Introduction
Breast cancer (BC) is one of the most common
malignancies affecting the health of women worldwide with an
estimated 2.3 million new cases and 685,000 deaths in 2020. It is
also the leading cause of cancer-related death in women (1). Early screening and diagnosis of BC has
positive impacts on treatment outcomes and the psychology of the
patient as well as decreasing the economic burden of this cancer
(2). Widespread BC screening in the
USA and other high-income countries has contributed to a decreased
number of mortalities from BC in these populations over recent
decades (3). It has also helped to
identify contraindications to medication, e.g. BC is a
contraindication for estrogen plus progestogen (4). However, there are ethical challenges
and economic and demographic differences that hinder early
screening in underdeveloped countries and regions, which, for
example, makes it difficult to systematically implement BC
screening in sub-Saharan Africa (5). Furthermore, the contradiction between
a large population and limited resources poses a huge challenge for
China to increase the national coverage of BC screening (3). For BC, breast self-examination (BSE)
and clinical breast examination (CBE) can catch the first physical
changes in the breasts and, subsequently, a mammography should be
performed (6). However, in
resource-limited settings, a mammography is assessed as not
cost-effective (5). In addition,
current research does not indicate that there is an improved
detection and diagnosis rate of early BC using BSE and CBE
(5,6). In recent decades, the serum
concentration of tumor markers has been used to detect tumor
activity, as suggested by the updated recommendations of the
American Society of Clinical Oncology (7). Tumor markers are minimally invasive,
readily available and low-cost, providing an alternative approach
to BC screening (7,8). However, the efficacy of mainstream
clinical tumor markers has been questioned due to their low
diagnostic sensitivity of the disease at early stages, such as
carcinoembryonic antigen (7). Thus,
there is a need for affordable, accurate and sensitive markers for
the monitoring of BC. Research on potential tumor markers may be of
significance for the screening of BC, especially in low- and
middle-income countries (9).
Cancer development is, among other factors, driven
by a tumor-mediated disorder of immunity, along with immune
disorders in all cell populations (10). There is evidence suggesting that the
neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio
(PLR) and lymphocyte-to-monocyte ratio (LMR), among those derived
from peripheral whole blood cell count, are useful indicators of BC
onset, development and prognosis (11–13).
Despite systematic reviews of peripheral whole blood cell
count-derived indicators of BC in the efficacy of drug therapy for
BC and the disease prognosis (13–15),
no meta-analyses have reported associations between peripheral
whole blood cell count-derived indicators (NLR, PLR and LMR) and
BC, to the best of our knowledge. Disordered neutrophils,
overactivated platelets and reduced lymphocytes create an optimal
environment for tumor growth, progression and metastasis (13,14,16,17).
NLR and PLR are positively associated with risk for multiple types
of cancer while LMR is negatively associated (18). In addition, these biomarkers change
prior to diagnosis, and they can be used to predict the presence of
malignancy (16,18). Moreover, these markers are low-cost,
accessible and sensitive, making them particularly suitable for BC
screening in underdeveloped countries and regions (3,5,16,18).
However, previous studies have come to different conclusions on the
differences in NLR, PLR and LMR between patients with BC, and
non-BC and healthy subjects and patients with benign breast disease
(17,19–22).
This difference has led to uncertainty on the diagnostic role of
NLR, PLR and LMR in BC screening and earlier identification.
Therefore, the present study performed a meta-analysis to assess
the current literature to evaluate the diagnostic role of NLR, PLR
and LMR in BC.
Materials and methods
Literature search
The methods of the present study were based on the
updated guidelines for systematic review reports of the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
2020 statement (23). ‘Breast
neoplasms’, ‘neutrophils and lymphocytes’, ‘NLR’,
‘neutrophil-lymphocyte ratio’, ‘blood platelets and lymphocytes’,
‘PLR’, ‘platelet-lymphocyte ratio’, ‘lymphocytes and monocytes’,
‘LMR’ and ‘lymphocyte-monocyte ratio’ were used as medical subject
headings terms and keywords to search in PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE
(https://www.embase.com/), Cochrane Library
(https://www.cochranelibrary.com/), China
National Knowledge Infrastructure (https://www.cnki.net/), Wanfang Database (https://www.wanfangdata.com.cn/), VIP database
(http://www.cqvip.com/) and China Biology Medicine
disc (http://www.sinomed.ac.cn/index.jsp), for a time frame
starting from database establishment to August 29, 2023 (24). Articles were limited to English and
Chinese versions only. Additional manual searches of relevant
journals were performed and the relevant documents were tracked in
the references. A total of two authors (DY and HW) independently
screened the research literature, and any differences were
discussed and resolved with a third author (DA).
Eligibility criteria
The inclusion criteria were as follows: ⅰ) Study
type: Observational studies, including cross-sectional studies,
cohort studies, case-control studies or case series; ⅱ) subjects:
Patients with BC that had received no treatment (including surgery,
drugs and radiation therapy); ⅲ) interventions: NLR, PLR and LMR;
ⅳ) controls: Healthy and benign controls; and ⅴ) outcomes:
Diagnosis.
The exclusion criteria were as follows: ⅰ) Cellular
experiments, in vitro studies; ⅱ) studies assessing NLR, PLR
and LMR data of patients with BC after treatment (surgery, drugs
and radiotherapy); ⅲ) literature reviews, comments, correspondence
letters and case reports; ⅳ) duplicate publications; ⅴ) literature
with unavailable full text, incomplete data, unavailable raw data
and unavailable synthetically extracted data; and ⅵ) relatively
low-quality literature [Newcastle-Ottawa scale (NOS) score <6]
(25).
Literature screening, quality
assessment and data extraction
A total of two investigators (DY and HW) reviewed
the titles, abstracts, keywords and full text of the literature
separately, and then screened and analyzed them and assessed their
quality against the inclusion and exclusion criteria. Any
differences arising during the study were resolved through
discussion with the third investigator (DA).
The NOS was used to assess the quality of each
cohort and case-control study based on the following components: i)
Selection of the cohort; ii) comparability of cohorts based on the
design or analysis; and iii) how the exposure was ascertained
(25). The cross-sectional study
evaluation criteria of the Agency for Healthcare Research and
Quality (AHRQ) was used (25). The
data were then extracted according to an independently pre-defined
information extraction form (15)
and reviewed by two investigators (DY and HW). Any discrepancy
between data extractions was resolved through discussion with the
third investigator (DA). The data extracted included the surname of
the first author, year of publication, country, age and sex of the
patient, as well as the sample size, disease stage, NLR, PLR and
LMR.
Statistical analysis
Statistical analysis was performed using RevMan 5.3
(https://www.cochrane.org/) and STATA
12.0 software (StataCorp LP). The mean and standard deviation
values were extrapolated from the median and interquartile
range/range values. NLR, PLR and LMR were analyzed using the
standardized mean difference (SMD) and 95% confidence intervals
(CI). A random-effects model was used in the present study
according to the Cochrane Handbook for Systematic Reviews of
Interventions, as a systematic review and meta-analysis including
multiple studies from different groups (26). P<0.05 was considered to indicate
a statistically significant difference. The I2 metric
and χ2 test were used to assess the heterogeneity among
studies. If there was significant heterogeneity (P<0.1,
I2≥50%), subgroup analysis was performed to identify the
causes of heterogeneity.
The command ‘metandi’ was used to calculate the
diagnostic odds ratio (DOR), pooled specificity, specificity,
positive likelihood ratio and negative likelihood ratio in STATA
12.0. A summary receiver operating characteristic (ROC) curve was
also generated. Sensitivity analysis was performed using STATA 12.0
using the ‘leave-one-out’ method. Publication bias was assessed
using funnel plots, Begg's test and Egger's test. The present study
is fully compliant with the PRISMA guidelines.
