Introduction
Pulmonary large cell neuroendocrine carcinoma
(LCNEC) is a rare and highly invasive lung cancer with a poor
prognosis, constituting 1–3% of primary lung tumor (1). For stage I LCNEC, the 5-year survival
rate is only 18% and the overall 5-year survival rate for LCNEC in
all stages is 13% (2). While
surgery is the primary treatment for early-stage LCNEC, adjuvant
chemotherapy post-lobar resection improves survival (3). However, there is a lack of large-scale
controlled clinical studies exploring the optimal treatment for
advanced LCNEC, and certain studies have shown that chemotherapy
remains the most common treatment option (4–6).
Immune checkpoint inhibitors (ICIs) have markedly
improved the prognosis of patients with small cell lung cancer
(SCLC) and non-small cell lung cancer (NSCLC) (7,8).
Furthermore, there have been case reports and retrospective studies
of clinical data on the use of ICIs in LCNEC treatment (9–17).
Nevertheless, reports on alternative treatment methods in patients
resistant to traditional programmed cell death protein-1 (PD-1) and
programmed cell death-ligand 1 (PD-L1) monoclonal antibodies are
limited.
Case report
In December 2020, a 68-year-old man with a
significant smoking history who underwent lung adenocarcinoma
resection and four cycles of adjuvant chemotherapy 15 years prior
at Affiliated Hospital of Guilin Medical University in Guilin,
(Guangxi Zhuang Autonomous Region, China), presented with a cough
and hemoptysis. Computed tomography (CT) revealed a neoplasm near
the carina and multiple enlarged lymph nodes in the right hilum and
mediastinum (data not shown). Pathological samples obtained through
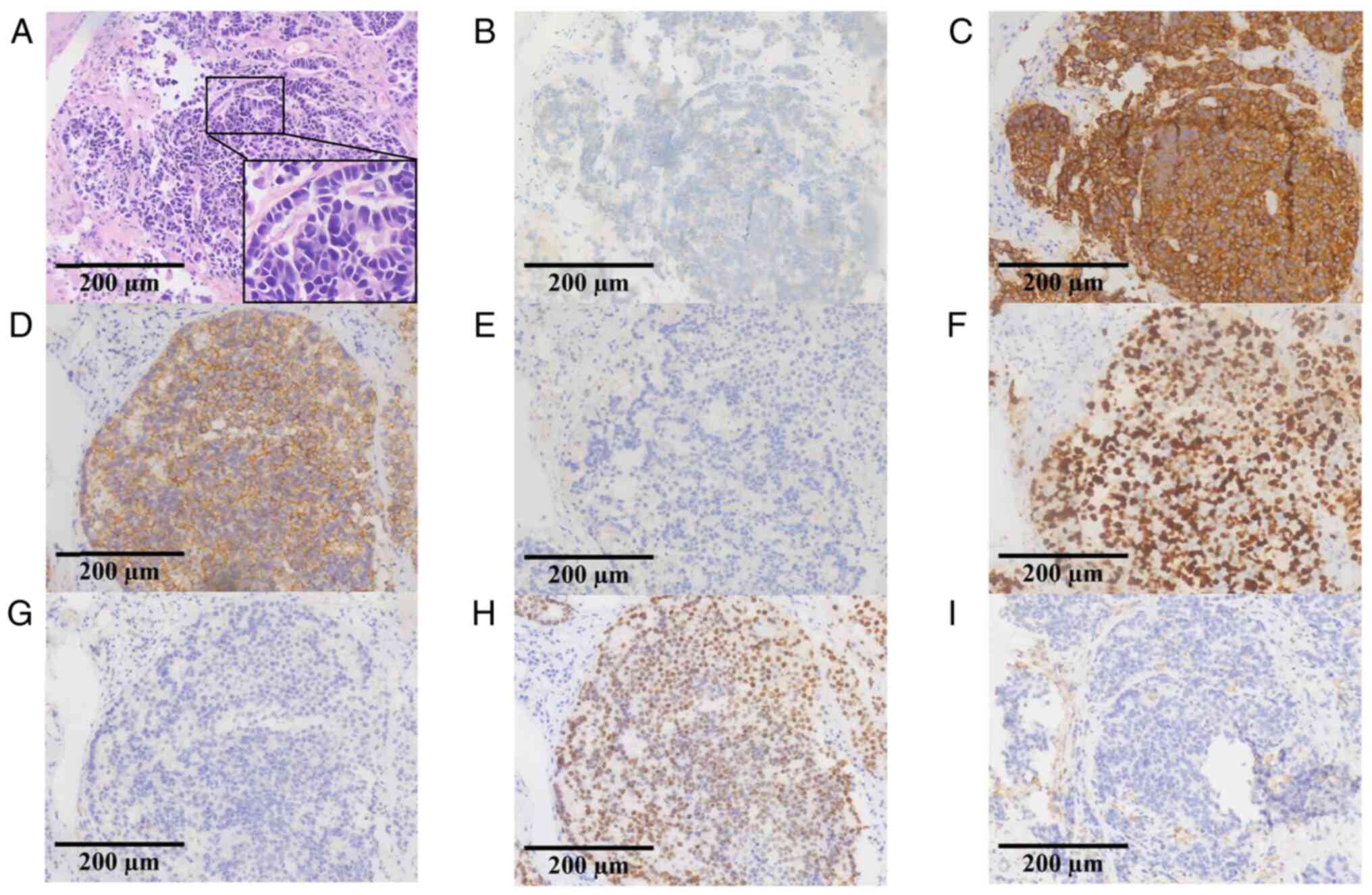
fiber bronchoscopy were stained and immunohistochemically analyzed
by the Department of Pathology, Affiliated Hospital of Guilin
Medical University, and the results showed that chromogranin A (+),
synaptophysin (+), CD56 (+), NapsinA (−), Ki-67 (+, 80%), P40 (−),
and thyroid transcription factor-1 (+) (Fig. 1), these results were consistent with
LCNEC. Subsequently, the biopsy tissue samples were used for
next-generation sequencing (NGS) targeting 139 cancer-relevant
genes, performed by Nanjing Geneseeq Technology Inc. The mean
coverage depth was 300× for controls and 1,000× for tissue samples.
