Introduction
Lung cancer is the top cause of cancer-related
mortality globally, accounting for 18.0% of the total
cancer-related deaths in 2020, and lung adenocarcinoma is one of
the most common lung cancer types (1,2).
Benefiting from improved surgical techniques, strategic progress
(such as novel, combined and personalized neoadjuvant therapies),
novel drug development and precise medicine realization, the
outcomes of patients with lung adenocarcinoma have continuously
improved over the past several decades (3–5).
However, a non-negligible proportion of patients with lung
adenocarcinoma are diagnosed at an advanced/metastatic stage of the
disease and are thus not eligible for surgery; even with
neoadjuvant therapies, these patients have an unfavorable prognosis
(6–8). With regard to patients with driver
gene-positive advanced-stage lung adenocarcinoma harboring
sensitizing epidermal growth factor receptor (EGFR), ALK receptor
tyrosine kinase (ALK) or ROS proto-oncogene 1 receptor tyrosine
kinase (ROS1) mutations, corresponding targeted therapy with or
without chemotherapy effectively improves patient outcomes
(9–11). As the most common driver gene, EGFR
has distinct expression and polymorphisms in different regions of
the world, which relate to the ethnic and demographic
characteristics of the studied population (12–15).
Meanwhile, the EGFR polymorphisms or variations help in predicting
the outcomes and adverse effects to some extent in patients
receiving tyrosine kinase inhibitors (16,17).
However, for patients with driver gene-negative advanced-stage lung
adenocarcinoma, only a limited number of treatment options are
available, which highlights the need for exploring further
treatment strategies.
Chemotherapeutic regimens (such as pemetrexed plus
cisplatin/carboplatin, gemcitabine plus cisplatin/carboplatin and
paclitaxel plus carboplatin regimens) are currently the cornerstone
for the treatment of patients with driver gene-negative
advanced-stage lung adenocarcinoma (18,19).
In addition to its use in combination with chemotherapy,
bevacizumab has been shown to further improve the prognosis of
patients with driver gene-negative advanced-stage lung
adenocarcinoma (20,21). However, conventional chemotherapy
affects antitumor immunosurveillance via its modification of
regulatory T lymphocytes (22), and
bevacizumab affects the immune system by repressing dendritic cell
maturation and regulating regulatory T-lymphocyte proliferation
(23–25). These findings suggest that the
combination of immune therapy with bevacizumab and chemotherapy
could further improve the outcomes of patients with driver
gene-negative advanced-stage lung adenocarcinoma.
Programmed cell death protein 1 (PD-1)/programmed
death-ligand 1 (PD-L1) inhibitors are immune checkpoint inhibitors
that repress the immune escape of cancer cells to achieve antitumor
activity and have been used in the treatment of various types of
cancer, including lung cancer (26–28).
In terms of advanced-stage lung adenocarcinoma, two previous trials
have demonstrated that in addition to bevacizumab and chemotherapy,
PD-1/PD-L1 inhibitors improve the progression-free survival (PFS)
and/or overall survival (OS) of patients with driver gene-negative
advanced-stage non-small cell lung cancer (NSCLC) (mostly patients
with lung adenocarcinoma) (29,30).
Nevertheless, to the best of our knowledge, only a limited number
of related studies under real-world clinical settings have been
reported.
Therefore, the present study retrospectively
analyzed patients with driver gene-negative advanced-stage lung
adenocarcinoma who were treated with the PD-1/PD-L1 inhibitor plus
bevacizumab and chemotherapy (PBC) regimen or only the BC regimen
to compare their treatment response, survival benefits and safety
profiles under real-world clinical conditions.
Patients and methods
Patients
Between January 2019 and January 2021, 65 patients
with driver gene-negative advanced-stage lung adenocarcinoma who
received the PBC or BC regimen in Sichuan Cancer Hospital and
Institute (Chengdu, China), and Sichuan Jianzhu Hospital (Chengdu,
China) were retrospectively analyzed in the present cohort study.
The eligible patients were screened from the database according to
the following criteria: i) A confirmed diagnosis of lung
adenocarcinoma; ii) age >18 years; iii) Tumor-Node-Metastasis
(TNM) stage IIIB-IV disease (31);
iv) confirmed driver gene-negative status (defined as patients
without sensitizing EGFR, ALK or ROS1 mutations); v) treatment with
the PBC or BC regimen; vi) available main clinical feature data,
response assessment data, follow-up data and safety data available
for analysis; and vii) no history of other fatal diseases. Among
the 65 patients, 27 patients received PBC and were classified as
the PBC group and 38 patients received BC without PD-1/PD-L1
inhibitors and were classified as the BC group. The driver gene
status was detected by the amplification refractory mutation
system-polymerase chain reaction method using the
AmoyDx® Pan Lung Cancer PCR Panel Kit (cat. no.
