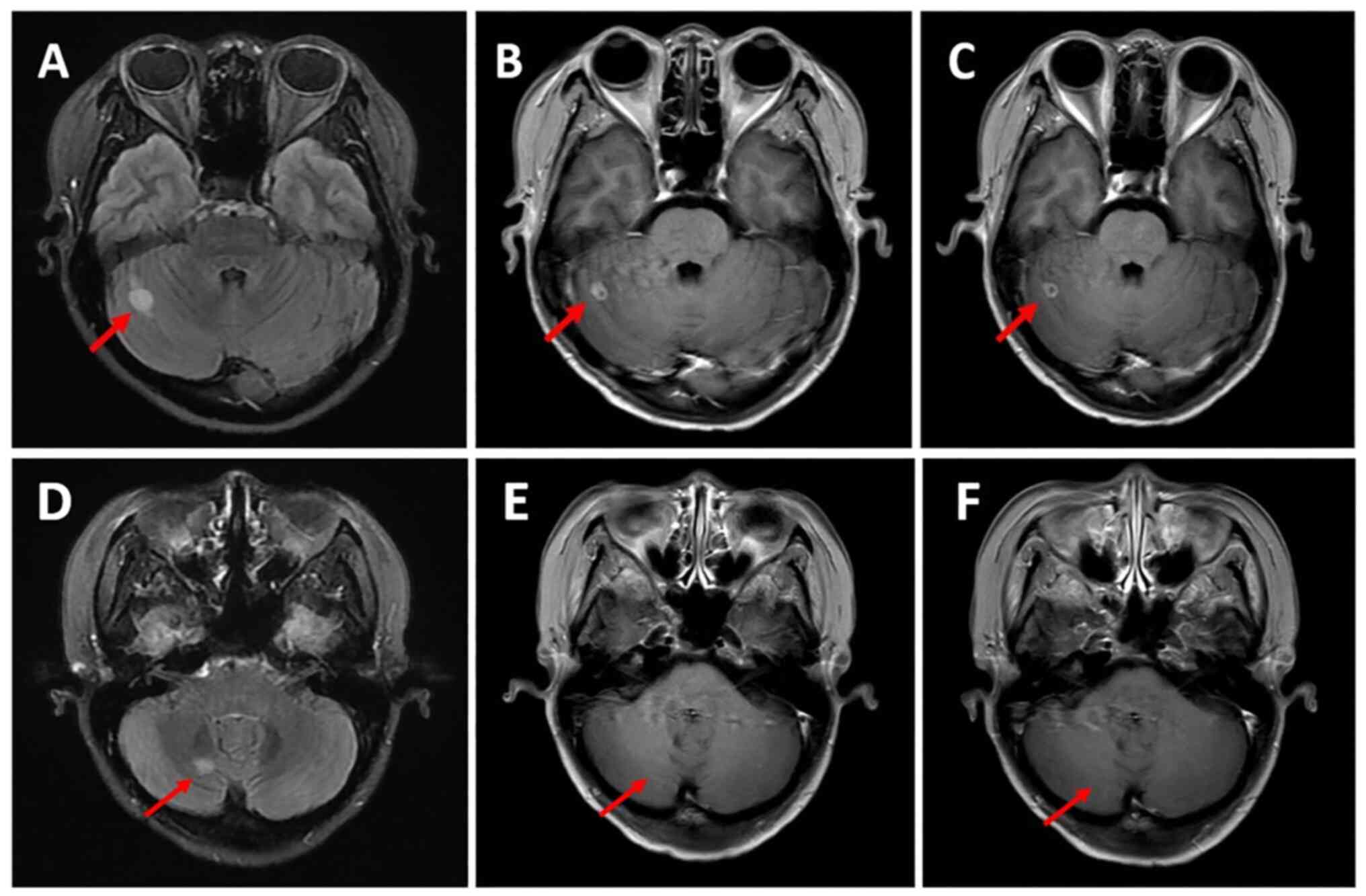
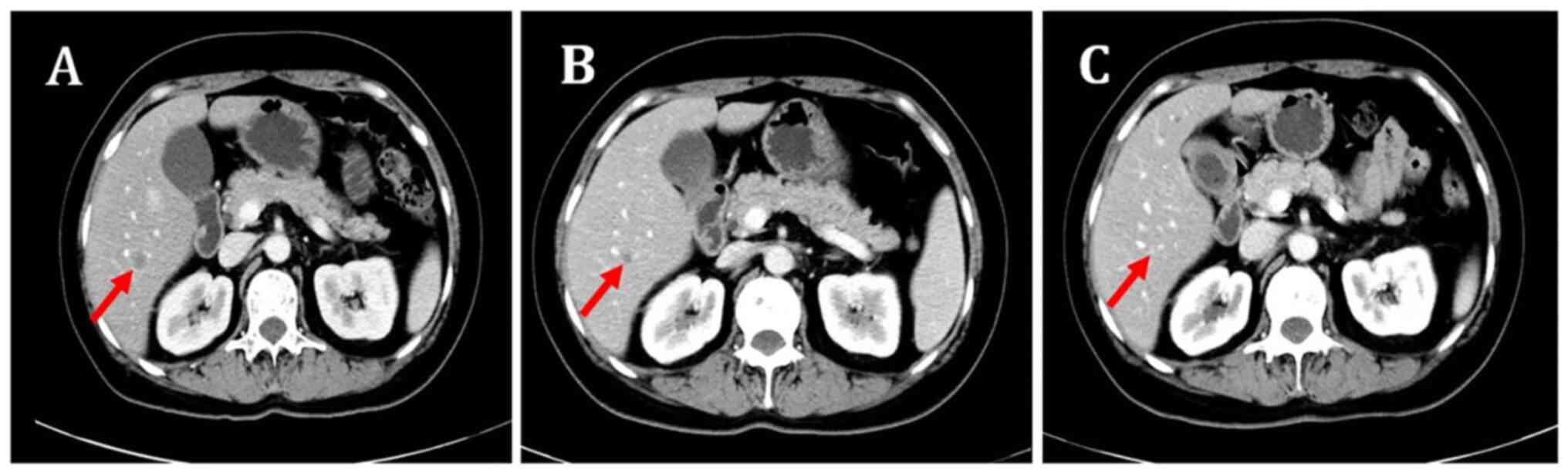
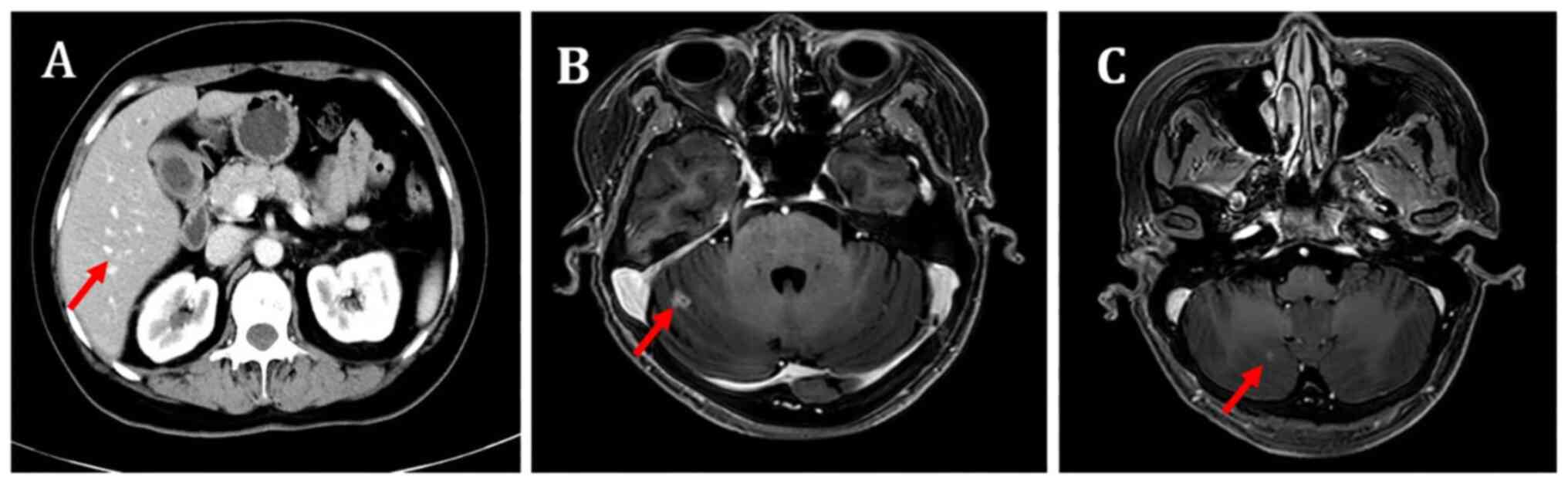
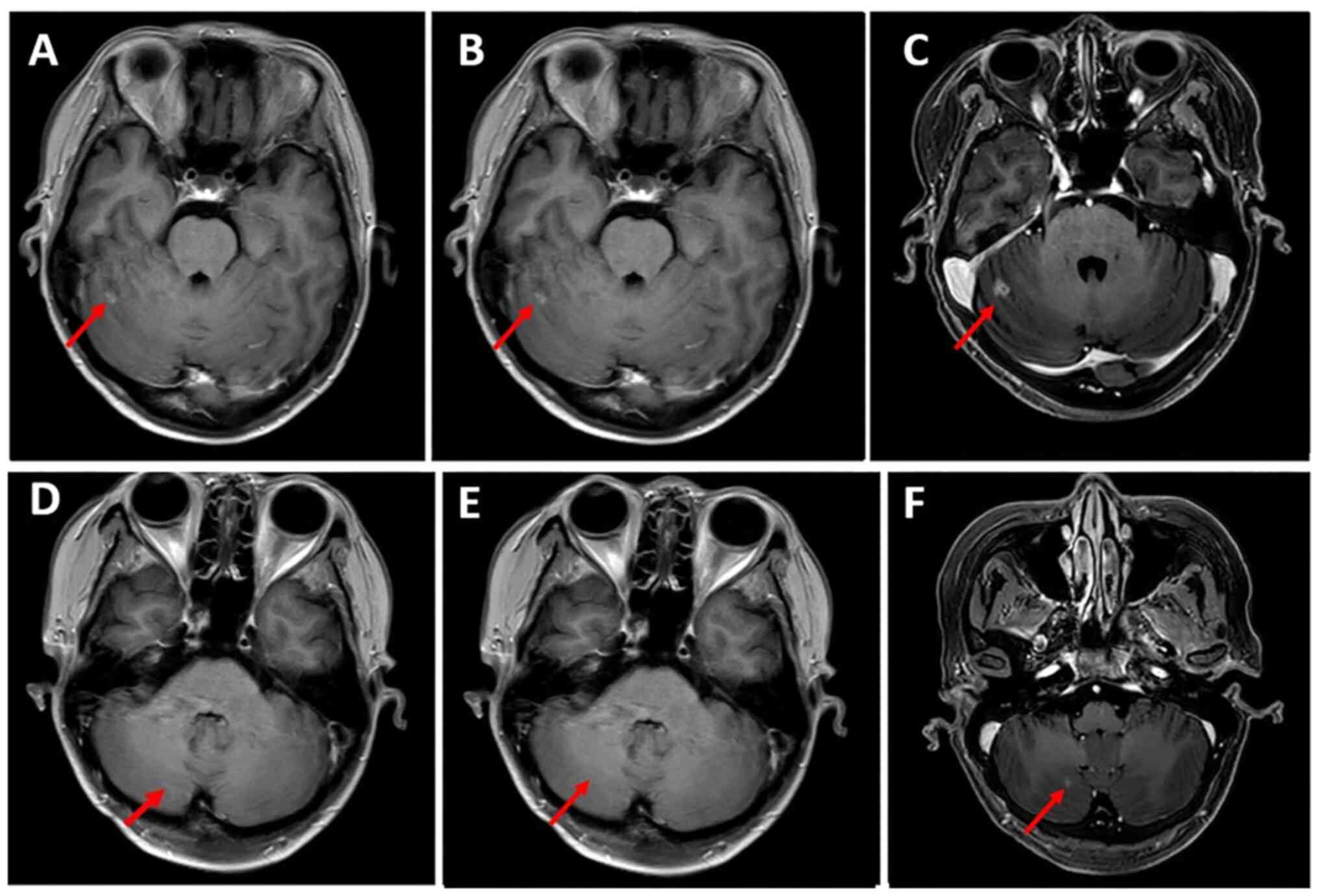
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
U.S. National Institutes Of Health,
National Cancer Institute, . SEER Cancer Statistics Review,
1975–2019. American Cancer Society; 2021, https://seer.cancer.gov/statfacts/html/breast.htm
|
|
3
|
Munzone E, Pagan E, Bagnardi V, Montagna
E, Cancello G, Dellapasqua S, Iorfida M, Mazza M and Colleoni M:
Systematic review and meta-analysis of post-progression outcomes in
ER+/HER2- metastatic breast cancer after CDK4/6 inhibitors within
randomized clinical trials. ESMO Open. 6:1003322021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marschner N, Harbeck N, Thill M, Stickeler
