Introduction
Breast cancer is the most common malignant tumor in
women. Female breast cancer surpassed lung cancer as the leading
cause of global cancer incidence in 2020, with an estimated 2.3
million new cases, representing 11.7% of all cancer cases (1). Although advancements in diagnostic and
therapeutic methods have significantly improved the prognosis of
breast cancer, the prognosis for patients with metastatic breast
cancer (mBC) remains poor, with a 5-year survival rate of only ~30%
(1,2). Therefore, extending the survival
period for patients with advanced-stage breast cancer remains a
major challenge in the field.
In patients with hormone receptor-positive
(HR+) and human epidermal growth factor receptor
2-negative (HER2−) mBC, CDK4/6 inhibitors combined with
endocrine therapy (ET) are the main treatment approach. However, at
present, there are no standardized guidelines for the treatment of
patients following progression on first-line CDK4/6 inhibitors
combined with ET. According to a meta-analysis of 8 studies,
including the MONALEESA-2/7, MONARCH-3 and PALOMA-1/2 clinical
trials, ~65% of patients receive ET-based treatments, 44% of
patients undergo chemotherapy, and 38% of patients continue to
receive CDK4/6 inhibitors (3).
There are various treatment approaches after the use of CDK4/6
inhibitors, but it remains unclear whether these significantly
impact patient prognosis (3).
Among patients who receive first-line CDK4/6
inhibitor treatment, ~20% experience rapid progression, and the
prognosis for these patients is generally poor (4). Therefore, it is necessary to focus
increased attention on the search for new treatment strategies for
patients with rapid progression. Chemotherapy, as a foundational
treatment method for mBC, has seen few new drug developments in the
past decade. This may imply that further innovation and research
are necessary to discover more effective therapeutic drugs and
treatment approaches for the treatment of mBC.
Utidelone is one of a new generation of
epothilone-class microtubule inhibitors. It was independently
developed in China, and is the only microtubule inhibitor with a
novel molecular structure approved globally in the past decade.
Notably, it serves as a breakthrough in China, which has not seen
the introduction of any novel chemotherapy drugs other than
paclitaxel for nearly 30 years, and serves as a new treatment
option for patients with mBC (5,6).
In mBC, depending on the molecular subtype, up to
49% of patients may develop brain metastases (BMs), and the
occurrence of BMs significantly shortens the survival duration of
the patient (7). In a retrospective
study of 4,118 patients from the French Epidemiological Strategy
and Medical Economics research program who were diagnosed with
breast cancer brain metastasis (BCBM) (7), the median overall survival (OS) time
was 7.1 months for HR+/HER2− cases. In this
study, the poorer prognosis observed for
HR+/HER2− disease compared with
HR+/HER2+ and HR−/HER2+
disease may be attributed to the development of BMs typically being
a late event as metastatic disease progression persists and tumors
are almost resistant to ET and chemotherapy (8). There is a clear requirement for an
effective clinical treatment for HR+/HER2−
BCBM. BMs derived from breast cancer are characterized by highly
vascular and morphologically malformed vessels. Anti-angiogenesis
therapy has the potential to normalize the blood vessels within
tumors, thereby facilitating the delivery of antitumor drugs.
Consequently, when combined with chemotherapy, it may be able to
reverse multidrug resistance (9,10).
Previous research has shown that a combination of an
anti-angiogenic drug and chemotherapy has synergistic effects in an
mBC cell line (11).
Anti-angiogenic drugs include both large-molecule biologics and
small molecules. The latter category includes apatinib and
anlotinib. Apatinib is a highly selective and potent VEGFR tyrosine
kinase inhibitor (TKI) with high affinity for VEGFR2. Its low
molecular weight facilitates its ability to cross the blood-brain
barrier (BBB), potentially offering superior efficacy for BMs
compared with other drugs (12).
Anlotinib is a small-molecule, multi-targeted TKI that was
developed in China and effectively inhibits VEGFR1-3,
platelet-derived growth factor receptor-α/β, fibroblast growth
factor receptors 1–4, c-Kit, and other targets. It has the ability
to inhibit tumor angiogenesis, growth and migration. Anlotinib has
also been suggested to be able to cross the BBB, and therefore, to
have potential in combating BMs (13).
In a preclinical study of utidelone, it was found
using animal models that utidelone has a higher concentration in
various tissues than in plasma, and can easily pass through the
BBB, maintaining a high concentration in brain tissue (Li et
al, unpublished data). However, studies on the use of utidelone
in BCBMs are lacking. In the present case report, a patient with
HR+ breast cancer that was refractory to CDK4/6
inhibitors combined with ET and progressed after
anthracycline/taxane chemotherapy, including the formation of BMs,
is described. The patient was treated with utidelone in combination
with apatinib/anlotinib, and good therapeutic efficacy was
achieved.
Case report
In August 2015, a 37-year-old Chinese female
underwent segmental mastectomy of the left breast and sentinel
lymph node biopsy of the left axilla at Sun Yat-sen University
Cancer Hospital (Guangzhou, China). Postoperative pathology
revealed invasive ductal carcinoma of the left breast, grade II,
with immunohistochemistry results as follows: Estrogen receptor
(ER; 90%+), progesterone receptor (PR;
100%+), HER2 (0) and Ki67 (15%+) (data not
shown). The patient received four cycles of adjuvant anthracycline
and cyclophosphamide chemotherapy, followed by 32 sessions of
radiotherapy and 5 years of tamoxifen as ET. Regular follow-ups
were performed, and no signs of metastasis were detected.
