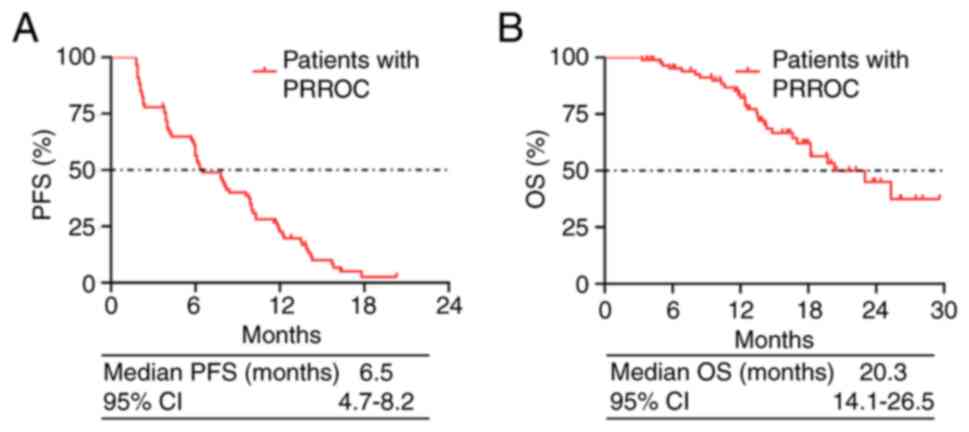
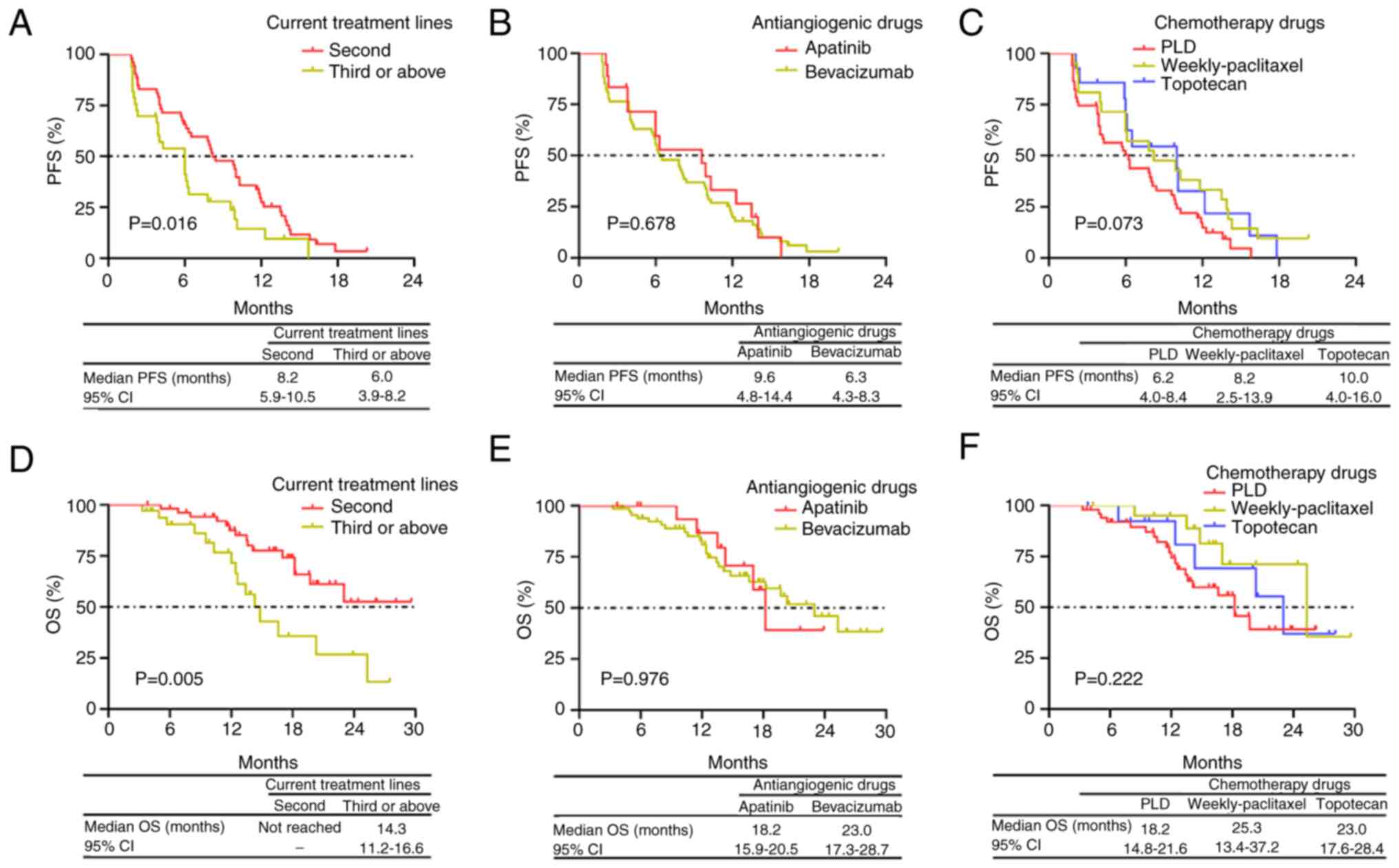
|
1
|
Momenimovahed Z, Tiznobaik A, Taheri S and
Salehiniya H: Ovarian cancer in the world: Epidemiology and risk
factors. Int J Womens Health. 11:287–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazidimoradi A, Momenimovahed Z, Allahqoli
L, Tiznobaik A, Hajinasab N, Salehiniya H and Alkatout I: The
global, regional and national epidemiology, incidence, mortality,
and burden of ovarian cancer. Health Sci Rep. 5:e9362022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ali AT, Al-Ani O and Al-Ani F:
Epidemiology and risk factors for ovarian cancer. Prz Menopauzalny.
22:93–104. 2023.PubMed/NCBI
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zamwar UM and Anjankar AP: Aetiology,
epidemiology, histopathology, classification, detailed evaluation,
and treatment of ovarian cancer. Cureus. 14:e305612022.PubMed/NCBI
|
|
6
|
Chandra A, Pius C, Nabeel M, Vishwanatha
JK, Ahmad S and Basha R: Ovarian cancer: Current status and
strategies for improving therapeutic outcomes. Cancer Med.
8:7018–7031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luvero D, Milani A and Ledermann JA:
Treatment options in recurrent ovarian cancer: Latest evidence and
clinical potential. Ther Adv Med Oncol. 6:229–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh N, Jayraj AS, Sarkar A, Mohan T,
Shukla A and Ghatage P: Pharmacotherapeutic treatment options for
recurrent epithelial ovarian cancer. Expert Opin Pharmacother.
24:49–64. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Havasi A, Cainap SS, Havasi AT and Cainap
C: Ovarian Cancer-Insights into platinum resistance and overcoming
it. Medicina (Kaunas). 59:5442023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Richardson DL, Eskander RN and O'Malley
DM: Advances in ovarian cancer care and unmet treatment needs for
patients with platinum resistance: A narrative review. JAMA Oncol.
