Introduction
Ovarian cancer (OC) commonly occurs in
postmenopausal women and accounts for more than two-thirds of
gynaecological cancer-related mortalities (1–3). In
2020, a total of 313,959 new cases and 207,252 mortalities due to
OC were recorded worldwide (4).
Cytoreductive surgery followed by platinum-based chemotherapy is
the standard treatment strategy for patients with OC (5,6).
Notably, 70.0–80.0% of patients with OC suffer from disease
recurrence and the majority of these patients will eventually
develop platinum resistance through multiple recurrences (7–10).
Currently, platinum resistance is a noteworthy obstacle that
negatively affects the survival of patients with OC (11).
Previously, studies report that antiangiogenic
therapy can inhibit neovascularisation and the supply of nutrients
and oxygen to cancer cells, thus suppressing tumour proliferation,
invasion and metastasis (12,13).
Emerging evidence also suggests that antiangiogenic therapy, such
as bevacizumab and apatinib, plus chemotherapy is a promising
treatment approach for patients with platinum-resistant recurrent
OC (PRROC) (14–16). For example, the randomised clinical
trial APPROVE indicates that apatinib plus pegylated liposomal
doxorubicin (PLD) increases progression-free survival (PFS) with
tolerable adverse effects compared with PLD alone in patients with
PRROC (14). Another study
demonstrates that bevacizumab plus chemotherapy (PLD,
weekly-paclitaxel or topotecan) increases the objective response
rate (ORR) and PFS, but does not increase overall survival (OS)
when compared with chemotherapy alone in patients with PRROC
(15). Additionally, a study
reveals that apatinib plus paclitaxel increases PFS and OS with
acceptable toxicity compared with paclitaxel monotherapy in the
same patients (16). Notably, the
aforementioned studies include patients with PRROC who are only
treated with one type of antiangiogenic drug, either apatinib or
bevacizumab, plus chemotherapy. Therefore, evaluating the efficacy
and safety of the different antiangiogenic drugs (bevacizumab or
apatinib) plus chemotherapy could provide further clinical evidence
for the application of this treatment modality.
The present study aimed to investigate the efficacy
and safety profile of antiangiogenic therapy (bevacizumab or
apatinib) plus chemotherapy in patients with PRROC.
Materials and methods
Patients
In the present retrospective study, a total of 86
female patients with PRROC that received antiangiogenic therapy
plus chemotherapy between July 2019 and May 2023 at the People's
Hospital of Zhongshan City (Zhongshan, China) were selected.
Patient data from the hospital records were accessed from April 19,
2024 to April 29, 2024. The present study obtained permission from
the Ethics Committee of People's Hospital of Zhongshan City
(approval no. 2024-033; Zhongshan, China). The patients signed the
informed consent form, or, if the patients were unable to sign,
their family members signed it on their behalf.
Inclusion and exclusion criteria
Inclusion criteria were as follows: i) Patients
diagnosed with epithelial ovarian, primary peritoneal or fallopian
tube cancer (17); ii) aged ≥18
years; iii) treated with antiangiogenic therapy plus chemotherapy;
iv) patients with an Eastern Cooperative Oncology Group (ECOG)
performance status (PS) score of ≤2; v) experienced disease
progression within 6 months after receiving platinum-based
chemotherapy; and vi) displayed at least one assessable lesion
according to the Response Evaluation Criteria in Solid Tumors
(RECIST) version 1.1 (18).
The exclusion criteria were as follows: i) Patients
with uncontrolled hypertension; ii) patients with coagulation
disorders; iii) patients with a history of bleeding; iv) patients
with gastrointestinal perforation; and v) patients with no
available follow-up data.
Treatment
Treatment data from patients with PRROC were
collected from the electronic medical system. The treatment
regimens were provided according to the National Comprehensive
Cancer Network (NCCN) guideline and the condition and requests of
the patient (19). The
antiangiogenic drugs used were bevacizumab (10 mg/kg once every 2
weeks) or apatinib (250 mg once every day). The chemotherapy drugs
were PLD (40 mg/m2 once every 4 weeks), paclitaxel (80
mg/m2 once every week) or gemcitabine (1,000
mg/m2 on days 1 and 8 every 3 weeks). The dose of each
drug was prescribed as recommended based on the NCCN guideline and
was adjusted based on the condition of the patient (19). Every cycle was defined as 4 weeks of
treatment with the aforementioned drugs.