In October 2020, a positron emission tomography
(PET)/computed tomography (CT) scan indicated the presence of a
mass next to the left internal thoracic artery, as well as multiple
bone metastases. On October 27, 2020, a biopsy of the mass
confirmed it comprised breast cancer tissue. Immunohistochemistry
results were as follows: ER (~70%+), PR
(<1%+), HER2 (0) and Ki67 (~70%+) (data
not shown). The disease-free survival time was 62 months.
From October 2020 to December 2020, the patient
received first-line treatment with palbociclib, fulvestrant and
goserelin, along with incadronate disodium to protect against bone
destruction. Two months later, a CT scan showed enlargement of the
mass on the left side of the internal thoracic artery. The efficacy
evaluation was progressive disease (PD), with a progression-free
survival 1 (PFS1) of 2 months.
In December 2020, the patient received one cycle of
second-line chemotherapy with carboplatin and nab-paclitaxel at
Fudan University Cancer Hospital (Shanghai, China), which
alleviated the pain symptoms. On January 30, 2021, the patient
first visited the National Cancer Center/National Clinical Research
Center for Cancer/Cancer Hospital and Shenzhen Hospital, Chinese
Academy of Medical Sciences and Peking Union Medical College
(Shenzhen, China; henceforth referred to as our hospital), and
continued the chemotherapy regimen for one more cycle. A subsequent
CT scan showed stable disease (SD), but due to serious side effects
and grade III transaminase elevation, carboplatin was discontinued.
From February 25, 2021 to May 13, 2021, the patient received four
cycles of single-agent nab-paclitaxel chemotherapy, achieving the
best overall response of SD.
From May 2021 to September 2021, the treatment was
switched to vinorelbine tartrate soft capsules (initially 60
mg/m2, then 80 mg/m2) on days 1 and 8, every
3 weeks for four cycles, while ET with goserelin and fulvestrant
was continued, and denosumab was regularly administered. On August
24, 2021, a magnetic resonance imaging (MRI) scan suggested liver
metastasis. On August 27, 2021, a PET/CT scan at our hospital
confirmed liver metastasis and multiple bone metastases. The PFS2
was 8 months.
In September 2021, the patient underwent liver
biopsy and ablation of the liver metastasis at The First Affiliated
Hospital of Sun Yat-sen University (Guangzhou, China).
Postoperative pathology showed the infiltrative growth of nest-like
atypical cells, consistent with mBC in the liver, with the
following immunohistochemistry results: ER (~50%+), PR (−), HER2
(0), CK19 (+) and GATA-3 (+).
In September 2021, third-line treatment was
initiated with abemaciclib 150 mg twice daily, along with
fulvestrant 0.5 g and goserelin 3.6 mg by subcutaneous injection
every 28 days. On November 3, 2021, the patient returned to our
hospital for a check-up, and a brain MRI scan indicated brain
metastasis and new liver metastasis. The PFS3 was 1.4 months. On
November 20, 2021, genetic testing revealed mutations in
phosphatidylinositol-4,5-bisphosphonate 3-kinase catalytic subunit
a, paired box 5, SUFU negative regulator of hedgehog signaling, and
tumor protein p53. Specifically, circulating tumor DNA (ctDNA)
samples were analyzed using the OncoD-C1021B next-generation
sequencing (NGS) platform (Beijing Geneplus Technology Co., Ltd.).
A 10-ml sample of whole blood was drawn into a standard stabilizing
tube (Streck LLC) and centrifuged within 72 h to separate the
plasma from peripheral blood cells. ctDNA extraction (QIAseq cfDNA
All-in-One Kit; cat. no. 180043; Qiagen GmbH) was performed in a
College of American Pathologists-accredited clinical laboratory,
according to the manufacturer's instructions. The DNA concentration
was measured using a Qubit™ fluorometer and Qubit dsDNA
HS Assay Kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Sequencing libraries were prepared from the ctDNA using KAPA DNA
Library Preparation Kits (Kapa Biosystems; Roche Diagnostics). NGS
was performed using a 1,021 gene panel mutation detection kit
(Beijing Geneplus Technology Co., Ltd.) for somatic single
nucleotide variants, insertions/deletions, structural variations
and copy number variations. The Integrative Genomics Viewer tool
(14) was used to verify the
candidate variants. Due to the clinical unavailability of PI3K
inhibitors, the patient consulted with neurosurgery and radiation
oncology specialists, but refused brain radiotherapy and
surgery.
