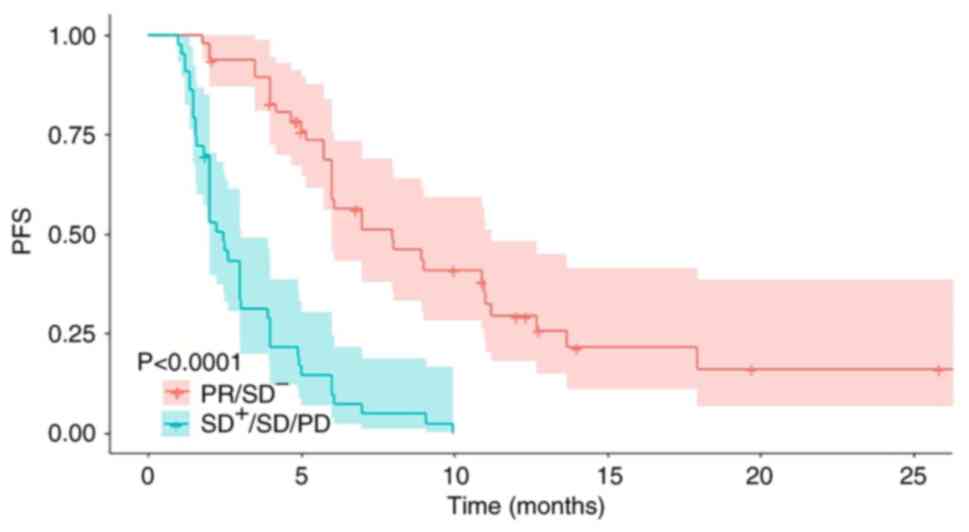
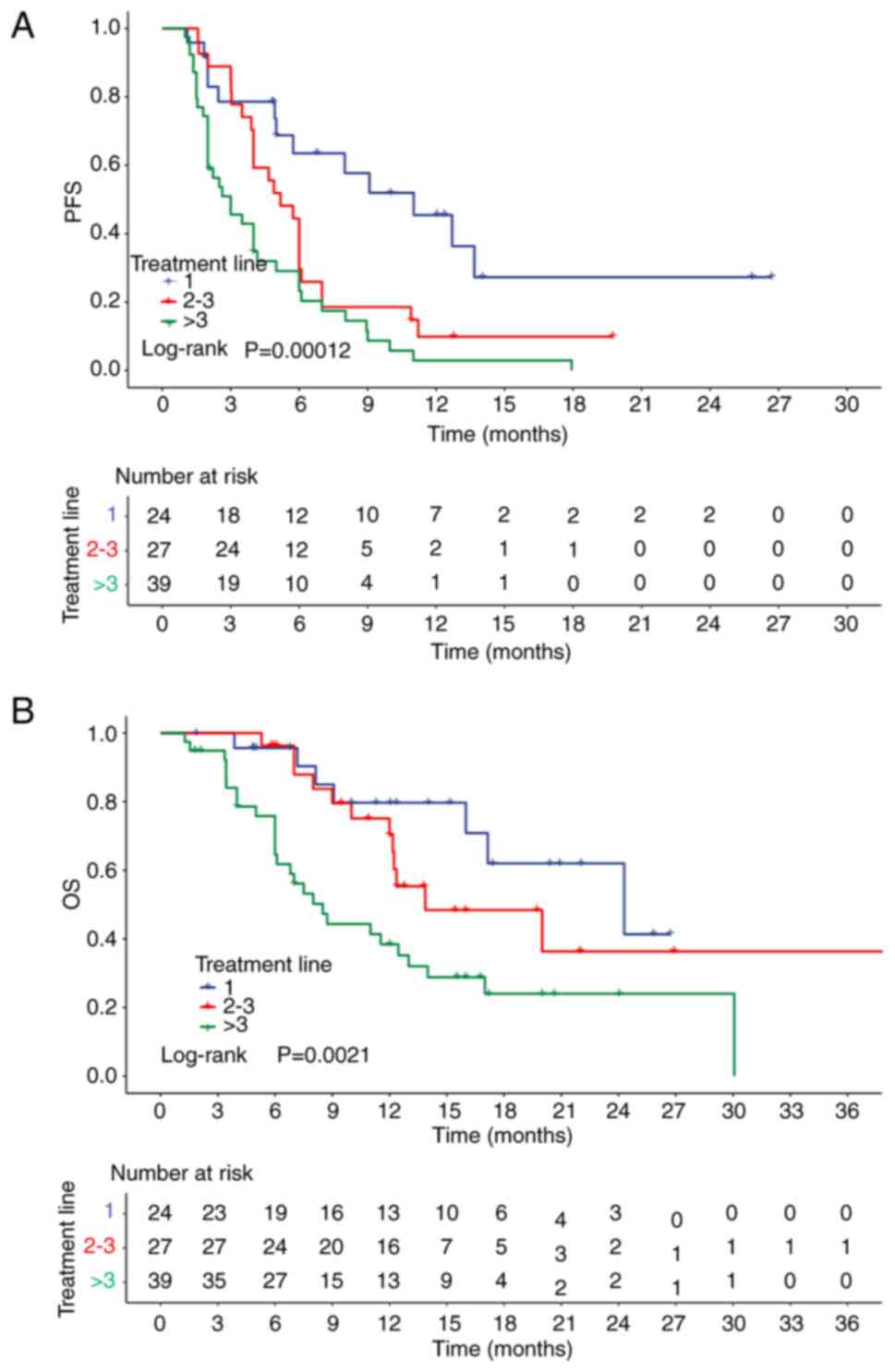
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Inst. 4:47–53. 2024.