Results
Search results and included
studies
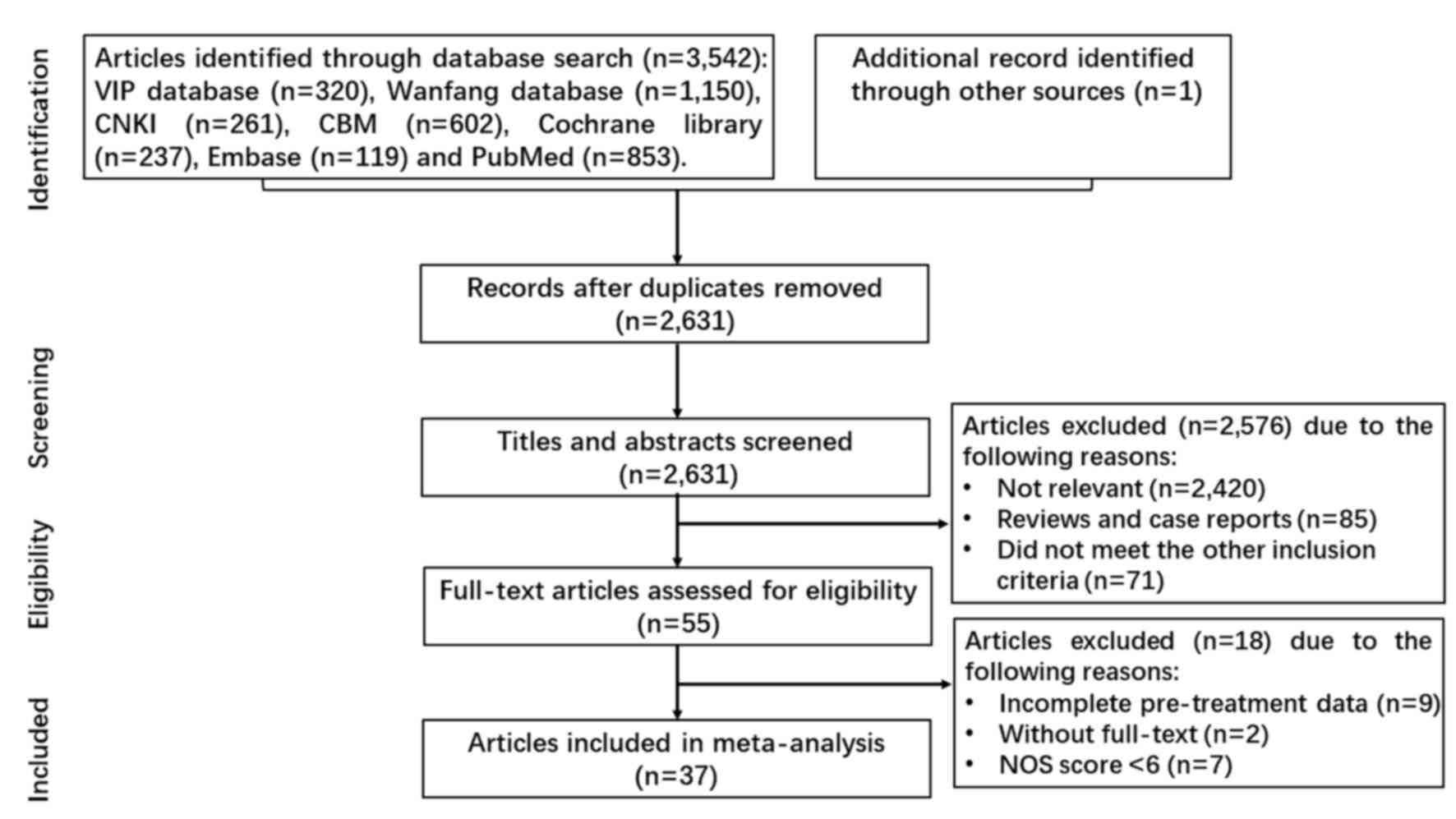
A total of 3,542 articles were retrieved through the
initial screening, and one was added by tracking references. After
removing 912 duplicates, 2,631 articles remained after the initial
screening. Following literature screening by title, abstract and
keywords, a total of 2,576 irrelevant studies were also excluded.
After full-text reading, an additional 18 studies were excluded due
to incomplete pre-treatment data (9 articles), without full-text (2
articles), and NOS score <6 points (7 articles). Finally, 37
articles were included in the meta-analysis (Fig. 1).
Characteristics of the population and
quality assessment
The 37 included studies in the present meta-analysis
involved in 8 countries: Greece (n=1), Iraq (n=1), Denmark (n=1),
Italy (n=1), Iran (n=2), Egypt (n=2), Turkey (n=4) and China (n=25)
(Table I). Of these studies, 37 had
cohort or case-control designs with NOS score 6–8, classifying them
as moderate or high-quality studies. The other two studies were
cross-sectional studies with AHRQ scores of 9 and 10 points,
respectively (Table I).
Furthermore, 16 studies analyzed ROC curves for NLR, seven for PLR
and two for LMR (Table II).
 | Table I.Characteristics of the enrolled
studies. |
Table I.
Characteristics of the enrolled
studies.
|
|
|
| BC | Control |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First author/s,
year | Region | Study design | n | Sex (M/F) | Age, years | Type | n | Sex (M/F) | Age, years | NOS/AHRQ
scores | Outcome | (Refs.) |
|---|
| Seretis et
al, 2012 | Greece |
Cross-sectional | 35 | 0/35 |
45.5±11.5a | Benignc | 44 | 0/44 | 60.2±12.5 | /9 | NLR | (21) |
| Ozyalvacli et
al, 2014 | Turkey | Case control | 120 | 0/120 | 54.02±13.45 | Benign | 50 | 0/50 | 51.90±10.26 | 6 | NLR | (28) |
| Okuturlar et
al, 2015 | Turkey | Case control | 178 | 0/178 | 53.8±11.5 | Healthy | 107 | 0/107 | 53.7±14.7 | 7 | NLR | (29) |
| Qian et al,
2015 | China | Case control | 82 | 0/82 |
53.5±10.5a | Healthy | 41 | 0/41 | - | 7 | NLR | (30) |
| Zhang et al,
2016 | China | Case control | 104 | 0/104 | 51.08±10.21 | Healthy | 50 | 0/50 | 45.68±11.37 | 7 | NLR/PLR | (43) |
| Sun et al,
2017 | China | Case control | 110 | 0/110 | 54.34±12.28 | Healthy | 78 | 0/78 | 51.54±10.37 | 7 | NLR | (17) |
| Wu et al,
2017 | China | Case control | 53 | 0/53 | 51.21±12.04 | Benign | 122 | 0/122 | 45.75±12.48 | 6 | NLR | (44) |
| Huan et al,
2018 | China | Case control | 126 | 0/126 | 56±9 | Benign | 102 | 0/102 | 52±9 | 7 | NLR | (45) |
| Pan et al,
2018 | China | Case control | 52 | 0/52 |
53.5±12a | Healthy | 47 | 0/47 | 53±11a | 7 | NLR | (46) |
| Fang et al,
2018 | China | Case control | 1540 | 0/1540 | 50.75±10.36 | Benign | 1540 | 0/1540 | 50.75±10.36 | 8 | NLR | (11) |
| Cao et al,
2018 | China | Case control | 118 | 0/118 | 51.25±9.24 | Healthy | 60 | 0/60 | 50.72±9.13 | 7 | NLR/PLR | (47) |
| Zhang et al,
2018 | China | Case control | 92 | 0/92 | 51±10 | Healthy | 50 | 0/50 | 51±12 | 7 | NLR | (48) |
| Zhong et al,
2018 | China | Case control | 115 | 0/115 |
50±7.25a | Healthy | 120 | 0/120 | - | 6 | PLR | (49) |
| Zhao et al,
2018 | China | Case control | 131 | 0/131 | 53±14a | i) Healthy; ii)
Benign | i) 95; ii) 120 | i) 0/95; ii)
0/120 | i)
48±9.75a; ii)
52.5±13.5a | 6 | NLR | (31) |
| Pei et al,
2019 | China | Case control | 412 | 0/402 | 48.17±11.09 | Benign | 412 | 0/412 | 47.67±10.33 | 6 | NLR | (50) |
| Alsaadi and Younus,
2019 | Iraq | Case control | 55 | 0/55 | 52.44±8.8 | Healthy | 28 | 0/28 | 47.13±12.79 | 8 | NLR/PLR | (51) |
| Xie et al,
2019 | China | Case control | 136 | 0/136 | 47.04±9.76 | Benign | 127 | 0/127 | 43.13±4.94 | 6 | NLR/PLR | (52) |
| Said, 2019 | Egypt | Case control | 84 | 0/84 | 32.7±18.3 | Healthy | 71 | 0/71 | 36.5±15.5 | 6 | PLR | (12) |
| Yan et al,
2019 | China | Case control | 86 | 6/80 | 56.14±11.98 | Benign | 167 | 2/165 | 41.77±11.77 | 6 | NLR | (32) |
| Gao et al,
2019 | China | Case control | 196 | 17/179 | 55.16±12.32 | Healthy | 392 | 34/358 | 55.53±12.54 | 7 | NLR/PLR | (53) |
| Liu et al,
2020 | China | Case control | 433 | 0/433 | 50b | Healthy | 631 | 0/631 | 44b | 6 | NLR/PLR | (54) |
| Chi et al,
2020 | China | Case control | 70 | 0/70 | 39±12 | Benign | 123 | 0/123 | 53±9 | 7 | NLR/PLR/LMR | (33) |
| Jørgensen et
al, 2021 | Denmark | Case control | 22 | 0/22 | 62.8