The results revealed no mutations in epidermal growth factor
receptor, anaplastic lymphoma kinase, ROS proto-oncogene 1,
receptor tyrosine kinase, MET proto-oncogene, receptor tyrosine
kinase, KRAS proto-oncogene, GTPase, ret proto-oncogene,
neurotrophic tropomyosin-receptor kinase, B-Raf proto-oncogene and
serine/threonine kinase or fusion. However, RB transcriptional
corepressor 1 (RB1; c.137 +1del; abundance, 55.1%) and tumor
protein P53 (TP53; c.404G>T; abundance,
64.2%/c.393C>G; abundance, 63.7%) mutations were identified, and
PD-L1 was positive (tumor proportion score, 2%). The clinical stage
was determined to be T1bN2M0 (IIIA), according to the Eighth
Edition Lung Cancer Stage Classification (18).
Despite the relatively small tumor size (29.5×19.1
mm), radical resection and conventional radiotherapy could not be
performed due to the presence of severe restrictive ventilation
disorder (diagnosed by pulmonary function testing). In January
2021, the patient received first-line treatment with etoposide
combined with cisplatin (EP), etoposide 100 mg/m2 and
cisplatin 25 mg/m2 were injected intravenously from day
1 to day 3, and repeated every 3 weeks. Partial response (PR) was
achieved compared with baseline according to the Response
Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1)
(19) (Fig. 2A and B). Tisleslizumab (an anti-PD-1
antibody drug), administered intravenously at a dose of 200 mg
every 3 weeks, was added to the EP regimen for another three
cycles. However, progressive disease (PD) was observed during an
imaging evaluation in May 2021 (Fig.
2C). Subsequently, anlotinib (an anti-vascular multi-targeted
tyrosine kinase receptor inhibitor), at a dose of 10 mg, was taken
orally from day 1 to day 14 every 3 weeks, in combination with
tisleslizumab (200 mg), serving as a second-line treatment for nine
cycles. The best of response (BOR) during the treatment was stable
disease (Fig. 2D). Unfortunately,
disease progression was again observed in February 2022 after seven
months of treatment (Fig. 2E).
Following this, temozolomide (150 mg/m2, taken orally
from day 10 to day 14, every 4 weeks) combined with capecitabine
(750 mg/m2 twice daily, taken orally from day 1 to day
14, every 4 weeks) were administered, with a PR as BOR (Fig. 2F), and PD was observed again in May
2022 (Fig. 2G). In June 2022,
radioactive iodine-125 seeds were implanted, followed by the
administration of vinorelbine (40 mg, administered orally every
Monday, Wednesday and Friday) as a beat regimen. PD was observed
again in October 2022 due to the development of retroperitoneal
lymph node metastases as new lesions (Fig. 2H and I). Subsequently, mediastinal
lymph node (4R) metastases were observed as new lesions following
irinotecan (60 mg/m2, administered intravenously on days
1, 8, and 15, every 4 weeks) treatment in November 2022 (Fig. 2J). Based on previous studies
(20,21), patient received intravenous infusion
of cadonilimab (250 mg) combined with oral administration of
anlotinib (10 mg, from day 1 to day 14) every 3 weeks (initiated in
November 2022).
