Introduction
Breast cancer poses a significant risk to women's
health worldwide (1–3). The recent estimates from the
International Agency for Research on Cancer demonstrated that
breast cancer represents 11.6% of all new cancer cases and 6.9% of
all cancer-associated mortalities, with 2.3 million new cases
diagnosed and 1.6 million mortalities annually (1). Although significant progress has been
made in the treatment of breast cancer, a considerable number of
patients experience disease recurrence and metastasis.
Consequently, strategies for improving treatment responses and
survival outcomes in patients with metastatic breast cancer (MBC)
are urgently needed.
The application of immune checkpoint inhibitors
(ICIs) is a novel and powerful treatment approach and its efficacy
has been verified in several types of solid tumors, such as lung,
esophageal and nasopharyngeal cancer and so on (4–6). The
available evidence suggests that combination immunotherapy using
programmed cell death-1 (PD-1) or programmed cell death-ligand 1
(PD-L1) inhibitors may be a promising treatment option for
metastatic triple-negative breast cancer (mTNBC) (7). KEYNOTE 119 trial reported suboptimal
efficacy of ICI monotherapy in patients with MBC, thus promoting
the investigation of novel therapeutic combinations, including ICIs
combined with chemotherapy and/or angiogenic inhibitors (8). In a number of clinical trials, the
overall response rate and median overall survival (OS) were
improved in patients with mTNBC treated with PD-1/PD-L1 inhibitors
in combination with chemotherapy compared with those treated with
chemotherapy alone (7,9,10). The
IMpassion130 and KEYNOTE-355 clinical trials show notable efficacy
of ICI combined with chemotherapy as a first-line therapy for mTNBC
(9,10). However, in the the IMpassion131
clinical trial atezolizumab plus paclitaxel failed to demonstrate a
significant improvement in progression-free survival (PFS) or OS in
mTNBC (11). In the second and
later-line settings, ICI combined with angiogenic inhibitors and
chemotherapy showed particular efficacy for patients that are
heavily pretreated (12). However,
clinical evidence for patients with MBC is currently limited. To
the best of our knowledge, there are no real-world reports on the
value of ICI-based therapy in patients with MBC. Although
real-world data lack a well-controlled efficacy design compared
with clinical trials, it can provide novel insights into authentic
clinical settings and treatment outcomes.
Therefore, in the present study, the characteristics
and prognoses of patients with MBC were recorded and the efficacy
and safety of ICI-based therapy in distinct treatment lines were
evaluated.
Materials and methods
Patient eligibility and data
collection
In the present study, the data from patients
diagnosed with MBC between August 1, 2018 and September 5, 2022, at
the Henan Provincial People's Hospital (Zhengzhou, China) and the
Affiliated Hospital of Xuzhou Medical University (Xuzhou, China),
were retrospectively analyzed. The medical records were collected
and assessed from November 2022 to May 2023 in the Henan Provincial
People's Hospital and from December 2022 to June 2023 in The
Affiliated Hospital of Xuzhou Medical University.
The full indications for ICI treatment in the
aforementioned hospitals were as follows: i) Patients with stage
II–III TNBC, ICI treatment was used in neoadjuvant setting; ii)
patients with TNBC who achieved pathological complete response
(pCR) continued to receive ICI treatment in adjuvant setting; iii)
patients with TNBC who did not achieve pCR, ICI treatment in
adjuvant setting was considered; iv) patients with PD-L1 positive,
locally advanced or MBC; v) patients with microsatellite
instability-high (MSI-H), locally advanced or MBC; vi) patients
with contraindications to chemotherapy and were strongly willing to
try ICI treatment; and vii) heavily pretreated patients (lines of
treatment, ≥3), with few alternative treatment options and were
strongly willing to try ICI treatment.
The inclusion criteria for eligible patients were as
follows: i) Patients with histologically confirmed breast cancer
according to the World Health Organization classification (13); and ii) patients who were treated
with ICI-based therapy in metastatic settings, including ICI plus
chemotherapy, ICI plus angiogenic inhibitors and ICI plus
chemotherapy and angiogenic inhibitors. The ICIs used included
pembrolizumab, atezolizumab, camrelizumab, sintilimab, toripalimab
and tislelizumab. The angiogenic inhibitors included anlotinib,
apatinib and bevacizumab; and iii) patients with complete clinical
and pathological data. The exclusion criteria were as follows: i)
Patients who did not receive ICI-based therapy in the metastatic
settings; ii) those whose clinical and pathological data were
incomplete; and iii) patients without follow-up data. The present
study was performed in compliance with the Declaration of Helsinki
and was approved by the Institutional Review Board of the Henan
Provincial People's Hospital and the Affiliated Hospital of Xuzhou
Medical University. Due to the retrospective nature of the study,
informed consent from the Institutional Ethics Committee was not
required. The clinicopathological data, including age, menstruation
status, Eastern Cooperative Oncology Group (ECOG) performance
status (PS) score, body mass index (BMI), histological grade,
molecular subtype, Ki-67 index, PD-L1 expression, and detailed
metastatic information (number of metastatic sites, site of
metastatic disease, lines of treatment) were collected. The
peripheral blood absolute neutrophil, lymphocyte and platelet (PLT)
counts were recorded at the baseline of ICI-based therapy. The
neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the
neutrophil count by that of lymphocytes. Consistently, the
platelet-to-lymphocyte ratio (PLR) was calculated by dividing the
platelet count by that of lymphocytes. Therapy strategies,
toxicity, response to therapy, date of relapse, death and last
follow-up were also recorded. All data were acquired from the
electronic medical records, follow-up visits and by telephone.
Tumor characteristics
The molecular subtype classification was based on
immunohistochemistry. Estrogen receptor (ER) and progesterone
receptor (PR) levels were assessed using immunohistochemistry and
were considered to be positive if ≥1% of cancer cells were stained.
HER2-positive tumors were defined as those with an
immunohistochemistry score of ≥3 or positively labeled using
fluorescence in situ hybridization (FISH) (14). TNBC was defined as ER−,
PR− and HER2-negative breast cancer. HER2-positive
breast cancer was defined as HER2-positive using
immunohistochemistry or FISH regardless of the ER and PR status,
and included luminal B and non-luminal HER2-positive breast cancer.
Luminal breast cancer was defined as ER+ or
PR+ and HER2-negative breast cancer.
Evaluation of tumor response and
adverse events
Tumor response was evaluated based on Response
Evaluation Criteria in Solid Tumors (RECIST, version 1.1) (15). The objective response rate (ORR) was
defined as the percentage of patients achieving complete response
(CR) or partial response (PR). The disease control rate (DCR) was
defined as the percentage of patients achieving CR, PR or stable
disease (SD). Patients assessed as SD with shrinkage target lesions
were documented as SD−, while patients categorized as SD
with enlarged target lesions were recorded as SD+.
Progression-free survival (PFS) was defined as the interval between
the initial ICI-based therapy and the time of progression or death.
Additionally, OS was defined as the interval between the initial
ICI-based therapy and the time of death from any cause. Finally,
toxicity was assessed according to the Common Terminology Criteria
for Adverse Events (CTCAE, version 5.0) (16).
Statistical analysis
The primary endpoint established was PFS and the
secondary endpoints included ORR, DCR, OS and toxicity. Efficacy
and safety were evaluated in all enrolled patients who were treated
with ≥2 cycles of ICI-based therapy. X-tile software (version
3.6.1, medicine.yale.edu/lab/rimm/research/software/, Yale
University) was used to obtain the optimal cut-off values for
lymphocyte count, PLT, NLR and PLR. PFS and OS curves were
calculated using Kaplan-Meier analysis and were compared using
log-rank tests. For univariate and multivariate analyses, a Cox
proportional hazards regression model was constructed. The
multivariate analysis included the significant characteristics from
the univariate analysis. The effects of independent prognostic
factors on PFS and OS were validated through C-index using the
Python lifelines library (version 3.10, python.org/, Python
Software Foundation). P<0.05 (two-sided) was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS Statistics (version 25.0, IBM
Corp.) and R software (http://www.r-project.org; version 3.6.2,
RStudio, Inc.).