ADX-LG01; Amoy Diagnostics Co., Ltd.) according to the
manufacturer's instructions. Briefly, the AmoyDx® tissue
DNA kit (cat. no. 8.02.0078; Amoy Diagnostics Co., Ltd.) and
AmoyDx® tissue RNA kit (cat. no. 8.02.0079; Amoy
Diagnostics Co., Ltd.) were used to extract DNA and RNA from
tissues. The AmoyDx® Pan Lung Cancer PCR Panel Kit (cat.
no. ADX-LG01, Amoy Diagnostics Co., Ltd., China) was applied to
analyze mutation. For reverse transcription, the RNA, LEG RT
reaction Mix and LEG Reverse Transcriptase were mixed. The mixture
was incubated at 42°C for 1 h and at 95°C for 5 min to generate
cDNA. For mutation detection, the cDNA and DNA were mixed with LEG
Reaction Mix A and LEG Reaction Mix B, respectively. Afterwards,
the PCR was conducted and parameters were as follows: 42°C for 5
min and 95°C for 5 min, for 1 cycle; 95°C for 25 sec, 64°C for 20
sec and 72°C for 20 sec, for 10 cycles; and 93°C for 25 sec, 60°C
for 35 sec and 72°C for 20 sec, for 36 cycles. A lightCycler480 II
(Roche Diagnostics) was applied to complete PCR and collect data.
The Institutional Review Board of Sichuan Cancer Hospital and
Institute, Sichuan Cancer Center, School of Medicine, University of
Electronic Science and Technology of China approved the study
protocol. Written informed consent was obtained at the start of the
retrospective study from each patient or from the patient's direct
relatives if they were deceased.
Treatment information
The treatment documents of the patients were
reviewed, and the treatment information was collected as follows:
In the PBC group (n=27), patients received one of the following
PD-1/PD-L1 inhibitors: Sintilimab (200 mg per 3-week cycle),
pembrolizumab (200 mg per 3-week cycle), camrelizumab (200 mg per
3-week cycle), durvalumab (10 mg/kg per 2-week cycle) or
atezolizumab (1,200 mg per 3-week cycle) until unbearable toxic
events or radiographically confirmed disease progression had
occurred. The patients received bevacizumab (7.5–15.0 mg/kg per
3-week cycle) until unbearable toxic events or radiographically
confirmed disease progression had occurred, plus chemotherapy with
paclitaxel (175 mg/m2 per 3-week cycle) and carboplatin
(AUC 5–6 per 3-week cycle) for approximately six cycles depending
on the patient's tolerance. In the BC group (n=38), patients
underwent chemotherapy with paclitaxel (175 mg/m2 per
3-week cycle) plus carboplatin (AUC 5–6 per 3-week cycle) for
approximately six cycles depending on the patient's tolerance and
continuously received bevacizumab (7.5–15.0 mg/kg per 3-week cycle)
without PD-1/PD-L1 Inhibitors until unbearable toxic events or
radiographically confirmed disease progression had occurred.
Efficacy, safety and survival
assessment
The treatment response data of the patients were
collected from the imaging assessment records, which were collected
every 4 to 8 weeks after commencing therapy. The optimal treatment
response was analyzed in the present study; patients were evaluated
referring to the Response Evaluation Criteria in Solid Tumors
guidelines (31) and were
concretely defined as having a complete response (CR), partial
response (PR), stable disease (SD), progressive disease (PD) or
unknown status (unpublished data or unevaluable data). The
objective response rate (ORR) and disease control rate (DCR) were
correspondingly calculated as follows: ORR=CR + PR and DCR=CR + PR
+ SD. The adverse events documented during the therapy were also
collected for safety analysis and were graded in terms of the
National Cancer Institute Common Terminology Criteria for Adverse
Events version 4.0 (32). In
addition, PFS and OS data were extracted from the follow-up
records. PFS was defined as the time from treatment initiation to
the time of cancer progression or death, and OS was defined as the
time from treatment initiation to the time of death.
Statistical analysis
Clinical features were compared using the unpaired
Student's t-test, the χ2 test and Fisher's exact test.
Treatment response was compared using the χ2 test. PFS
and OS were demonstrated using Kaplan-Meier curves and compared
using the log-rank test. PFS- and OS-related factors were analyzed
using multivariate Cox proportional hazards regression. Adverse
events were compared using the χ2 test or Fisher's exact
test, as appropriate. SPSS 24.0 (IBM Corp.) and GraphPad Prism 7.02
(Dotmatics) software were used for the statistical analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Among the 65 enrolled patients with driver
gene-negative, advanced-stage lung adenocarcinoma, the mean ages
were 63.9±9.1 years (range, 43.0–80.0 years) and 64.9±8.2 years
(range, 49.0–79.0 years) (P=0.643), and the male/female ratios were
70.4/29.6 and 78.9/21.1% (P=0.429), in the PBC group and the BC
group, respectively. Moreover, no marked differences were found in
the other characteristics such as TNM stage and lactate
dehydrogenase (LDH) level between the PBC and BC groups (Table I).
 | Table I.Clinical characteristics. |
Table I.