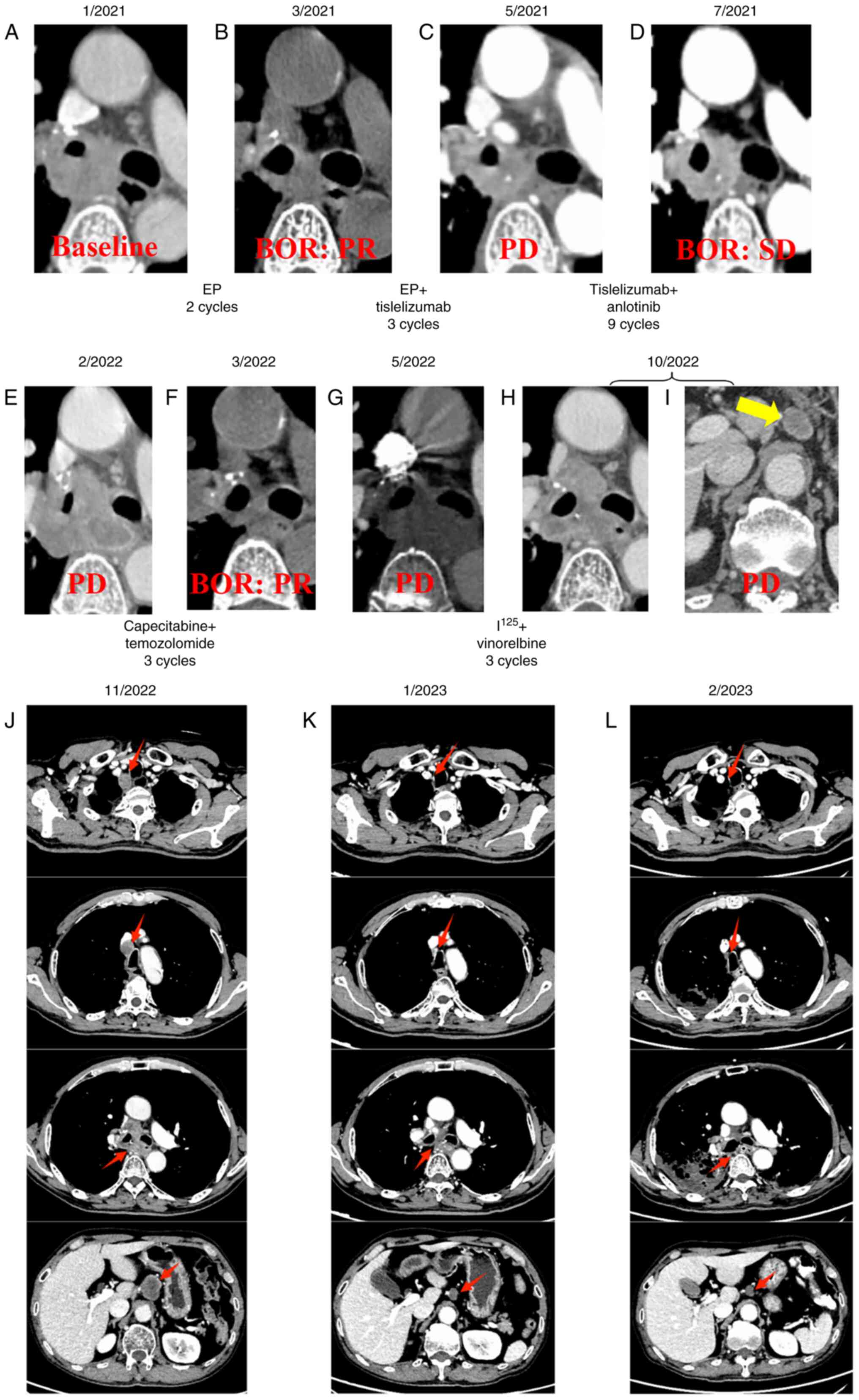 | Figure 2.Therapy timeline of the patient. CT
images of the patient in (A) January, 2021, (B) March, 2021, (C)
May, 2021, (D) July, 2021, (E) February, 2022, (F) March, 2022, (G)
May, 2022, (H and I) October, 2022. (J) CT findings before
treatment with cadonilimab + anlotinib in November 2022. CT
findings after 3 and 5 cycles of treatment with cadonilimab +
anlotinib in (K) January 2023, (L) March 2023, respectively. The
new lesion of retroperitoneal lymph node metastases is indicated by
a yellow arrows, and red arrows indicate the changes in primary
tumor and lymph node metastases before and after treatment with
cadonilimab + anlotinib. BOR, best of response; PR, partial
response; SD, stable disease; PD, progressive disease; EP,
etoposide + cisplatin. |
The treatment demonstrated efficacy and met PR
criteria according to the RECIST 1.1 (19) after three treatment cycles (Fig. 2K), resulting in the resolution of
the patient's coughing and dyspnea. After five treatment cycles,
the primary mass and lymph node metastases continued to shrink
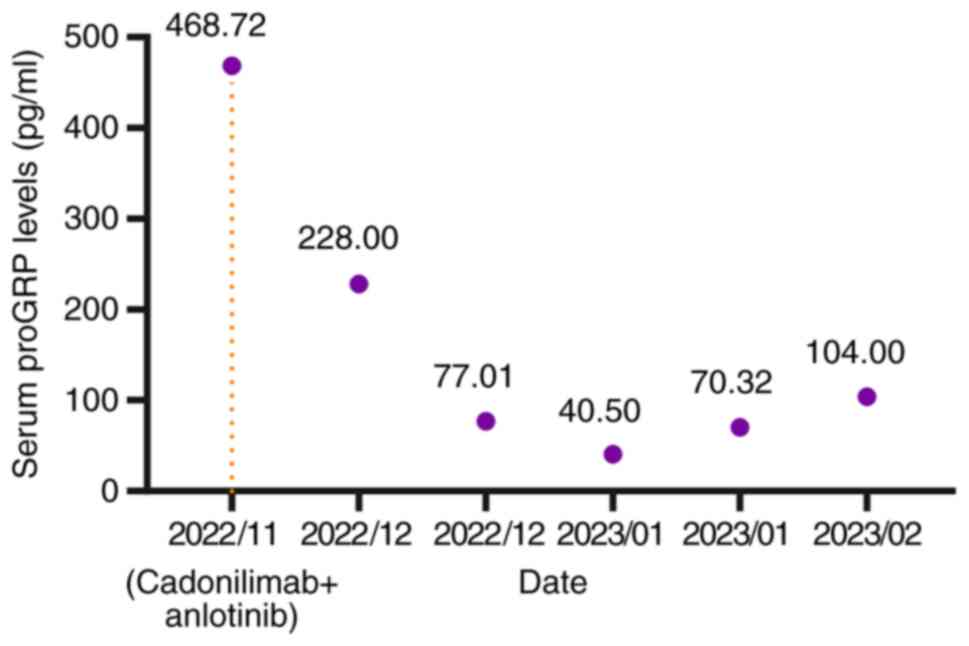
(Fig. 2L). Notably, serum levels of
gastrin-releasing peptide precursor (reference value: 3–77.8 pg/ml)
demonstrated a marked decline (Fig.