Results
Demographics and clinical
characteristics
A total of 90 patients treated with ICI-based
therapy in the metastatic stage were enrolled and evaluated.
Patient demographics and clinical characteristics are shown in
Table I. All patients were female.
The median age of the patients was 50 years (range, 27–76 years).
Among all patients, 80 (88.9%) had an ECOG PS of 0–1, while 39
patients (43.3%) were premenopausal. The predominant tumor subtypes
were TNBC (53.3%) and luminal breast cancer (31.1%), while only
7.8% were HER2-positive. In total, 22 (24.4%) patients harbored
data on PD-L1 expression, with 13 patients being PD-L1 positive. In
addition, the majority of patients (61.1%) were heavily pretreated
(lines of treatment, ≥3). Approximately half of patients (46.7%)
exhibited ≥3 metastatic sites. The rate of each therapeutic
combination was different. More specifically, 44.4% of patients
received chemoimmunotherapy, 13.3% received immunotherapy plus
angiogenic inhibitors, 28.9% received chemoimmunotherapy plus
angiogenic inhibitors, while the remaining 13.3% of patients
received a combination of immunotherapy with endocrine or anti-HER2
therapy. In total, 33 patients were confirmed as PR and 38 as SD,
while 19 patients were confirmed with progressive disease (PD),
yielding an ORR and a DCR of 36.7 and 78.9%, respectively (Table II). No patient was confirmed as CR.
The median follow-up time of the total patient cohort was 16.0
months. In the metastatic settings the median lines of treatment
was 3, the overall median PFS was 4.9 months (95% CI, 3.8–6.1),
whereas the overall median OS was 13.9 months (95% CI, 9.5–18.2).
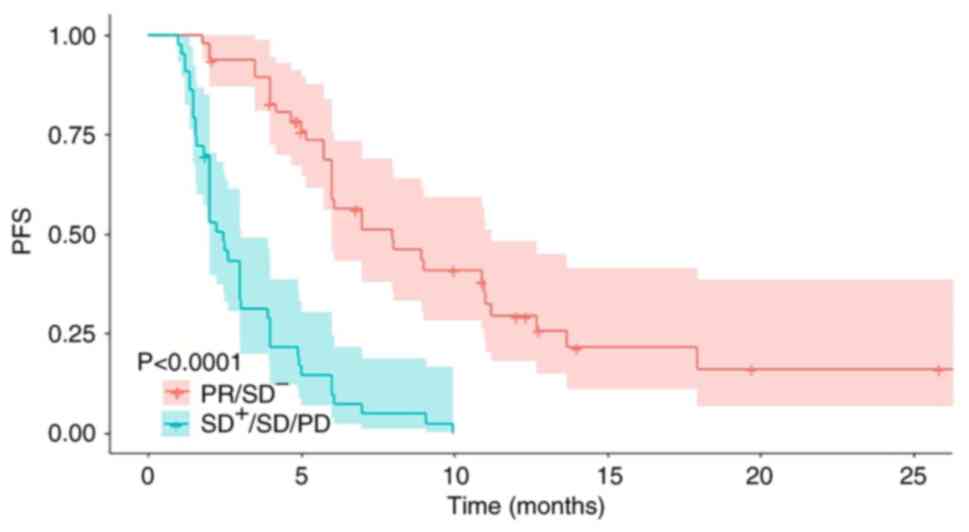
The effect of tumor response on long-term efficacy is shown in
Fig. 1. Patients who achieved PR or
SD− displayed a median PFS of 6.1 months, which was
significantly longer compared with patients classed as
SD+ or SD or PD [median PFS, 2.1 months; hazard ratio
(HR)=5.42; 95% CI, 3.22–9.12; P<0.001].
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
| Baseline
characteristics | Patients |
|---|
| Median age, years
(range) | 50 (27–76) |
| Age group, n
(%) |
|
| ≤47
years | 37 (41.1) |
| >47
years | 53 (58.9) |
| ECOG PS, n (%) |
|
|
0-1 | 80 (88.9) |
| 2 | 10 (11.1) |
| Body mass index, n
(%) |
|
|
≤21.5 | 26 (28.9) |
|
>21.5 | 64 (71.1) |
| Menopausal state, n
(%) |
|
|
Postmenopausal | 51 (56.7) |
|
Premenopausal | 39 (43.3) |
| Pathology, n
(%) |
|
|
Invasive ductal carcinoma | 85 (94.4) |
|
Metaplastic carcinoma | 4 (4.4) |
|
Neuroendocrine carcinoma | 1 (1.1) |
| Histology type, n
(%) |
|
| I | 1 (1.1) |
| II | 22 (24.4) |
|
III | 36 (40.0) |
|
Unknown | 31 (34.4) |
| Molecular subtypes,
n (%) |
|
|
Triple-negative breast
cancer | 48 (53.3) |
|
Luminala | 28 (31.1) |
|
HER2-positiveb | 7 (7.8) |
|
Unknown | 7 (7.8) |
| Ki-67 index, n
(%) |
|
|
≤50% | 32 (35.6) |
|
>50% | 51 (56.7) |
|
Unknown | 7 (7.8) |
| No. of metastatic
sites, n (%) |
|
|
1-2 | 48 (53.3) |
| ≥3 | 42 (46.7) |
| Site of metastatic
disease, n (%) |
|
|
Brain | 21 (23.3) |
|
Lung | 51 (56.7) |
|
Liver | 33 (36.7) |
|
Bone | 40 (44.4) |
| Lymphocyte count, n
(%) |
|
|
≤1.16 | 47 (52.2) |
|
>1.16 | 42 (46.7) |
| Platelet count, n
(%) |
|
|
<325 | 72 (80.0) |
|
≥325 | 17 (18.9) |
| Neutrophil to
lymphocyte ratio, n (%) |
|
|
≤3.16 | 55 (61.1) |
|
>3.16 | 34 (37.8) |
|
Platelet-to-lymphocyte ratio, n (%) |
|
|
≤171 | 34 (37.8) |
|
>171 | 55 (61.1) |
| Lines of treatment,
n (%) |
|
| 1 | 24 (26.7) |
|
2-3 | 27 (30.0) |
|
>3 | 39 (43.3) |
| Choice of systemic
treatment, n (%) |
|
| ICI
plus chemotherapy | 40 (44.4) |
| ICI
plus angiogenic inhibitors | 12 (13.3) |
| ICI
plus chemotherapy and angiogenic inhibitors | 26 (28.9) |
| ICI
plus others | 12 (13.3) |
| PD-L1 expression, n
(%) |
|
|
<1% | 9 (10.0) |
|
≥1% | 13 (14.4) |
|
Unknown | 68 (75.6) |
 | Table II.Efficacy of distinct lines of
treatment. |
Table II.