Clinical characteristics.
| Items | BC group
(n=38) | PBC group
(n=27) | P-value |
|---|
| Mean age ± SD,
years | 64.9±8.2 | 63.9±9.1 | 0.643 |
| Sex, n (%) |
|
| 0.429 |
|
Female | 8 (21.1) | 8 (29.6) |
|
|
Male | 30 (78.9) | 19 (70.4) |
|
| Smoking status, n
(%) |
|
| 0.658 |
|
Never | 12 (31.6) | 6 (22.2) |
|
|
Former | 21 (55.3) | 16 (59.3) |
|
|
Current | 5 (13.2) | 5 (18.5) |
|
| ECOG PS score, n
(%) |
|
| 0.750 |
| 0 | 14 (36.8) | 11 (40.7) |
|
| 1 | 24 (63.2) | 16 (59.3) |
|
| TNM stage, n
(%) |
|
| 0.260 |
|
IIIB | 3 (7.9) | 5 (18.5) |
|
| IV | 35 (92.1) | 22 (81.5) |
|
| Bone metastasis, n
(%) | 6 (15.8) | 7 (25.9) | 0.314 |
| Brain metastasis, n
(%) | 6 (15.8) | 6 (22.2) | 0.510 |
| CEA level, n
(%) |
|
| 0.564 |
|
Normal | 8 (21.1) | 7 (25.9) |
|
|
Abnormal | 29 (76.3) | 18 (66.7) |
|
| UK | 1 (2.6) | 2 (7.4) |
|
| CA125 level, n
(%) |
|
| 0.520 |
|
Normal | 13 (34.2) | 11 (40.7) |
|
|
Abnormal | 24 (63.2) | 14 (51.9) |
|
| UK | 1 (2.6) | 2 (7.4) |
|
| LDH level, n
(%) |
|
| 0.681 |
|
≤ULN | 22 (57.9) | 17 (63.0) |
|
|
>ULN | 16 (42.1) | 10 (37.0) |
|
| PD-L1 TPS, n
(%) |
|
| 0.450 |
|
<0% | 11 (28.9) | 10 (37.0) |
|
|
≥1% | 13 (34.2) | 11 (40.7) |
|
| UK | 14 (36.8) | 6 (22.2) |
|
Treatment response
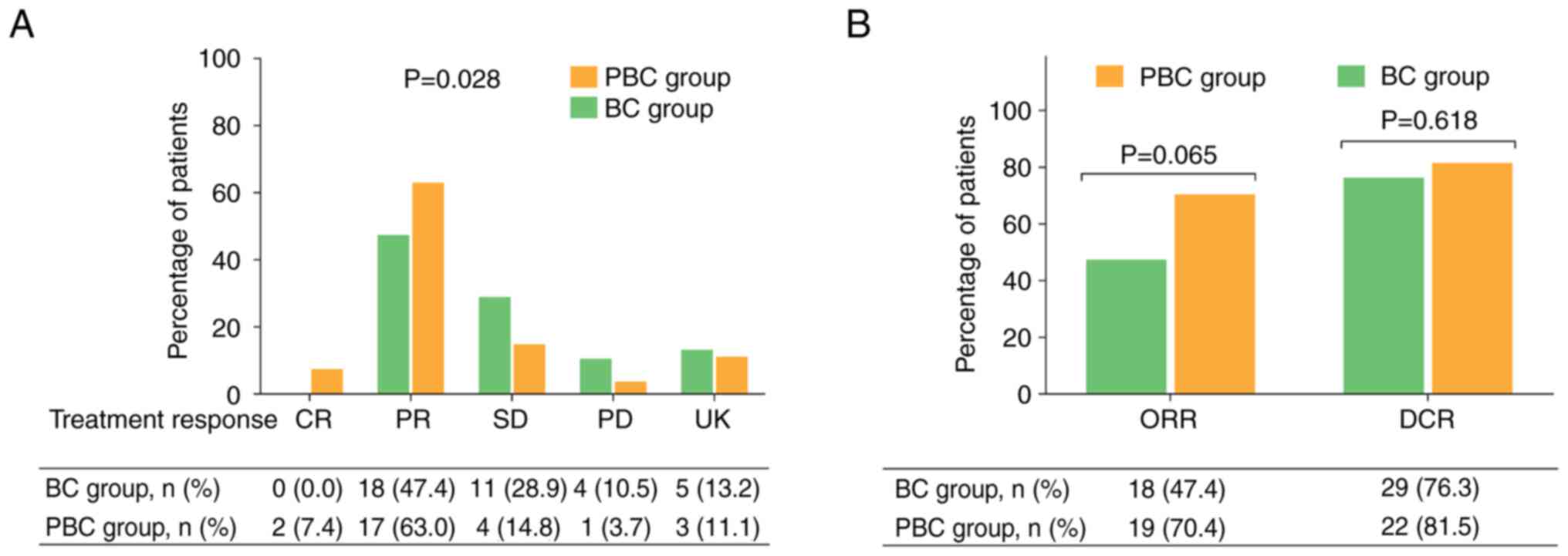
The CR, PR, SD and PD rates were 7.4, 63.0, 14.8 and
3.7%, respectively, in the PBC group, while the rates were 0.0,
47.4, 28.9 and 10.5%, respectively, in the BC group. Further
comparisons revealed that the treatment response (comparison of CR,
PR, SD and PD rates between two groups) was higher in the PBC group
than in the BC group (P=0.028; Fig.