3). However, during the fourth and fifth treatment cycles, the
patient experienced moderate dyspnea during the infusion of
cadonilimab but after intravenous injection of 40 mg of
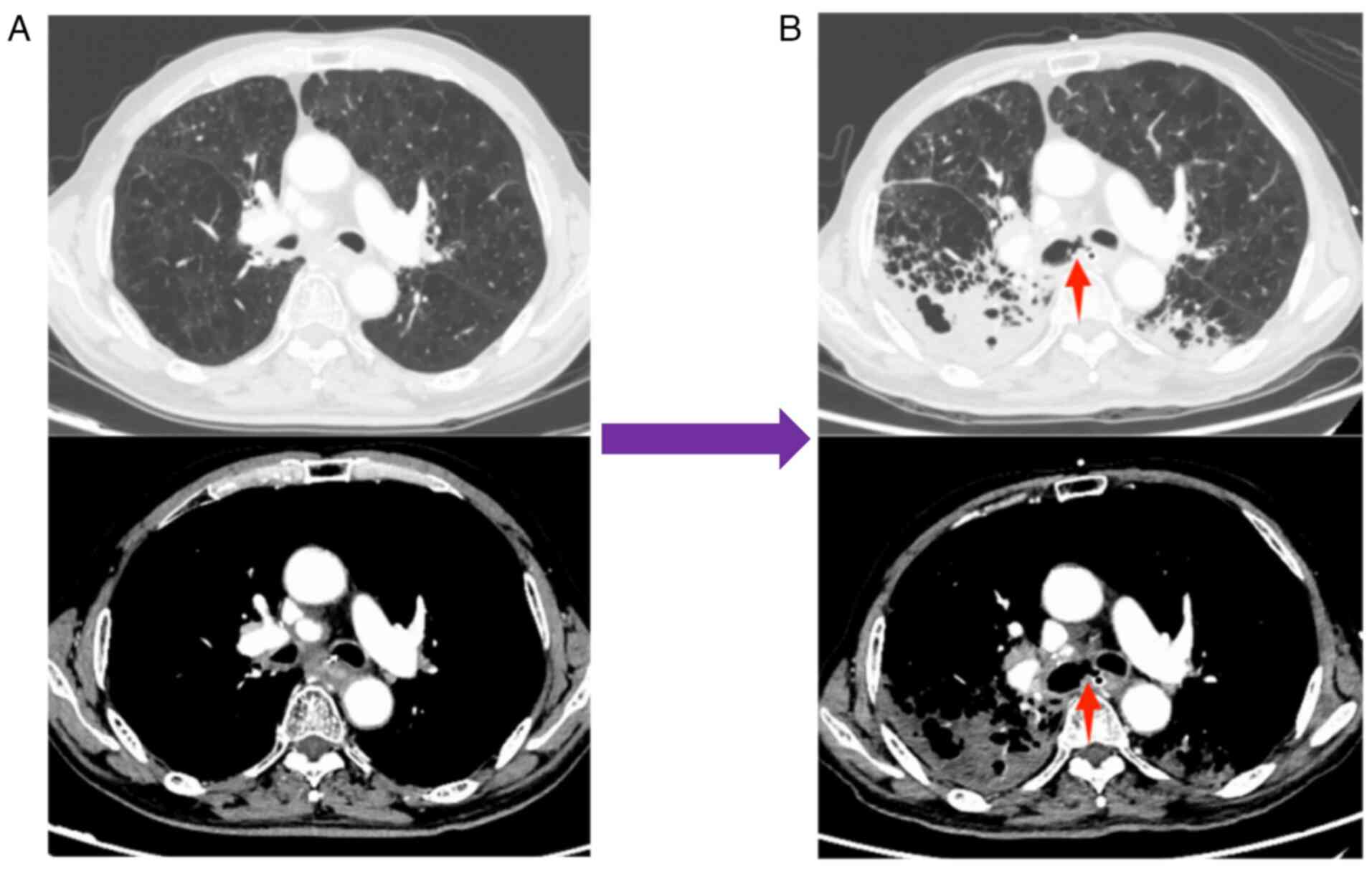
methylprednisolone, the dyspnea symptoms improved. In February
2023, the patient reported a severe cough lasting 2 days, prompting
a return to the Affiliated Hospital of Guilin Medical University
(Guangxi Zhuang Autonomous Region, China). A tracheoesophageal
fistula was identified using CT (Fig.