Efficacy of distinct lines of
treatment.
| Treatment line | No. of
patients | Median follow-up
time (months) | PFS (median, 95%
CI) (months) | OS (median, 95% CI)
(months) | ORR, % | DCR, % |
|---|
| Total patient
cohort | 90 | 16.0 | 4.9 (3.8–6.1) | 13.9
(9.5–18.2) | 36.7 (33/90) | 78.9 (71/90) |
| First-line | 24 | 15.2 | 11.0
(6.0–16.0) | 24.3
(11.4–37.2) | 50.0 (12/24) | 91.7 (22/24) |
| Second and
third-line | 27 | 15.4 | 5.2 (3.4–7.0) | 13.9
(4.9–22.8) | 55.6 (15/27) | 81.5 (22/27) |
| Fourth or
later-line | 39 | 17.2 | 3.0 (1.5–4.5 | 8.5 (6.1–11.0) | 38.5 (15/39) | 66.7 (26/39) |
Efficacy of distinct lines of
treatment
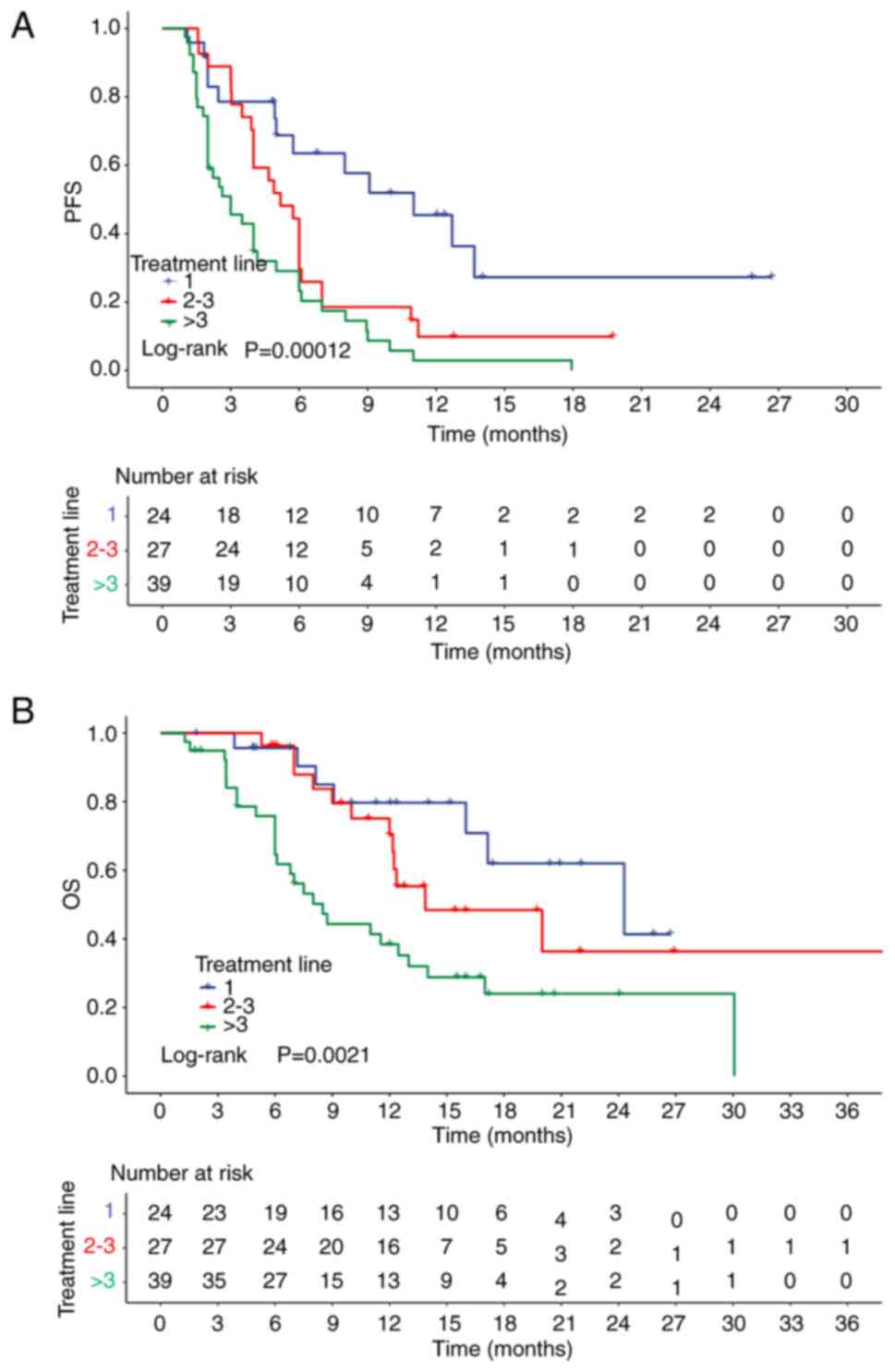
Efficacy of the first-line treatment
A total of 24 patients with breast cancer received
ICI-based therapy as first-line therapy. The majority of the
patients (23/24) were treated with chemoimmunotherapy, while one
patient received immunotherapy plus interleukin-7. The ORR and DCR
of each different line of therapy are listed in Table II. In the first-line therapy group,
the ICI-based therapy was effective in half of the patients (50.0%;
12/24), as evidenced by the corresponding ORR of 50.0%. In
addition, the DCR in the first-line treatment was 91.7% (22/24).
The median follow-up time was 15.2 months. The first-line median
PFS and OS were 11.0 months (95% CI, 6.0–16.0) and 24.3 months (95%
CI, 11.4–37.2), respectively. The PFS and OS of patients treated
with distinct lines of ICI-based therapy are shown in Fig. 2.
Efficacy of the second- and third-line
treatment
A total of 27 patients received ICI-based therapy in
the second- or third-line setting, including 11 patients in the
second-line setting and 16 in the third-line setting. Among them,
10 patients received chemoimmunotherapy, 10 received
chemoimmunotherapy plus angiogenic inhibitors and 6 received
immunotherapy plus angiogenic inhibitors, while 1 patient was
treated with immunotherapy plus endocrine therapy. The overall ORR
and DCR in the second- and third-line groups were 55.6 (15/27) and
81.5% (22/27), respectively (Table
II). The median follow-up time was 15.4 months, with a median
PFS of 5.2 months (95% CI, 3.4–7.0) and a median OS of 13.9 months
(95% CI, 4.9–22.8) (Table II).
Efficacy in later-line treatment
A total of 39 patients were treated with ICI-based
therapy in the fourth or later treatment lines. Among them, 16
patients received chemoimmunotherapy plus angiogenic inhibitors,
seven received chemoimmunotherapy, seven received immunotherapy
plus angiogenic inhibitors, eight received immunotherapy in
combination with anti-HER2 agents, while the remaining patients
received immunotherapy in combination with endocrine therapy. The
overall ORR in these heavily pretreated patients was 38.5% (15/39)
and the DCR was 66.7% (26/39) (Table
II). Additionally, a median follow-up time of 17.2 months was
recorded. The median PFS was 3.0 months (95% CI, 1.5–4.5), while
the median OS was 8.5 months (95% CI, 6.1–11.0) (Table II).
Efficacy of different therapy
strategies
A total of 40 patients received immunotherapy
combined with chemotherapy. The most commonly used chemotherapy
regimen was taxane with or without platinum (75.0%; 30/40;
Supplementary Table I). In
addition, 57.5% (23/40) of patients were treated with first-line
therapy, and 67.5% (27/40) had one or two metastatic sites. The ORR
and DCR in the aforementioned patients were 47.5% (19/40) and 82.5%
(33/40), respectively. With a median follow-up time of 15.2 months,
a median PFS and OS of 7.0 (95% CI, 5.6–8.4) and 24.3 (95% CI,
14.3–34.3) months, respectively, were recorded. Furthermore, a
total of 26 patients received chemoimmunotherapy plus angiogenic
inhibitors, with a median of four lines of treatment. Among them,
76.9% (20/26) had ≥3 metastatic sites. The ORR and DCR in these
heavily pretreated patients were 23.1% (6/26) and 80.8% (21/26),
respectively. With a median follow-up time of 16.0 months, a median
PFS of 4.0 months (95% CI, 2.9–4.9) and a median OS of 12.0 months
(95% CI, 7.9–16.1) were recorded. Additionally, a total of 12
patients were treated with immunotherapy plus angiogenic
inhibitors, with a median of four lines of treatment. The most
commonly used angiogenic inhibitor was anlotinib (58.3%; 7/12;
Table SI). The ORR and DCR were
33.3% (4/12) and 50.0% (6/12), respectively. A median follow-up
time of 17.2 months was also recorded, with a median PFS of 2.0
months (95% CI, 0.2–3.8) and a median OS of 12.2 months (95% CI,
11.1–13.3) (Table SII).