9:851–859. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elyashiv O, Aleohin N, Migdan Z, Leytes S,
Peled O, Tal O and Levy T: The poor prognosis of acquired secondary
platinum resistance in ovarian cancer patients. Cancers (Basel).
16:6412024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mei C, Gong W, Wang X, Lv Y, Zhang Y, Wu S
and Zhu C: Anti-angiogenic therapy in ovarian cancer: Current
understandings and prospects of precision medicine. Front
Pharmacol. 14:11477172023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang T, Tang J, Yang H, Yin R, Zhang J,
Zhou Q, Liu Z, Cao L, Li L, Huang Y, et al: Effect of apatinib plus
pegylated liposomal doxorubicin vs pegylated liposomal doxorubicin
alone on platinum-resistant recurrent ovarian cancer: The APPROVE
randomized clinical trial. JAMA Oncol. 8:1169–1176. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA Open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geng A, Yang H, Wang Z and Wu C: Apatinib
plus paclitaxel versus paclitaxel monotherapy for
platinum-resistant recurrent ovarian cancer treatment: A
retrospective cohort study. J Clin Pharm Ther. 47:2264–2273. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Armstrong DK, Alvarez RD, Bakkum-Gamez JN,
Barroilhet L, Behbakht K, Berchuck A, Berek JS, Chen LM, Cristea M,
DeRosa M, et al: NCCN guidelines insights: Ovarian cancer, version
1.2019. J Natl Compr Canc Netw. 17:896–909. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz LH, Litiere S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Armstrong DK, Alvarez RD, Bakkum-Gamez JN,
Barroilhet L, Behbakht K, Berchuck A, Chen LM, Cristea M, DeRosa M,
Eisenhauer EL, et al: Ovarian cancer, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
19:191–226. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berek JS, Renz M, Kehoe S, Kumar L and
Friedlander M: Cancer of the ovary, fallopian tube, and peritoneum:
2021 update. Int J Gynaecol Obstet. 155 (Suppl 1):S61–S85. 2021.