Follow-up and assessment data
Follow-up data from patients with PRROC were
obtained from the electronic medical system. Patients were followed
up every two cycles of therapy and their condition was evaluated
using magnetic resonance imaging or computed tomography (20). The median duration of follow-up was
13.7 months and the final follow-up date was August 2023. The
treatment response of patients with PRROC was assessed based on
RECIST version 1.1 and the best response was selected for
evaluation. ORR and disease control rate (DCR) were also measured.
PFS and OS were determined based on the follow-up data.
Additionally, the adverse events were retrieved from the medical
records of patients with PRROC.
Statistical analysis
All statistical analyses were performed using SPSS
version 24.0 software (IBM Corp). For sample size calculation, an
ORR of 0.3 and a confidence interval width of 0.2 was assumed.
Assuming a dropout rate of 5%, the minimum sample size required was
86. The characteristics, treatment response and adverse events of
patients with PRROC were assessed using descriptive statistics. The
association between the current treatments, antiangiogenic drugs
and chemotherapy drugs with PFS and OS was examined using
Kaplan-Meier curves. All data were analysed using a log-rank test.
Univariate and multivariate (stepwise forward) Cox regression
analyses were performed to determine the factors that affect PFS
and OS. P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline features of patients with
PRROC
In the present study, the median age of patients
with PRROC was 60.5 years [interquartile range (IQR), 51.0–67.0
years). A total of 32 (37.2%) patients presented with ascites.
Moreover, 53 (61.6%) patients received antiangiogenic therapy plus
chemotherapy as a second-line treatment and the remaining 33
(38.4%) patients received antiangiogenic therapy plus chemotherapy
as a third-line or above treatment. Regarding the antiangiogenic
drugs, 68 (79.1%) patients were treated with bevacizumab and 18
(20.9%) patients received apatinib. Finally, in terms of
chemotherapy, 51 (59.3%) patients received PLD, 21 (24.4%) received
weekly-paclitaxel and 14 (16.3%) received gemcitabine. The detailed
data of patients with PRROC are presented in Table I.
 | Table I.Characteristics of patients with
PRROC. |
Table I.
Characteristics of patients with
PRROC.
| Characteristics | Patients with PRROC
(n=86) |
|---|
| Age, years [median
(IQR)] | 60.5 (51.0–67.0) |
| Age, n (%) |
|
| <65
years | 57 (66.3) |
| ≥65
years | 29 (33.7) |
| Origin of cancer, n
(%) |
|
|
Ovary | 77 (89.5) |
| Fallopian
tube | 6 (7.0) |
|
Peritoneum | 3 (3.5) |
| Histology subtype, n
(%) |
|
| HGSC | 66 (76.7) |
| LGSC | 3 (3.5) |
|
Endometrioid carcinoma | 8 (9.3) |
| Clear
cell carcinoma | 9 (10.5) |
| ECOG PS, n (%) |
|
| 0 | 33 (38.3) |
| 1 | 47 (54.7) |
| 2 | 6 (7.0) |
| FIGO stage, n
(%) |
|
| I | 4 (4.7) |
| II | 7 (8.1) |
| III | 64 (74.4) |
| IV | 11 (12.8) |
| Platinum-free
interval ≤3 months, n (%) | 27 (31.4) |
| Ascites, n (%) | 32 (37.2) |
| CA-125 ≥100 U/ml, n
(%) | 61 (70.9) |
| Prior chemotherapy,
n (%) | 86 (100.0) |
| Prior
antiangiogenic therapy, n (%) | 9 (10.5) |
| Current treatment
lines, n (%) |
|
|
Second | 53 (61.6) |
| Third
or above | 33 (38.4) |
| Antiangiogenic
drugs, n (%) |
|
|
Bevacizumab | 68 (79.1) |
|
Apatinib | 18 (20.9) |
| Chemotherapy drugs,
n (%) |
|
|
PLD | 51 (59.3) |
|
Weekly-paclitaxel | 21 (24.4) |
|
Gemcitabine | 14 (16.3) |
Best response, ORR and DCR in patients
with PRROC
In terms of the treatment response of patients with
PRROC that received antiangiogenic therapy plus chemotherapy, no
(0.0%) patients achieved a complete response (CR) and 29 (33.7%)
patients achieved a partial response (PR). Furthermore, the ORR and
DCR of patients with PRROC were 33.7 and 77.9%, respectively
(Table II).
 | Table II.Treatment response of patients with
PRROC. |
Table II.