A fourth-line chemotherapy regimen with utidelone
combined with anti-angiogenic drug therapy was then established,
starting in November 2021. The patient received utidelone 50 mg
intravenously on days 1–5 and apatinib 0.25 g orally daily from day
1 to 21 every 21 days for one cycle. The patient experienced grade
I peripheral neurotoxicity, grade I hypertension, grade I
hyperbilirubinemia and elevated transaminases, which were
considered to be associated with apatinib. In the second cycle,
which started in December 2021, apatinib was replaced with
anlotinib. The patient received utidelone 50 mg intravenously on
days 1–5 and anlotinib 8 mg orally daily from day 1 to 21 every 21
days for one cycle, and experienced grade II muscle soreness and
numbness of the hands and feet. After two cycles, the efficacy
evaluation indicated a partial response to the treatment. Due to
muscle soreness, the dose of utidelone was reduced, and the
treatment was continued. From January 2022 to February 2022,
chemotherapy with the utidelone plus anlotinib regimen was
continued, with utidelone 40 mg intravenously on days 1–5 and
anlotinib 8 mg orally daily from day 1 to 21 every 21 days for two
cycles. After four cycles, the efficacy evaluation indicated that
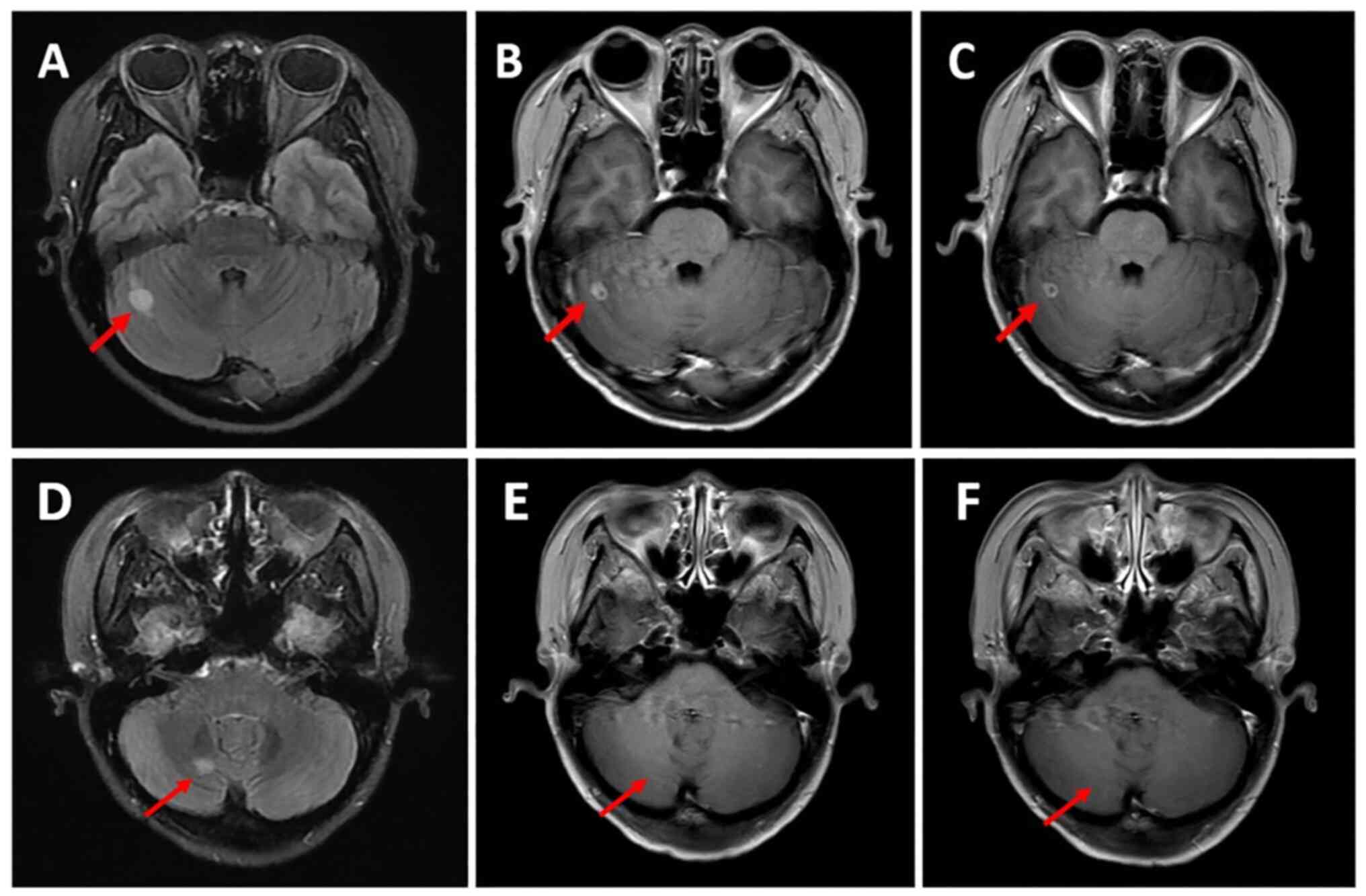
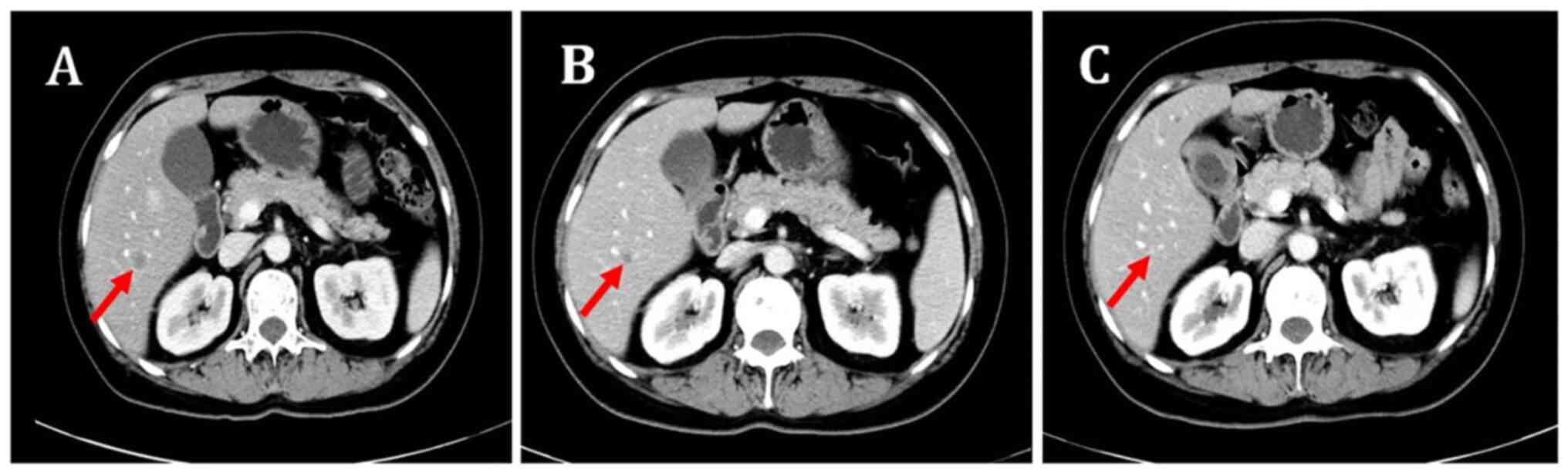
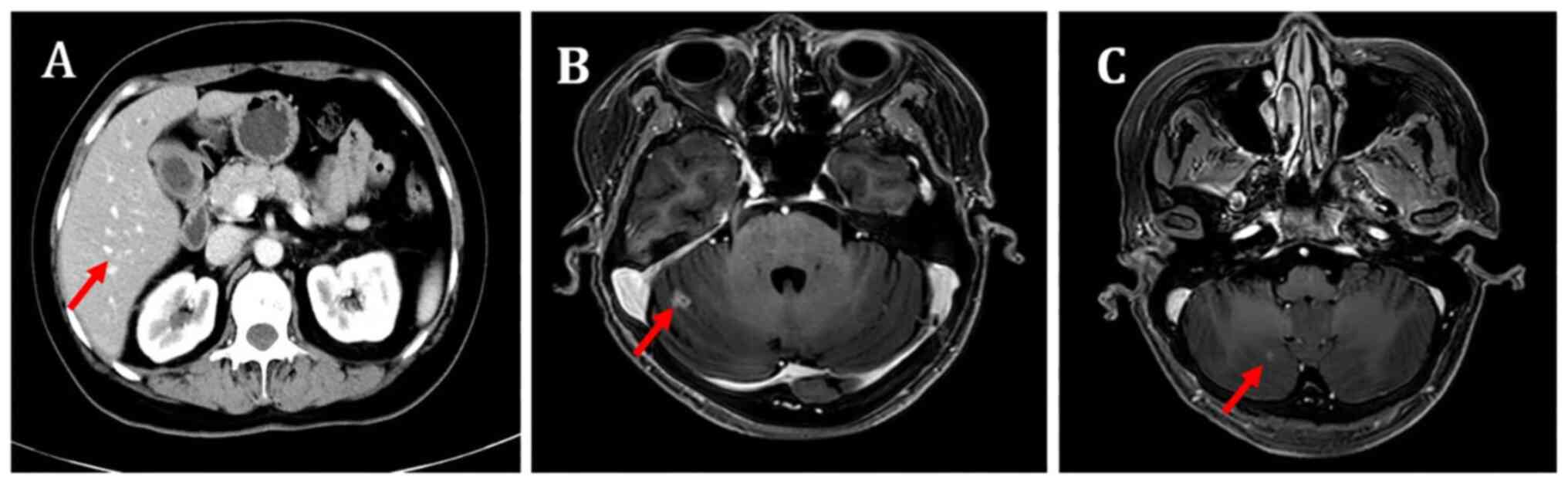
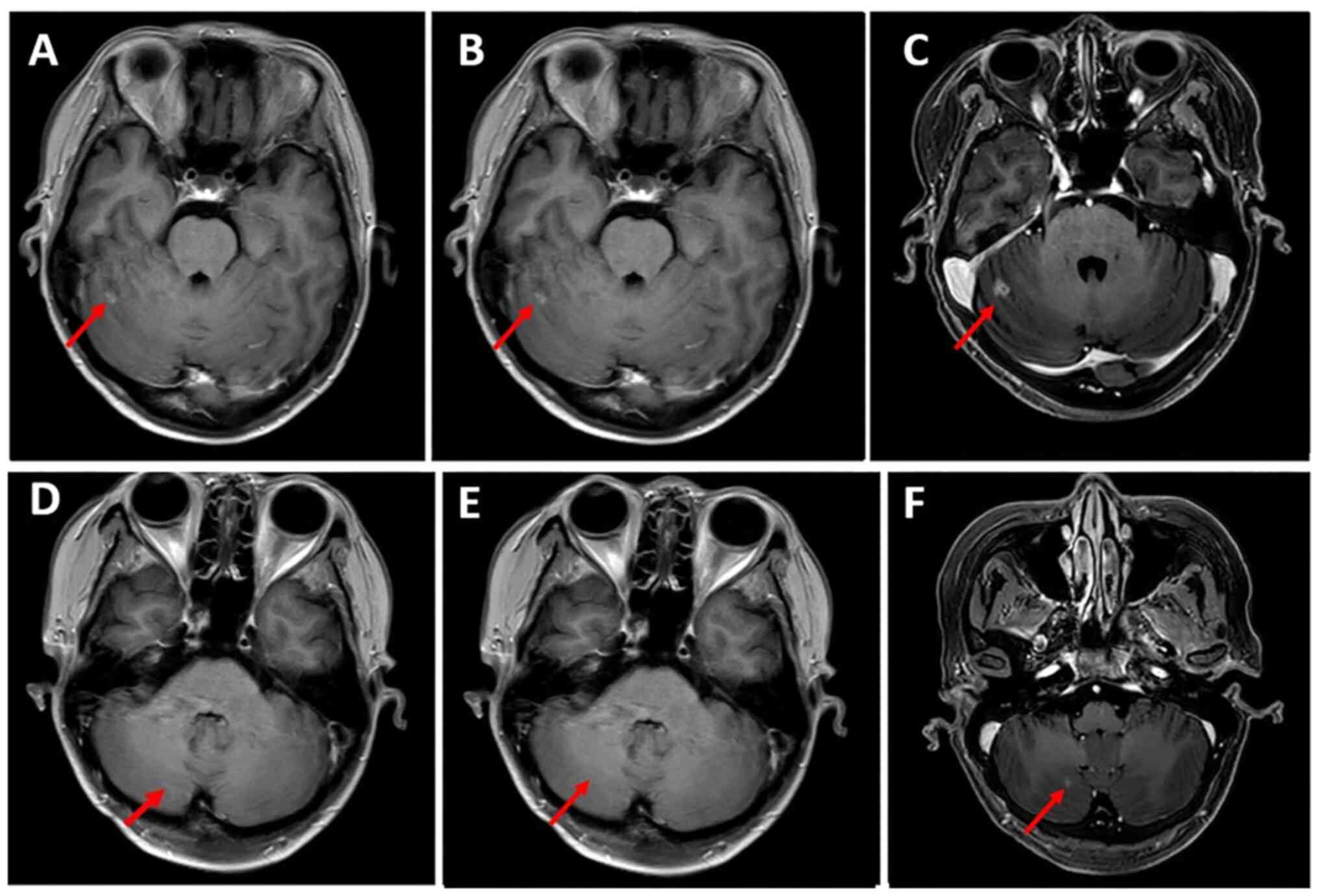
the partial response was maintained (Figs. 1 and 2). However, the patient also had grade II
limb soreness, numbness of the hands and feet, abnormal sensations
in a ‘glove and sock’ distribution, and fatigue. The patient, who
had a high demand for quality of life and poor compliance,