1A). In addition, the ORR was higher in the PBC group compared
with that in the BC group (70.4 vs. 47.4%; P=0.065), although this
did not reaching statistical significance. Furthermore, the DCR did
not differ markedly between the two groups (81.5 vs. 76.3%;
P=0.618) (Fig. 1B).
Survival profiles
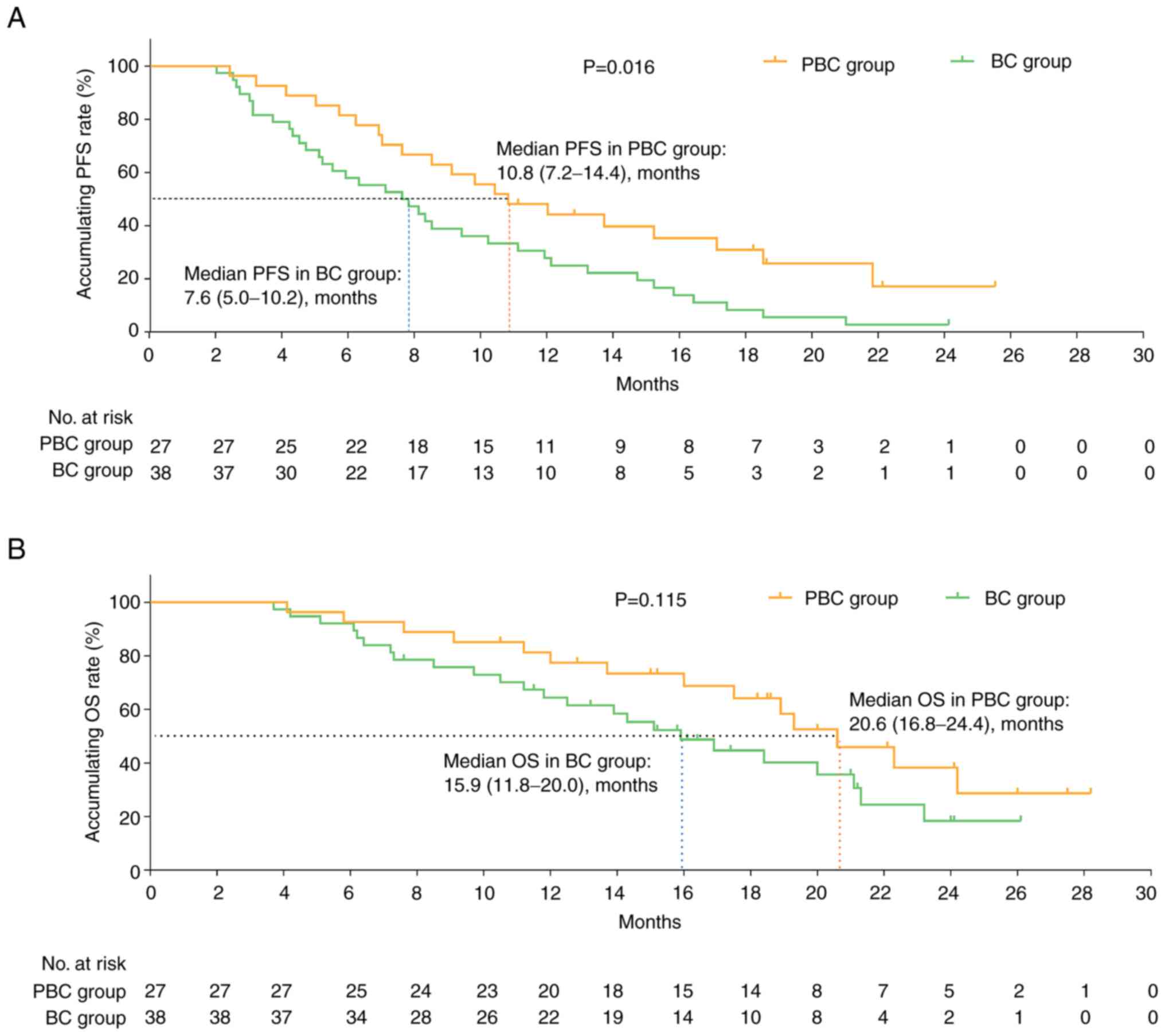
PFS times was prolonged in the PBC group compared
with that in the BC group [median PFS: 10.8 months (95% confidence
interval (CI), 7.2–14.4) vs. 7.6 months (95% CI, 5.0–10.2);
P=0.016; Fig. 2A], while the OS
exhibited a non-significant trend for improvement in the PBC group
compared with that in the BC group [median OS: 20.6 months (95% CI,
16.8–24.4) vs. 15.9 months (95% CI, 11.8–20.0); P=0.115; Fig. 2B].
Adjustment by multivariate Cox
analyses
To reduce the influence of compounding factors,
multivariate Cox analyses were performed, which confirmed that the
PBC regimen (vs. the BC regimen) was independently associated with
an improved PFS time [hazard ratio (HR)=0.566; P=0.045], but not OS
time (Table II). In addition, an
increased TNM stage was independently associated with both a worse
PFS (HR=5.092; P=0.008) and OS (HR=4.363; P=0.043) time (Table II).
 | Table II.Multivariate Cox's proportional
hazards regression analysis for PFS and OS. |
Table II.