Treatment response of patients with
PRROC.
| Treatment
response | Patients with PRROC
(n=86) |
|---|
| Best response, n
(%) |
|
| CR | 0 (0.0) |
| PR | 29 (33.7) |
| SD | 38 (44.2) |
| PD | 19 (22.1) |
| ORR, n (%) |
|
|
Yes | 29 (33.7) |
| No | 57 (66.3) |
| DCR, n (%) |
|
|
Yes | 67 (77.9) |
| No | 19 (22.1) |
PFS and OS in patients with PRROC with
different treatment information
Results of the present study revealed that the
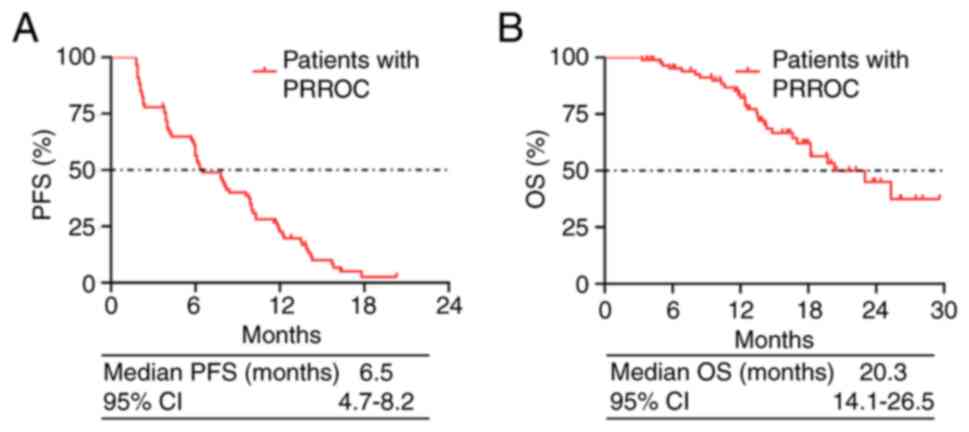
overall median PFS in patients with PRROC was 6.5 months [95%
confidence interval (CI), 4.7–8.2 months; Fig. 1A]. The overall median OS was 20.3
months (95% CI, 14.1–26.5 months; Fig.
1B).
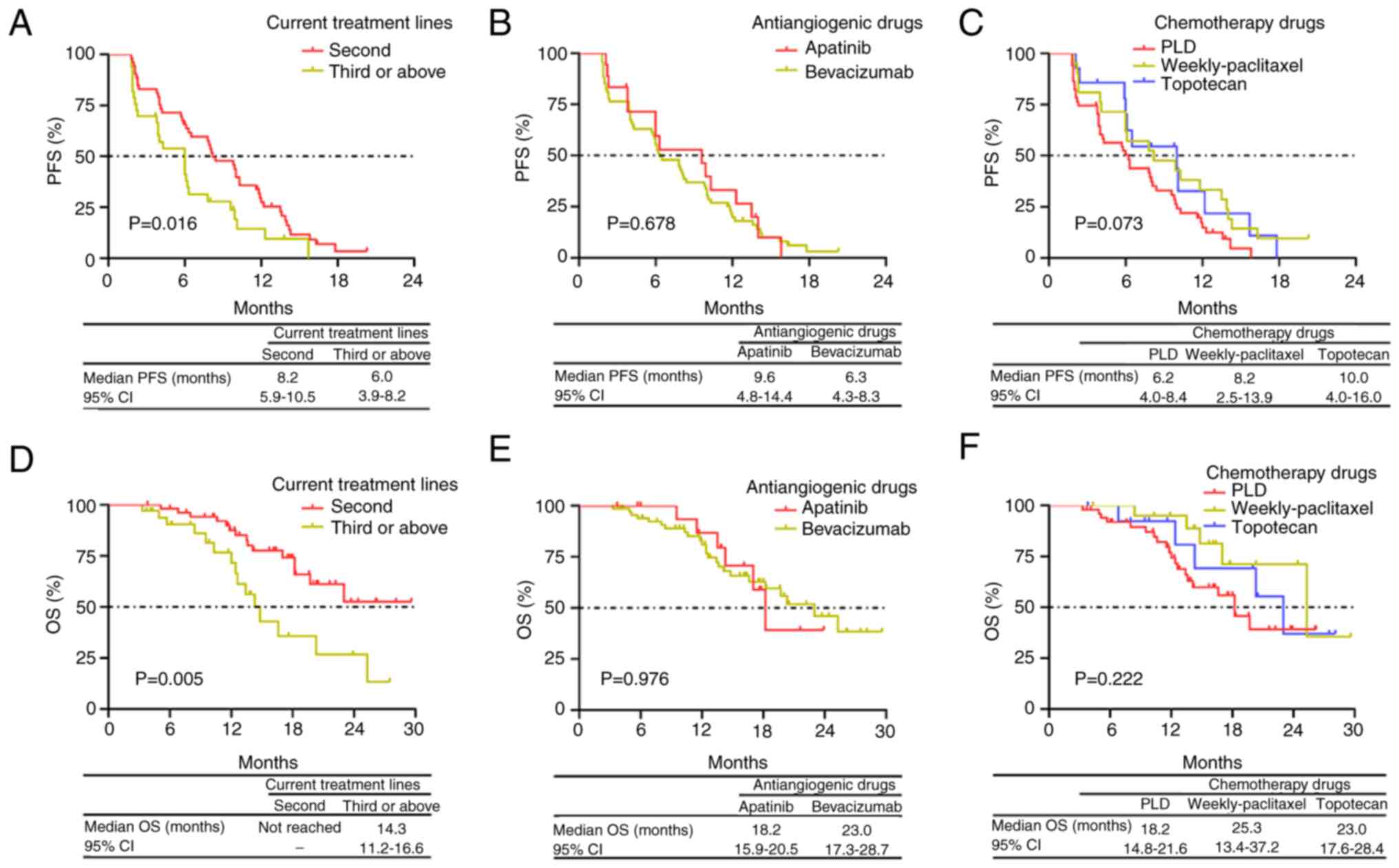
PFS was prolonged in patients with PRROC who
received antiangiogenic therapy plus chemotherapy as a second-line
treatment compared with patients who received the aforementioned
regimen as a third-line or above treatment (P=0.016; Fig. 2A). However, no significant
difference in PFS between patients with PRROC who received
bevacizumab and patients who received apatinib was observed
(P=0.678; Fig. 2B). Additionally,
no significant difference in PFS was observed between patients who
were treated with PLD, weekly-paclitaxel or gemcitabine (P=0.073;
Fig. 2C).
OS was also increased in patients with PRROC who
received antiangiogenic therapy plus chemotherapy as a second-line
treatment vs. patients who received it as a third-line or above
treatment (P=0.005; Fig. 2D). OS