Predictive factors of PFS and OS in
ICI-based therapy
The univariate and multivariate analysis results of
the factors that were associated with PFS in patients treated with
ICI-based therapy are shown in Table
III. The univariate analysis demonstrated that BMI, number of
metastatic sites, liver metastases, brain metastases, lymphocyte
count, PLR, lines of treatment and the choice of systemic treatment
were significantly associated with PFS. Due to the overlap between
lymphocyte count and PLR, only PLR was included in the multivariate
analysis. Finally, liver metastases (HR=2.234; 95% CI, 1.196–4.171;
P=0.012), PLR (HR=2.406; 95% CI, 1.325–4.370; P=0.004) and lines of
treatment (3 vs. 1; HR=3.531; 95% CI, 1.311–9.512; P=0.013) were
identified as independent risk factors in multivariate analysis.
The C-index value of the effect of the aforementioned independent
prognostic factors on PFS was 0.783 (Table III). Furthermore, the univariate
and multivariate analysis results of the factors associated with OS
are shown in Table IV. The
univariate analysis showed that a higher BMI (>21.5) and
lymphocyte count (>1.16), lower PLT (<325) and PLR (≤190), no
liver metastases, <3 metastatic sites and <3 treatment lines
were notably associated with improved OS. Additionally, the
multivariate analysis demonstrated that PLR was a significant
covariate for OS (HR=2.376; 95% CI, 1.059–5.328; P=0.036). Finally,
the C-index of the effect of the aforementioned independent
prognostic factors on OS was 0.723 (Table IV).
 | Table III.Univariable and multivariate analysis
of predictive factors for PFS. |
Table III.
Univariable and multivariate analysis
of predictive factors for PFS.
|
| Univariate
analysis | Multivariate
analysis |
|
|---|
|
|
|
|
|
|---|
| Baseline
characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value | C-index |
|---|
| Age group,
years |
|
|
|
| 0.783 |
|
≤47 | Reference |
|
|
|
|
|
>47 | 0.945
(0.574–1.505) | 0.813 |
|
|
|
| ECOG PS |
|
|
|
|
|
|
0-1 | Reference |
|
|
|
|
| 2 | 1.228
(0.758–1.989) | 0.404 |
|
|
|
| Body mass
index |
|
|
|
|
|
|
≤21.5 | Reference |
| Reference |
|
|
|
>21.5 | 0.490
(0.292–0.823) | 0.007 | 0.844
(0.424–1.677) | 0.628 |
|
| Menopausal
state |
|
|
|
|
|
|
Postmenopausal | Reference |
|
|
|
|
|
Premenopausal | 0.861
(0.541–1.371) | 0.529 |
|
|
|
| Pathology |
|
|
|
|
|
|
Invasive ductal carcinoma | Reference |
|
|
|
|
|
Non-invasive ductal
carcinoma | 2.157
(0.862–5.398) | 0.100 |
|
|
|
| Histology type |
|
|
|
|
|
|
I–II | Reference |
|
|
|
|
|
III | 1.205
(0.658–2.205) | 0.546 |
|
|
|
| Molecular
subtypes |
|
|
|
|
|
|
Non-TNBC | Reference |
|
|
|
|
|
TNBC | 0.807
(0.765–2.009) | 0.384 |
|
|
|
| Ki-67 index |
|
|
|
|
|
|
≤50% | Reference |
|
|
|
|
|
>50% | 1.525
(0.926–2.511) | 0.097 |
|
|
|
| No. of metastatic
sites |
|
|
|
|
|
|
1-2 | Reference |
| Reference |
|
|
| ≥3 | 2.050
(1.261–3.333) | 0.004 | 0.945
(0.43–2.075) | 0.888 |
|
| Lung
metastasis |
|
|
|
|
|
| No | Reference |
|
|
|
|
|
Yes | 1.506
(0.935–2.424) | 0.092 |
|
|
|
| Liver
metastasis |
|
|
|
|
|
| No | Reference |
| Reference |
|
|
|
Yes | 2.373
(1.461–3.854) | <0.001 | 2.234
(1.196–4.171) | 0.012 |
|
| Brain
metastasis |
|
|
|
|
|
| No | Reference |
| Reference |
|
|
|
Yes | 1.791
(1.054–3.041) | 0.031 | 1.403
(0.662–2.976) | 0.377 |
|
| Lymphocyte
count |
|
|
|
|
|
|
≤1.16 | Reference |
|
|
|
|
|
>1.16 | 0.425
(0.264–0.685) | <0.001 |
|
|
|
| Platelet count |
|
|
|
|
|
|
<325 | Reference |
|
|
|
|
|
≥325 | 1.310
(0.743–2.311) | 0.351 |
|
|
|
|
Neutrophil-to-lymphocyte ratio |
|
|
|
|
|
|
≤3.16 | Reference |
|
|
|
|
|
>3.16 | 1.566
(0.984–2.494) | 0.059 |
|
|
|
|
Platelet-to-lymphocyte ratio |
|
|
|
|
|
|
≤190 | Reference |
| Reference |
|
|
|
>190 | 2.309
(1.426–3.739) | 0.001 | 2.406
(1.325–4.370) | 0.004 |
|
| No. of lines of
treatment |
|
|
|
|
|
| 1 | Reference |
| Reference |
|
|
|
2-3 | 2.163
(1.092–4.283) | 0.027 | 1.855
(0.799–4.307) | 0.151 |
|
|
>3 | 3.646
(1.907–6.972) | <0.001 | 3.531
(1.311–9.512) | 0.013 |
|
| Choice of systemic
treatment |
|
|
|
|
|
| ICI
plus chemotherapy | Reference |
| Reference |
|
|
| ICI
plus angiogenic inhibitors | 3.386
(1.645–6.969) | 0.001 | 2.376
(0.977–5.779) | 0.056 |
|
| ICI
plus chemotherapy and angiogenic inhibitors | 2.482
(1.387–4.441) | 0.002 | 0.821
(0.361–1.866) | 0.638 |
|
| PD-L1 expression,
% |
|
|
|
|
|
|
<1 | Reference |
|
|
|
|
| ≥1 | 0.867
(0.331–2.268) | 0.771 |
|
|
|
 | Table IV.Uni- and multivariate analysis of
predictive factors for OS. |
Table IV.
Uni- and multivariate analysis of
predictive factors for OS.