E, Zaiss M, Nusch A, Rauh J, Schulz H, Engelken K, Kruggel L, et
al: 232P Second-line therapies of patients with early progression
under CDK4/6-inhibitor in first-line-data from the registry
platform OPAL. Ann Oncol. 33 (Suppl 7):S643–S644. 2022. View Article : Google Scholar
|
|
5
|
Xu B, Sun T, Zhang Q, Zhang P, Yuan Z,
Jiang Z, Wang X, Cui S, Teng Y, Hu XC, et al: Efficacy of utidelone
plus capecitabine versus capecitabine for heavily pretreated,
anthracycline- and taxane-refractory metastatic breast cancer:
Final analysis of overall survival in a phase III randomised
controlled trial. Ann Oncol. 32:218–228. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bi P, Wang X, Liu R, Li X, Wei S, Zhao J,
Tan X, Zhang F, Mao Q, Zhang Y, et al: Efficacy and safety of
utidelone plus capecitabine in advanced first-line therapy for
metastatic breast cancer: A multicenter real-world study. Surg Open
Sci 16: 171–183, 2023. Liu MC, Cortés J and O'Shaughnessy J:
Challenges in the treatment of hormone receptor-positive,
HER2-negative metastatic breast cancer with brain metastases.
Cancer Metastasis Rev. 35:323–332. 2016.PubMed/NCBI
|
|
7
|
Labidi SI, Bachelot T, Ray-Coquard I,
Mosbah K, Treilleux I, Fayette J, Favier B, Galy G, Blay JY and
Guastalla JP: Bevacizumab and paclitaxel for breast cancer patients
with central nervous system metastases: A case series. Clin Breast
Cancer. 9:118–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leone JP, Emblem KE, Weitz M, Gelman RS,
Schneider BP, Freedman RA, Younger J, Pinho MC, Sorensen AG,
Gerstner ER, et al: Phase II trial of carboplatin and bevacizumab
in patients with breast cancer brain metastases. Breast Cancer Res.
22:1312020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Deng S, Zhang Y, Wang C, Hu X,
Kong D, Liang G, Yuan X, Li Y and Wang X: Apatinib enhances the
anti-tumor effect of paclitaxel via the PI3K/p65/Bcl-xl pathway in
triple-negative breast cancer. Ann Transl Med. 9:10012021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y,
Li J and Lou L: YN968D1 is a novel and selective inhibitor of
vascular endothelial growth factor receptor-2 tyrosine kinase with
potent activity in vitro and in vivo. Cancer Sci. 102:1374–1380.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Syed YY: Anlotinib: First global approval.