±12a | Healthy | 30 | 0/30 |
51.8±9.25a | 6 | NLR | (10) |
| Velidedeoglu et
al, 2021 | Turkey | Case control | 50 | 0/50 | 44.3±7.55 | Healthy | 50 | 0/50 | 44.92±8.02 | 8 | NLR/PLR | (20) |
| Peng et al,
2021 | China | Case control | 49 | 0/49 | - | Benign | 48 | 0/48 | - | 6 | NLR | (34) |
| Divsalar et
al, 2021 | Iran | Case control | 160 | 0/160 | 51±12 | Healthy | 160 | 0/160 | 50±13 | 7 | NLR | (35) |
| Baselice et
al, 2021 | Italy | Case control | 77 | 0/77 |
63.06±11.8a | Benign | 50 | 0/50 |
33.39±12.7a | 6 | NLR | (55) |
| Youssry et
al, 2022 | Egypt | Case control | 82 | 0/82 | 49.58±7.7 | i) Healthy; ii)
Benign | i) 40; ii) 44 | i) 0/40; ii)
0/44 | i) 48.97±7.1; ii)
47.36±8.43 | 7 | NLR/PLR | (41) |
| Dal et al,
2022 | Turkey | Case control | 28 | 28/0 | 60.6±10.6 | Healthy | 22 | 22/0 | 61.0±8.3 | 8 | NLR/PLR/LMR | (19) |
| Xu et al,
2022 | China | Case control | 102 | 0/102 | 47.61±9.25 | Healthy | 48 | 0/48 | 48.12±9.47 | 7 | NLR | (56) |
| Wang et al,
2022 | China | Case control | 174 | 0/174 |
50±11.5a | Healthy | 181 | 0/181 | 48±10b | 7 | NLR/PLR/LMR | (36) |
| Zou et al,
2022 | China | Case control | 653 | 1/652 |
49±15.75b | Benign | 100 | 0/100 | - | 6 | NLR | (37) |
| Ding et al,
2022 | China | Cohort study | 286 | 0/286 | 52.0±12.2 | Benign | 143 | 0/143 | 42.5±14.7 | 6 | NLR | (57) |
| Alizamir et
al, 2022 | Iran |
Cross-sectional | 103 | 0/103 | - | Benign | 94 | 0/94 | - | 10 | NLR/PLR | (16) |
| Guo et al,
2022 | China | Case control | 278 | 0/278 | 50.79±11.03 | Healthy | 278 | 0/278 | 50.79±11.03 | 7 | NLR | (38) |
| Li et al,
2023 | China | Case control | 1224 | 0/1224 |
54±10.37b | Healthy | 1180 | 0/1180 |
56±8.89b | 7 | NLR/PLR/LMR | (22) |
| Tang et al,
2023 | China | Case control | 62 | 0/62 | 47.25±9.56 | i) Healthy; ii)
Benign | i) 60; ii) 104 | i) 0/60; ii)
0/104 | i) 46.23±10.98; ii)
45.39±10.36 | 7 | NLR | (39) |
 | Table II.General sensitivity and specificity
of the includes studies. |
Table II.
General sensitivity and specificity
of the includes studies.
|
| NLR | PLR | LMR |
|
|---|
|
|
|
|
|
|
|---|
| First author/s,
year | Cut-off point | Sensitivity | Specificity | Cut-off point | Sensitivity | Specificity | Cut-off point | Sensitivity | Specificity | (Refs.) |
|---|
| Ozyalvacli et
al, 2014 | 2.96 | 76 | 80 | - | - | - | - | - | - | (28) |
| Okuturlar et
al, 2015 | 2.56 | 30 | 85 | - | - | - | - | - | - | (29) |
| Qian et al,
2015 | 4.5 | 71 | 81 | - | - | - | - | - | - | (30) |
| Wu et al,
2017 | 1.9 | 77 | 67 | - | - | - | - | - | - | (44) |
| Zhao et al,
2018 | 1.995 | 85 | 87 | - | - | - | - | - | - | (31) |
| Yan et al,
2019 | 1.713 | 79 | 60 | - | - | - | - | - | - | (32) |
| Chi et al,
2020 | 1.659 | 63 | 77 | 144.339 | 30 | 93 | - | - | - | (33) |
| Peng et al,
2021 | 1.78 | 69 | 90 | 143.57 | 35 | 98 | - | - | - | (34) |
| Divsalar et
al, 2021 | 2.29 | 32 | 91 | 98.5 | 37 | 84 | - | - | - | (35) |
| Baselice et
al, 2021 | 1.598 | 84 | 38 | - | - | - | - | - | - | (55) |
| Wang et al,
2022 | 1.85 | 74 | 62 | 131.62 | 74 | 68 | 1.56 | 100 | 2.2 | (36) |
| Zou et al,
2022 | 1.58 | 69 | 74 | - | - | - | - | - | - | (37) |
| Alizamir et
al, 2022 | 1.24 | 74 | 81 | 96 | 85 | 99 | - | - | - | (16) |
| Guo et al,
2022 | 1.742 | 55 | 60 | - | - | - | - | - | - | (38) |
| Li et al,
2023 | - | - | - | 119.43 | 59 | 30 | 5.64 | 38 | 55 | (22) |
| Tang et al,
2023 | 2 | 58 | 76 | - | - | - | - | - | - | (39) |
Differences in NLR level between
patients with BC, and non-BC and healthy subjects or patients with
benign breast disease
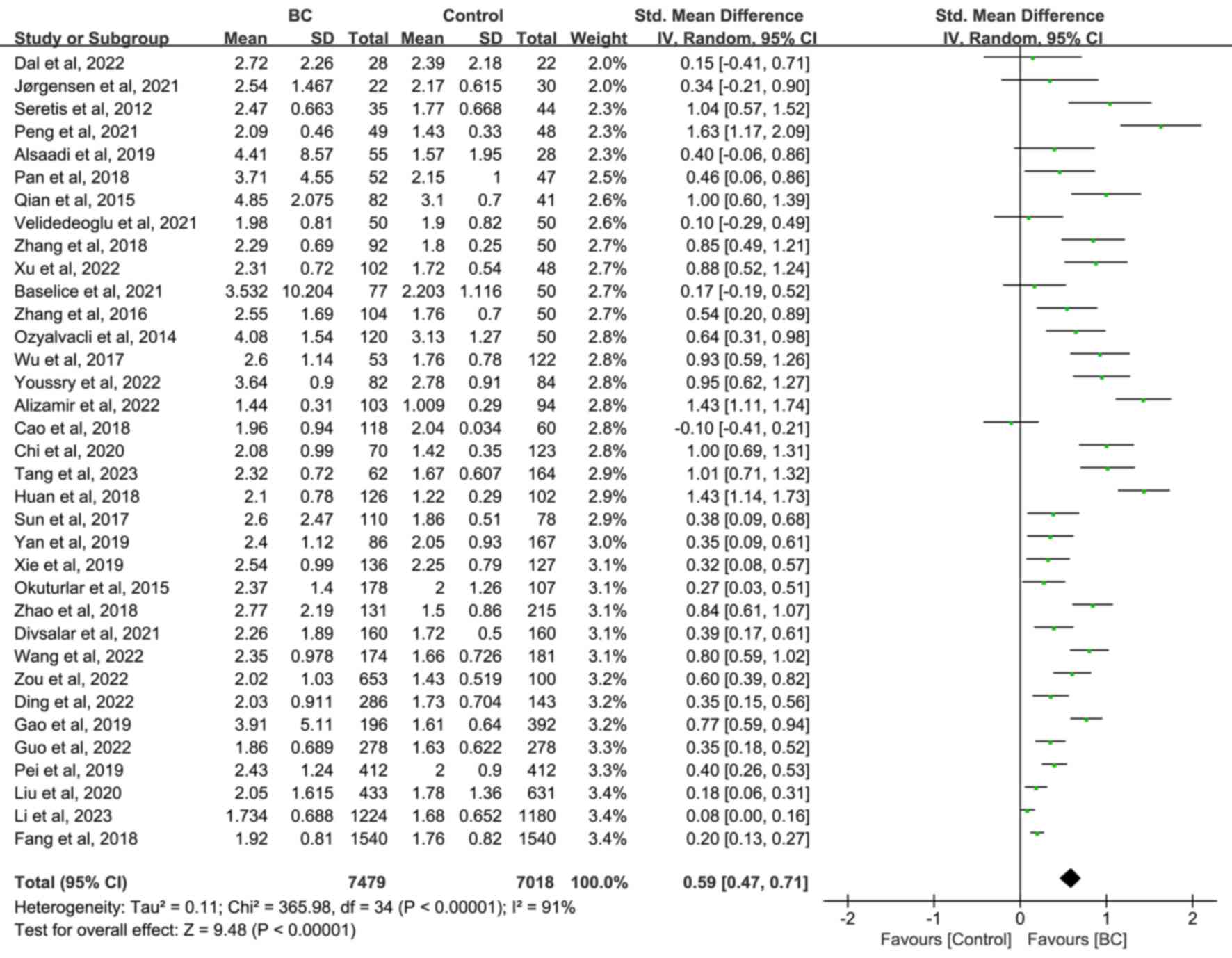
A total of 7,479 patients with BC vs. 7,018 with
non-BC (3,628 healthy and 3,390 patients with benign breast
disease) subjects were included in the meta-analysis. The random
effect analysis revealed that NLR was significantly higher in the
BC group compared with the non-BC (SMD=0.59; 95% CI, 0.47–0.71;
P<0.00001; Fig. 2), healthy
(SMD=0.56; 95% CI, 0.39, 0.73; P<0.00001; Fig. S1) and patients with benign breast
disease (SMD=0.70; 95% CI, 0.51, 0.90; P<0.00001; Fig. S2) groups. Due to heterogeneity,
further subgroup analysis was performed and the results
demonstrated that the hematology analyzer (in non-BC and healthy
subjects, and patients with benign breast disease) and study design
and NOS score (in non-BC subjects and patients with benign breast
disease) were the sources of heterogeneity (Table SI, Table SII, Table SIII).
