Introduction
In total, 20–50% of patients with diffuse large
B-cell lymphoma (DLBCL) will become resistant to initial
immuno-chemotherapy or relapse after achieving a complete metabolic
response (CMR), thus predicting a poor prognosis (1). Chimeric antigen receptor (CAR) T-cell
therapy targeting CD19 has resulted in tremendous progress with
regard to increasing survival rates in the past two decades
(2–5). In the ZUMA-1 trial, patients with
refractory large B-cell lymphoma received axicabtagene ciloleucel
autologous anti-CD19 CAR T-cell therapy and the long-term survival
analysis yielded an estimated 5-year OS rate of 42.6% (5), suggesting that further investigations
are warranted to maintain long-term clinical remission, as well as
to manage the potential infusion-related toxicities, namely
cytokine release syndrome (CRS; grade ≥3 reported in 2–22% of
patients) and immune effector cell-associated neurotoxicity
syndrome (grade ≥3 reported in 5.1–28% of patients).
Cytokine release syndrome (CRS) is a potentially
life threatening toxicity with a prevalence that can be as high as
93% (6,7). Currently, the commonly used treatment
regimens for CRS include tocilizumab and corticosteroids (8). In patients with CRS who respond to
tocilizumab, symptoms such as fever and hypotension typically
resolve within a few hours. However, a subset of patients may show
no improvement in symptoms or response. In these cases, the
consideration of other drugs, such as corticosteroids, is warranted
(9,10). Nevertheless, some studies have
indicate that corticosteroids may adversely affect CAR T-cell
function, and the cumulative dose could be linked to reduced
survival rates (9,11,12).
Additionally, some patients exhibit poor responses to both
treatment regimens, resulting in limited options. Consequently,
there is an urgent need to explore other medications for more
effective management (10,13). The present study presented two cases
of patients with relapsed DLBCL who received CAR T-cell therapy and
aimed to investigate the efficacy of the interferon-γ (IFN-γ)
antibody emapalumab in treating CAR T-cell induced CRS.
Case report
Patient 1
A 56-year-old male patient was diagnosed with
germinal center B-cell (GCB) like DLBCL, Ann Arbor stage IV, an
International Prognostic Index score (14) of 3 and MYC-BCL2 double expression in
January 2022 at the Department of Oncology, The First Affiliated
Hospital of Soochow University. The patient received 8 cycles
(every 21 days for a cycle) of R-CHOP (iv rituximab, 375
mg/m2 on day 0; iv cyclophosphamide, 750
mg/m2 on day 1; iv doxorubicin, 50 mg/m2 on
day 1; iv vincristine, 1.4 mg/m2, dose cap of 2 mg on
day 1; and oral prednisone, 100 mg daily on days 1–5). The
fluorodeoxyglucose-positron emission tomography (FDG-PET) scan
showed progression in both lungs, with a Deauville score (DS)
(15) of 5 and an abdominal node
with a DS of 4. A salvage chemotherapy comprising 2 cycles (every
21 days for a cycle) of R-GDP (iv rituximab, 375 mg/m2
on day 0; iv gemcitabine, 1,000 mg/m2 on days 1 and 8;
iv dexamethasone, 40 mg on days 1–4; and iv cisplatin, 25
mg/m2 on days 1–3) was administered. The patient was
later referred to the National Clinical Research Center for
Hematological Diseases, The First Affiliated Hospital of Soochow
University for CD19-directed CAR T-cell therapy (16,17) in
October 2022. The patient underwent lymphodepleting conditioning
with cyclophosphamide 0.3 g/m2 day-5-2 and fludarabine
30 mg/m2 d-5-2. The patient was infused with
2×106 axicabtagene ciloleucel cells/kg. On day 4, the
patient experienced a fever of 38.0°C with tachycardia, but was
normotensive with a normal respiratory exam. The patient had
repeated negative blood cultures and was diagnosed with CRS (grade
1) based on the American Society for Transplantation and Cellular
Therapy grading scale (18). Blood
cultures testing for pathogenic microorganisms were obtained which
was later reported to be negative, and broad-spectrum antibiotics
(iv meropenem 1g q8h, and oral voriconazole 4 mg/kg) were
administered. On day 6, the patient developed grade 3 CRS with
hypotension (85/54 mmHg) and hypoxia. Supplemental oxygen was
supplied at 6 l/min, as well as norepinephrine infusions initially
at 0.15 µg/kg/min. Two doses of 8 mg/kg tocilizumab were
administered intravenously every 12 h, but the patient did not
exhibit a notable and persistent change in the fever or blood
pressure. The temperature climbed back up to 39.6°C and the blood
pressure was 101/62 mmHg treated with norepinephrine infusions.