|
| Univariate
analysis | Multivariate
analysis |
|
|---|
|
|
|
|
|
|---|
| Baseline
characteristics | HR (95% CI) | P-value | HR (95%CI) | P-value | C-index |
|---|
| Age group,
years |
|
|
|
| 0.723 |
|
≤47 | Reference |
|
|
|
|
|
>47 | 0.605
(0.338–1.082) | 0.090 |
|
|
|
| ECOG PS |
|
|
|
|
|
|
0-1 | Reference |
|
|
|
|
| 2 | 1.271
(0.693–2.333) | 0.439 |
|
|
|
| Body mass
index |
|
|
|
|
|
|
≤21.5 | Reference |
| Reference |
|
|
|
>21.5 | 0.407
(0.225–0.738) | 0.003 | 0.726
(0.334–1.579) | 0.419 |
|
| Menopausal
state |
|
|
|
|
|
|
Postmenopausal | Reference |
|
|
|
|
|
Premenopausal | 0.868
(0.478–1.578) | 0.643 |
|
|
|
| Pathology |
|
|
|
|
|
|
Invasive ductal carcinoma | Reference |
|
|
|
|
|
Non-invasive ductal
carcinoma | 1.508
(0.466–4.878) | 0.492 |
|
|
|
| Histology type |
|
|
|
|
|
|
I–II | Reference |
|
|
|
|
|
III | 0.667
(0.322–1.380) | 0.275 |
|
|
|
| Molecular
subtypes |
|
|
|
|
|
|
Non-TNBC | Reference |
|
|
|
|
|
TNBC | 0.886
(0.483–1.626) | 0.699 |
|
|
|
| Ki-67 index |
|
|
|
|
|
|
≤50% | Reference |
|
|
|
|
|
>50% | 1.719
(0.909–3.254) | 0.096 |
|
|
|
| No. of metastatic
sites |
|
|
|
|
|
|
1-2 | Reference |
| Reference |
|
|
| ≥3 | 2.267
(1.248–4.119) | 0.007 | 1.092
(0.504–2.363) | 0.823 |
|
| Lung
metastasis |
|
|
|
|
|
| No | Reference |
|
|
|
|
|
Yes | 1.576
(0.864–2.872) | 0.138 |
|
|
|
| Liver
metastasis |
|
|
|
|
|
| No | Reference |
| Reference |
|
|
|
Yes | 2.342
(1.298–4.223) | 0.005 | 2.163
(0.976–4.797) | 0.058 |
|
| Brain
metastasis |
|
|
|
|
|
| No | Reference |
|
|
|
|
|
Yes | 1.601
(0.833–3.078) | 0.158 |
|
|
|
| Lymphocyte
count |
|
|
|
|
|
|
≤1.16 | Reference |
|
|
|
|
|
>1.16 | 0.447
(0.244–0.817) | 0.009 |
|
|
|
| Platelet count |
|
|
|
|
|
|
<325 | Reference |
|
|
|
|
|
≥325 | 1.978
(1.016–3.853) | 0.045 |
|
|
|
|
Neutrophil-to-lymphocyte ratio |
|
|
|
|
|
|
≤3.16 | Reference |
|
|
|
|
|
>3.16 | 1.553
(0.867–2.782) | 0.139 |
|
|
|
|
Platelet-to-lymphocyte ratio |
|
|
|
|
|
|
≤190 | Reference |
| Reference |
|
|
|
>190 | 2.203
(1.174–4.134) | 0.014 | 2.376
(1.059–5.328) | 0.036 |
|
| No. of lines of
treatment |
|
|
|
|
|
| 1 | Reference |
| Reference |
|
|
|
2-3 | 1.558
(0.610–3.974) | 0.354 | 1.784
(0.559–5.692) | 0.328 |
|
|
>3 |
3.480(1.506–8.043) | 0.040 | 2.784
(0.775–9.992) | 0.116 |
|
| Choice of systemic
treatment |
|
|
|
|
|
| ICI
plus chemotherapy | Reference |
| Reference |
|
|
| ICI
plus angiogenic inhibitors | 2.942
(1.285–6.734) | 0.011 | 1.497
(0.504–4.453) | 0.468 |
|
| ICI
plus chemotherapy and angiogenic inhibitors | 1.807
(0.865–3.777) | 0.116 | 0.566
(0.191–1.676) | 0.304 |
|
| PD-L1 expression,
% |
|
|
|
|
|
|
<1 | Reference |
|
|
|
|
| ≥1 | 1.296
(0.322–5.220) | 0.715 |
|
|
|
Breast cancer brain metastases
In total, 21 patients had brain metastases (Table I) and 81.0% of them were heavily
pretreated (lines of treatment, ≥3). The ORR was 14.3% (3/21) and
the DCR was 71.4% (15/21). The median PFS was 3.9 months (95% CI,
2.8–5.2) and the median OS was 11 months (95% CI, 6.2–15.8)
(Table SIII).
Toxicity
The treatment-related adverse events are listed in
Table V. Treatment-related
mortality was not recorded. The most common adverse events were
nausea (44.4%), neutropenia (42.0%) and enhanced alanine
aminotransferase/aspartate aminotransferase levels (22.2%). A total
of two patients developed immune-related pneumonia (grade 2), while
one patient was diagnosed with immune-related myositis (grade 1).
All three patients recovered following supportive therapy and
continued ICI-based therapy.
 | Table V.Treatment-related toxicity events
reported in the patient cohort. |
Table V.
Treatment-related toxicity events
reported in the patient cohort.
| Toxicity event | All grades, n
(%) | Grade 3 or 4, n
(%) |
|---|
| Nausea | 40 (44.4) | 0 (0) |
| Peripheral
neuropathy | 7 (7.8) | 0 (0) |
| Hypothyroidism | 2 (2.2) | 0 (0) |
| Hypertension | 3 (3.3) | 2 (2.2) |
| Neutropenia | 38 (42) | 18 (20) |
|
Thrombocytopenia | 3 (3.3) | 1 (1.1) |
| ALT/AST
elevation | 20 (22.2) | 3(3.3) |
| Pneumonia | 2 (2.2) | 0 (0) |
| Myositis | 1 (1.1) | 0 (0) |
Discussion
Emerging evidence has suggested that ICIs exhibit
encouraging antitumor effects on patients with lung cancer,
melanoma and other solid carcinomas (17–19).
However, breast cancer is commonly classified as a cold immunogenic
cancer (20). To the best of our
knowledge, the present study was the first multi-center real-world
objective report that evaluated the efficacy and safety of ICIs
combined with angiogenic inhibitors and/or chemotherapy in China.
The results indicated that ICIs could provide positive clinical
outcomes with manageable toxicity in patients with MBC in
real-world settings. Additionally, the most favorable results were
observed in the first-line treatment scenarios.
A previous review evaluated different treatment
strategies for metastatic TNBC and summarized that in numerous
clinical trials, the ORR and survival were improved in patients
treated with ICI treatment combinations compared with those treated
with chemotherapy alone (7). The
ICI combination may be a promising treatment option for metastatic
TNBC. The present study reported the efficacy of ICI treatment
combinations in MBC and the clinical outcomes were also improved
compared with trials of the aforementioned review where patients
were treated with chemotherapy alone, which was consistent with the
conclusion of the present review.
The present study assessed the efficacy of ICI-based
therapy in patients with MBC among patient groups with various
lines of treatment. In the first-line group, the majority of
patients (95.8%) underwent immunochemotherapy and reached an ORR of
50.0% and a median PFS of 11.0 months (95% CI, 6.0–16.0), which was
consistent with the previous studies (7,9,10). The
outcomes were improved compared with later lines of treatment. This
may be due to the fact that the immune system could be more
effective in patients with primary metastasis compared with heavily
pretreated patients (21). Previous
preclinical and clinical studies demonstrated that tumor burden is
a negative predictor of immunotherapy efficacy, since larger tumors
tend to exert a more suppressive microenvironment (22–25).
Other clinical studies also verified the efficacy of
immunochemotherapy in previously untreated patients with MBC
(9,10). Therefore, immunochemotherapy may be
a promising alternative in the first-line treatment of MBC. There
are limited treatment options for heavily pretreated patients with
multiple metastases. Preclinical studies indicate that the
angiogenic inhibitor-induced tumor vascular normalization can
sensitize programmed cell death PD-1/PD-L1 blockade in several
solid tumor models, including breast cancer (26,27).
Therefore, immunotherapy combined with angiogenic inhibitors has
gained increasing attention as a novel therapeutic strategy.