Multivariate Cox's proportional
hazards regression analysis for PFS and OS.
| A, Forward stepwise
multivariate Cox's regression analysis for PFS |
|---|
|
|---|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Items | P-value | HR | Lower | Upper |
|---|
| Treatment (PBC vs.
BC) | 0.045 | 0.566 | 0.325 | 0.986 |
| TNM stage (IV vs.
IIIB) | 0.008 | 5.092 | 1.524 | 17.013 |
|
| B, Forward
stepwise multivariate Cox's regression analysis for OS |
|
|
|
|
| 95% CI |
|
|
|
|
|
| Items | P-value | HR | Lower | Upper |
|
| TNM stage (IV vs.
IIIB) | 0.043 | 4.363 | 1.046 | 18.191 |
Adverse events
The most common adverse events were neutropenia
(44.4 vs. 28.9%), alopecia (40.7 vs. 36.8%), leukopenia (37.0 vs.
31.6%), nausea and vomiting (37.0 vs. 28.9%) and fatigue (33.3 vs.
28.9%) in the PBC and BC groups (Table III). The majority of the adverse
events were grade 1–2, and only a few adverse events were grade
3–4, such as leukopenia, neutropenia, anemia and peripheral
neuropathy.
 | Table III.Adverse events. |
Table III.
Adverse events.
|
| BC group | PBC group |
|
|---|
|
|
|
|
|
|---|
| Items | Total | Grade 1–2 | Grade 3–4 | Total | Grade 1–2 | Grade 3–4 | P-value |
|---|
| Neutropenia, n
(%) | 11 (28.9) | 7 (18.4) | 4 (10.5) | 12 (44.4) | 9 (33.3) | 3 (11.1) | 0.365 |
| Alopecia, n
(%) | 14 (36.8) | 14 (36.8) | 0 (0.0) | 11 (40.7) | 11 (40.7) | 0 (0.0) | 0.750 |
| Leukopenia, n
(%) | 12 (31.6) | 9 (23.7) | 3 (7.9) | 10 (37.0) | 6 (22.2) | 4 (14.8) | 0.674 |
| Nausea and
vomiting, n (%) | 11 (28.9) | 9 (23.7) | 2 (5.3) | 10 (37.0) | 9 (33.3) | 1 (3.7) | 0.682 |
| Fatigue, n (%) | 11 (28.9) | 11 (28.9) | 0 (0.0) | 9 (33.3) | 9 (33.3) | 0 (0.0) | 0.706 |
| Anemia, n (%) | 6 (15.8) | 6 (15.8) | 0 (0.0) | 8 (29.6) | 6 (22.2) | 2 (7.4) | 0.169 |
| Peripheral
neuropathy, n (%) | 3 (7.9) | 3 (7.9) | 0 (0.0) | 6 (22.2) | 5 (18.5) | 1 (3.7) | 0.099 |
| Anorexia, n
(%) | 7 (18.4) | 6 (15.8) | 1 (2.6) | 6 (22.2) | 4 (14.8) | 2 (7.4) | 0.664 |
| Elevated
transaminase, n (%) | 7 (18.4) | 7 (18.4) | 0 (0.0) | 6 (22.2) | 5 (18.5) | 1 (3.7) | 0.488 |
| Rash, n (%) | 7 (18.4) | 5 (13.2) | 2 (5.3) | 5 (18.5) | 3 (11.1) | 2 (7.4) | 0.918 |
| Thrombopenia, n
(%) | 6 (15.8) | 5 (13.2) | 1 (2.6) | 5 (18.5) | 4 (14.8) | 1 (3.7) | 0.949 |
| Elevated bilirubin,
n (%) | 5 (13.2) | 5 (13.2) | 0 (0.0) | 5 (18.5) | 5 (18.5) | 0 (0.0) | 0.729 |
| Diarrhea, n
(%) | 5 (13.2) | 5 (13.2) | 0 (0.0) | 5 (18.5) | 5 (18.5) | 0 (0.0) | 0.729 |
| Increased blood
pressure, n (%) | 7 (18.4) | 7 (18.4) | 0 (0.0) | 4 (14.8) | 4 (14.8) | 0 (0.0) | 0.751 |
| Constipation, n
(%) | 3 (7.9) | 3 (7.9) | 0 (0.0) | 3 (11.1) | 3 (11.1) | 0 (0.0) | 0.686 |
Notably, the general incidence of each adverse event
did not differ significantly between the PBC and BC groups
(Table III). However, neutropenia
(44.4 vs. 28.9%), anemia (29.6 vs. 15.8%), and peripheral
neuropathy (22.2 vs. 7.9%) were more common in the PBC group than
in the BC group, without reaching statistical significance (all
P>0.05). In addition, the incidence of grade 3–4 adverse events
was also greater in the PBC group than in the BC group; however,
these differences were not statistically significant (all
P>0.05).
Discussion
In contrast to the multiple treatment options
available for patients with driver gene-positive advanced-stage
lung adenocarcinoma, surrogate treatment modalities for patients
with driver gene-negative advanced-stage lung adenocarcinoma are
limited, and mainly include platinum-doublet chemotherapy with or
without bevacizumab (33–35). However, the emergence of
immunotherapy has provided an alternative option for patients with
driver gene-negative advanced-stage lung adenocarcinoma (26–28).