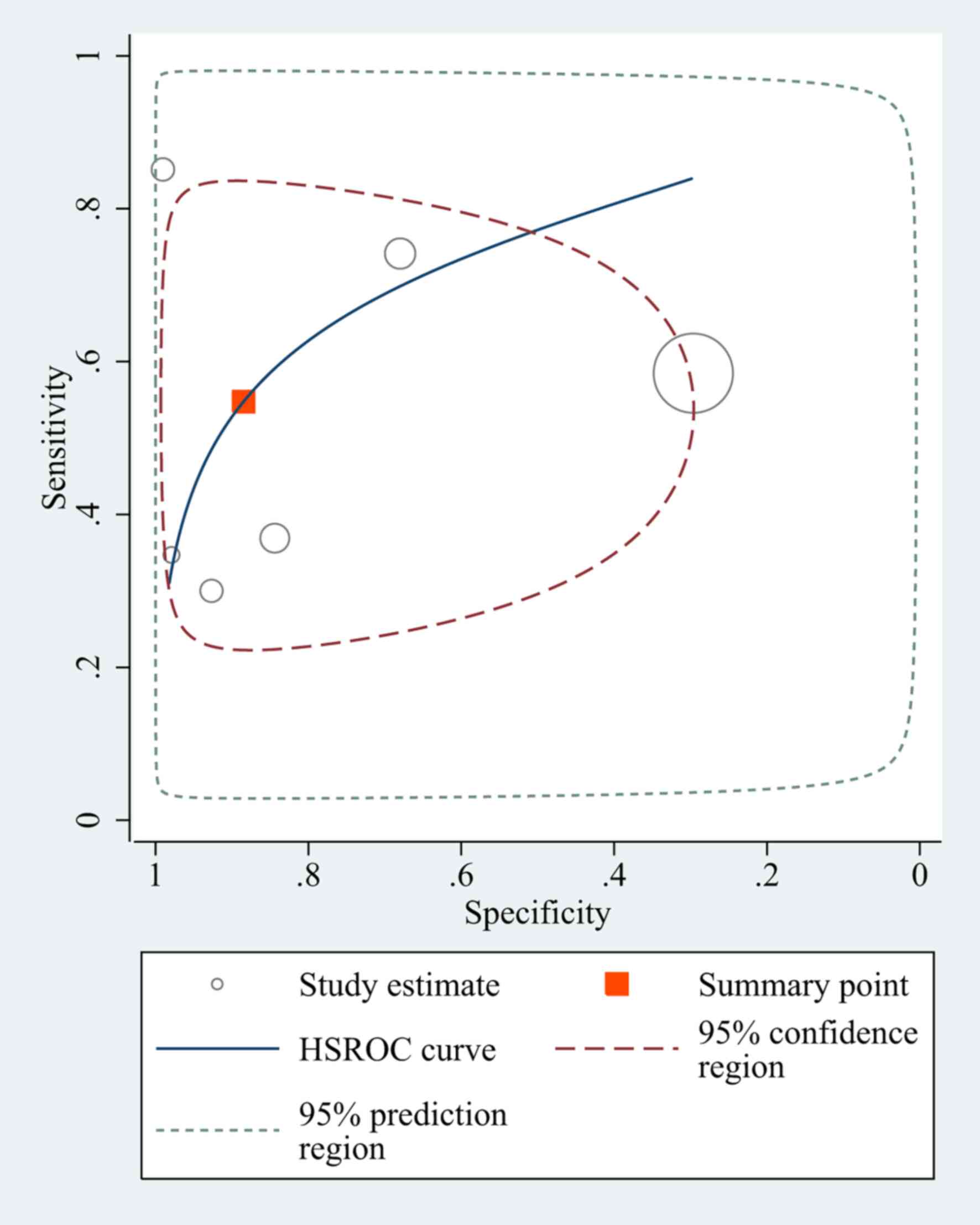
Diagnostic value of NLR for
differentiating between patients with BC and non-BC subjects
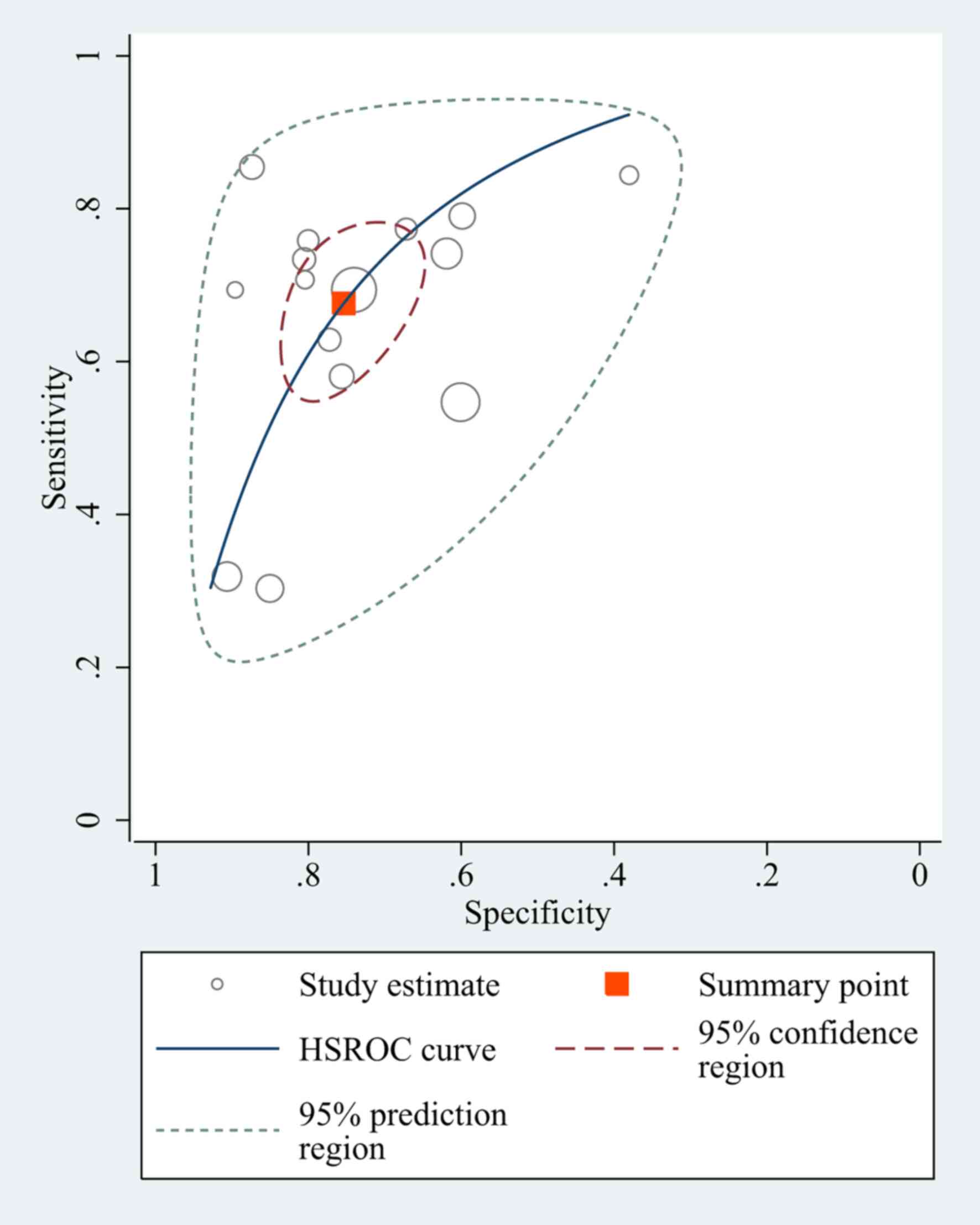
A total of 15 studies had a pooled sensitivity of
0.68 (95% CI, 0.59–0.75), and a pooled specificity of 0.75 (95% CI,
0.68–0.81). The pooled positive likelihood ratio, negative
likelihood ratio and DOR of NLR were 2.75 (95% CI, 2.15–3.51), 0.43
(95% CI, 0.34–0.54) and 6.39 (95% CI, 4.31–9.48), respectively
(Fig. 3).