|
|
3
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kojima T, Shah MA, Muro K, Francois E,
Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, et al:
Randomized phase III KEYNOTE-181 study of pembrolizumab versus
chemotherapy in advanced esophageal cancer. J Clin Oncol.
38:4138–4148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JC, Durbeck J, Boudadi K, Ho WJ and
Kang H: The efficacy of anti-PD-1 immune checkpoint inhibitor in
nasopharyngeal carcinoma. Oral Oncol. 108:1049352020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elizabeth MS, Cristina SBJ and Christian
CG: Immunotherapy in combination with chemotherapy for
triple-negative breast cancer. Mini Rev Med Chem. 24:431–439. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmid P, Lipatov O, Im SA, Goncalves A,
Muñoz-Couselo E, Lee KS, Tamura K, Testa L, Witzel I, Ohtani S, et
al: Impact of pembrolizumab versus chemotherapy on health-related
quality of life in patients with metastatic triple-negative breast
cancer: Results from the phase 3 randomised KEYNOTE-119 study. Eur
J Cancer. 195:133932023. View Article : Google Scholar
|
|
9
|
Schmid P, Rugo HS, Adams S, Schneeweiss A,
Barrios CH, Iwata H, Dieras V, Henschel V, Molinero L, Chui SY, et
al: Atezolizumab plus nab-paclitaxel as first-line treatment for
unresectable, locally advanced or metastatic triple-negative breast
cancer (IMpassion130): Updated efficacy results from a randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
21:44–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cortes J, Cescon DW, Rugo HS, Nowecki Z,
Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et
al: Pembrolizumab plus chemotherapy versus placebo plus
chemotherapy for previously untreated locally recurrent inoperable
or metastatic triple-negative breast cancer (KEYNOTE-355): A
randomised, placebo-controlled, double-blind, phase 3 clinical
trial. Lancet. 396:1817–1828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miles D, Gligorov J, Andre F, Cameron D,
Schneeweiss A, Barrios C, Xu B, Wardley A, Kaen D, Andrade L, et
al: Primary results from IMpassion131, a double-blind,
placebo-controlled, randomised phase III trial of first-line
paclitaxel with or without atezolizumab for unresectable locally
advanced/metastatic triple-negative breast cancer. Ann Oncol.
32:994–1004. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Wang Y, Tian Z, Lin Y, Li H, Zhu Z,
Liu Q, Su S, Zeng Y, Jia W, et al: Multicenter phase II trial of
Camrelizumab combined with Apatinib and Eribulin in heavily
pretreated patients with advanced triple-negative breast cancer.
Nat Commun. 13:30112022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan PH, Ellis I, Allison K, Brogi E, Fox
SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The
2019 world health organization classification of tumours of the
breast. Histopathology. 77:181–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Cancer Institute, . Common
terminology criteria for adverse events (CTCAE) Version 5.0. U.S.
Department of Health and Human Services, 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
|
|
17
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robert C, Carlino MS, McNeil C, Ribas A,
Grob JJ, Schachter J, Nyakas M, Kee D, Petrella TM, Blaustein A, et
al: Seven-year follow-up of the phase III KEYNOTE-006 study:
Pembrolizumab versus ipilimumab in advanced melanoma. J Clin Oncol.
41:3998–4003. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Y, Qin X, Peng X, Zeng A, Li J, Chen
C, Qiu S, Pan S, Zheng Y, Cai J, et al: Efficacy and safety of
KL-A167 in previously treated recurrent or metastatic
nasopharyngeal carcinoma: A multicenter, single-arm, phase 2 study.