In addition, inspired by the mutual effects of chemotherapy,
bevacizumab and PD-1/PD-L1 on immunosurveillance, regimens that
involve the combination of PD-1/PD-L1 inhibitors, bevacizumab and
chemotherapy have been applied in the treatment of patients with
driver gene-negative advanced-stage lung adenocarcinoma and have
subsequently been recommended as first-line therapies (23–25,29,36–40).
For example, the IMpower150 study revealed that the addition of
atezolizumab to bevacizumab plus chemotherapy achieved an ORR of
63.5% (CR of 3.7% and PR of 59.8%) in patients with driver
gene-negative advanced-stage non-squamous NSCLC (29). In addition, in the
ONO-4538-52/TASUKI-52 study, the addition of nivolumab to
platinum-doublet chemotherapy plus bevacizumab was associated with
a notably greater ORR of 61.5% (vs. 50.5% in the placebo
combination group) for patients with driver gene-negative
metastatic non-squamous NSCLC (30). However, as PD-1/PD-L1 inhibitor
therapy has not been used in China for that long, and as previous
studies involve randomized controlled trials, the relevant efficacy
and safety data for PD-1/PD-L1 inhibitors in the Chinese population
are not sufficient, particularly in real-world clinical settings.
Thus, the present real-world study was conducted with the aim of
determining the efficacy and safety of the PBC regimen in Chinese
patients with driver gene-negative advanced-stage lung
adenocarcinoma. The PBC regimen achieved an ORR of 70.4%, which was
greater than the 47.4% ORR in patients treated with the BC regimen;
however, their DCR did not differ. These findings are similar to
those of previous studies, except that in the present study, there
was only an upward trend, not a significant difference, in the ORR
in patients who received the PBC regimen compared with the patients
who received the BC regimen alone. These findings may be explained
by the small sample size of the present study, which caused a lower
statistical power; thus, further studies with larger sample sizes
are warranted. Another notable finding of the present study was
that the ORR of 70.4% in patients treated with the PBC regimen was
numerically greater than that reported in previous studies
(29,30). These findings may be explained by
the fact that the response rate evaluated in the present study was
the optimal response rate, which corresponded to a longer
evaluation period than that used in previous studies and was thus
associated with a numerically elevated ORR.
Previous trials have revealed that the PBC regimen
can achieve a promising survival profile compared with BC regimens
alone in patients with driver gene-negative advanced-stage lung
adenocarcinoma (29,30,41,42). A
previous phase Ib study demonstrated that nivolumab combined with
platinum-doublet chemotherapy plus bevacizumab was associated with
a long PFS time (41,42). Moreover, in the IMpower150 study,
the addition of atezolizumab to bevacizumab plus chemotherapy
prolonged the PFS time by 1.5 months (median PFS time: 8.3 vs. 6.8
months) and OS by 4.5 months (median OS time: 19.2 vs. 14.7 months)
compared with the bevacizumab plus chemotherapy alone in patients
with driver gene-negative advanced-stage non-squamous NSCLC
(29). In addition, in the
ONO-4538-52/TASUKI-52 study, the addition of nivolumab to
platinum-doublet chemotherapy plus bevacizumab was associated with
an even longer PFS time (median PFS time: 12.1 vs. 8.1 months)
compared with platinum-doublet chemotherapy plus bevacizumab for
patients with driver gene-negative metastatic non-squamous NSCLC
(30). In the present study, it was
found that the PFS time was longer in the PBC group than that in
the BC group, while the OS time did not differ between these two
groups. A possible explanation for this may be that due to the
relatively short follow-up period, a significant difference in OS
time was not detected between these two groups. Moreover, the OS
could be affected by the treatment regimens after tumor
progression, which weakened the difference in the OS time between
the PBC and BC groups in the current study. For instance, some of
the patients in the BC group received PD-1/PD-L1 inhibitor after
the tumor progression, which affected the comparison of OS between
the PBC and BC groups. However, since a proportion of patients
received subsequent treatment in other hospitals, the detailed
information of further treatment regimens after tumor progression
was not analyzed in the study (the accurate information of
treatment regimen after progression was confirmed in only 19
patients, while the data for the majority if patients was not
available or could not be accurately confirmed).
In terms of safety profiles, preceding studies have
demonstrated that treatment with the PBC regimen is well tolerated
and that no new adverse events have occurred compared with those
for the BC regimen, but only in patients with driver gene-negative
advanced-stage lung adenocarcinoma (29,30,41,42).
In line with the findings of these previous studies (29,30,41,42),
the present study revealed that the adverse event rates did not
differ significantly between the PBC and BC groups. The most common
adverse events of the PBC regimen included neutropenia, alopecia,
leukopenia, nausea and vomiting, fatigue, anemia, peripheral
neuropathy, anorexia, elevated transaminase levels, rash,
thrombopenia, elevated bilirubin levels, diarrhea, increased blood
pressure and constipation. Furthermore, the majority of the adverse
events were grade 1 or 2. These data indicated that the PBC regimen
had acceptable safety profiles in patients with driver
gene-negative advanced-stage lung adenocarcinoma.
