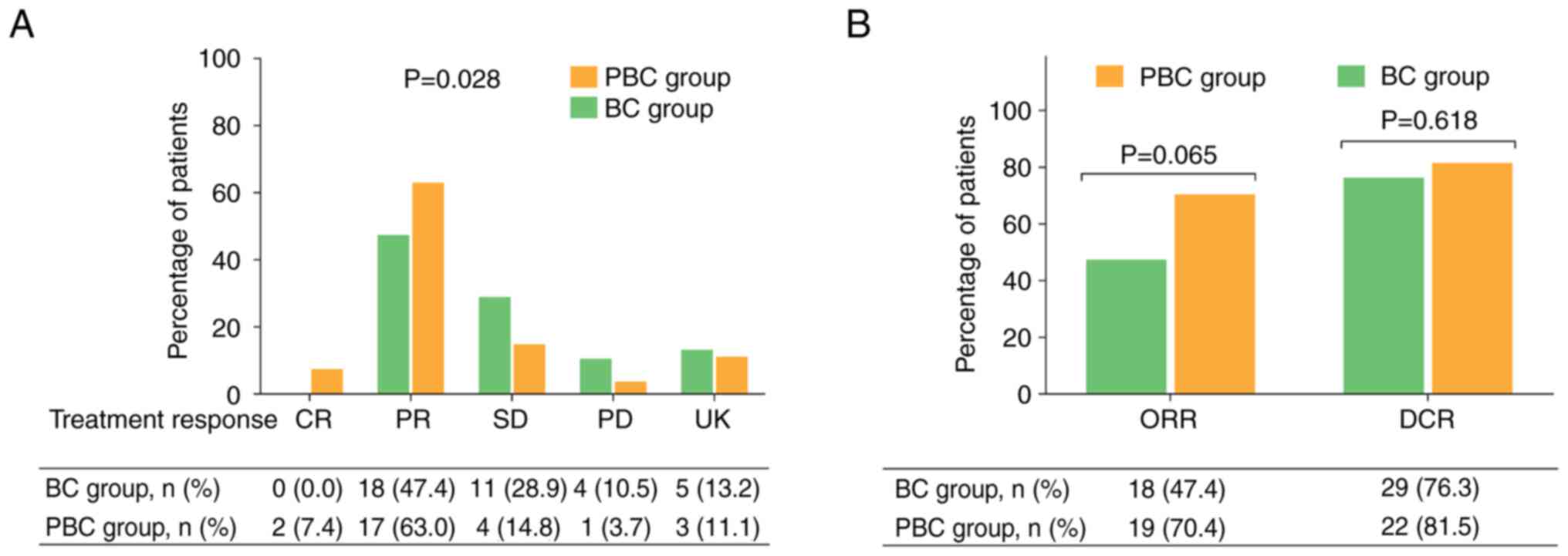
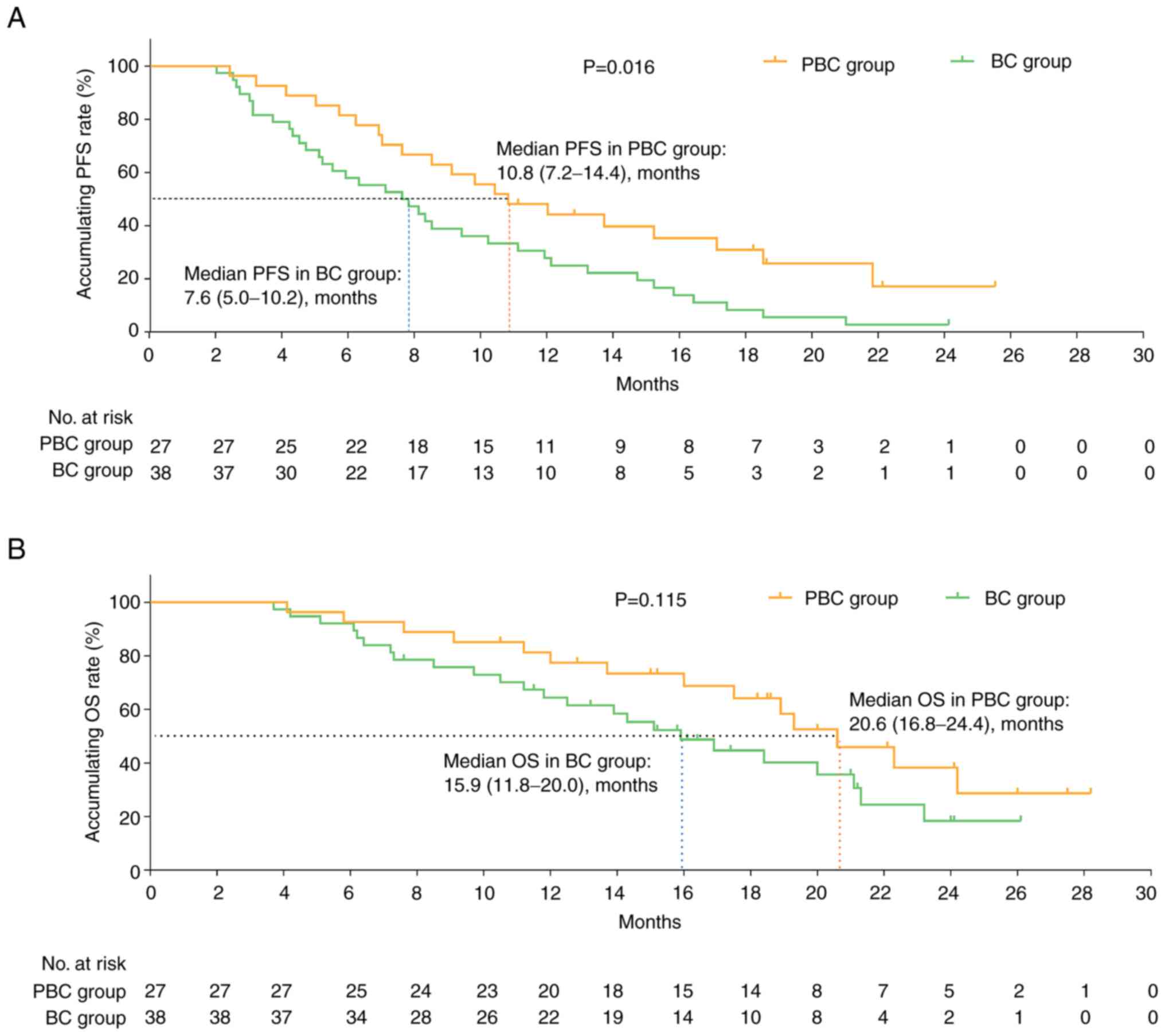
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jenkins R, Walker J and Roy UB: 2022
cancer statistics: Focus on lung cancer. Future Oncol. 5:1–11.
2023. View Article : Google Scholar
|
|
3
|
Chen LN, Wei AZ and Shu CA: Neoadjuvant
immunotherapy in resectable non-small-cell lung cancer. Ther Adv
Med Oncol. 15:175883592311637982023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seguin L, Durandy M and Feral CC: Lung
adenocarcinoma tumor origin: A guide for personalized medicine.
Cancers (Basel). 14:17592022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mithoowani H and Febbraro M:
Non-small-cell lung cancer in 2022: A review for general
practitioners in oncology. Curr Oncol. 29:1828–1839. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miao D, Zhao J, Han Y, Zhou J, Li X, Zhang
T, Li W and Xia Y: Management of locally advanced non-small cell
lung cancer: State of the art and future directions. Cancer Commun
(Lond). 44:23–46. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong J, Bai H, Wang Z, Duan J, Zhuang W,
Wang D, Wan R, Xu J, Fei K, Ma Z, et al: Treatment of advanced
non-small cell lung cancer with driver mutations: Current
applications and future directions. Front Med. 17:18–42. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peters S, Camidge DR, Shaw AT, Gadgeel S,
Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, et al:
Alectinib versus crizotinib in untreated ALK-positive
non-small-cell lung cancer. N Engl J Med. 377:829–838. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drilon A, Siena S, Dziadziuszko R, Barlesi