did not significantly differ between patients who received
bevacizumab and patients who received apatinib (P=0.976; Fig. 2E). Additionally, no significant
difference in OS was observed between patients with PRROC who were
treated with PLD, weekly-paclitaxel or gemcitabine (P=0.222;
Fig. 2F).
Independent factors associated with
PFS in patients with PRROC
Stepwise forward multivariate Cox regression model
analysis revealed that ascites (yes vs. no) [hazard ratio
(HR)=1.804; P=0.018] and current treatment lines (third or above
vs. second) (HR=1.921; P=0.010) were independently associated with
a reduced PFS in patients with PRROC (Table III).
 | Table III.Univariate and stepwise forward
multivariate COX regression models of PFS. |
Table III.
Univariate and stepwise forward
multivariate COX regression models of PFS.
| A, Univariate COX
regression model |
|---|
|
|---|
| Factors | HR (95% CI) | P-value |
|---|
| Age, ≥65 years vs.
<65 years | 1.371
(0.841–2.234) | 0.205 |
| Origin of
cancer |
|
|
|
Ovary | Reference |
|
|
Fallopian tube | 1.401
(0.601–3.265) | 0.435 |
|
Peritoneum | 1.078
(0.337–3.451) | 0.899 |
| Histology
subtype |
|
|
|
HGSC | Reference |
|
|
LGSC | 0.322
(0.045–2.335) | 0.263 |
|
Endometrioid carcinoma | 0.519
(0.223–1.207) | 0.128 |
| Clear
cell carcinoma | 0.684
(0.326–1.438) | 0.317 |
| ECOG PS | 1.459
(0.997–2.135) | 0.052 |
| FIGO stage | 1.592
(1.071–2.366) | 0.021 |
| Platinum-free
interval, >3 months vs. ≤3 months | 1.126
(0.686–1.847) | 0.639 |
| Ascites, yes vs.