Differences in PLR levels between
patients with BC, and non-BC and healthy subjects or patients with
benign breast disease
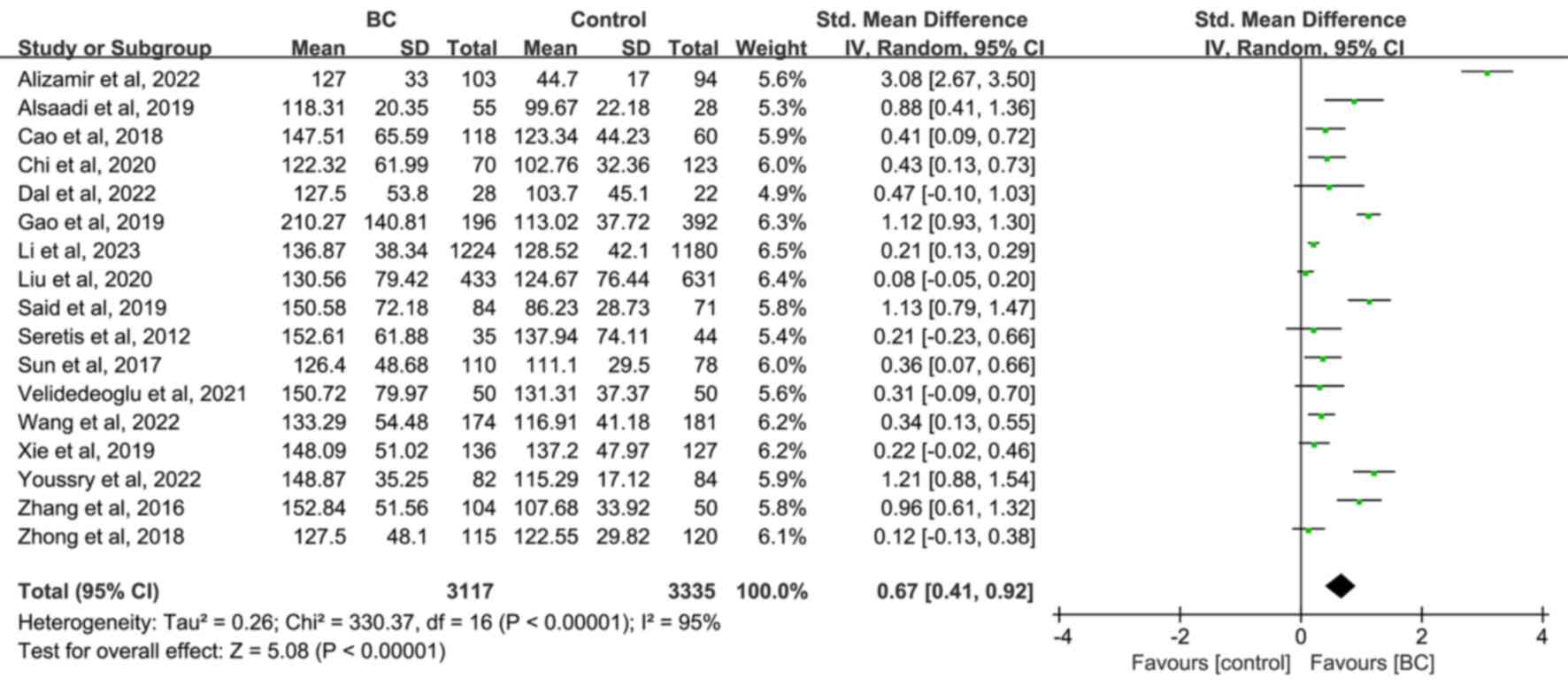
A total of 3,117 patients with BC compared with
3,335 non-BC subjects (2,903 healthy subjects and 432 patients with
benign breast disease) from 17 publications were included. The
random effect analysis revealed that PLR was significantly higher
in the BC group compared with the non-BC (SMD=0.67; 95% CI,
0.41–0.92; P<0.00001; Fig. 4),
and healthy (SMD=0.58; 95% CI, 0.35–0.81; P<0.00001; Fig. S3) groups, however it was not
significantly higher compared with the benign breast disease group
(SMD=0.95; 95% CI, 0.02–1.88; P=0.05; Fig. S4). Further subgroup analysis showed
that the hematology analyzer (in non-BC and healthy subjects),
study design, NOS score (in non-BC and healthy subjects) and region
(in patients with benign breast disease) were the sources of
heterogeneity (Table SIV, Table SV, Table SVI), whereas the study by Alizamir
et al (16) was the source
of the heterogeneity in benign subjects, with the results remaining
unchanged after exclusion (SMD=0.45; 95% CI, 0.27–0.63;
P<0.0001).
Diagnostic value of PLR for
differentiating between patients with BC and non-BC subjects
A total of sixstudies had a pooled sensitivity of
0.55 (95% CI, 0.36–0.72) and a pooled specificity of 0.88 (95% CI,
0.62–0.97). The pooled positive likelihood ratio, negative
likelihood ratio and DOR of NLR were 4.76 (95% CI, 1.17–19.39),
0.51 (95% CI, 0.32–0.81), and 9.30 (95% CI-1.65–56.3), respectively
(Fig. 5).
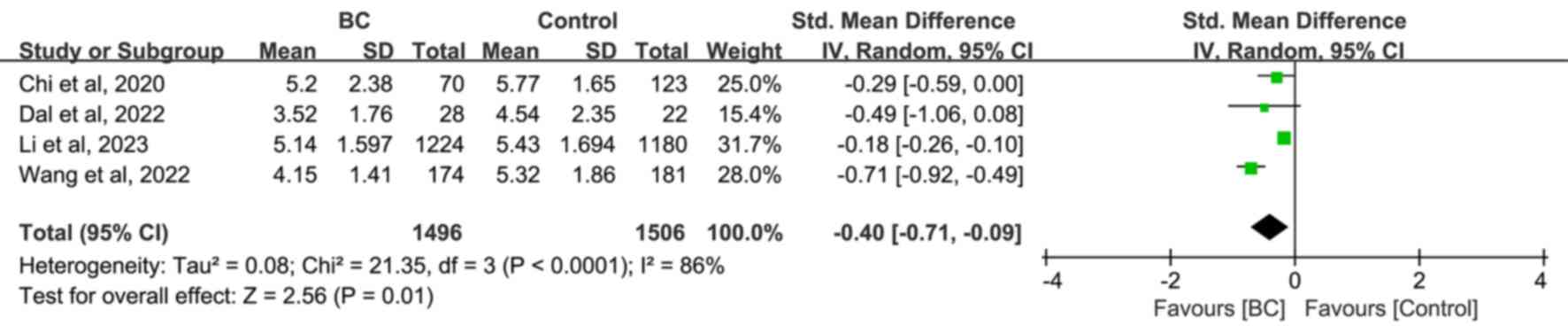
Differences in LMR levels between
patients with BC, and non-BC and healthy subjects or patients with
benign breast disease
The analysis of the pooled results from four studies
revealed that LMR was significantly lower in the BC group compared
with the non-BC [SMD=−0.40; 95% CI, -(0.71–0.09); P=0.001; Fig. 6], healthy [SMD=−0.44; 95% CI,
-(0.87–0.02); P=0.004; Fig. S5]
groups, but but was not significantly higher compared with the
benign breast disease group [SMD=−0.29; 95% CI, -(0.49–0.00);
P=0.06; Fig. S6] groups. Further
subgroup analysis demonstrated that the hematology analyzer and NOS
score were the sources of heterogeneity in non-BC and healthy
subjects, whilst patients with benign breast disease was only
included in one study (Tables SVII
and SVIII). Only two studies
analyzed both sensitivity and specificity, which meant it was not
possible to evaluate the diagnostic value of LMR. More research on
LMR is required to assess its value.