View Article : Google Scholar
|
|
21
|
Vergote I, Gonzalez-Martin A, Lorusso D,
Gourley C, Mirza MR, Kurtz JE, Okamoto A, Moore K, Kridelka F,
McNeish I, et al: Clinical research in ovarian cancer: Consensus
recommendations from the Gynecologic Cancer InterGroup. Lancet
Oncol. 23:e374–e384. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le T, Hopkins L, Baines KA, Rambout L, Al
Hayki M and Kee Fung MF: Prospective evaluations of continuous
weekly paclitaxel regimen in recurrent platinum-resistant
epithelial ovarian cancer. Gynecol Oncol. 102:49–53. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukumura D, Kloepper J, Amoozgar Z, Duda
DG and Jain RK: Enhancing cancer immunotherapy using
antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol.
15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu H, Li J, Liu Q, Tang M and Wang Y:
Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor
growth in cervical cancer and synergizes with Paclitaxel. Cell
Cycle. 17:1235–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Volk LD, Flister MJ, Chihade D, Desai N,
Trieu V and Ran S: Synergy of nab-paclitaxel and bevacizumab in
eradicating large orthotopic breast tumors and preexisting
metastases. Neoplasia. 13:327–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rapisarda A, Hollingshead M, Uranchimeg B,
Bonomi CA, Borgel SD, Carter JP, Gehrs B, Raffeld M, Kinders RJ,
Parchment R, et al: Increased antitumor activity of bevacizumab in
combination with hypoxia inducible factor-1 inhibition. Mol Cancer
Ther. 8:1867–1877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cesca M, Morosi L, Berndt A, Fuso Nerini
I, Frapolli R, Richter P, Decio A, Dirsch O, Micotti E, Giordano S,
et al: Bevacizumab-Induced inhibition of angiogenesis promotes a
more homogeneous intratumoral distribution of paclitaxel, improving
the antitumor response. Mol Cancer Ther. 15:125–135. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang H, Geng A, Wang Z and Wu C: Efficacy
and safety of apatinib combined with liposomal doxorubicin or
paclitaxel versus liposomal doxorubicin or paclitaxel monotherapy
in patients with recurrent platinum-resistant ovarian cancer. J
Obstet Gynaecol Res. 49:1611–1619. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fukuda T, Noda T, Uchikura E, Awazu Y,
Tasaka R, Imai K, Yamauchi M, Ichimura T, Yasui T and Sumi T:
Real-world efficacy and safety of bevacizumab for advanced or
recurrent mullerian cancer: A Single-institutional experience.
Anticancer Res. 43:3097–3105. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ford CE, Werner B, Hacker NF and Warton K:
The untapped potential of ascites in ovarian cancer research and
treatment. Br J Cancer. 123:9–16. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simion L, Rotaru V, Cirimbei C, Stefan DC,
Gherghe M, Ionescu S, Tanase BC, Luca DC, Gales LN and Chitoran E:
Analysis of efficacy-to-safety ratio of angiogenesis-inhibitors
based therapies in ovarian cancer: A systematic review and
Meta-analysis. Diagnostics (Basel). 13:10402023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Lin H, Ou Y, Fu H, Tsai C, Chien
CC and Wu C: Safety analysis of bevacizumab in ovarian cancer
patients. J Clin Med. 12:20652023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verschraegen CF, Czok S, Muller CY, Boyd
L, Lee SJ, Rutledge T, Blank S, Pothuri B, Eberhardt S and Muggia
F: Phase II study of bevacizumab with liposomal doxorubicin for
patients with Platinum- and taxane-resistant ovarian cancer. Ann
Oncol. 23:3104–3110. 2012. View Article : Google Scholar : PubMed/NCBI
|