F, Krebs MG, Shaw AT, de Braud F, Rolfo C, Ahn MJ, Wolf J, et al:
Entrectinib in ROS1 fusion-positive non-small-cell lung cancer:
Integrated analysis of three phase 1–2 trials. Lancet Oncol.
21:261–270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jurisic V, Obradovic J, Nikolic N, Javorac
J, Perin B and Milasin J: Analyses of P16(INK4a) gene promoter
methylation relative to molecular, demographic and clinical
parameters characteristics in non-small cell lung cancer patients:
A pilot study. Mol Biol Rep. 50:971–979. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Obradovic J, Djordjevic N, Tosic N,
Mrdjanović J, Stanković B, Stanić J, Zarić B, Perin B, Pavlović S
and Jurišić V: Frequencies of EGFR single nucleotide polymorphisms
in non-small cell lung cancer patients and healthy individuals in
the Republic of Serbia: A preliminary study. Tumour Biol.
37:10479–10486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Yang H, Teo ASM, Amer LB, Sherbaf
FG, Tan CQ, Alvarez JJS, Lu B, Lim JQ, Takano A, et al: Genomic
landscape of lung adenocarcinoma in East Asians. Nat Genet.
52:177–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitsudomi T: Molecular epidemiology of
lung cancer and geographic variations with special reference to
EGFR mutations. Transl Lung Cancer Res. 3:205–211. 2014.PubMed/NCBI
|
|
16
|
Obradovic J, Todosijevic J and Jurisic V:
Side effects of tyrosine kinase inhibitors therapy in patients with
non-small cell lung cancer and associations with EGFR
polymorphisms: A systematic review and meta-analysis. Oncol Lett.