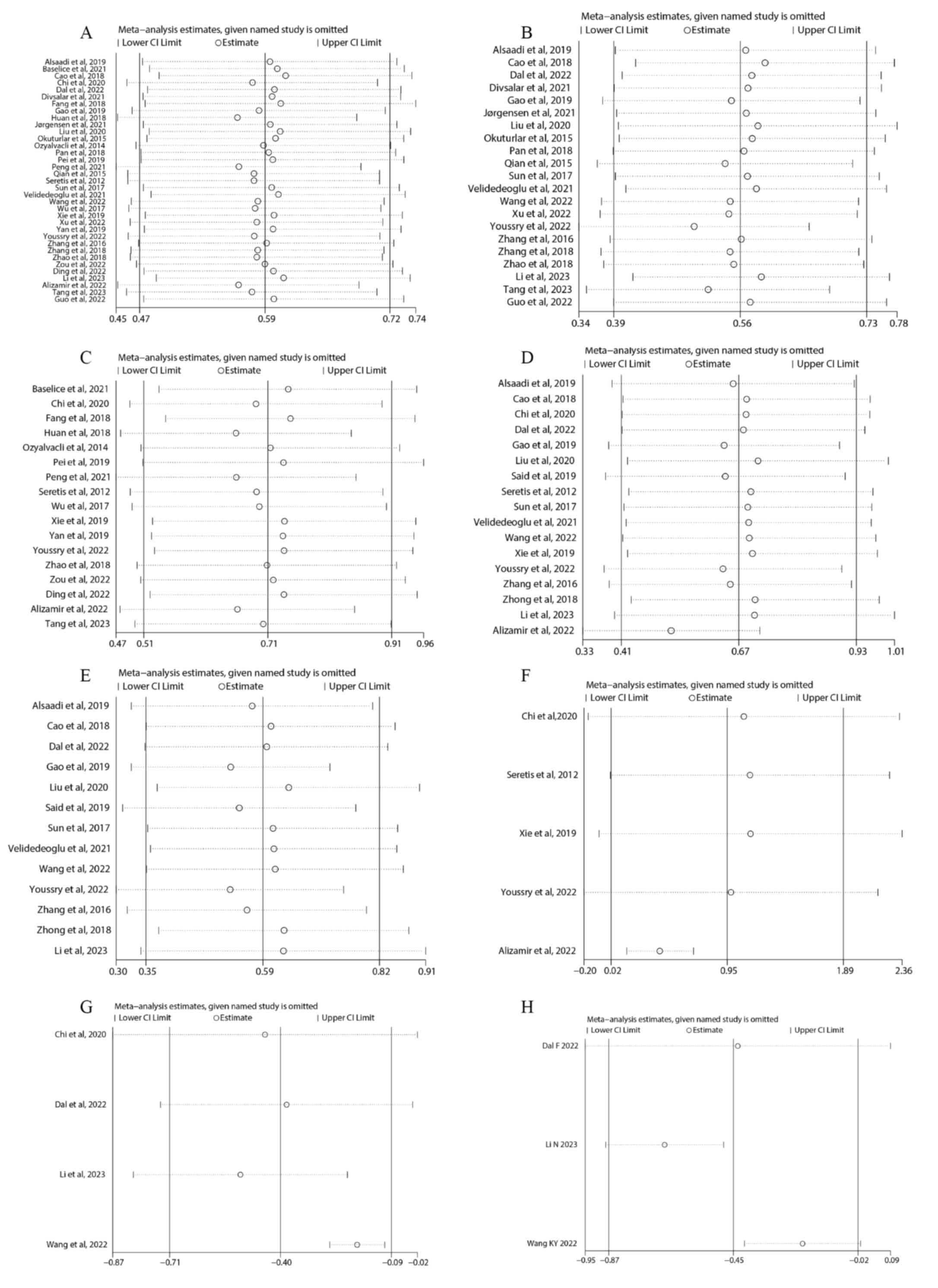
Sensitivity analysis
The present study performed a sensitivity analysis
to evaluate the robustness of the results. The pooled SMD values
did not significantly differ when single studies were removed,
suggesting that the results of the meta-analysis were stable
(Fig. 7 and Table SIX).
Publication bias
Begg's and Egger's tests and funnel plots were used
to determine publication bias. The results demonstrated that there
was no publication bias for PLR between BC and benign subjects
(Fig. S7 and Table SX). The other asymmetric funnel
plots were further processed by trimming and filling, respectively,
with no significant differences observed (Fig. S8 and Table SXI), indicating stable results. As
≤5 studies were included, the level of publication bias for LMR was
not assessed.
Discussion
The underlying mechanisms of BC are currently
unknown, but a notable number of studies have reported that tumor
initiation, progression and metastasis are influenced by the host
cancer-related inflammatory response as well as tumor
microenvironment (6,7,11,20).
Therefore, as the derived parameters of peripheral whole blood cell
counts are less invasive, more readily available and less expensive
compared with mainstream tumor markers (7), their role in cancer-associated
inflammatory responses and tumors has become a research topic of
interest. Previous systematic reviews and meta-analyses have
demonstrated that peripheral blood cell-derived parameters are
notably associated with the efficacy of neoadjuvant chemotherapy
for BC and its prognosis (6,13–15).
A cohort study also reported that NLR and PLR are associated with
an increased incidence of multiple types of cancer, including BC,
after 10 years of follow-up (27).
Researchers have retrospectively assessed the use of the NLR
(17,22,28–39)
and PLR (22,34–36) in
differentiating between BC, and healthy subjects and patients with
benign breast disease, with different conclusions. However, to the
best of our knowledge, no study has performed a systematic review
and meta-analysis of the association between BC and peripheral
blood cell-derived parameters. Therefore, the present study was
performed to address the varying results.
The current meta-analysis demonstrated that patients
with BC are associated with a higher NLR and PLR, to a medium or
large effect, and with lower LMR, to a small effect compared with
non-BC individuals (40). The
results suggest that NLR, PLR and LMR levels may influence the
pathogenesis of BC. As reported by Youssry et al (41), altered peripheral blood cells and
the cytokines they release may result in a disordered immune
response in patients with BC.
Neutrophils are associated with the release of
ectopic interleukin-8 in tumor proliferation, progression and
metastasis, whereas cancer-associated cytokines, such as tumor
necrosis factor-α and interleukin-6, contribute to neutrophilia in
solid cancers (7). Neutrophils
inhibit the cytotoxic activity of immune cells, such as
lymphocytes, natural killer cells and T cells, and reduce
regulatory T cells, leading to immune escape (7,10).
Activated platelets stimulate cancer-associated inflammation by
regulating the migration of hematopoietic and immune cells to the
tumor site and promoting metastasis (16). In contrast, lymphocytes activate the
host immune response to malignancy by inducing cancer cell death
and inhibiting proliferation and migration (17). It has been reported that elevated
NLR and PLR and lowered LMR may have potential as biomarkers for
predicting the presence of malignancy (22,38),
which may help to improve the diagnostic sensitivity for early BC
on the basis of common clinical tumor markers, and use of this data
may facilitate and improve clinical decision-making for treatment
(17). Therefore, NLR and PLR are
prospective biomarkers for predicting the pathogenesis of BC.