Considering the lack of improvement in CRS symptoms, additional
treatment is needed. Due to an increase in the level of IFN-γ,
emapalumab, an anti-IFN-γ antibody, was administered at a dose of 1
mg/kg on day 7 after informed consent was obtained. The temperature
of the patient rapidly normalized and the norepinephrine dose was
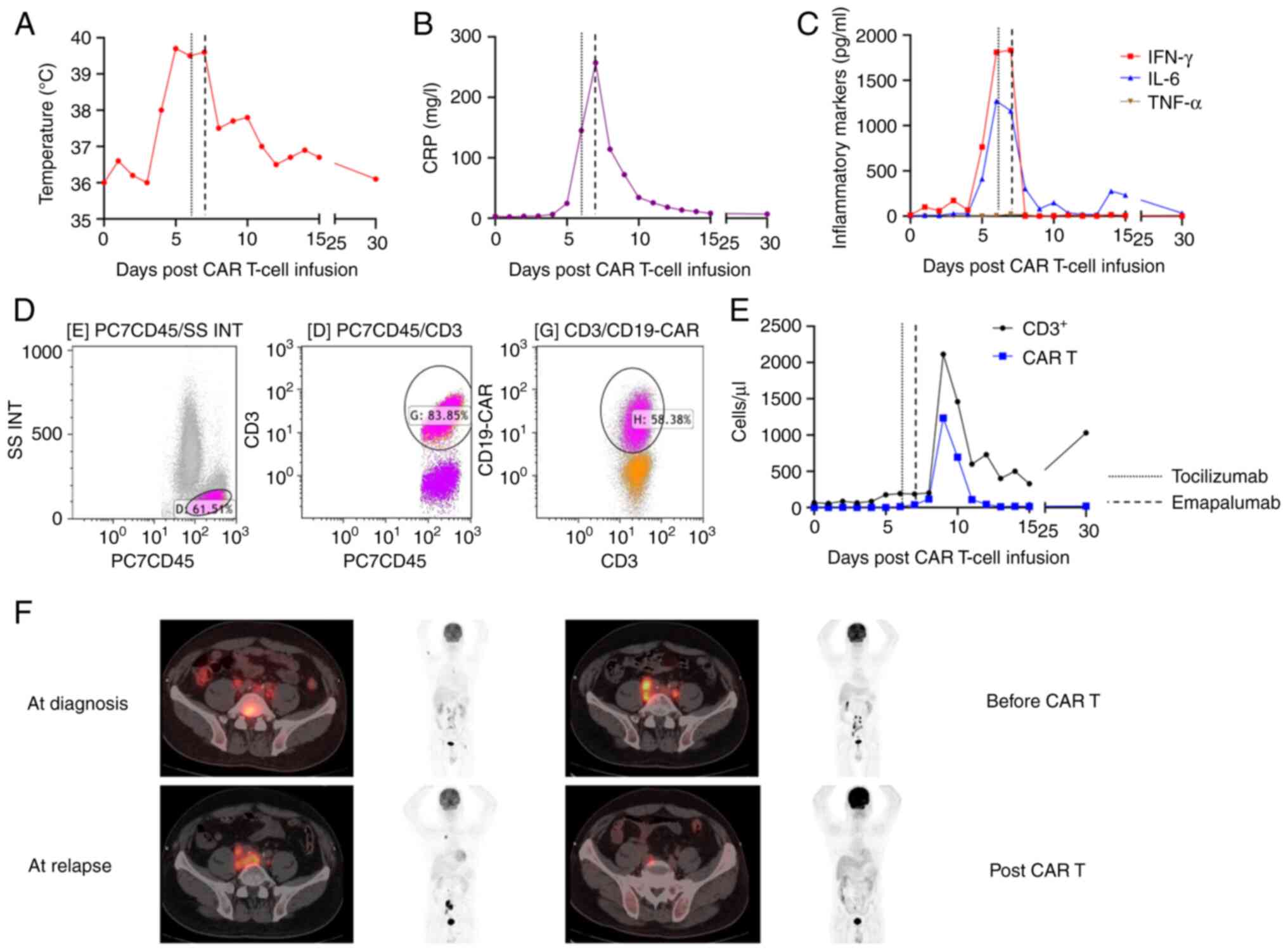
gradually reduced (Fig. 1A). The
levels of C-reactive protein (CRP) and inflammatory cytokines
significantly decreased, with IFN-γ level particularly decreased to
0 pg/ml (Fig. 1B and C). The level
of interleukin 6 (IL-6) descended from 1,162 on day 7 to 304 pg/ml
on day 8 and continued to fall (Fig.