requested adjustment of the medication. The duration of therapy was
3.7 months.
The fifth-line chemotherapy regimen comprising
apatinib and eribulin was administered from March 2022 to April
2022. Eribulin 2 mg was dosed intravenously on days 1 and 8, and
apatinib 0.25 g was given orally once daily from day 1 to 21 every
21 days for two cycles. The partial response was maintained
following this treatment. In April 2022, due to nausea and
vomiting, apatinib was switched to oral anlotinib. On May 7, 2022,
chemotherapy with the anlotinib and eribulin regimen was initiated,
comprising eribulin 2 mg intravenously on days 1 and 8, and
anlotinib 8 mg orally daily from day 1 to 21 every 21 days for two
cycles. In July 2022, CT and MRI scans showed controlled
extracranial lesions but progressive intracranial lesions (Figs. 3 and 4). The PFS5 was 3.9 months.
The sixth-line treatment plan was adjusted to a
combination of darolutamide, apatinib and exemestane. In August
2022, a follow-up scan suggested progression of the intracranial
lesions, with a PFS6 of 1.6 months.
The seventh-line treatment was a combination of
seribantam, anlotinib and exemestane. From September 2022 to
October 2022, the patient received whole-brain palliative
radiotherapy and local boost radiotherapy at our hospital. The PFS7
was 2.1 months. In November 2022, the patient visited Luohu
District People's Hospital (Shenzhen, China) due to pain in the
lower limbs and waist, and an eighth-line treatment with
seribantam, bevacizumab and exemestane was started, but the pain
did not markedly improve. In November 2022, a follow-up at our
hospital revealed multiple nodules in the liver, which were larger
and more numerous than before, with an elevated metabolism,