no | 1.671
(1.033–2.702) | 0.036 |
| CA-125, ≥100 U/ml
vs. <100 U/ml | 0.945
(0.569–1.571) | 0.828 |
| Prior
antiangiogenic therapy, yes vs. no | 1.685
(0.801–3.542) | 0.169 |
| Current treatment
lines, third or above vs. second | 1.788
(1.100–2.905) | 0.019 |
| Antiangiogenic
drugs, bevacizumab vs. apatinib | 1.127
(0.638–1.991) | 0.681 |
| Chemotherapy
drugs |
|
|
|
PLD | Reference |
|
|
Weekly-paclitaxel | 0.569
(0.325–0.995) | 0.048 |
|
Gemcitabine | 0.585
(0.298–1.147) | 0.119 |
|
| B, Stepwise
forward multivariate COX regression model |
|
| Ascites, yes vs.
no | 1.804
(1.106–2.942) | 0.018 |
| Current treatment
lines, third or above vs. second | 1.921
(1.172–3.150) | 0.010 |
Independent factors associated with OS
in patients with PRROC
Stepwise forward multivariate Cox regression model
analysis indicated that ascites (yes vs. no) (HR=2.439; P=0.026)
and current treatment lines (third or above vs. second) (HR=2.991;
P=0.003) were independently associated with a reduced OS in
patients with PRROC (Table
IV).
 | Table IV.Univariate and stepwise forward
multivariate COX regression models of OS. |
Table IV.
Univariate and stepwise forward
multivariate COX regression models of OS.
| A, Univariate COX
regression model |
|---|
|
|---|
| Factors | HR (95% CI) | P-value |
|---|
| Age, ≥65 years vs.
<65 years | 2.078
(1.012–4.267) | 0.046 |
| Origin of
cancer |
|
|
|
Ovary | Reference |
|
|
Fallopian tube | 0.886
(0.209–3.750) | 0.869 |
|
Peritoneum | 1.744
(0.411–7.403) | 0.451 |
| Histology
subtype |
|
|
|
HGSC | Reference |
|
|
LGSC | 1.949
(0.252–15.097) | 0.523 |
|
Endometrioid carcinoma | 0.220
(0.030–1.630) | 0.138 |
| Clear
cell carcinoma | 0.539
(0.162–1.794) | 0.314 |
| ECOG PS | 1.475
(0.832–2.614) | 0.183 |
| FIGO stage | 2.031
(1.016–4.063) | 0.045 |
| Platinum-free
interval, >3 months vs. ≤3 months | 1.363
(0.647–2.875) | 0.415 |
| Ascites, yes vs.
no | 2.133
(0.999–4.552) | 0.050 |
| CA-125, ≥100 U/ml
vs. <100 U/ml | 1.726
(0.704–4.231) | 0.233 |
| Prior
antiangiogenic therapy, yes vs. no | 2.185
(0.742–6.436) | 0.156 |
| Current treatment
lines, third or above vs. second | 2.729
(1.325–5.621) | 0.006 |
| Antiangiogenic
drugs, bevacizumab vs. apatinib | 1.014
(0.411–2.504) | 0.976 |
| Chemotherapy
drugs |
|
|
|
PLD | Reference |
|
|
Weekly-paclitaxel | 0.443
(0.166–1.185) | 0.105 |
|
Gemcitabine | 0.665
(0.246–1.793) | 0.420 |
|
| B, Stepwise
forward multivariate COX regression model |
|
| Ascites, yes vs.
no | 2.439
(1.115–5.333) | 0.026 |
| Current treatment
lines, third or above vs. second | 2.991
(1.436–6.232) | 0.003 |
Adverse events in patients with
PRROC
In the present cohort, the most common adverse
events in patients with PRROC were leukopenia (34.9%), hypertension
(30.2%), fatigue (30.2%), neutropenia (29.1%) as well as nausea and
vomiting (27.9%). The specific adverse events are presented in
Table V.
 | Table V.Adverse events of patients with PRROC
(n=86). |
Table V.
Adverse events of patients with PRROC
(n=86).
| Adverse events | Patients with
PRROC, n (%) |
|---|
| Leukopenia | 30 (34.9) |
| Hypertension | 26 (30.2) |
| Fatigue | 26 (30.2) |
| Neutropenia | 25 (29.1) |
| Nausea and
vomiting | 24 (27.9) |
| Anaemia | 21 (24.4) |
| Hand-foot
syndrome | 17 (19.8) |
| Diarrhoea | 14 (16.3) |
| Dysregulated liver
function | 14 (16.3) |
| Oral ulcer | 14 (16.3) |
|
Thrombocytopenia | 13 (15.1) |
| Proteinuria | 10 (11.6) |
| Bleeding | 5 (5.8) |
| Gastrointestinal
perforation | 2 (2.3) |
Discussion
Platinum-based chemotherapy is the mainstay of OC
treatment, but the occurrence of platinum resistance remains a
challenge (9,10). Currently, single-agent non-platinum
chemotherapy is one of the most commonly used treatment approaches
for patients with PRROC (21). The
efficacy of single-agent non-platinum chemotherapy in patients with
PRROC has been investigated previously (14,22).