Notably, a recent study observed that the
measurement of blood inflammatory cytokines helped to predict the
efficacy and adverse events of PD-1 inhibitor treatment in patients
with metastatic melanoma (43),
which could also be evaluated in the future in patients with driver
gene-negative advanced-stage lung adenocarcinoma. It has also been
reported that an LDH measurement is able to predict the response
and outcome in patients with lung cancer receiving PD-1 inhibitor
or PD-L1 inhibitor treatment (44,45);
however, the present study observed that LDH proportion did not
differed between the PBC and BC groups, indicating that it would
not affect the findings.
The present study had several limitations that
should be mentioned: i) As a real-world study, the sample size of
65 patients was insufficient; ii) the follow-up duration was short
and could be further prolonged to determine the difference in OS
time between the PBC and BC regimens; and iii) selection bias may
have been present due to the study being in a single institute, and
more centers could be invited to join a real-world study in the
future to reduce this.
In conclusion, the present study demonstrated that
the PBC regimen serves as a superior treatment option with
promising efficacy and tolerable safety in patients with driver
gene-negative advanced-stage lung adenocarcinoma. However, further
large-scale, multicenter real-world studies with longer follow-up
periods are required to verity the findings presented.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XY, XL, KH and XZ contributed to the study
conception and design. XY, XL and KH performed material
preparation, and data collection and analysis. The first draft of
the manuscript was written by XY, and all authors commented on
previous versions of the manuscript. XZ and XY confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of Sichuan Cancer
Hospital and Institute, Sichuan Cancer Center, School of Medicine,
University of Electronic Science and Technology of China (Chengdu,
China) approved the protocol (approval no. SCCHEC-01A-2020-010).
Written informed consent was obtained from the patient or the
patient's direct relative.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jenkins R, Walker J and Roy UB: 2022
cancer statistics: Focus on lung cancer. Future Oncol. 5:1–11.
2023. View Article : Google Scholar
|
|
3
|
Chen LN, Wei AZ and Shu CA: Neoadjuvant
immunotherapy in resectable non-small-cell lung cancer. Ther Adv
Med Oncol. 15:175883592311637982023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seguin L, Durandy M and Feral CC: Lung
adenocarcinoma tumor origin: A guide for personalized medicine.
Cancers (Basel). 14:17592022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mithoowani H and Febbraro M:
Non-small-cell lung cancer in 2022: A review for general
practitioners in oncology. Curr Oncol. 29:1828–1839. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miao D, Zhao J, Han Y, Zhou J, Li X, Zhang
T, Li W and Xia Y: Management of locally advanced non-small cell
lung cancer: State of the art and future directions. Cancer Commun
(Lond). 44:23–46. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong J, Bai H, Wang Z, Duan J, Zhuang W,
Wang D, Wan R, Xu J, Fei K, Ma Z, et al: Treatment of advanced
non-small cell lung cancer with driver mutations: Current
applications and future directions. Front Med. 17:18–42. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peters S, Camidge DR, Shaw AT, Gadgeel S,
Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, et al:
Alectinib versus crizotinib in untreated ALK-positive
non-small-cell lung cancer. N Engl J Med. 377:829–838. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drilon A, Siena S, Dziadziuszko R, Barlesi
F, Krebs MG, Shaw AT, de Braud F, Rolfo C, Ahn MJ, Wolf J, et al:
Entrectinib in ROS1 fusion-positive non-small-cell lung cancer:
Integrated analysis of three phase 1–2 trials. Lancet Oncol.
21:261–270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jurisic V, Obradovic J, Nikolic N, Javorac
J, Perin B and Milasin J: Analyses of P16(INK4a) gene promoter
methylation relative to molecular, demographic and clinical
parameters characteristics in non-small cell lung cancer patients:
A pilot study. Mol Biol Rep. 50:971–979. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Obradovic J, Djordjevic N, Tosic N,
Mrdjanović J, Stanković B, Stanić J, Zarić B, Perin B, Pavlović S
and Jurišić V: Frequencies of EGFR single nucleotide polymorphisms
in non-small cell lung cancer patients and healthy individuals in
the Republic of Serbia: A preliminary study. Tumour Biol.
37:10479–10486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Yang H, Teo ASM, Amer LB, Sherbaf
FG, Tan CQ, Alvarez JJS, Lu B, Lim JQ, Takano A, et al: Genomic
landscape of lung adenocarcinoma in East Asians. Nat Genet.
52:177–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitsudomi T: Molecular epidemiology of
lung cancer and geographic variations with special reference to
EGFR mutations. Transl Lung Cancer Res. 3:205–211. 2014.PubMed/NCBI
|
|
16
|
Obradovic J, Todosijevic J and Jurisic V:
Side effects of tyrosine kinase inhibitors therapy in patients with
non-small cell lung cancer and associations with EGFR
polymorphisms: A systematic review and meta-analysis. Oncol Lett.