suggesting liver metastasis. The PFS8 was 0.3 months.
Subsequently, the patient was admitted to hospice
care at Cihai Hospital (Shenzhen, China) and ultimately succumbed
to the disease in April 2023. The entire treatment process is
depicted in Table I.
 | Table I.Treatments received by the patient
after recurrence and metastasis. |
Table I.
Treatments received by the patient
after recurrence and metastasis.
| Lines of therapy | Treatment dates | Therapy | Best therapeutic
evaluation, extracranial lesions/intracranial lesions | PFS or duration of
therapya, months |
|---|
| First | October 2020-December
2020 | Palbociclib +
fulvestrant | PD/NA | PFS1, 2.8 |
| Second | December 2020-August
2021 | Carboplatin +
nab-paclitaxel, 2 cycles; nab-paclitaxel, 4 cycles; vinorelbine, 4
cycles | SD/NA | PFS2, 8 |
| Third | September
2021-November 2021 | Abemaciclib +
fulvestran | PD/PD | PFS3, 1.4 |
| Fourth | November 2021-March
2022 | Utidelone + apatinib,
1 cycle; utidelone + anlotinib, 3 cycles | PR/PR | PFS4, 3.7 |
| Fifth | March 2022-July
2022 | Eribulin + apatinib,
2 cycles; eribulin + anlotinib, 2 cycles | PR/PR | PFS5, 3.9 |
| Sixth | July 2022-August
2022 | Darolutamide +
apatinib + exemestane | PD/PD | PFS6, 1.6 |
| Seventh | September
2022-October 2022 | Seribantam +
anlotinib + exemestane | PD/PD | PFS7, 2.1 |
| Eighth | November 2022 | Seribantam +
bevacizumab + exemestane | PD/PD | PFS8, 0.3 |
Discussion
Currently, the treatment options for BCBM encompass
local therapies and/or systemic treatments. Local therapies include
surgery, stereotactic radiosurgery (SRS) and whole-brain
radiotherapy (WBRT), while systemic therapies comprise chemotherapy
and targeted therapies (15).