However, these results should be interpreted with caution due to
heterogeneity. Given that these indicators are simple, inexpensive,
readily available and less invasive, they are especially suitable
for BC screening in underdeveloped countries.
The present study has certain limitations: i) The
funnel plot and Egger's tests indicate a slight publication bias,
with no significant change in direction or magnitude, suggesting
that the results are still acceptable after trimming and filling;
ii) the meta-analysis had high heterogeneity, and the hematology
analyzer was the most important source of heterogeneity, but it had
no impact on the robustness of the results. The direction and
significance of results for NLR, PLR and LMR did not change in
subgroups of hematology analysis, but PLR did not show significance
when compared with the benign group. The possible reason is the use
of different measurement methods to measure blood cell counts
(42), but still provide evidence
of a meaningful benefit of a higher NLR and PLR, and a lower LMR in
BC as possible potential markers; iii) the geographic concentration
of the literature was skewed towards the East Asian region, which
may limit the generalizability of the findings. However, in
subgroup analysis, the direction of the results did not change,
regardless of whether the focus was on East Asian populations.
Furthermore, the consistency of the results makes the findings more
generalizable; and iv) most of the included studies excluded
patients with diseases affecting indices, such as acute or chronic
infection, hepatic and renal dysfunction, steroid therapy,
inflammatory diseases and hematological disorders. This exclusion
criterion increases the validity of the present results. Meanwhile,
this exclusion may limit the generalizability of the present
findings. Based on the study populations, the NLR and PLR may be
used in clinical practice to distinguish patients with BC; however,
more real-world application data are still required to support this
conclusion.
In summary, the present systematic review and
meta-analyses demonstrated that higher NLR and PLR and lower LMR
were associated with the presence of BC. These findings indicate
that NLR and PLR may be potential blood-based biomarkers for the
differentiation of BC. However, further research is needed to
validate their clinical applicability and use.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the Health
Commission of Xinjiang Uygur Autonomous Region's ‘Tianshan Ying
Cai’ medical and health high level personnel training project
(grant no. TSYC202301B154) and The Science & Technology
Department of Xinjiang Uygur Autonomous Region's major science and
technology project (grant no. 2022A02013-3).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DY, HW, DA, JZ, QZ, JL, HL and XG contributed to the
conception and design of the study. Material preparation and data
collection and analysis were performed by DY, HW, DA, QZ, XG and
JZ. The first draft of the manuscript was written by DY, QZ, JL and
HL, and all authors commented on previous versions of the
manuscript. DY and HW confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
PLR
|
platelet-to-lymphocyte ratio
|
|
LMR
|
lymphocyte-to-monocyte ratio
|
|
BC
|
breast cancer
|
|
SMD
|
standardized mean differences
|
|
CI
|
confidence interval
|
|
PRISMA
|
Preferred Reporting Items for
Systematic Review and Meta-Analysis
|
|
NOS
|
Newcastle-Ottawa Scale
|
|
ROC
|
receiver operating characteristic
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pashayan N, Antoniou AC, Ivanus U,
Esserman LJ, Easton DF, French D, Sroczynski G, Hall P, Cuzick J,
Evans DG, et al: Personalized early detection and prevention of
breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol.
17:687–705. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xia C, Basu P, Kramer BS, Li H, Qu C, Yu
XQ, Canfell K, Qiao Y, Armstrong BK and Chen W: Cancer screening in
China: A steep road from evidence to implementation. Lancet Public
Health. 8:e996–e1005. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Santen RJ and Yue W: Cause or prevention
of breast cancer with estrogens: Analysis from tumor biologic data,
growth kinetic model and Women's Health Initiative study.
Climacteric. 22:3–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martei YM, Dauda B and Vanderpuye V:
Breast cancer screening in sub-Saharan Africa: A systematic review
and ethical appraisal. BMC Cancer. 22:2032022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
The Society of Breast Cancer China
Anti-Cancer Association, Breast Oncology Group of the Oncology
Branch of the Chinese Medical Association, . Guidelines for breast
cancer diagnosis and treatment by China Anti-cancer Association
(2024 edition). China Oncol. 33:1092–1187. 2023.(In Chinese).
|
|
7
|
Ahmed M and Kabel: Tumor markers of breast
cancer: New prospectives. J Oncol Sci. 3:5–11. 2017. View Article : Google Scholar
|
|
8
|
Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W
and Deng M: Prognostic value of neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio for breast cancer patients: An updated
meta-analysis of 17079 individuals. Cancer Med. 8:4135–4148. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oktay K, Santaliz-Casiano A, Patel M,
Marino N, Storniolo AMV, Torun H, Acar B and Madak Erdogan Z: A
Computational statistics approach to evaluate blood biomarkers for
breast cancer risk stratification. Horm Cancer. 11:17–33. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jørgensen N, Lænkholm AV, Sækmose SG,
Hansen LB and Hviid TVF: Peripheral blood immune markers in breast
cancer: Differences in regulatory T cell abundance are related to
clinical parameters. Clin Immunol. 232:1088472021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang Q, Tong YW, Wang G, Zhang N, Chen WG,
Li YF, Shen KW, Wu BW and Chen XS: Neutrophil-to-lymphocyte ratio,
obesity, and breast cancer risk in Chinese population. Medicine
(Baltimore). 97:e116922018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Said NM: Three gold indicators for breast
cancer prognosis: A case-control study with ROC analysis for novel
ratios related to CBC with (ALP and LDH). Mol Biol Rep.
46:2013–2027. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Q, Dong J, Sun Q, Lu N, Pan Y and Han
X: Role of neutrophil-to-lymphocyte ratio as a prognostic biomarker
in patients with breast cancer receiving neoadjuvant chemotherapy:
A meta-analysis. BMJ Open. 11:e0479572021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, He M, Wang C, Zhang X and Cai S:
Prognostic value of neutrophil-to-lymphocyte ratio for patients
with triple-negative breast cancer: A meta-analysis. Medicine
(Baltimore). 101:e298872022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cullinane C, Creavin B, O'Leary DP,
O'Sullivan MJ, Kelly L, Redmond HP and Corrigan MA: Can the
neutrophil to lymphocyte ratio predict complete pathologic response
to neoadjuvant breast cancer treatment? A Systematic Review and
Meta-analysis. Clin Breast Cancer. 20:e675–e681. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alizamir A, Dehghan Azad S, Pirdehghan A
and Moradi A: Preoperative neutrophil: Lymphocyte ratio, platelet:
Lymphocyte ratio, and C-reactive protein levels predictive value in
determining the severity of breast mass. Iran J Pathol. 17:413–418.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun H, Yin CQ, Liu Q, Wang F and Yuan CH:
Clinical significance of routine blood test-associated inflammatory
index in breast cancer patients. Med Sci Monit. 23:5090–5095. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nøst TH, Alcala K, Urbarova I, Byrne KS,
Guida F, Sandanger TM and Johansson M: Systemic inflammation
markers and cancer incidence in the UK Biobank. Eur J Epidemiol.
36:841–848. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dal F, Ökmen H, Ulusan K, Havare SB, Orhan
B, Çolak Ş, Ferlengez E and Sari S: Hemogram index parameters in
the evaluation of male breast cancer and inflammatory response: A
case-control study. Rev Assoc Med Bras. 68:94–99. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Velidedeoglu M, Kundaktepe BP, Aksan H and
Uzun H: Preoperative fibrinogen and hematological indexes in the
differential diagnosis of idiopathic granulomatous mastitis and
breast cancer. Medicina (Kaunas). 57:6982021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seretis C, Seretis F, Lagoudianakis E,
Politou M, Gemenetzis G and Salemis NS: Enhancing the accuracy of
platelet to lymphocyte ratio after adjustment for large platelet
count: A pilot study in breast cancer patients. Int J Surg Oncol.
2012:6536082012.PubMed/NCBI
|
|
22
|
Li N, Cao L, Zhao K and Feng Y:
Development and validation of a nomogram to predict Chinese breast
cancer risk based on clinical serum biomarkers. Biomark Med.