Lancet Reg Health West Pac. 31:1006172022.PubMed/NCBI
|
|
20
|
Yang L, Hu Q and Huang T: Breast cancer
treatment strategies targeting the tumor microenvironment: How to
convert ‘Cold’ tumors to ‘Hot’ tumors. Int J Mol Sci. 25:72082024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esteva FJ, Hubbard-Lucey VM, Tang J and
Pusztai L: Immunotherapy and targeted therapy combinations in
metastatic breast cancer. Lancet Oncol. 20:e175–e186. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guisier F, Cousse S, Jeanvoine M,
Thiberville L and Salaun M: A rationale for surgical debulking to
improve anti-PD1 therapy outcome in non-small cell lung cancer. Sci
Rep. 9:169022019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chardin D, Paquet M, Schiappa R, Darcourt
J, Bailleux C, Poudenx M, Sciazza A, Ilie M, Benzaquen J, Martin N,
et al: Baseline metabolic tumor volume as a strong predictive and
prognostic biomarker in patients with non-small cell lung cancer
treated with PD1 inhibitors: A prospective study. J Immunother
Cancer. 8:e0006452020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Colak S and Ten Dijke P: Targeting TGF-β
signaling in cancer. Trends Cancer. 3:56–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishino M, Giobbie-Hurder A, Ramaiya NH
and Hodi FS: Response assessment in metastatic melanoma treated
with ipilimumab and bevacizumab: CT tumor size and density as
markers for response and outcome. J Immunother Cancer. 2:402014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Wang Y, Jia W, Deng H, Li G, Deng W,
Chen J, Kim BYS, Jiang W, Liu Q and Liu J: Low-dose anti-angiogenic
therapy sensitizes breast cancer to PD-1 blockade. Clin Cancer Res.
26:1712–1724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmittnaegel M, Rigamonti N, Kadioglu E,
Cassara A, Rmili CW, Kiialainen A, Kienast Y, Mueller HJ, Ooi CH,
Laoui D and De Palma M: Dual angiopoietin-2 and VEGFA inhibition
elicits antitumor immunity that is enhanced by PD-1 checkpoint
blockade. Sci Transl Med. 9:eaak96702017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, Liu Q, Li Y, Li Q, Su F, Yao H, Su
S, Wang Q, Jin L, Wang Y, et al: Efficacy and safety of
camrelizumab combined with apatinib in advanced triple-negative
breast cancer: An open-label phase II trial. J Immunother Cancer.
8:e0006962020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lwin Z, Gomez-Roca C, Saada-Bouzid E,
Yáñez E, Longo F, Im SA, Castanon E, Senellart H, Graham D, Voss M,
et al: LBA41 LEAP-005: Phase II study of lenvatinib (len) plus
pembrolizumab (pembro) in patients (pts) with previously treated
advanced solid tumours. Ann Oncol. 31:S11702020. View Article : Google Scholar
|
|
30
|
Wang J, Sun T, Ouyang Q, Han Y and Xu B: A
phase Ib study of TQB2450 plus anlotinib in patients with advanced
triple-negative breast cancer. iScience. 26:1068762023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borghaei H, Gettinger S, Vokes EE, Chow
LQM, Burgio MA, de Castro Carpeno J, Pluzanski A, Arrieta O,
Frontera OA, Chiari R, et al: Five-year outcomes from the
randomized, phase III trials CheckMate 017 and 057: Nivolumab
versus docetaxel in previously treated non-small-cell lung cancer.
J Clin Oncol. 39:723–733. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Bragagnoli AC, et al: First-line nivolumab plus chemotherapy versus
chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng Y, Tang L, Wang H, Liu Y, Yang S, Lin
L, Hu X and Shi Y: Immune checkpoint inhibitors combined with
angiogenic inhibitors in the treatment of locally advanced or
metastatic lung adenocarcinoma patients. Cancer Immunol Immunother.
72:449–459. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brahmer JR, Lee JS, Ciuleanu TE, Caro RB,
Nishio M, Urban L, Audigier-Valette C, Lupinacci L, Sangha R,
Pluzanski A, et al: Five-year survival outcomes with nivolumab plus
ipilimumab versus chemotherapy as first-line treatment for
metastatic non-small-cell lung cancer in CheckMate 227. J Clin
Oncol. 41:1200–1212. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raskov H, Orhan A, Agerbæk MØ and Gögenur
I: The impact of platelets on the metastatic potential of tumour
cells. Heliyon. 10:e343612024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou L, Zhang Z, Tian Y, Li Z, Liu Z and
Zhu S: The critical role of platelet in cancer progression and
metastasis. Eur J Med Res. 28:3852023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M and Degen JL:
Platelets and fibrin(ogen) increase metastatic potential by
impeding natural killer cell-mediated elimination of tumor cells.