Although surgery has been demonstrated to improve the OS of
patients with BCBM, it is typically reserved for those with
pronounced symptoms, who are in good general condition and have
limited BMs (16,17). The majority of patients with BCBM
receive radiotherapy, with patients having a good performance
status and localized BMs undergoing SRS, and those with a poorer
performance or extensive BMs typically receiving WBRT. However,
surgery and radiotherapy can cause serious physical harm to
patients. In addition, after local palliative surgery or
radiotherapy of the brain, systemic treatment remains necessary
(18). Therefore, effective
systemic therapy may enable patients with BCBM to avoid the trauma
of surgery and the neurotoxicity associated with radiotherapy
during advanced treatment.
The role of ET in the treatment of BMs from
HR+ breast cancer is currently supported by limited
research and literature. Despite ET being one of the primary
treatment modalities for HR+ breast cancer, further
clinical data and studies are required to confirm its application
and efficacy in the context of BM. In terms of systemic treatment,
the BBB limits the penetration of numerous chemotherapeutic agents,
which has limited research into chemotherapy for BCBM (19). Small-molecule anti-angiogenic drugs,
such as apatinib and anlotinib, have shown some efficacy in the
systemic treatment of triple negative breast cancer with BMs
(9,20–24).
However, the treatment of patients with HR+ advanced
BCBM remains a considerable clinical challenge. In the present
case, the patient had a mass adjacent to the left side of the
internal thoracic artery, suspected of being a metastasis, with
multiple bone metastases. First-line treatment with the CDK4/6
inhibitor palbociclib combined with fulvestrant resulted in rapid
disease progression, with a PFS1 of 2 months. Continued ET provided
limited benefit, leading to chemotherapy being considered.
Second-line chemotherapy with nab-paclitaxel was administered for
six cycles, which achieved SD, followed by oral vinorelbine soft
capsule maintenance therapy for four cycles. Following this, liver
metastases were detected, with a PFS2 of 8 months. Third-line
treatment involved another CDK4/6 inhibitor, abemaciclib, for
‘cross-line’ use, combined with fulvestrant therapy. Unfortunately,
the patient developed new BMs, with a PFS3 of 1.4 months. Due to
refusal of brain palliative radiotherapy and surgery by the
patient, the selection of drugs that can control BMs by crossing
the BBB was crucial. Previous studies have shown that utidelone,
apatinib and anlotinib are all able to cross the BBB and may be of
benefit in the treatment of intracranial tumors (9,10,12,13).
Utidelone is a recently launched drug of the
epothilone class that not only possesses the antitumor effects of
traditional microtubule inhibitors but also has a unique mechanism
of action that avoids cross-resistance with traditional microtubule
inhibitors. The mechanism of paclitaxel resistance has been
demonstrated to include the overexpression of P-glycoprotein (P-gp)
and changes in microtubule β and α subunits (5). Although the mechanism of action of
utidelone is similar to that of paclitaxel, its binding site
differs and it does not bind to P-gp on the surface of tumor cells;
thus, it effectively evades paclitaxel resistance mechanisms
(6).
In the present case, a patient with refractory
HR+ BCBMs was treated with utidelone combined with a
small-molecule anti-angiogenic drug, namely apatinib/anlotinib,
achieving a PR after two cycles and sustained response after four
cycles of treatment. A brain MRI scan showed a marked reduction in
the BMs, likely due to the concerted antitumor effect of utidelone
and small-molecule anti-angiogenic drugs crossing the BBB. During
treatment, the patient experienced grade II limb soreness,
hand-foot syndrome and paresthesias, accompanied by fatigue. Due to
the patient demanding to maintain a high quality of life and having
poor compliance, medication adjustment was requested. Therefore,
the treatment was adjusted to eribulin combined with a
small-molecule anti-angiogenic drug. After two cycles, the PR was
sustained, but after four cycles, the disease progressed, with a
therapeutic evaluation of PD. According to preclinical research,
eribulin has limited ability to cross the BBB (25). Therefore, it may be inferred that
the PR was achieved through the effects of the utidelone and
small-molecule anti-angiogenic drug therapy. The continued PR after
switching to eribulin and small-molecule anti-angiogenic therapy
may have been due to the continued antitumor effect of the
anti-angiogenic drug crossing the BBB, although only a single drug
reaching the brain is likely to have been less potent than the
combined therapy. A previous study indicated that tumor
invasiveness may increase in patients who fail CDK4/6 inhibitor
therapy, making them more likely to develop resistance to
subsequent treatments, ultimately leading to disease progression
(26).
Data from the phase III clinical trial of utidelone
(registered at ClinicalTrials.gov as trial no. NCT02253459) showed
that in comparison with patients treated with capecitabine alone,
patients treated with utidelone plus capecitabine had a significant
extension in PFS from 4.11 to 8.57 months (27). In this trial, 87% of the 251
HR+ patients had previously received ET. In the present
case report, the PFS of the patient with the fourth-line treatment
was 3.7 months, which was lower than the PFS of 8.57 months in the
clinical trial. This was mainly because the patient could not
tolerate neurotoxicity and stopped taking the drug. The efficacy
evaluation was PR. Although combinations of utidelone with
anti-angiogenic agents have shown promising efficacy, particularly
in patients with BMs, their neurotoxicity should not be ignored. A
combination of utidelone and capecitabine was not selected for
treatment of the present case due to the lack of imported
capecitabine, and the obvious adverse reactions observed clinically
for domestically produced capecitabine, including hand-foot
syndrome, abnormal aminotransferase and gastrointestinal reactions.