A phase II study, enrolling 46 patients, reported
that combination therapy with camrelizumab, apatinib and eribulin
in patients with heavily pretreated advanced TNBC results in an ORR
of 37.0%, a DCR of 87.0% and a median PFS of 8.1 months,
accompanied by manageable toxicity profile (12). The aforementioned study demonstrated
that the novel triplet rationale of ICI, angiogenic inhibitor and
chemotherapy warrants further investigation. In the present study,
26 patients received the aforementioned triplet rationale. However,
the outcomes of PFS and OS were not as beneficial as that reported
in the aforementioned study. This could be due to the difference in
the patient population between these two studies. While in the
phase II study, all patients were diagnosed with TNBC, in the
present study, only 50.0% (13/26) of patients were diagnosed with
TNBC. Additionally, in the present study, more patients (43.3%)
received ≥3 lines of treatment in the metastatic settings prior
enrolment compared with the previous study (37.0%). Overall, the
aforementioned findings suggested that the triplet rationale of
immunotherapy combined with angiogenic inhibitors and chemotherapy
could be a novel and promising strategy for patients with heavily
pretreated MBC who underwent multiple lines of unsuccessful
systemic therapies. However, the patient population that could
benefit most from the aforementioned therapy combinations should be
further explored.
Given the synergistic effect of ICIs and angiogenic
inhibitors, numerous studies have investigated the efficacy of the
combination of ICIs and angiogenic inhibitors. Previous clinical
trials showed that the aforementioned chemotherapy-free regimen
displayed a particular efficacy, with an ORR of 43.3% for
camrelizumab plus apatinib and 29% for pembrolizumab plus
lenvatinib (28,29). A phase Ib study, including 34
patients, explored the efficacy of the PD-L1 inhibitor TQB2450
combined with anlotinib. In the aforementioned study, ~80% of
patients were treated with a chemotherapy-free regimen as a first-
or second-line therapy. The results demonstrated an ORR of 26.5%
and a median PFS of 5.6 months (30). Considering the lower toxicity, the
chemotherapy-free regimen may be an alternative for patients with
poor tolerance.
The present study results showed that the short-term
tumor response to immunotherapy could, to a certain extent, predict
long-term efficacy. Patients with a shrinkage of target lesions
(PR/SD) during immunotherapy exhibited a significantly reduced risk
of recurrence compared with others, thus verifying the durable
response of immunotherapy. It has been also reported that
immunotherapy can prompt a long-term benefit in multiple solid
tumors. In a pooled analysis of the CheckMate 017 and 057 trials,
78% of nivolumab-treated patients with non-small-cell lung cancer
(NSCLC), who survived for 5 years, showed CR or PR (31). Additionally, the CheckMate-649 trial
also demonstrated notably improved outcomes in patients with
gastric cancer (32). Therefore,
the 3-year OS rate of a subgroup of Chinese patients with PD-L1
combined positive score of 5 in the nivolumab combination with
chemotherapy arm was 31%, which was notably increased compared with
that obtained in the chemotherapy arm (11%). Numerous studies have
also highlighted the unique characteristics of immunotherapy
action, namely the ‘tail effect’. Therefore, once the treatment is
effective, the long-term survival can be improved, thus providing
novel insights into the clinical application of immunotherapy
(33,34).
Subgroup analysis demonstrated that high
pretreatment blood PLR was associated with poor PFS and OS. Given
that peripheral blood testing is simple and easy to perform, PLR
could be used as a prognostic factor in patients with MBC treated
with ICI-based therapy. In terms of the underlying mechanism,
preclinical studies indicated that platelet activation could
suppress the functions of natural killer cells and could play a
significant role in promoting tumor proliferation, invasion and
angiogenic signaling (35,36). Additionally, lymphocytes can inhibit
tumor cell growth and improve the prognosis of patients with
malignant tumors via secreting IFN-g and TNF (37–41).
Emerging evidence has also suggested that PLR is an informative
marker reflecting the changes in platelet and lymphocyte counts,
while it is associated with the degree of inflammation (42). Therefore, PLR may potentially
exhibit a negative effect on the immune system of the host
(43). Other studies have also
suggested that a high PLR may be a negative immunotherapy
prognostic factor in several types of solid tumors, such as NSCLC
and gastric cancer (44,45). A previous study by Onagi et
al (43) demonstrated that in
patients with TNBC, a high PLR predominantly includes more
CD3+CD4+FOXP3+ T-cells (regulatory
T-cells), thus suggesting that local tumor immunity can be
suppressed in these patients, eventually leading to poor
immunotherapy outcomes.
Of note, two real-world studies also explored the
efficacy of ICIs in MBC. The ANASTASE study (46), which retrospectively enrolled 52
patients with PD-L1-positive metastatic TNBC treated with
first-line atezolizumab plus nab-paclitaxel, achieved an ORR of
42.3% and a median PFS of 6.3 months; while in the present study,
24 patients of first-line therapy, regardless of PD-L1 level,
reached an ORR of 50% and a median PFS of 11 months. In contrast to
the ANASTASE study, the present study not only included patients
who received Nab-paclitaxel + immunotherapy (12 patients), but also
stronger combinations, such as platinum-based chemotherapy or
anthracyclines plus taxane, which might be the reason of longer
PFS. Another real-world study (47)
in China included 81 patients with metastatic TNBC, of which 9.9%
patients received ICI monotherapy and 90.1% patients received
combined treatment. The ORR was 32.1% and the DCR was 64.2%. The
median PFS was 4.2 months and the median OS was 11.0 months. The
combined drugs were not described specifically. The present study
classified therapy strategies and reported their outcomes
respectively, which shed light on the exploration of different
combination of ICIs. A systematic review (48) concluded that up to 45% of patients
with TNBC will develop breast cancer brain metastases (BCBM) but
only 3.3% of patients were included in the clinical trials, and
only two clinical trials have reported the BCBM-specific outcomes.
Evaluation of the efficacy of ICIs in patients with BCBM is greatly
needed. The present study reported the survival outcomes of
patients with BCBM and provided more evidence to patients with
BCBM.
However, the present study has a number of
limitations. Due to its retrospective nature, potential biases
should be taken into consideration, including no controlled-arm,
patient selection and over-enthusiasm, which describes a potential
bias introduced when clinical physicians pay more attention to
positive outcomes or improvements in patients receiving treatment
and may inadvertently overlook or underreport adverse events or
negative outcomes. In addition, clinical physicians potentially
might pay more attention to positive outcomes in patients receiving
ICIs, inadvertently overlooking or underreporting adverse events or
negative outcomes.
Breast cancer was previously not considered a
particularly immunogenic tumor due to relatively lower levels of
tumor infiltrating lymphocytes, tumor mutational burden and PD-L1
expression (49–51). Nevertheless, there is increasing
evidence in recent years to suggest the presence of variable
immunogenic activity in different breast cancer subtypes, with TNBC
likely exhibiting the strongest immunogenicity (52,53).
However, as the ICIs approved by the Food and Drug Administration
(FDA) for the treatment of breast cancer, namely atezolizumab and
pembrolizumab, are expensive and not covered by medical insurance,
immunotherapy for the treatment of breast cancer has not been
prioritized clinically. As a result, the application of
immunotherapy is rare in the real world. Therefore, the present
study included a heterogeneous patient population and certain
confounding factors were unavoidable, such as patients using a
variety of ICIs, including pembrolizumab, atezolizumab,
camrelizumab, sintilimab, toripalimab and tislelizumab. By
contrast, this allowed the analysis of the comprehensive subgroups
of different combinations, such as ICI combined with chemotherapy,
ICI with angiogenic inhibitors and ICI with chemotherapy and
angiogenic inhibitors.
However, not all patients have finished PD-L1
detection, for a number of reasons including limited biopsy tissue,
low quality tissue samples as samples that must comprise sufficient
amounts of tumor cells, lymphocytes and phagocytes and as certain
patients started immunotherapy before PD-L1 was recognized as a
clinically relevant biomarker for ICIs in November 2020, when the
US FDA accelerated the approval of pembrolizumab in combination
with chemotherapy based on the positive outcomes of KEYNOTE 355.