4), and the patient died following respiratory failure caused
by a lung infection in March 2023.
Discussion
LCNEC was first described by Travis et al in
1991 (22) and is similar to SCLC
in terms of its high invasiveness, proliferation and neuroendocrine
gene expression patterns (23).
Therefore, the 2021 World Health Organization Classification of
Lung Tumours defines LCNEC as a neuroendocrine carcinoma subtype
(24). PD-1 and PD-L1 ICIs are
standard treatment options for advanced NSCLC and SCLC. However,
only a few studies have been published on the application of ICIs
in advanced LCNEC (Table I).
Furthermore, there is no consensus among clinicians on how to
effectively and rationally treat patients with LCNEC, especially
for the subsequent treatment of advanced LCNEC, and this issue
needs more attention.
 | Table I.Summary of studies of patients with
advanced large cell neuroendocrine carcinoma treated with immune
checkpoint inhibitors. |
Table I.
Summary of studies of patients with
advanced large cell neuroendocrine carcinoma treated with immune
checkpoint inhibitors.
| First author/s,
year | Journal | Type of
research | Findings | (Refs.) |
|---|
| Wang et al,
2017 | Journal for
ImmunoTherapy of Cancer | Case report | A patient with
TMB-H LCNEC who was resistant to first-line platinum doublet
achieved a marked and durable response with pembrolizumab. | (9) |
| Qin et al,
2020 | Immunotherapy | Case report | A case of advanced
lung LCNEC treated with combined radiotherapy and nivolumab
resulted in a durable response. | (10) |
| Sherman et
al, 2020 | Lung Cancer | Retrospective
cohort | Patients with
advanced LCNEC treated with ICIs alone, ORR and median PFS were 33%
and 4.2 months, respectively (n=23). | (11) |
| Chauhan et
al, 2018 | Oncotarget | Case report | A total of 3
patients with metastatic LCNEC were treated with nivolunmab on
≥2-line therapy, of which 1 patient achieved CR, 1 patient achieved
clinical and radiological response and 1 patient achieved stable
disease. | (12) |
| Takimoto Sato et
al, 2020 | Molecular and
Clinical Oncology | Case report | A case of stage IVB
LCNEC treated with-nivolunmab as a three-line treatment resulted in
stable disease for ≥6 months. | (13) |
| Oda et al,
2020 | Thoracic
Cancer | Case report | A case of stage IV
LCNEC was treated with nivolumab, all the metastatic lesions shrunk
and a partial response was maintained for >3 years. | (14) |
| Giaj Levra et
al, 2017 | Journal of Thoracic
Oncology | Retrospective
cohort | A total of 10 LCNEC
patients were treated with nivolumab or pembrolizumab, of which
6/10 achieved partial response and 1 had stable disease. The median
PFS was reported to be 57 weeks. | (15) |
| Zhang et al,
2020 | OncoTargets and
Therapy | Case report | A patient with
TMB-H advanced LCNEC who had received nivolunmab had achieved CR
for >20 months. | (16) |
| Dudnik et
al, 2021 | Journal for
ImmunoTherapy of Cancer | Retrospective
cohort | Survival benefit
was reported in patients with advanced LCNEC treated with ICIs
(n=41) compared with those treated with no ICIs (n=84) in an
analysis of real-world data. | (17) |
Cadonilimab is a tetravalent PD-1/cytotoxic T
lymphocyte-associated antigen-4 (CTLA-4) bispecific antibody that
specifically binds PD-1 and CTLA-4. It exhibits superior antitumor
effects compared with mono-specific anti-PD-1 antibody (25) and was approved in China in June 2022
for patients with relapsed or metastatic cervical cancer who have
progressed or after platinum-based chemotherapy (26). Previous studies have reported that
certain patients with LCNEC may benefit from targeted or
traditional PD-1 monoclonal antibody therapies (27,28).
In the present case, an elderly male patient was treated with and
responded to cadonilimab combined with anlotinib after developing
resistance to PD-1 monoclonal antibodies and several
chemotherapeutic drugs. However, considering the complexity of the
treatment process, the anlotinib and radioactive iodine-125 seeds
combination may have affected the efficacy of the ICIs (29,30).
Nonetheless, cadonilimab exhibited considerable short-term efficacy
and markedly improved the quality of life of the patient. However,
it is still unclear whether CTLA-4 monoclonal antibodies will
benefit patients who are resistant to conventional PD-1 monoclonal
antibodies, and to the best of our knowledge, cadonilimab does not
have fragment crystallizable which is necessary for
antibody-dependent cell-mediated cytotoxicity (31). It may exert antitumor effects by
other means or combined blocking of immune checkpoints (32,33).
Although the patient in the present report
experienced grade 2 cadonilimab-related infusion reactions, the
treatment plan was successfully completed with routine management.
During nearly 3 months of cadonilimab treatment, no immune-related
adverse event was observed. After the multi-disciplinary team
discussion, it was speculated that the formation of a
tracheoesophageal fistula may have been related to the radioactive
seed implantation, antitumor therapy or the therapeutic side
effects. Considering the excessive cumulative dose of radiation in
the esophagus, the fistula was most likely caused by the
radioactive seeds. Previous studies have mixed views on the effect
of antiangiogenic tyrosine kinase inhibitors (TKIs) on
tracheoesophageal fistula, with certain studies suggesting that
TKIs can cause this condition, whilst others indicating they may
improve it (34,35). Moreover, the pneumonia that led to
the death of the patient in the present report had definite
evidence of infection, and no imaging features typical of
immune-related pneumonitis were observed during treatment.