25:622023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jurisic V, Vukovic V, Obradovic J,
Gulyaeva LF, Kushlinskii NE and Djordjevic N: EGFR polymorphism and
survival of NSCLC patients treated with TKIs: A systematic review
and meta-analysis. J Oncol. 2020:19732412020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paz-Ares LG, de Marinis F, Dediu M, Thomas
M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et
al: PARAMOUNT: Final overall survival results of the phase III
study of maintenance pemetrexed versus placebo immediately after
induction treatment with pemetrexed plus cisplatin for advanced
nonsquamous non-small-cell lung cancer. J Clin Oncol. 31:2895–2902.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, phase III study of
first-line Carboplatin/Paclitaxel plus bevacizumab or placebo in
chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chu T, Lu J, Bi M, Zhang H, Zhuang W, Yu
Y, Shi J, Chen Z, Zhang X, Guo Q, et al: Equivalent efficacy study
of QL1101 and bevacizumab on untreated advanced non-squamous
non-small cell lung cancer patients: A phase 3 randomized,
double-blind clinical trial. Cancer Biol Med. 18:816–824. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zitvogel L, Galluzzi L, Smyth MJ and
Kroemer G: Mechanism of action of conventional and targeted
anticancer therapies: Reinstating immunosurveillance. Immunity.
39:74–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martino EC, Misso G, Pastina P, Costantini
S, Vanni F, Gandolfo C, Botta C, Capone F, Lombardi A, Pirtoli L,
et al: Immune-modulating effects of bevacizumab in metastatic
non-small-cell lung cancer patients. Cell Death Discov.
2:160252016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alfaro C, Suarez N, Gonzalez A, Solano S,
Erro L, Dubrot J, Palazon A, Hervas-Stubbs S, Gurpide A,
Lopez-Picazo JM, et al: Influence of bevacizumab, sunitinib and
sorafenib as single agents or in combination on the inhibitory
effects of VEGF on human dendritic cell differentiation from
monocytes. Br J Cancer. 100:1111–1119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wada J, Suzuki H, Fuchino R, Yamasaki A,
Nagai S, Yanai K, Koga K, Nakamura M, Tanaka M, Morisaki T and
Katano M: The contribution of vascular endothelial growth factor to
the induction of regulatory T-cells in malignant effusions.
Anticancer Res. 29:881–888. 2009.PubMed/NCBI
|
|
26
|
Gan J, Huang Y, Fang W and Zhang L:
Research progress in immune checkpoint inhibitors for lung cancer
in China. Ther Adv Med Oncol. 13:175883592110298262021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li K, Zhang A, Li X, Zhang H and Zhao L:
Advances in clinical immunotherapy for gastric cancer. Biochim
Biophys Acta Rev Cancer. 1876:1886152021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Alessio A, Rimassa L, Cortellini A and
Pinato DJ: PD-1 blockade for hepatocellular carcinoma: Current
research and future prospects. J Hepatocell Carcinoma. 8:887–897.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sugawara S, Lee JS, Kang JH, Kim HR, Inui
N, Hida T, Lee KH, Yoshida T, Tanaka H, Yang CT, et al: Nivolumab
with carboplatin, paclitaxel, and bevacizumab for first-line
treatment of advanced nonsquamous non-small-cell lung cancer. Ann
Oncol. 32:1137–1147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumors:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
U.S. Department Of Health and Human
Services: National Institutes of Health and National Cancer
Institute: Common terminology criteria for adverse events (CTCAE)
version 4.02. Available from:. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.02_2009-09-15_QuickReference_8.5×11.pdf
|
|
33
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Novello S, Barlesi F, Califano R, Cufer T,
Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al:
Metastatic non-small-cell lung cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
27:v1–v27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Network NCC: NCCN Clinical Practice
Guidelines in Oncology: Non-small cell lung cancer. 2018.PubMed/NCBI
|
|
36
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-Positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gandhi L, Rodriguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Şenler FC, Csőszi T, Fülöp A, et al:
Pembrolizumab plus chemotherapy for squamous non-small-cell lung
cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kanda S, Goto K, Shiraishi H, Kubo E,
Tanaka A, Utsumi H, Sunami K, Kitazono S, Mizugaki H, Horinouchi H,
et al: Safety and efficacy of nivolumab and standard chemotherapy
drug combination in patients with advanced non-small-cell lung
cancer: A four arms phase Ib study. Ann Oncol. 27:2242–2250. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kanda S, Ohe Y, Goto Y, Horinouchi H,
Fujiwara Y, Nokihara H, Yamamoto N, Yamamoto T and Tamura T:
Five-year safety and efficacy data from a phase Ib study of
nivolumab and chemotherapy in advanced non-small-cell lung cancer.
Cancer Sci. 111:1933–1942. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Martinovic KM, Vuletic A, Miletic NT,
Žižak IB, Milovanović J, Matković S and Jurišić V: Circulating
cytokine dynamics as potential biomarker of response to anti-PD-1
immunotherapy in BRAFwt MM patients. Transl Oncol. 38:1017992023.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen X, Li Z, Zhou J, Wei Q, Wang X and
Jiang R: Identification of prognostic factors and nomogram model
for patients with advanced lung cancer receiving immune checkpoint
inhibitors. PeerJ. 10:e145662022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jurisic V, Radenkovic S and Konjevic G:
The actual role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–124. 2015. View Article : Google Scholar : PubMed/NCBI
|