Drugs. 78:1057–1062. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watase C, Shiino S, Shimoi T, Noguchi E,
Kaneda T, Yamamoto Y, Yonemori K, Takayama S and Suto A: Breast
cancer brain metastasis-overview of disease state, treatment
options and future perspectives. Cancers (Basel). 13:10782021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases to the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thorvaldsdóttir H, Robinson JT and Mesirov
JP: Integrative genomics viewer (IGV): High-performance genomics
data visualization and exploration. Brief Bioinform. 14:178–192.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalkanis SN, Kondziolka D, Gaspar LE,
Burri SH, Asher AL, Cobbs CS, Ammirati M, Robinson PD, Andrews DW,
Loeffler JS, et al: The role of surgical resection in the
management of newly diagnosed brain metastases: A systematic review
and evidence-based clinical practice guideline. J Neurooncol.
96:33–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bailleux C, Eberst L and Bachelot T:
Treatment strategies for breast cancer brain metastases. Br J
Cancer. 124:142–155. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sperduto PW, Kased N, Roberge D, Xu Z,
Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al: Effect
of tumor subtype on survival and the graded prognostic assessment
for patients with breast cancer and brain metastases. Int J Radiat
Oncol Biol Phys. 82:2111–2117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong C, Yu S, Qian P, Song X, Wen J, Jiang
M, Zhu J, Xu J, Zhao L, Guo Z, et al: Anlotinib combined with
whole-brain radiotherapy in non-small cell lung cancer with
multiple brain metastases that progressed or developed after at
least one line of prior treatment. Front Oncol. 13:11693332023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Xu J, Ye W, Zhong W, Zhang X, Mao J
and Wu D: Whole-brain radiotherapy combined with anlotinib for
multiple brain metastases from non-small cell lung cancer without
targetable driver mutation: A single-arm, phase II study. Clin Med
Insights Oncol. 16:117955492210791852022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng Y, Wang Q, Li K, Shi J, Han B, Wu L,
Chen G, He J, Wang J, Qin H and Li X: Third-line or above anlotinib
in relapsed and refractory small cell lung cancer patients with
brain metastases: A post hoc analysis of ALTER1202, a randomized,
double-blind phase 2 study. Cancer Innov. 2:181–190. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Y, Liu B, Guan M and Liu M:
Successful treatment using apatinib in intractable brain edema: A
case report and literatures review. Cancer Biol Ther. 19:1093–1096.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Bai X, Xie X, Huang J, Chen L,
Song L, Lan X, Zhang Q, Guo J and Du C: The anti-tumor efficiency
of low-dose apatinib-based chemotherapy in pretreated HER2-negative
breast cancer with brain metastases. Ann Med. 55:22186472023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bollag DM, McQueney PA, Zhu J, Hensens O,
Koupal L, Liesch J, Goetz M, Lazarides E and Woods CM: Epothilones,
a new class of microtubule-stabilizing agents with a taxol-like
mechanism of action. Cancer Res. 55:2325–2333. 1995.PubMed/NCBI
|
|
24
|
Zhang P, Tong Z, Tian F, Wang Y, Yang J,
Li W, Di L, Liu W, Tang L, Qiu R and Xu B: Phase II trial of
utidelone as monotherapy or in combination with capecitabine in
heavily pretreated metastatic breast cancer patients. J Hematol
Oncol. 9:682016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garrone O, Miraglio E, Vandone AM, Vanella
P, Lingua D and Merlano MC: Eribulin in advanced breast cancer:
Safety, efficacy and new perspectives. Future Oncol. 13:2759–2769.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Portman N, Alexandrou S, Carson E, Wang S,
Lim E and Caldon CE: Overcoming CDK4/6 inhibitor resistance in
ER-positive breast cancer. Endocr Relat Cancer. 26:R15–R30. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang P, Sun T, Zhang Q, Yuan Z, Jiang Z,
Wang XJ, Cui S, Teng Y, Hu XC, Yang J, et al: Utidelone plus
capecitabine versus capecitabine alone for heavily pretreated
metastatic breast cancer refractory to anthracyclines and taxanes:
A multicentre, open-label, superiority, phase 3, randomised
controlled trial. Lancet Oncol. 18:371–383. 2017. View Article : Google Scholar : PubMed/NCBI
|