Moreover, NGS results have reported that LCNEC is a
biologically heterogeneous group of tumors comprising distinct
subsets of SCLC, NSCLC and highly proliferative carcinoids with
distinct genomic signatures (36).
Although LCNEC is rare, few randomized controlled clinical trials
have been reported. The NGS results of the patient in the present
report revealed RB1 and TP53 mutations at baseline.
Based on previous reports (36,37),
the present case is characterized as the SCLC-like subtype of
LCNEC. It was reported that a case of a patient with advanced LCNEC
who received cadonilimab as first-line treatment and achieved
complete response with duration of response over 20 months
(20). In the present study, it was
notable that progression-free survival was still reached >3
months after multiple drug resistance and objective remission was
achieved. The short-term efficacy of the cadonilimab and anlotinib
combination observed suggests that combined inhibition of PD-1 and
CTLA-4 may have efficacy in treating certain neuroendocrine cancers
and may be a potential treatment option for advanced LCNEC after
resistance to PD-1/PD-L1 monoclonal antibodies. However, additional
biochemical and clinical studies are required to assess the
efficacy of this combination.
Currently, several registered clinical studies are
using PD-1 or PD-L1 monoclonal antibodies to treat LCNEC (Table II), whilst certain clinical studies
have concentrated on subsequent treatment options after initial ICI
resistance, possibly due to the low incidence of LCNEC and the
difficulty in recruiting patients.
 | Table II.Current trials involving immune
checkpoint inhibitors for the treatment of large cell
neuroendocrine carcinoma. |
Table II.
Current trials involving immune
checkpoint inhibitors for the treatment of large cell
neuroendocrine carcinoma.
| Trial | Phase | Tumors | n | Experimental
arm | Primary
endpoint | Status |
|---|
| NCT05470595
(LCNEC-ALPINE) | II | Locally advanced or
metastatic LCNEC | 67 | Atezolizumab | OS | Recruiting |
| NCT03591731
(NIPINEC) | II |
Gastroenteropancreatic or pulmonary poorly
differentiated neuroendocrine tumors (including LCNEC) | 185 | Arm A: Nivolumab;
Arm B: Nivolumab + ipilimumab | ORR | Active, not
recruiting |
| NCT03976518
(CHANCE) | II | Advanced non-small
cell lung cancer with rare histologies (including LCNEC) | 43 | Atezolizuma b | DCR | Recruiting |
| NCT03728361 | II | Small cell lung
cancer or advanced neuroendocrine cancer (including LCNEC) | 55 | Nivolumab +
temozolomide | ORR | Active, not
recruiting |
| EudraCT
2020-005942-41 (DUPLE) | II | Metastatic
LCNEC | 49 | Durvalumab with
carboplatin + etoposide followed by durvalumab maintenance | OS rate at 1
year | Ongoing |
| EudraCT
2020-002683-31 | II | Advanced LCNEC | 67 | Atezolizumab with
platinum + etoposide | OS | Ongoing |
| ChiCTR
2300068111 | II | Locally
advanced/metastatic LCNEC | 64 | Serplulimab with
carboplatin + etoposide | ORR | Prospective
registration |
In a patient with advanced LCNEC who was resistant
to traditional PD-1 antibody and multiple lines of chemotherapy,
the application of cadonilimab and anlotinib showed impressive
efficacy. Systemic treatment of advanced LCNEC requires additional
biochemical studies and clinical data.
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Natural Science
Foundation of China (grant nos. 82260498 and 82160471), Quzhou City
Qujiang District Life Oasis Public Welfare Service Center; Health
Development Promotion Project-Cancer Research Project (grant no.
BJHA-CRP-033), Guangxi Medical and Health Key Discipline
Construction Project, 2021 Guilin City Science Research and
Technology Development Plan Project (grant no. 20210227-7-9) and
Beijing Xisike Clinical Oncology Research Foundation (grant no.
Y-QL202101-0214).
Availability of data and materials
The NGS data generated in the present study may be
found in the China National Center for Bioinformation under
accession number HRA008073 or at the following URL: https://ngdc.cncb.ac.cn/gsa-human/browse/HRA008073.
Other data generated in the present study may be requested from the
corresponding author.
Authors' contributions
XQ and YL participated in study design and data
collection, data analysis and writing of the manuscript. LZ
contributed to data collection, and was involved in drafting and
revising the manuscript. LX contributed to acquisition, analysis
and interpretation of image data. JL, YM and MK contributed to the
conception and design of the study. FX was involved in drafting the
manuscript, revising it critically for important intellectual
content, data analysis and gave final approval of the version to be
published. FX and XQ confirmed the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present case report was approved by the Medical
Ethics Committee of the Affiliated Hospital of Guilin Medical
University (Guilin, China; approval no. 2023QTLL-17).