Blood. 105:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Egan K, Crowley D, Smyth P, O'Toole S,
Spillane C, Martin C, Gallagher M, Canney A, Norris L, Conlon N, et
al: Platelet adhesion and degranulation induce pro-survival and
pro-angiogenic signalling in ovarian cancer cells. PLoS One.
6:e261252011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bambace NM and Holmes CE: The platelet
contribution to cancer progression. J Thromb Haemost. 9:237–249.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mijic S and Dabrosin C: Platelet
activation in situ in breasts at high risk of cancer: Relationship
with mammographic density and estradiol. J Clin Endocrinol Metab.
106:485–500. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Deng H and Wang J: LTBP1 promotes
the progression of triple negative breast cancer via activating the
RhoA/ROCK signaling pathway. Cancer Insight. 3:37–48. 2023.
View Article : Google Scholar
|
|
42
|
Tao Y, Zhou Y, Chen H, Qin Y, He X, Liu P,
Zhou S, Yang J, Zhou L, Zhang C, et al: Prognostic role of red
blood cell distribution width and platelet/lymphocyte ratio in
early-stage classical hodgkin lymphoma. Future Oncol. 18:1817–1827.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Onagi H, Horimoto Y, Sakaguchi A, Ikarashi
D, Yanagisawa N, Nakayama T, Nakatsura T, Ishizuka Y, Sasaki R,
Watanabe J, et al: High platelet-to-lymphocyte ratios in
triple-negative breast cancer associates with immunosuppressive
status of TILs. Breast Cancer Res. 24:672022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gou M and Zhang Y: Pretreatment
platelet-to-lymphocyte ratio (PLR) as a prognosticating indicator
for gastric cancer patients receiving immunotherapy. Discov Oncol.
13:1182022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Platini H, Ferdinand E, Kohar K, Prayogo
SA, Amirah S, Komariah M and Maulana S: Neutrophil-to-lymphocyte
ratio and platelet-to-lymphocyte ratio as prognostic markers for
advanced non-small-cell lung cancer treated with immunotherapy: A
systematic review and meta-analysis. Medicina (Kaunas).
58:10692022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fabi A, Carbognin L, Botticelli A, Paris
I, Fuso P, Savastano MC, La Verde N, Strina C, Pedersini R, Guarino
S, et al: Real-world ANASTASE study of atezolizumab+nab-paclitaxel
as first-line treatment of PD-L1-positive metastatic
triple-negative breast cancer. NPJ Breast Cancer. 9:732023.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Z, Zhang Y, Liu C, Shao J, Chen Y,
Zhu Y, Zhang L, Qin B, Kong Z, Wang X, et al: A real-world study of
immune checkpoint inhibitors in advanced triple-negative breast
cancer. Cancer Innov. 2:172–180. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schlam I and Gatti-Mays ME: Immune
checkpoint inhibitors in the treatment of breast cancer brain
metastases. Oncologist. 27:538–547. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
De La Cruz LM and Czerniecki BJ:
Immunotherapy for breast cancer is finally at the doorstep:
Immunotherapy in breast cancer. Ann Surg Oncol. 25:2852–2857. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Plitas G, Konopacki C, Wu K, Bos PD,
Morrow M, Putintseva EV, Chudakov DM and Rudensky AY: Regulatory T
cells exhibit distinct features in human breast cancer. Immunity.
45:1122–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sisirak V, Faget J, Gobert M, Goutagny N,
Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI,
Goddard-Leon S, et al: Impaired IFN-α production by plasmacytoid
dendritic cells favors regulatory T-cell expansion that may
contribute to breast cancer progression. Cancer Res. 72:5188–5197.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miller LD, Chou JA, Black MA, Print C,
Chifman J, Alistar A, Putti T, Zhou X, Bedognetti D, Hendrickx W,
et al: Immunogenic subtypes of breast cancer delineated by gene
classifiers of immune responsiveness. Cancer Immunol Res.
4:600–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu Z, Li M, Jiang Z and Wang X: A
comprehensive immunologic portrait of triple-negative breast
cancer. Transl Oncol. 11:311–329. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang T, Gradus JL and Rosellini AJ:
Supervised machine learning: A brief primer. Behav Ther.
51:675–687. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Greener JG, Kandathil SM, Moffat L and
Jones DT: A guide to machine learning for biologists. Nat Rev Mol
Cell Biol. 23:40–55. 2022. View Article : Google Scholar : PubMed/NCBI
|