The present case had liver metastasis and mild abnormal
aminotransferase. Considering the treatment tolerance and
compliance of the patient, combination therapy with apatinib was
selected. As both apatinib and utidelone can be neurotoxic, in
order to minimize peripheral neurotoxicity, low-dose apatinib was
selected for use in the combination therapy. Whether the
combination of utidelone with capecitabine or apatinib has greater
efficacy and lower toxic side effects requires investigation in
future clinical studies.
The most common adverse reactions of utidelone are
peripheral neuropathy, hand-foot syndrome, hematological toxicity
and gastrointestinal toxicity, with peripheral neuropathy being the
primary adverse reaction. The hematological and gastrointestinal
toxicities of utidelone are lower than those of other chemotherapy
drugs (5,27). Clinical research and practice have
demonstrated that utidelone has good safety, with the longest
reported use being 34 cycles, which exceeds the use-cycle
limitations of traditional chemotherapeutic agents and promotes
patient adherence to the medication, thus providing long-term
sustained benefits (5). The present
patient discontinued utidelone due to peripheral neurotoxicity,
primarily because during the year prior to the use of utidelone
they had received six cycles of nab-paclitaxel chemotherapy, which
also has significant peripheral neurotoxicity, and secondly,
because efforts to prevent and manage peripheral neurotoxicity in
this patient were inadequate. Strengthening this management in the
future will allow patients to benefit from the efficacy of
utidelone while also ensuring their quality of life.
As a new-generation microtubule inhibitor, utidelone
has been shown in a previous study to evade paclitaxel resistance
mechanisms and have the characteristics of high efficacy and low
toxicity (5). This makes it a
particularly suitable treatment for patients with mBC who have
received paclitaxel therapy. To the best of our knowledge, there
have been no previous case reports on utidelone combined with
small-molecule anti-angiogenic drug therapy in patients with
HR+ BCBM, particularly those with refractory advanced
BCBM after two lines of CDK4/6 inhibitors combined with ET and
anthracycline/paclitaxel therapy.
In summary, the present case report describes the
use of utidelone combined with small-molecule anti-angiogenic drugs
to treat a patient with HR+ advanced BCBMs who was
refractory to CDK4/6 inhibitors and progressed after
anthracycline/paclitaxel therapy. The effects observed in this
patient demonstrate the efficacy of utidelone, even after
progression on prior paclitaxel therapy. The present case
illustrates the potential of utidelone combined with small-molecule
anti-angiogenic drugs in the treatment of BCBM. On this basis,
clinical research to elucidate the role of utidelone combined with
small-molecule anti-angiogenic drugs in the treatment of patients
with BCBM has been initiated.
Acknowledgements
Not applicable.
Funding
The study was supported by Shenzhen Key Medical Discipline
Construction Fund (grant no. SZXK013).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The raw sequencing data
generated in the present study have been deposited in the Genome
Sequence Archive (Genomics, Proteomics and Bioinformatics 2021) in
National Genomics Data Center (Nucleic Acids Res 2022), China
National Center for Bioinformation/Beijing Institute of Genomics,
Chinese Academy of Sciences under accession number HRA008225 or at
the following URL: https://ngdc.cncb.ac.cn/gsa-human/browse/HRA008225.
Authors' contributions
XB and CD designed the study and wrote the
manuscript. XX, XL, LC and JH gathered medical images and examined
patient information. XB, XX, ML, JH, XC, LS, QS, JZ, XL and LC
contributed to the conceptualization, general design and quality
assurance of the study. XB, ML, XC, LS, JZ and QS confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Since the patient is deceased, her husband provided
written informed consent for the publication of this case report
and the associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
U.S. National Institutes Of Health,
National Cancer Institute, . SEER Cancer Statistics Review,
1975–2019. American Cancer Society; 2021, https://seer.cancer.gov/statfacts/html/breast.htm
|
|
3
|
Munzone E, Pagan E, Bagnardi V, Montagna
E, Cancello G, Dellapasqua S, Iorfida M, Mazza M and Colleoni M:
Systematic review and meta-analysis of post-progression outcomes in
ER+/HER2- metastatic breast cancer after CDK4/6 inhibitors within
randomized clinical trials. ESMO Open. 6:1003322021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marschner N, Harbeck N, Thill M, Stickeler
E, Zaiss M, Nusch A, Rauh J, Schulz H, Engelken K, Kruggel L, et
al: 232P Second-line therapies of patients with early progression
under CDK4/6-inhibitor in first-line-data from the registry
platform OPAL. Ann Oncol. 33 (Suppl 7):S643–S644. 2022. View Article : Google Scholar
|
|
5
|
Xu B, Sun T, Zhang Q, Zhang P, Yuan Z,
Jiang Z, Wang X, Cui S, Teng Y, Hu XC, et al: Efficacy of utidelone
plus capecitabine versus capecitabine for heavily pretreated,
anthracycline- and taxane-refractory metastatic breast cancer:
Final analysis of overall survival in a phase III randomised
controlled trial. Ann Oncol. 32:218–228. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bi P, Wang X, Liu R, Li X, Wei S, Zhao J,
Tan X, Zhang F, Mao Q, Zhang Y, et al: Efficacy and safety of
utidelone plus capecitabine in advanced first-line therapy for
metastatic breast cancer: A multicenter real-world study. Surg Open
Sci 16: 171–183, 2023. Liu MC, Cortés J and O'Shaughnessy J:
Challenges in the treatment of hormone receptor-positive,
HER2-negative metastatic breast cancer with brain metastases.