Patient consent for publication
The patient in the present report provided written
informed consent.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LCNEC
|
large cell neuroendocrine
carcinoma
|
|
ICIs
|
immune checkpoint inhibitors
|
|
SCLC
|
small cell lung cancer
|
|
NSCLC
|
non-small cell lung cancer
|
|
PD-1
|
programmed cell death protein-1
|
|
PD-L1
|
programmed cell death-ligand 1
|
|
CT
|
computed tomography
|
|
RECIST 1.1
|
Response Evaluation Criteria in Solid
Tumors version 1.1
|
|
CTLA-4
|
cytotoxic T lymphocyte-associated
antigen-4
|
|
TKIs
|
antiangiogenic tyrosine kinase
inhibitors
|
|
NGS
|
next-generation sequencing
|
References
|
1
|
Gustafsson BI, Kidd M, Chan A,
Malfertheiner MV and Modlin IM: Bronchopulmonary neuroendocrine
tumors. Cancer. 113:5–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dresler CM, Ritter JH, Patterson GA, Ross
E, Bailey MS and Wick MR: Clinical-pathologic analysis of 40
patients with large cell neuroendocrine carcinoma of the lung. Ann
Thorac Surg. 63:180–185. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raman V, Jawitz OK, Yang CJ, Tong BC,
D'Amico TA, Berry MF and Harpole DH Jr: Adjuvant therapy for
patients with early large cell lung neuroendocrine cancer: A
National Analysis. Ann Thorac Surg. 108:377–383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le Treut J, Sault MC, Lena H, Souquet PJ,
Vergnenegre A, Le Caer H, Berard H, Boffa S, Monnet I, Damotte D
and Chouaid C: Multicentre phase II study of cisplatin-etoposide
chemotherapy for advanced large-cell neuroendocrine lung carcinoma:
the GFPC 0302 study. Ann Oncol. 24:1548–1552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niho S, Kenmotsu H, Sekine I, Ishii G,
Ishikawa Y, Noguchi M, Oshita F, Watanabe S, Nakajima R, Tada H and
Nagai K: Combination chemotherapy with irinotecan and cisplatin for
large-cell neuroendocrine carcinoma of the lung: A multicenter
phase II study. J Thorac Oncol. 8:980–984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia L, Wang L, Zhou Z and Han S: Treatment
outcome and prognostic analysis of advanced large cell
neuroendocrine carcinoma of the lung. Sci Rep. 12:165622022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng Y, Han L, Wu L, Chen J, Sun H, Wen
G, Ji Y, Dvorkin M, Shi J, Pan Z, et al: Effect of First-Line
Serplulimab vs Placebo Added to Chemotherapy on Survival in
Patients With Extensive-Stage Small Cell Lung Cancer: The
ASTRUM-005 Randomized Clinical Trial. JAMA. 328:1223–1232. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab Plus
Carboplatin and Pemetrexed as First-Line Treatment for Advanced
Nonsquamous NSCLC: Extended Follow-Up of CameL Phase 3 Trial. J
Thorac Oncol. 18:628–639. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang VE, Urisman A, Albacker L, Ali S,
Miller V, Aggarwal R and Jablons D: Checkpoint inhibitor is active
against large cell neuroendocrine carcinoma with high tumor
mutation burden. J Immunother Cancer. 5:752017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin Y, Yu M, Zhou L, Jiang L and Huang M:
Durable response to combination radiotherapy and immunotherapy in
EP-resistant lung large-cell neuroendocrine carcinoma with B2M and
STK11 mutations: A case report. Immunotherapy. 12:223–227. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sherman S, Rotem O, Shochat T, Zer A,
Moore A and Dudnik E: Efficacy of immune check-point inhibitors
(ICPi) in large cell neuroendocrine tumors of lung (LCNEC). Lung
Cancer. 143:40–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chauhan A, Arnold SM, Kolesar J, Thomas
HE, Evers M and Anthony L: Immune checkpoint inhibitors in large
cell neuroendocrine carcinoma: current status. Oncotarget.
9:14738–14740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takimoto Sato M, Ikezawa Y, Sato M, Suzuki
A and Kawai Y: Large cell neuroendocrine carcinoma of the lung that
responded to nivolumab: A case report. Mol Clin Oncol. 13:43–47.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oda R, Okuda K, Yamashita Y, Sakane T,
Tatematsu T, Yokota K, Endo K and Nakanishi R: Long-term survivor
of pulmonary combined large cell neuroendocrine carcinoma treated
with nivolumab. Thorac Cancer. 11:2036–2039. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giaj Levra M, Mazieres J, Audigier Valette
C, Molinier O, Planchard D, Frappat V, Ferrer L, Claire Toffart A
and Moro-Sibilot D: Efficacy of immune checkpoint inhibitors in
large cell neuroendocrine lung cancer: Results from a French
Retrospective Cohort: Topic: Drug treatment alone and in
combination with radiotherapy. J Thor Oncol. 12:1556–0864.
2017.
|
|
16
|
Zhang X, Sun Y, Miao Y and Xu S: Immune
checkpoint inhibitor therapy achieved complete response for
drug-sensitive EGFR/ALK mutation-negative metastatic pulmonary
large-cell neuroendocrine carcinoma with high tumor Mutation
Burden: A case report. Onco Targets Ther. 13:8245–8250. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dudnik E, Kareff S, Moskovitz M, Kim C,
Liu SV, Lobachov A, Gottfried T, Urban D, Zer A, Rotem O, et al:
Real-world survival outcomes with immune checkpoint inhibitors in
large-cell neuroendocrine tumors of lung. J Immunother Cancer.