For example, a previous study reveals that the median PFS and OS in
patients with PRROC who received weekly-paclitaxel are 6.1 and 10.4
months, respectively (22). Another
study demonstrates that patients with PRROC who are treated with
PLD yield an ORR and DCR of 10.9 and 53.1%, respectively, with a
median PFS and OS of 3.3 and 14.4 months, respectively (14). In the present study, the ORR and DCR
of patients with PRROC who received antiangiogenic therapy plus
chemotherapy were 33.7 and 77.9%, respectively, with a PFS of 6.5
months and an OS of 20.3 months. The aforementioned values were all
increased compared with those reported in previous studies
(14,22). A possible reason for this
discrepancy might be that patients with PRROC in the present study
received antiangiogenic therapy in addition to single-agent
non-platinum chemotherapy, while the previous aforementioned
studies only use single-agent non-platinum chemotherapy.
Antiangiogenic drugs inhibit the vascular endothelial growth factor
signalling pathway, which subsequently inhibits the delivery of
nutrients and oxygen to tumour cells, suppressing tumour growth and
metastasis (12,13). Additionally, antiangiogenic drugs
normalise the abnormal tumour vasculature, increasing vascular
perfusion and promoting the infiltration of immune effector cells
into tumours, which may promote anticancer immunity (23). Previous studies also report that
antiangiogenic drugs have synergistic effects with chemotherapy in
killing tumour cells (24–27). In addition, a number of previous
studies reveal that patients with PRROC who receive antiangiogenic
therapy plus chemotherapy have ORRs and DCRs ranging from 27.3–43.1
and 57.1–84.4%, respectively. Furthermore, in these previous
studies, the median PFS and OS range from 5.0–7.1 and 16.6–23.0
months, respectively (14–16,28,29).
Therefore, the results of the present study were similar to those
of the aforementioned studies, which suggested that the results of
the present study were valid (14–16,28,29).
Notably, the advantage of the present study was that it included
patients with PRROC who received different antiangiogenic drugs
(bevacizumab or apatinib) plus chemotherapy, while previous studies
only included one type of antiangiogenic drug. The results of the
present study may provide further comprehensive evidence for the
application of antiangiogenic drugs plus chemotherapy in patients
with PRROC.
The present study demonstrated that administering
antiangiogenic therapy plus chemotherapy as a third-line or above
therapy was independently associated with a reduced PFS and OS in
patients with PRROC compared with patients who received the therapy
as a second-line treatment. A possible explanation for this may be
that patients with PRROC are administered antiangiogenic drugs plus
chemotherapy as a third-line or above therapy after their first-
and second-line treatments fail; thus, these patients may be more
resistant to multiple types of chemotherapy drugs compared with
those who received the therapy as a second-line treatment.
Additionally, in patients with PRROC who received antiangiogenic
therapy plus chemotherapy, ascites also independently predicted
reduced PFS and OS values compared with patients that did not have
ascites. This may be explained as the ascites themselves represent
the metastasis of malignant cells into the peritoneal cavity and
thus indicate the aggravated progression of the tumour (30).
The safety of antiangiogenic therapy plus
chemotherapy in treating patients with PRROC is also a noteworthy
issue (31,32). A previous study discloses that the
most common adverse events in patients with PRROC who receive
apatinib plus PLD are decreased white blood cell count (60.8%),
decreased neutrophil count (59.5%) and oral ulcers (28.4%)
(14). Another study demonstrates
that the most common adverse events in patients with PRROC who were
treated with bevacizumab plus PLD are mucositis (including
dysphagia; 64.0%), palmar-plantar erythroderma/ulceration (52.0%)
and asthenia (52.0%) (33). In the
present study, the most frequent adverse events were leukopenia
(34.9%), hypertension (30.2%) and fatigue (30.2%). The incidences
of the aforementioned adverse events were reduced compared with
those reported in previous studies, which could be attributed to
the underestimation of adverse events due to the retrospective
nature of the present study. Moreover, only previously reported
adverse events were observed. The findings of the present study
support the safety of antiangiogenic therapy plus chemotherapy in
patients with PRROC.