17:273–286. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Y, Xu D, Song H, Qiu B, Tian D, Li
Z, Ji Y and Wang J: Inflammation and nutrition-based biomarkers in
the prognosis of oesophageal cancer: A systematic review and
meta-analysis. BMJ Open. 11:e0483242021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhadauria US, Purohit B, Nilima N and
Priya H: Oral health in individuals with bleeding disorders: A
systematic review and meta-analysis. Haemophilia. 30:658–670. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deeks JJ and Higgins JPT; Altman DG
(eds.); on behalf of the Cochrane Statistical Methods Group, :
Chapter 10: Analysing data and undertaking meta-analyses In:
Cochrane Handbook for Systematic Reviews of Interventions.
https://training.cochrane.org/handbook/current/chapter-10#section-10-10-4-1August
22–2023
|
|
27
|
Rimini M, Casadei-Gardini A, Ravaioli A,
Rovesti G, Conti F, Borghi A, Dall'Aglio AC, Bedogni G, Domenicali
M, Giacomoni P, et al: Could inflammatory indices and metabolic
syndrome predict the risk of cancer development? Analysis from the
bagnacavallo population study. J Clin Med. 9:11772020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ozyalvacli G, Yesil C, Kargi E, Kizildag
B, Kilitci A and Yilmaz F: Diagnostic and prognostic importance of
the neutrophil lymphocyte ratio in breast cancer. Asian Pac J
Cancer Prev. 15:10363–10366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okuturlar Y, Gunaldi M, Tiken EE, Oztosun
B, Inan YO, Ercan T, Tuna S, Kaya AO, Harmankaya O and Kumbasar A:
Utility of peripheral blood parameters in predicting breast cancer
risk. Asian Pac J Cancer Prev. 16:2409–2412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian P, Yun HZ, Zhu L and Zhang YY: The
value of inflammatory markers in preoperative diagnosis and
prognostic evaluation of breast cancer. Lab Med Clin. 12:3765–3767.
2015.
|
|
31
|
Weiwei Z, Linlin X, Xiufen L, You P, Huang
dingding, Yuping W and Jing F: Preoperative peripheral blood
neutrophil-to-lymphocyte ratio in the diagnosis of breast cancer.
Lab Med. 33:209–212. 2018.(In Chinese).
|
|
32
|
Yan X, Zhang HZ, Yan J, Peng HW and Wu X:
The value of preoperative peripheral blood NLR in the differential
diagnosis of benign and malignant breast masses. Jiangsu Med J.
45:638–641. 2019.(In Chinese).
|
|
33
|
Tao C, Mingming Y and Zhiqi H: Application
value of preoperative peripheral blood multi-index combined
detection and analysis in the diagnosis of breast cancer. Chin J
Cancer Prev Treat. 27:730–734. 2020.(In Chinese).
|
|
34
|
Peng F, Luo P and Li L: Correlation
analysis of preoperative inflammatory indicators and
clinicopathological features in breast cancer patients. J Bengbu
Med Coll. 46:1208-1211-1215. 2021.(In Chinese).
|
|
35
|
Divsalar B, Heydari P, Habibollah G and
Tamaddon G: Hematological parameters changes in patients with
breast Cancer. Clin Lab. 672021.PubMed/NCBI
|
|
36
|
Wang K, Zhang P, Su B, Wang H, Dong X and
Yang Q: Analysis of blood routine test and PLR, NLR, LMR in breast
cancer patients. Lab Med Clin. 19:84–89. 2022.(In Chinese).
|
|
37
|
Zou H, Liu SH, Yang R, Wu XJ, Cao YP and
Huang HF: Combination of Neutrophil-to-Lymphocyte ratio and red
cell distribution width with serum tumor markers for the
differential diagnosis of breast cancer and its association with
pathological features and molecular types. Clin Breast Cancer.
22:e526–e535. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo M, Bai Y and Zhang J: Application of
peripheral blood NLR expression level in the adjuvant diagnosis of
breast cancer. Systems Medi. 7:14–17+22. 2022.
|
|
39
|
Tang CL, Li Y and Zhang XF: Diagnostic
value of preoperative neutrophil lymphocyte count ratio combined
with carbohydrate antigen 153 in breast cancer. Lab Med Clin.
38:237–240. 2023.(In Chinese).
|
|
40
|
Schober P, Mascha EJ and Vetter TR:
Statistics from A (Agreement) to Z (z Score): A Guide to
interpreting common measures of association, agreement, diagnostic
accuracy, effect size, heterogeneity, and reliability in medical
research. Anesth Analg. 133:1633–1641. 2021.PubMed/NCBI
|
|
41
|
Youssry S, Hussein A, Ramadan R,
Alkarmouty A and Elsheredy A: The association of human
cytomegalovirus with biomarkers of inflammation and immune
activation in breast cancer. Breast Dis. 41:229–239. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Velizarova M, Yacheva T, Genova M and
Svinarov D: Evaluation of automated hematology analyzer DYMIND DH76
compared to SYSMEX XN 1000 system. J Med Biochem. 40:367–377. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Zhao R, Gu G, Wu L, Li X, Huang B
and Peng Q: Change and significance of partial peripheral blood
biochemical indicators in breast cancer patients. Lab Med Clin.
13:2153–2155. 2016.(In Chinese).
|
|
44
|
Wei W, Wen T, Xin M, Shaoping S, Yongliang
Z and Yanjun Z: Diagnostic value of neutrophil lymphocyte ratio for
intraductal papillary neoplasms of breast. Acad J Chin PLA Med Sch.
38:628–630+661. 2017.
|
|
45
|
Yu H, Xushan C, Jiajun J and Chunli Z: The
Predictive value of preoperative neutrophil-to lymphocyte ratio for
breast cancer and its relationship with Beclin1. Lab Med Clin.
15:3667–3669+3673. 2018.
|
|
46
|
Zhenzhen P, Minmin Y, Yingge C, Jiuling D,
Meiqiu Y and Jianli G: Abnormal granulocyte differentiation and the
paradoxical switch of transforming growth factor-β1 in breast
cancer patients. J South Med Univ. 38:856–860. 2018.
|
|
47
|
Cao L, Peng X, Jin L and Su Z: Clinical
significance of peripheral blood related indexes in patients with
breast cancer. Medicine and Health 4. 192018.(In Chinese).
|
|
48
|
Lulu Z, Yun L, Wenbing D, Wen T, Xiaoying
L, Wanhui Z and Bingchang Z: Diagnostic values of peripheral blood
indexes and tumor markers for breast cancer. China Med. 13:421–425.
2018.
|
|
49
|
Tianhua Z, Wenqiang W, Zhenhui C and
Xiaoping M: Clinical significance of preoperative platelet to
lymphocyte ratio and red cell distribution width in patients with
breast cancer. Hainan Med J. 29:2284–2287. 2018.
|
|
50
|
Pei Y and Qian YQ: Analysis of the
relationship between body mass index, neutrophil-to-lymphocyte
ratio and the risk of breast cancer. Baojianwenhui. 9:226–227.
2019.(In Chinese).
|
|
51
|
Alsaadi JHH and Younus BM: Study of some
biochemical and blood parameters as screening markers for breast
cancer patients before adjuvant therapy in Thi Qar
Government-southern Iraq. J Global Pharma Technol. 11:236–244.
2009.
|
|
52
|
Xiaolin X, Xiaoqun Y, Menglu L and Bing L:
Analysis of hematological indexes and clinical features i breast
cancer patients. Hainan Med J. 30:186–188, (In Chinese).
|
|
53
|
Gao X, Yin J, Wang X, Petersen F and Yu X:
A comprehensive comparison of hematological parameters among 39
common diseases. Scand J Clin Lab Invest. 79:251–259. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Congfang L, Baoxiang W, Yuhui W, Zhi X,
Wei Z and Sufang W: Relationship between NLR, PLR and breast
cancer. J Clin Res. 37:1184–1187. 2020.(In Chinese).
|
|
55
|
Baselice S, Castaldo R, Giannatiempo R,
Casaretta G, Franzese M, Salvatore M and Mirabelli P: Impact of
breast tumor onset on blood count, Carcinoembryonic antigen, cancer
antigen 15-3 and lymphoid subpopulations supported by automatic
classification approach: A pilot study. Cancer Control.
28:107327482110486122021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Meng X, Bo S and Hongquan C: Changes of
some peripheral blood indexes and diagnostic value of serum CA125,
CA153 and CEA in patients with breast cancer. Chinese Foreign Med
Res. 20:66–70. 2022.(In Chinese).
|
|
57
|
Ding H, Xu J, Wang F, Zhang Q, Pan H, Mu
Y, Gu CR, Miao SX, Li XN, Ju HY, et al: Differential diagnosis
model of benign and malignant breast BI-RADS category 4 nodules
based on serum SP70 and conventional laboratory indicators. Chin J
Prev Med. 56:1774–1783. 2022.(In Chinese).
|