Several patients did not assess PD-L1 but still received ICIs. The
reasons were as follows: i) Several patients harbored high
mutational burden or microsatellite instability, which might make
ICIs a reasonable treatment option; ii) there might be
patient-specific factors that made ICIs a preferred treatment
option, such as comorbidities that make other treatments less
suitable; and iii) in certain cases, ICIs might be used off-label
based on the judgment of the clinical physician, considering the
potential benefits and risks, especially for patients whose cancer
has progressed after other treatments.
Machine learning was not applied in the present
study, as machine learning often cannot achieve sufficiently
reliable results when the sample data is not large enough and also
involves a large number of parameters. For a model with multiple
factors, it often requires thousands or even tens of thousands of
data points to generate a model with some effectiveness. In cases
where the data volume is small, the data dimensions are high,
statistical methods tend to be more efficient and effective than
machine learning (54,55).
Despite the aforementioned limitations, the findings
of the present study could still be considered meaningful as it
provided real-world outcomes of ICI-based therapy in MBC and
offered insight on the role and application of ICIs in MBC.
However, to enforce global policy changes, large-scale phase III
clinical trials are needed. Multi-centered, randomized controlled
prospective trials could be conducted to confirm the efficacy and
safety of ICIs in the first-line treatment of MBC, particularly for
ICIs developed in China, such as camrelizumab, sintilimab,
toripalimab and tislelizumab. Further investigation on the role of
baseline PLR as a prognostic biomarker for ICI treatment and
additional biomarkers or factors that could predict response to ICI
treatment are also warranted. In addition, investigations of
combination therapies that could enhance the effectiveness of ICIs
should also be considered in the future.
In conclusion, the present study demonstrated that
ICI-based therapy could achieve promising clinical outcomes with
manageable toxicity for patients with MBC in real-world settings.
The most appropriate time for ICIs was the first-line treatment.
Furthermore, baseline PLR could serve as an independent prognostic
factor for ICI treatment. In addition, it was demonstrated that
once the ICI treatment was effective, long-term survival could be
improved. However, although the present study had also a number of
limitations, the preliminary findings of this real-world cohort
warrant further prospective research and clinical practice of
treating MBC in future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Shenzhen Key Medical
Discipline Construction Fund and Guangdong Basic and Applied Basic
Research Foundation (grant no. 2023A1515220051).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL contributed to the study concept and design. XQ
and YT participated in patient management, acquisition of data and
confirm the authenticity of all the raw data. XX, SL, HYC, HZC and
SC contributed to data analysis and interpretation. XQ and HZC
drafted the manuscript. SC revised the manuscript. XL and YW
contributed to data collection. All authors approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was performed in compliance with
the Declaration of Helsinki and approved by the Institutional
Review Board of the Henan Provincial People's Hospital [approval
no. 84 (2022); Zhengzhou, China] and the Affiliated Hospital of
Xuzhou Medical University (approval no. XYEY2022-KL428-01; Xuzhou,
China). Written informed consent for participation was not required
for the present study in accordance with national legislation and
institutional requirements. A waiver of individual informed consent
from the Ethics Committee was granted as the present study was a
retrospective analysis. Patient information was anonymized and
protected.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Inst. 4:47–53. 2024.
|
|
3
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kojima T, Shah MA, Muro K, Francois E,
Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, et al:
Randomized phase III KEYNOTE-181 study of pembrolizumab versus
chemotherapy in advanced esophageal cancer. J Clin Oncol.
38:4138–4148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JC, Durbeck J, Boudadi K, Ho WJ and
Kang H: The efficacy of anti-PD-1 immune checkpoint inhibitor in
nasopharyngeal carcinoma. Oral Oncol. 108:1049352020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elizabeth MS, Cristina SBJ and Christian
CG: Immunotherapy in combination with chemotherapy for
triple-negative breast cancer. Mini Rev Med Chem. 24:431–439. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmid P, Lipatov O, Im SA, Goncalves A,
Muñoz-Couselo E, Lee KS, Tamura K, Testa L, Witzel I, Ohtani S, et
al: Impact of pembrolizumab versus chemotherapy on health-related
quality of life in patients with metastatic triple-negative breast
cancer: Results from the phase 3 randomised KEYNOTE-119 study. Eur
J Cancer. 195:133932023. View Article : Google Scholar
|
|
9
|
Schmid P, Rugo HS, Adams S, Schneeweiss A,
Barrios CH, Iwata H, Dieras V, Henschel V, Molinero L, Chui SY, et
al: Atezolizumab plus nab-paclitaxel as first-line treatment for
unresectable, locally advanced or metastatic triple-negative breast
cancer (IMpassion130): Updated efficacy results from a randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
21:44–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cortes J, Cescon DW, Rugo HS, Nowecki Z,
Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et
al: Pembrolizumab plus chemotherapy versus placebo plus
chemotherapy for previously untreated locally recurrent inoperable
or metastatic triple-negative breast cancer (KEYNOTE-355): A
randomised, placebo-controlled, double-blind, phase 3 clinical
trial. Lancet. 396:1817–1828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miles D, Gligorov J, Andre F, Cameron D,
Schneeweiss A, Barrios C, Xu B, Wardley A, Kaen D, Andrade L, et
al: Primary results from IMpassion131, a double-blind,
placebo-controlled, randomised phase III trial of first-line
paclitaxel with or without atezolizumab for unresectable locally
advanced/metastatic triple-negative breast cancer. Ann Oncol.
32:994–1004. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Wang Y, Tian Z, Lin Y, Li H, Zhu Z,
Liu Q, Su S, Zeng Y, Jia W, et al: Multicenter phase II trial of
Camrelizumab combined with Apatinib and Eribulin in heavily
pretreated patients with advanced triple-negative breast cancer.
Nat Commun. 13:30112022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan PH, Ellis I, Allison K, Brogi E, Fox
SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The
2019 world health organization classification of tumours of the
breast. Histopathology. 77:181–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Cancer Institute, . Common
terminology criteria for adverse events (CTCAE) Version 5.0. U.S.
Department of Health and Human Services, 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
|
|
17
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robert C, Carlino MS, McNeil C, Ribas A,
Grob JJ, Schachter J, Nyakas M, Kee D, Petrella TM, Blaustein A, et
al: Seven-year follow-up of the phase III KEYNOTE-006 study:
Pembrolizumab versus ipilimumab in advanced melanoma. J Clin Oncol.
41:3998–4003. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Y, Qin X, Peng X, Zeng A, Li J, Chen
C, Qiu S, Pan S, Zheng Y, Cai J, et al: Efficacy and safety of
KL-A167 in previously treated recurrent or metastatic
nasopharyngeal carcinoma: A multicenter, single-arm, phase 2 study.
Lancet Reg Health West Pac. 31:1006172022.PubMed/NCBI
|
|
20
|
Yang L, Hu Q and Huang T: Breast cancer
treatment strategies targeting the tumor microenvironment: How to
convert ‘Cold’ tumors to ‘Hot’ tumors. Int J Mol Sci. 25:72082024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esteva FJ, Hubbard-Lucey VM, Tang J and
Pusztai L: Immunotherapy and targeted therapy combinations in
metastatic breast cancer. Lancet Oncol. 20:e175–e186. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guisier F, Cousse S, Jeanvoine M,
Thiberville L and Salaun M: A rationale for surgical debulking to
improve anti-PD1 therapy outcome in non-small cell lung cancer. Sci
Rep. 9:169022019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chardin D, Paquet M, Schiappa R, Darcourt
J, Bailleux C, Poudenx M, Sciazza A, Ilie M, Benzaquen J, Martin N,
et al: Baseline metabolic tumor volume as a strong predictive and
prognostic biomarker in patients with non-small cell lung cancer
treated with PD1 inhibitors: A prospective study. J Immunother
Cancer. 8:e0006452020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Colak S and Ten Dijke P: Targeting TGF-β
signaling in cancer. Trends Cancer. 3:56–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishino M, Giobbie-Hurder A, Ramaiya NH
and Hodi FS: Response assessment in metastatic melanoma treated
with ipilimumab and bevacizumab: CT tumor size and density as
markers for response and outcome. J Immunother Cancer. 2:402014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Wang Y, Jia W, Deng H, Li G, Deng W,
Chen J, Kim BYS, Jiang W, Liu Q and Liu J: Low-dose anti-angiogenic
therapy sensitizes breast cancer to PD-1 blockade. Clin Cancer Res.