Cancer Metastasis Rev. 35:323–332. 2016.PubMed/NCBI
|
|
7
|
Labidi SI, Bachelot T, Ray-Coquard I,
Mosbah K, Treilleux I, Fayette J, Favier B, Galy G, Blay JY and
Guastalla JP: Bevacizumab and paclitaxel for breast cancer patients
with central nervous system metastases: A case series. Clin Breast
Cancer. 9:118–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leone JP, Emblem KE, Weitz M, Gelman RS,
Schneider BP, Freedman RA, Younger J, Pinho MC, Sorensen AG,
Gerstner ER, et al: Phase II trial of carboplatin and bevacizumab
in patients with breast cancer brain metastases. Breast Cancer Res.
22:1312020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Deng S, Zhang Y, Wang C, Hu X,
Kong D, Liang G, Yuan X, Li Y and Wang X: Apatinib enhances the
anti-tumor effect of paclitaxel via the PI3K/p65/Bcl-xl pathway in
triple-negative breast cancer. Ann Transl Med. 9:10012021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y,
Li J and Lou L: YN968D1 is a novel and selective inhibitor of
vascular endothelial growth factor receptor-2 tyrosine kinase with
potent activity in vitro and in vivo. Cancer Sci. 102:1374–1380.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Syed YY: Anlotinib: First global approval.
Drugs. 78:1057–1062. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watase C, Shiino S, Shimoi T, Noguchi E,
Kaneda T, Yamamoto Y, Yonemori K, Takayama S and Suto A: Breast
cancer brain metastasis-overview of disease state, treatment
options and future perspectives. Cancers (Basel). 13:10782021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases to the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thorvaldsdóttir H, Robinson JT and Mesirov
JP: Integrative genomics viewer (IGV): High-performance genomics
data visualization and exploration. Brief Bioinform. 14:178–192.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalkanis SN, Kondziolka D, Gaspar LE,
Burri SH, Asher AL, Cobbs CS, Ammirati M, Robinson PD, Andrews DW,
Loeffler JS, et al: The role of surgical resection in the
management of newly diagnosed brain metastases: A systematic review
and evidence-based clinical practice guideline. J Neurooncol.
96:33–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bailleux C, Eberst L and Bachelot T:
Treatment strategies for breast cancer brain metastases. Br J
Cancer. 124:142–155. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sperduto PW, Kased N, Roberge D, Xu Z,
Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al: Effect
of tumor subtype on survival and the graded prognostic assessment
for patients with breast cancer and brain metastases. Int J Radiat
Oncol Biol Phys. 82:2111–2117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong C, Yu S, Qian P, Song X, Wen J, Jiang
M, Zhu J, Xu J, Zhao L, Guo Z, et al: Anlotinib combined with
whole-brain radiotherapy in non-small cell lung cancer with
multiple brain metastases that progressed or developed after at
least one line of prior treatment. Front Oncol. 13:11693332023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Xu J, Ye W, Zhong W, Zhang X, Mao J
and Wu D: Whole-brain radiotherapy combined with anlotinib for
multiple brain metastases from non-small cell lung cancer without
targetable driver mutation: A single-arm, phase II study. Clin Med
Insights Oncol. 16:117955492210791852022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng Y, Wang Q, Li K, Shi J, Han B, Wu L,
Chen G, He J, Wang J, Qin H and Li X: Third-line or above anlotinib
in relapsed and refractory small cell lung cancer patients with
brain metastases: A post hoc analysis of ALTER1202, a randomized,
double-blind phase 2 study. Cancer Innov. 2:181–190. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Y, Liu B, Guan M and Liu M:
Successful treatment using apatinib in intractable brain edema: A
case report and literatures review. Cancer Biol Ther. 19:1093–1096.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Bai X, Xie X, Huang J, Chen L,
Song L, Lan X, Zhang Q, Guo J and Du C: The anti-tumor efficiency
of low-dose apatinib-based chemotherapy in pretreated HER2-negative
breast cancer with brain metastases. Ann Med. 55:22186472023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bollag DM, McQueney PA, Zhu J, Hensens O,
Koupal L, Liesch J, Goetz M, Lazarides E and Woods CM: Epothilones,
a new class of microtubule-stabilizing agents with a taxol-like
mechanism of action. Cancer Res. 55:2325–2333. 1995.PubMed/NCBI
|
|
24
|
Zhang P, Tong Z, Tian F, Wang Y, Yang J,
Li W, Di L, Liu W, Tang L, Qiu R and Xu B: Phase II trial of
utidelone as monotherapy or in combination with capecitabine in
heavily pretreated metastatic breast cancer patients. J Hematol
Oncol. 9:682016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garrone O, Miraglio E, Vandone AM, Vanella
P, Lingua D and Merlano MC: Eribulin in advanced breast cancer:
Safety, efficacy and new perspectives. Future Oncol. 13:2759–2769.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Portman N, Alexandrou S, Carson E, Wang S,
Lim E and Caldon CE: Overcoming CDK4/6 inhibitor resistance in
ER-positive breast cancer. Endocr Relat Cancer. 26:R15–R30. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang P, Sun T, Zhang Q, Yuan Z, Jiang Z,
Wang XJ, Cui S, Teng Y, Hu XC, Yang J, et al: Utidelone plus
capecitabine versus capecitabine alone for heavily pretreated
metastatic breast cancer refractory to anthracyclines and taxanes:
A multicentre, open-label, superiority, phase 3, randomised
controlled trial. Lancet Oncol. 18:371–383. 2017. View Article : Google Scholar : PubMed/NCBI
|