However, the present study had limitations. Firstly,
the sample size in the present study was small. Therefore, studies
with a larger sample size are required to verify the results of the
present study. Secondly, the present study was a single-arm study;
therefore, the results should be further verified by randomised,
controlled studies. Finally, the follow-up period of the present
study was short. Therefore, further studies with a longer follow-up
period should be carried out to investigate the efficacy and safety
of antiangiogenic therapy plus chemotherapy in patients with
PRROC.
In conclusion, antiangiogenic therapy plus
chemotherapy achieved an ORR of 33.7%, a median PFS of 6.5 months
and a median OS of 20.3 months with tolerable toxicity in patients
with PRROC. Moreover, ascites and administering treatment as a
third-line or above therapy independently predicted an unfavourable
survival rate in these patients. Future studies should consider
increasing the sample size, including a control group and extending
the follow-up period to further investigate the long-term efficacy
and safety of antiangiogenic therapy plus chemotherapy in patients
with PRROC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL contributed to the study conception and design.
JX performed data collection and analysis. MT was responsible for
the interpretation of data. All authors contributed to drafting the
article. HL and MT confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study obtained permission from the Ethics
Committee of People's Hospital of Zhongshan City (approval no.
2024-033; Zhongshan, China). The patients signed the informed
consent form, or if the patient were unable to sign, their family
members signed the informed consent form on their behalf.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Momenimovahed Z, Tiznobaik A, Taheri S and
Salehiniya H: Ovarian cancer in the world: Epidemiology and risk
factors. Int J Womens Health. 11:287–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazidimoradi A, Momenimovahed Z, Allahqoli
L, Tiznobaik A, Hajinasab N, Salehiniya H and Alkatout I: The
global, regional and national epidemiology, incidence, mortality,
and burden of ovarian cancer. Health Sci Rep. 5:e9362022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ali AT, Al-Ani O and Al-Ani F:
Epidemiology and risk factors for ovarian cancer. Prz Menopauzalny.
22:93–104. 2023.PubMed/NCBI
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zamwar UM and Anjankar AP: Aetiology,
epidemiology, histopathology, classification, detailed evaluation,
and treatment of ovarian cancer. Cureus. 14:e305612022.PubMed/NCBI
|
|
6
|
Chandra A, Pius C, Nabeel M, Vishwanatha
JK, Ahmad S and Basha R: Ovarian cancer: Current status and
strategies for improving therapeutic outcomes. Cancer Med.
8:7018–7031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luvero D, Milani A and Ledermann JA:
Treatment options in recurrent ovarian cancer: Latest evidence and
clinical potential. Ther Adv Med Oncol. 6:229–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh N, Jayraj AS, Sarkar A, Mohan T,
Shukla A and Ghatage P: Pharmacotherapeutic treatment options for
recurrent epithelial ovarian cancer. Expert Opin Pharmacother.
24:49–64. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Havasi A, Cainap SS, Havasi AT and Cainap
C: Ovarian Cancer-Insights into platinum resistance and overcoming
it. Medicina (Kaunas). 59:5442023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Richardson DL, Eskander RN and O'Malley
DM: Advances in ovarian cancer care and unmet treatment needs for
patients with platinum resistance: A narrative review. JAMA Oncol.
9:851–859. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elyashiv O, Aleohin N, Migdan Z, Leytes S,
Peled O, Tal O and Levy T: The poor prognosis of acquired secondary
platinum resistance in ovarian cancer patients. Cancers (Basel).
16:6412024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mei C, Gong W, Wang X, Lv Y, Zhang Y, Wu S
and Zhu C: Anti-angiogenic therapy in ovarian cancer: Current
understandings and prospects of precision medicine. Front
Pharmacol. 14:11477172023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang T, Tang J, Yang H, Yin R, Zhang J,
Zhou Q, Liu Z, Cao L, Li L, Huang Y, et al: Effect of apatinib plus
pegylated liposomal doxorubicin vs pegylated liposomal doxorubicin
alone on platinum-resistant recurrent ovarian cancer: The APPROVE
randomized clinical trial. JAMA Oncol. 8:1169–1176. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA Open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geng A, Yang H, Wang Z and Wu C: Apatinib
plus paclitaxel versus paclitaxel monotherapy for
platinum-resistant recurrent ovarian cancer treatment: A
retrospective cohort study. J Clin Pharm Ther. 47:2264–2273. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Armstrong DK, Alvarez RD, Bakkum-Gamez JN,
Barroilhet L, Behbakht K, Berchuck A, Berek JS, Chen LM, Cristea M,
DeRosa M, et al: NCCN guidelines insights: Ovarian cancer, version
1.2019. J Natl Compr Canc Netw. 17:896–909. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz LH, Litiere S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Armstrong DK, Alvarez RD, Bakkum-Gamez JN,
Barroilhet L, Behbakht K, Berchuck A, Chen LM, Cristea M, DeRosa M,
Eisenhauer EL, et al: Ovarian cancer, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
19:191–226. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berek JS, Renz M, Kehoe S, Kumar L and
Friedlander M: Cancer of the ovary, fallopian tube, and peritoneum:
2021 update. Int J Gynaecol Obstet. 155 (Suppl 1):S61–S85. 2021.