26:1712–1724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmittnaegel M, Rigamonti N, Kadioglu E,
Cassara A, Rmili CW, Kiialainen A, Kienast Y, Mueller HJ, Ooi CH,
Laoui D and De Palma M: Dual angiopoietin-2 and VEGFA inhibition
elicits antitumor immunity that is enhanced by PD-1 checkpoint
blockade. Sci Transl Med. 9:eaak96702017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, Liu Q, Li Y, Li Q, Su F, Yao H, Su
S, Wang Q, Jin L, Wang Y, et al: Efficacy and safety of
camrelizumab combined with apatinib in advanced triple-negative
breast cancer: An open-label phase II trial. J Immunother Cancer.
8:e0006962020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lwin Z, Gomez-Roca C, Saada-Bouzid E,
Yáñez E, Longo F, Im SA, Castanon E, Senellart H, Graham D, Voss M,
et al: LBA41 LEAP-005: Phase II study of lenvatinib (len) plus
pembrolizumab (pembro) in patients (pts) with previously treated
advanced solid tumours. Ann Oncol. 31:S11702020. View Article : Google Scholar
|
|
30
|
Wang J, Sun T, Ouyang Q, Han Y and Xu B: A
phase Ib study of TQB2450 plus anlotinib in patients with advanced
triple-negative breast cancer. iScience. 26:1068762023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borghaei H, Gettinger S, Vokes EE, Chow
LQM, Burgio MA, de Castro Carpeno J, Pluzanski A, Arrieta O,
Frontera OA, Chiari R, et al: Five-year outcomes from the
randomized, phase III trials CheckMate 017 and 057: Nivolumab
versus docetaxel in previously treated non-small-cell lung cancer.
J Clin Oncol. 39:723–733. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Bragagnoli AC, et al: First-line nivolumab plus chemotherapy versus
chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng Y, Tang L, Wang H, Liu Y, Yang S, Lin
L, Hu X and Shi Y: Immune checkpoint inhibitors combined with
angiogenic inhibitors in the treatment of locally advanced or
metastatic lung adenocarcinoma patients. Cancer Immunol Immunother.
72:449–459. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brahmer JR, Lee JS, Ciuleanu TE, Caro RB,
Nishio M, Urban L, Audigier-Valette C, Lupinacci L, Sangha R,
Pluzanski A, et al: Five-year survival outcomes with nivolumab plus
ipilimumab versus chemotherapy as first-line treatment for
metastatic non-small-cell lung cancer in CheckMate 227. J Clin
Oncol. 41:1200–1212. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raskov H, Orhan A, Agerbæk MØ and Gögenur
I: The impact of platelets on the metastatic potential of tumour
cells. Heliyon. 10:e343612024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou L, Zhang Z, Tian Y, Li Z, Liu Z and
Zhu S: The critical role of platelet in cancer progression and
metastasis. Eur J Med Res. 28:3852023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M and Degen JL:
Platelets and fibrin(ogen) increase metastatic potential by
impeding natural killer cell-mediated elimination of tumor cells.
Blood. 105:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Egan K, Crowley D, Smyth P, O'Toole S,
Spillane C, Martin C, Gallagher M, Canney A, Norris L, Conlon N, et
al: Platelet adhesion and degranulation induce pro-survival and
pro-angiogenic signalling in ovarian cancer cells. PLoS One.
6:e261252011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bambace NM and Holmes CE: The platelet
contribution to cancer progression. J Thromb Haemost. 9:237–249.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mijic S and Dabrosin C: Platelet
activation in situ in breasts at high risk of cancer: Relationship
with mammographic density and estradiol. J Clin Endocrinol Metab.
106:485–500. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Deng H and Wang J: LTBP1 promotes
the progression of triple negative breast cancer via activating the
RhoA/ROCK signaling pathway. Cancer Insight. 3:37–48. 2023.
View Article : Google Scholar
|
|
42
|
Tao Y, Zhou Y, Chen H, Qin Y, He X, Liu P,
Zhou S, Yang J, Zhou L, Zhang C, et al: Prognostic role of red
blood cell distribution width and platelet/lymphocyte ratio in
early-stage classical hodgkin lymphoma. Future Oncol. 18:1817–1827.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Onagi H, Horimoto Y, Sakaguchi A, Ikarashi
D, Yanagisawa N, Nakayama T, Nakatsura T, Ishizuka Y, Sasaki R,
Watanabe J, et al: High platelet-to-lymphocyte ratios in
triple-negative breast cancer associates with immunosuppressive
status of TILs. Breast Cancer Res. 24:672022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gou M and Zhang Y: Pretreatment
platelet-to-lymphocyte ratio (PLR) as a prognosticating indicator
for gastric cancer patients receiving immunotherapy. Discov Oncol.
13:1182022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Platini H, Ferdinand E, Kohar K, Prayogo
SA, Amirah S, Komariah M and Maulana S: Neutrophil-to-lymphocyte
ratio and platelet-to-lymphocyte ratio as prognostic markers for
advanced non-small-cell lung cancer treated with immunotherapy: A
systematic review and meta-analysis. Medicina (Kaunas).
58:10692022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fabi A, Carbognin L, Botticelli A, Paris
I, Fuso P, Savastano MC, La Verde N, Strina C, Pedersini R, Guarino
S, et al: Real-world ANASTASE study of atezolizumab+nab-paclitaxel
as first-line treatment of PD-L1-positive metastatic
triple-negative breast cancer. NPJ Breast Cancer. 9:732023.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Z, Zhang Y, Liu C, Shao J, Chen Y,
Zhu Y, Zhang L, Qin B, Kong Z, Wang X, et al: A real-world study of
immune checkpoint inhibitors in advanced triple-negative breast
cancer. Cancer Innov. 2:172–180. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schlam I and Gatti-Mays ME: Immune
checkpoint inhibitors in the treatment of breast cancer brain
metastases. Oncologist. 27:538–547. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
De La Cruz LM and Czerniecki BJ:
Immunotherapy for breast cancer is finally at the doorstep:
Immunotherapy in breast cancer. Ann Surg Oncol. 25:2852–2857. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Plitas G, Konopacki C, Wu K, Bos PD,
Morrow M, Putintseva EV, Chudakov DM and Rudensky AY: Regulatory T
cells exhibit distinct features in human breast cancer. Immunity.
45:1122–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sisirak V, Faget J, Gobert M, Goutagny N,
Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI,
Goddard-Leon S, et al: Impaired IFN-α production by plasmacytoid
dendritic cells favors regulatory T-cell expansion that may
contribute to breast cancer progression. Cancer Res. 72:5188–5197.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miller LD, Chou JA, Black MA, Print C,
Chifman J, Alistar A, Putti T, Zhou X, Bedognetti D, Hendrickx W,
et al: Immunogenic subtypes of breast cancer delineated by gene
classifiers of immune responsiveness. Cancer Immunol Res.
4:600–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu Z, Li M, Jiang Z and Wang X: A
comprehensive immunologic portrait of triple-negative breast
cancer. Transl Oncol. 11:311–329. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang T, Gradus JL and Rosellini AJ:
Supervised machine learning: A brief primer. Behav Ther.
51:675–687. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Greener JG, Kandathil SM, Moffat L and
Jones DT: A guide to machine learning for biologists. Nat Rev Mol
Cell Biol. 23:40–55. 2022. View Article : Google Scholar : PubMed/NCBI
|