25:622023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jurisic V, Vukovic V, Obradovic J,
Gulyaeva LF, Kushlinskii NE and Djordjevic N: EGFR polymorphism and
survival of NSCLC patients treated with TKIs: A systematic review
and meta-analysis. J Oncol. 2020:19732412020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paz-Ares LG, de Marinis F, Dediu M, Thomas
M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et
al: PARAMOUNT: Final overall survival results of the phase III
study of maintenance pemetrexed versus placebo immediately after
induction treatment with pemetrexed plus cisplatin for advanced
nonsquamous non-small-cell lung cancer. J Clin Oncol. 31:2895–2902.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, phase III study of
first-line Carboplatin/Paclitaxel plus bevacizumab or placebo in
chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chu T, Lu J, Bi M, Zhang H, Zhuang W, Yu
Y, Shi J, Chen Z, Zhang X, Guo Q, et al: Equivalent efficacy study
of QL1101 and bevacizumab on untreated advanced non-squamous
non-small cell lung cancer patients: A phase 3 randomized,
double-blind clinical trial. Cancer Biol Med. 18:816–824. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zitvogel L, Galluzzi L, Smyth MJ and
Kroemer G: Mechanism of action of conventional and targeted
anticancer therapies: Reinstating immunosurveillance. Immunity.
39:74–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martino EC, Misso G, Pastina P, Costantini
S, Vanni F, Gandolfo C, Botta C, Capone F, Lombardi A, Pirtoli L,
et al: Immune-modulating effects of bevacizumab in metastatic
non-small-cell lung cancer patients. Cell Death Discov.
2:160252016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alfaro C, Suarez N, Gonzalez A, Solano S,
Erro L, Dubrot J, Palazon A, Hervas-Stubbs S, Gurpide A,
Lopez-Picazo JM, et al: Influence of bevacizumab, sunitinib and
sorafenib as single agents or in combination on the inhibitory
effects of VEGF on human dendritic cell differentiation from
monocytes. Br J Cancer. 100:1111–1119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wada J, Suzuki H, Fuchino R, Yamasaki A,
Nagai S, Yanai K, Koga K, Nakamura M, Tanaka M, Morisaki T and
Katano M: The contribution of vascular endothelial growth factor to
the induction of regulatory T-cells in malignant effusions.
Anticancer Res. 29:881–888. 2009.PubMed/NCBI
|
|
26
|
Gan J, Huang Y, Fang W and Zhang L:
Research progress in immune checkpoint inhibitors for lung cancer
in China. Ther Adv Med Oncol. 13:175883592110298262021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li K, Zhang A, Li X, Zhang H and Zhao L:
Advances in clinical immunotherapy for gastric cancer. Biochim
Biophys Acta Rev Cancer. 1876:1886152021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Alessio A, Rimassa L, Cortellini A and
Pinato DJ: PD-1 blockade for hepatocellular carcinoma: Current
research and future prospects. J Hepatocell Carcinoma. 8:887–897.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sugawara S, Lee JS, Kang JH, Kim HR, Inui
N, Hida T, Lee KH, Yoshida T, Tanaka H, Yang CT, et al: Nivolumab
with carboplatin, paclitaxel, and bevacizumab for first-line
treatment of advanced nonsquamous non-small-cell lung cancer. Ann
Oncol. 32:1137–1147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumors:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
U.S. Department Of Health and Human
Services: National Institutes of Health and National Cancer
Institute: Common terminology criteria for adverse events (CTCAE)
version 4.02. Available from:. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.02_2009-09-15_QuickReference_8.5×11.pdf
|
|
33
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Novello S, Barlesi F, Califano R, Cufer T,
Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al:
Metastatic non-small-cell lung cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
27:v1–v27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Network NCC: NCCN Clinical Practice
Guidelines in Oncology: Non-small cell lung cancer. 2018.PubMed/NCBI
|
|
36
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-Positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gandhi L, Rodriguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Şenler FC, Csőszi T, Fülöp A, et al:
Pembrolizumab plus chemotherapy for squamous non-small-cell lung
cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kanda S, Goto K, Shiraishi H, Kubo E,
Tanaka A, Utsumi H, Sunami K, Kitazono S, Mizugaki H, Horinouchi H,
et al: Safety and efficacy of nivolumab and standard chemotherapy
drug combination in patients with advanced non-small-cell lung
cancer: A four arms phase Ib study. Ann Oncol. 27:2242–2250. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kanda S, Ohe Y, Goto Y, Horinouchi H,
Fujiwara Y, Nokihara H, Yamamoto N, Yamamoto T and Tamura T:
Five-year safety and efficacy data from a phase Ib study of
nivolumab and chemotherapy in advanced non-small-cell lung cancer.
Cancer Sci. 111:1933–1942. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Martinovic KM, Vuletic A, Miletic NT,
Žižak IB, Milovanović J, Matković S and Jurišić V: Circulating
cytokine dynamics as potential biomarker of response to anti-PD-1
immunotherapy in BRAFwt MM patients. Transl Oncol. 38:1017992023.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen X, Li Z, Zhou J, Wei Q, Wang X and
Jiang R: Identification of prognostic factors and nomogram model
for patients with advanced lung cancer receiving immune checkpoint
inhibitors. PeerJ. 10:e145662022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jurisic V, Radenkovic S and Konjevic G:
The actual role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–124. 2015. View Article : Google Scholar : PubMed/NCBI
|