9:e0019992021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frentzas S, Kwek KY, Konpa A and Jin X:
Efficacy and safety of AK104, an anti-PD-1/CTLA-4 bispecific
antibody, in a patient with large cell neuroendocrine carcinoma of
the lung. J Thor Oncol. 16:5062020. View Article : Google Scholar
|
|
21
|
Wu L, Chen B, Yao W, Li X, Xiao Z, Liu H,
Kong Y, Liu L, Xu Y, Wang Q, et al: A phase Ib/II trial of AK104
(PD-1/CTLA-4 bispecific antibody) in combination with Anlotinib in
advanced NSCLC. Ann Oncol. 32:10062021. View Article : Google Scholar
|
|
22
|
Travis WD, Linnoila RI, Tsokos MG,
Hitchcock CL, Cutler GB Jr, Nieman L, Chrousos G, Pass H and
Doppman J: Neuroendocrine tumors of the lung with proposed criteria
for large-cell neuroendocrine carcinoma. An ultrastructural,
immunohistochemical, and flow cytometric study of 35 cases. Am J
Surg Pathol. 15:529–553. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gazdar AF, Savage TK, Johnson JE, Berns A,
Sage J, Linnoila RI, MacPherson D, McFadden DG, Farago A, Jacks T,
et al: The comparative pathology of genetically engineered mouse
models for neuroendocrine carcinomas of the lung. J Thorac Oncol.
10:553–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicholson AG, Tsao MS, Beasley MB, Borczuk
AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S,
et al: The 2021 WHO Classification of Lung Tumors: Impact of
Advances Since 2015. J Thorac Oncol. 17:362–387. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pang X, Huang Z, Zhong T, Zhang P, Wang
ZM, Xia M and Li B: Cadonilimab, a tetravalent PD-1/CTLA-4
bispecific antibody with trans-binding and enhanced target binding
avidity. MAbs. 15:21807942023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keam SJ: Cadonilimab: First Approval.
Drugs. 82:1333–1339. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Pas TM, Giovannini M, Manzotti M,
Trifirò G, Toffalorio F, Catania C, Spaggiari L, Labianca R and
Barberis M: Large-cell neuroendocrine carcinoma of the lung
harboring EGFR mutation and responding to gefitinib. J Clin Oncol.
29:e819–e822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mauclet C, Duplaquet F, Pirard L, Rondelet
B, Dupont M, Pop-Stanciu C, Vander Borght T, Remmelink M, D'Haene
N, Lambin S, et al: Complete tumor response of a locally advanced
lung large-cell neuroendocrine carcinoma after palliative thoracic
radiotherapy and immunotherapy with nivolumab. Lung Cancer.
128:53–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng
Y, Gao Y and Li K: Anlotinib alters tumor immune microenvironment
by downregulating PD-L1 expression on vascular endothelial cells.
Cell Death Dis. 11:3092020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weichselbaum RR, Liang H, Deng L and Fu
YX: Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev
Clin Oncol. 14:365–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du X, Tang F, Liu M, Su J, Zhang Y, Wu W,
Devenport M, Lazarski CA, Zhang P, Wang X, et al: A reappraisal of
CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res.
28:416–432. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mitsui J, Nishikawa H, Muraoka D, Wang L,
Noguchi T, Sato E, Kondo S, Allison JP, Sakaguchi S, Old LJ, et al:
Two distinct mechanisms of augmented antitumor activity by
modulation of immunostimulatory/inhibitory signals. Clin Cancer
Res. 16:2781–2791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pentcheva-Hoang T, Simpson TR,
Montalvo-Ortiz W and Allison JP: Cytotoxic T lymphocyte antigen-4
blockade enhances antitumor immunity by stimulating
melanoma-specific T-cell motility. Cancer Immunol Res. 2:970–980.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He L, Han Q, Zhao M, Ma H, Cheng P, Yang H
and Zhao Y: Case report of radiotherapy combined with anlotinib and
immunotherapy for a patient with esophageal cancer and esophageal
fistula. Appl Radiat Isot. 205:1111622024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Adeoye O and Kozyreva O: Case of
tracheoesophageal fistula formation as a rare complication of
antiangiogenic tyrosine kinase inhibitor therapy for metastatic
hepatocellular carcinoma. Cureus. 15:e417832023.PubMed/NCBI
|
|
36
|
Rekhtman N, Pietanza MC, Hellmann MD,
Naidoo J, Arora A, Won H, Halpenny DF, Wang H, Tian SK, Litvak AM,
et al: Next-Generation sequencing of pulmonary large cell
neuroendocrine carcinoma reveals small cell carcinoma-like and
non-small cell carcinoma-like subsets. Clin Cancer Res.
22:3618–3629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhuo M, Guan Y, Yang X, Hong L, Wang Y, Li
Z, Chen R, Abbas HA, Chang L, Gong Y, et al: The prognostic and
therapeutic role of genomic subtyping by sequencing tumor or
Cell-Free DNA in pulmonary large-cell neuroendocrine carcinoma.
Clin Cancer Res. 26:892–901. 2020. View Article : Google Scholar : PubMed/NCBI
|