View Article : Google Scholar
|
|
21
|
Vergote I, Gonzalez-Martin A, Lorusso D,
Gourley C, Mirza MR, Kurtz JE, Okamoto A, Moore K, Kridelka F,
McNeish I, et al: Clinical research in ovarian cancer: Consensus
recommendations from the Gynecologic Cancer InterGroup. Lancet
Oncol. 23:e374–e384. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le T, Hopkins L, Baines KA, Rambout L, Al
Hayki M and Kee Fung MF: Prospective evaluations of continuous
weekly paclitaxel regimen in recurrent platinum-resistant
epithelial ovarian cancer. Gynecol Oncol. 102:49–53. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukumura D, Kloepper J, Amoozgar Z, Duda
DG and Jain RK: Enhancing cancer immunotherapy using
antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol.
15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu H, Li J, Liu Q, Tang M and Wang Y:
Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor
growth in cervical cancer and synergizes with Paclitaxel. Cell
Cycle. 17:1235–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Volk LD, Flister MJ, Chihade D, Desai N,
Trieu V and Ran S: Synergy of nab-paclitaxel and bevacizumab in
eradicating large orthotopic breast tumors and preexisting
metastases. Neoplasia. 13:327–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rapisarda A, Hollingshead M, Uranchimeg B,
Bonomi CA, Borgel SD, Carter JP, Gehrs B, Raffeld M, Kinders RJ,
Parchment R, et al: Increased antitumor activity of bevacizumab in
combination with hypoxia inducible factor-1 inhibition. Mol Cancer
Ther. 8:1867–1877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cesca M, Morosi L, Berndt A, Fuso Nerini
I, Frapolli R, Richter P, Decio A, Dirsch O, Micotti E, Giordano S,
et al: Bevacizumab-Induced inhibition of angiogenesis promotes a
more homogeneous intratumoral distribution of paclitaxel, improving
the antitumor response. Mol Cancer Ther. 15:125–135. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang H, Geng A, Wang Z and Wu C: Efficacy
and safety of apatinib combined with liposomal doxorubicin or
paclitaxel versus liposomal doxorubicin or paclitaxel monotherapy
in patients with recurrent platinum-resistant ovarian cancer. J
Obstet Gynaecol Res. 49:1611–1619. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fukuda T, Noda T, Uchikura E, Awazu Y,
Tasaka R, Imai K, Yamauchi M, Ichimura T, Yasui T and Sumi T:
Real-world efficacy and safety of bevacizumab for advanced or
recurrent mullerian cancer: A Single-institutional experience.
Anticancer Res. 43:3097–3105. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ford CE, Werner B, Hacker NF and Warton K:
The untapped potential of ascites in ovarian cancer research and
treatment. Br J Cancer. 123:9–16. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simion L, Rotaru V, Cirimbei C, Stefan DC,
Gherghe M, Ionescu S, Tanase BC, Luca DC, Gales LN and Chitoran E:
Analysis of efficacy-to-safety ratio of angiogenesis-inhibitors
based therapies in ovarian cancer: A systematic review and
Meta-analysis. Diagnostics (Basel). 13:10402023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Lin H, Ou Y, Fu H, Tsai C, Chien
CC and Wu C: Safety analysis of bevacizumab in ovarian cancer
patients. J Clin Med. 12:20652023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verschraegen CF, Czok S, Muller CY, Boyd
L, Lee SJ, Rutledge T, Blank S, Pothuri B, Eberhardt S and Muggia
F: Phase II study of bevacizumab with liposomal doxorubicin for
patients with Platinum- and taxane-resistant ovarian cancer. Ann
Oncol. 23:3104–3110. 2012. View Article : Google Scholar : PubMed/NCBI
|