ml in
Chinese men. Urol Oncol. 31:744–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carter HB, Ferrucci L, Kettermann A,
Landis P, Wright EJ, Epstein JI, Trock BJ and Metter EJ: Detection
of life-threatening prostate cancer with prostate-specific antigen
velocity during a window of curability. J Natl Cancer Inst.
98:1521–1527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lepor A, Catalona WJ and Loeb S: The
prostate health index: Its utility in prostate cancer detection.
Urol Clin North Am. 43:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parekh DJ, Punnen S, Sjoberg D, Asroff SW,
Bailen JL, Cochran JS, Concepcion R, David RD, Deck KB, Dumbadze I,
et al: A multi-institutional prospective trial in the USA confirms
that the 4Kscore accurately identifies men with high-grade prostate
cancer. Eur Urol. 68:464–470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei JT, Feng Z, Partin AW, Brown E,
Thompson I, Sokoll L, Chan DW, Lotan Y, Kibel AS, Busby JE, et al:
Can urinary PCA3 supplement PSA in the early detection of prostate
cancer? J Clin Oncol. 32:4066–4072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKiernan J, Donovan MJ, O'Neill V,
Bentink S, Noerholm M, Belzer S, Skog J, Kattan MW, Partin A,
Andriole G, et al: A novel urine exosome gene expression assay to
predict high-grade prostate cancer at initial biopsy. JAMA Oncol.
2:882–889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Neste L, Hendriks RJ, Dijkstra S,
Trooskens G, Cornel EB, Jannink SA, de Jong H, Hessels D, Smit FP,
Melchers WJ, et al: Detection of high-grade prostate cancer using a
urinary molecular biomarker-based risk score. Eur Urol. 70:740–748.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de la Calle C, Patil D, Wei JT, Scherr DS,
Sokoll L, Chan DW, Siddiqui J, Mosquera JM, Rubin MA and Sanda MG:
Multicenter evaluation of the prostate health index to detect
aggressive prostate cancer in biopsy Naïve men. J Urol. 194:65–72.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davis JW, Nakanishi H, Kumar VS,
Bhadkamkar VA, McCormack R, Fritsche HA, Handy B, Gornet T and
Babaian RJ: Circulating tumor cells in peripheral blood samples
from patients with increased serum prostate specific antigen:
Initial results in early prostate cancer. J Urol. 179:2187–2191;
discussion 2191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lau E, McCoy P, Reeves F, Chow K, Clarkson
M, Kwan EM, Packwood K, Northen H, He M, Kingsbury Z, et al:
Detection of ctDNA in plasma of patients with clinically localised
prostate cancer is associated with rapid disease progression.
Genome Med. 12:722020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho H, Oh CK, Cha J, Chung JI, Byun SS,
Hong SK, Chung JS and Han KH: Association of serum
prostate-specific antigen (PSA) level and circulating tumor
cell-based PSA mRNA in prostate cancer. Prostate Int. 10:14–20.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McKiernan J, Donovan MJ, Margolis E,
Partin A, Carter B, Brown G, Torkler P, Noerholm M, Skog J, Shore
N, et al: A prospective adaptive utility trial to validate
performance of a novel urine exosome gene expression assay to
predict high-grade prostate cancer in patients with
prostate-specific antigen 2–10 ng/ml at initial biopsy. Eur Urol.
74:731–738. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manoj A, Ahmad MK, Prasad G, Kumar D,
Mahdi A and Kumar M: Screening and validation of novel serum panel
of microRNA in stratification of prostate cancer. Prostate Int.
11:150–158. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Glinge C, Clauss S, Boddum K, Jabbari R,
Jabbari J, Risgaard B, Tomsits P, Hildebrand B, Kääb S, Wakili R,
et al: Stability of circulating blood-based MicroRNAs-pre-analytic
methodological considerations. PLoS One. 12:e01679692017.
View Article : Google Scholar : PubMed/NCBI
|