1C). The expansion of CAR-T cells were monitored by flow
cytometry (Fig. 1D): The PE-labeled
monoclonal anti-FMC63 antibody (ACROBiosystems, FM3-HPY53),
CD3-FITC-conjugated antibody (Immunotech S.A.S, A07746), and
CD45-PC7-conjugated antibody (Immunotech S.A.S, IM3548) were used
to label CAR-T cells. The Beckman Coulter Navios (Beckman Coulter,
Inc.) was used to acquire the data, and Kaluza Analysis Software
(v2.1; Beckman Coulter, Inc.) was employed for data analysis. The
number of CAR-T cells reached a peak less than 10 days after cell
infusion (Fig. 1E). This also
suggested that neutralizing IFN-γ may not affect the normal
activity of CAR-T cells (19). The
patient was followed up at 1, 3, and 6 months after treatment. At 3
months, the patient underwent an FDG-PET scan, showing a partial
response (PR) status (16)
(Fig. 1F). The response status
remained stable at the last follow-up (at 6 months).
Patient 2
A 49-year-old female patient was diagnosed with
DLBCL transformed from follicular lymphoma (FL; grade 3B, WHO
grading system for FL) (20) in
November 2020 at the First People's Hospital of Changzhou. The
patient achieved a complete metabolic response (CMR) after 4 cycles
of R-CHOP (as above) and 4 cycles of R-CHOP (as above) with
zanubrutinib (oral, 160 mg, bid), but relapsed 6 months later. She
was referred to the National Clinical Research Center for
Hematological Diseases, The First Affiliated Hospital of Soochow
University, in January 2023. The refractory disease was confirmed
with a biopsy of the inguinal lymph node. Next-generation
sequencing (NGS) showed an MCD subtype (a genetic subtype in DLBCL,
based on the co-occurrence of MYD88L265P and
CD79B mutations) (21), with
positivity for protein coding gene CD79B, CDKN2A (multiple
tumor suppressor l), CCND3 (protein coding gene), FAS
(protein coding gene), APC (tumor suppressor gene), B-cell
lymphoma 6 (BCL6) and CREBBP (protein coding gene).
NGS was performed using a NovaSeq 6000 S1 Reagent Kit (cat. no.
20028319; Illumina, Inc.) and a GeneJET Genomic DNA Purification
Kit (cat. no. K0721; Thermo Fisher Scientific, Inc.) to prepare the
DNA sample. A Bioanalyzer 2100 (Agilent Technologies, Inc.) was
used to verify the quality of the processed samples. The purified
library was quantified using the KAPA Library Quantification Kit
(KAPA Biosystems; cat. no. 07960140001; Roche Diagnostics).
Enriched libraries were amplified and subjected to NGS on Illumina
novaseq 6000 platforms (150 cycles; cat. no. 2002746; Illumina,
Inc.). The quantitative concentration of the final library was 2
nM. The 2×150 bp paired-end sequencing was performed in a testing
laboratory (Geneseeq Technology, Inc.) accredited by the Clinical
Laboratory Improvement Amendments and the College of American
Pathologists. Data analysis was performed using Trimmomatic 0.39
(https://github.com/usadellab/Trimmomatic).
After oral treatment with 3 cycles of R-ICE (iv
rituximab, 375 mg/m2 on day 0; iv ifosfamide, 5,000
mg/m2 on day 2; iv etoposide, 100 mg/m2 on
days 1–3; carboplatin area under the curve 5, maximum dose of 800
mg, iv on day 2) and zanubrutinib (as above), the patient proceeded
to undergo autologous stem cell transplant (ASCT) followed by CAR
T-cell therapy. Pre-treatment with CEAC regimen (oral lomustine,
200 mg/m2 on day 6; iv etoposide, 100 mg/m2
q12h on days-5 to −2; iv cytarabine, 200 mg/m2 q12h on
days-5 to −2; and iv cyclophosphamide 1.5 g/m2 on days-5
to −2) and iv cladribine (50 mg/m2 on days-5 to −2) was
administered, and autologous hematopoietic stem cells were infused
(mononuclear cells, 6.8×108/kg; CD34+ cells,
8.0×106/kg). After 7 days, the patient received a total
amount of 100×106 relmacabtagene autoleucel cells/kg. A
fever (39.6°C) and hypoxemia developed on day 2 after CAR T-cell
infusion, with negative repeat blood cultures, and the patient was
diagnosed with rapid progression to grade 2 CRS (18). The serum IL-6 level reached a
maximum of 506 pg/ml and the IFN-γ level reached 888 pg/ml. One
dose of emapalumab (1 mg/kg) was administered after informed
consent was obtained, as well as antifebrile therapy and
supplemental oxygen. The body temperature rapidly decreased to
38.7°C (Fig. 2A), with IFN-γ and
IL-6 levels rapidly decreasing to 0 pg/ml and 141 pg/ml on day 4
after CAR T-cell infusion, respectively (Fig. 2B and C). The CAR T-cells continued
to expand (Fig. 2D and E). The
patient was followed up at 1, 3, and 6 months after treatment. The
1-month FDG-PET scan showed a CMR (Fig.
2F), and circulating tumor DNA monitoring at 2 months revealed
a negative disease state (Fig. 2G).
The patient was still alive, taking zanubrutinib 160 mg bid orally,
with no signs of occurrence at the 6-month follow-up.
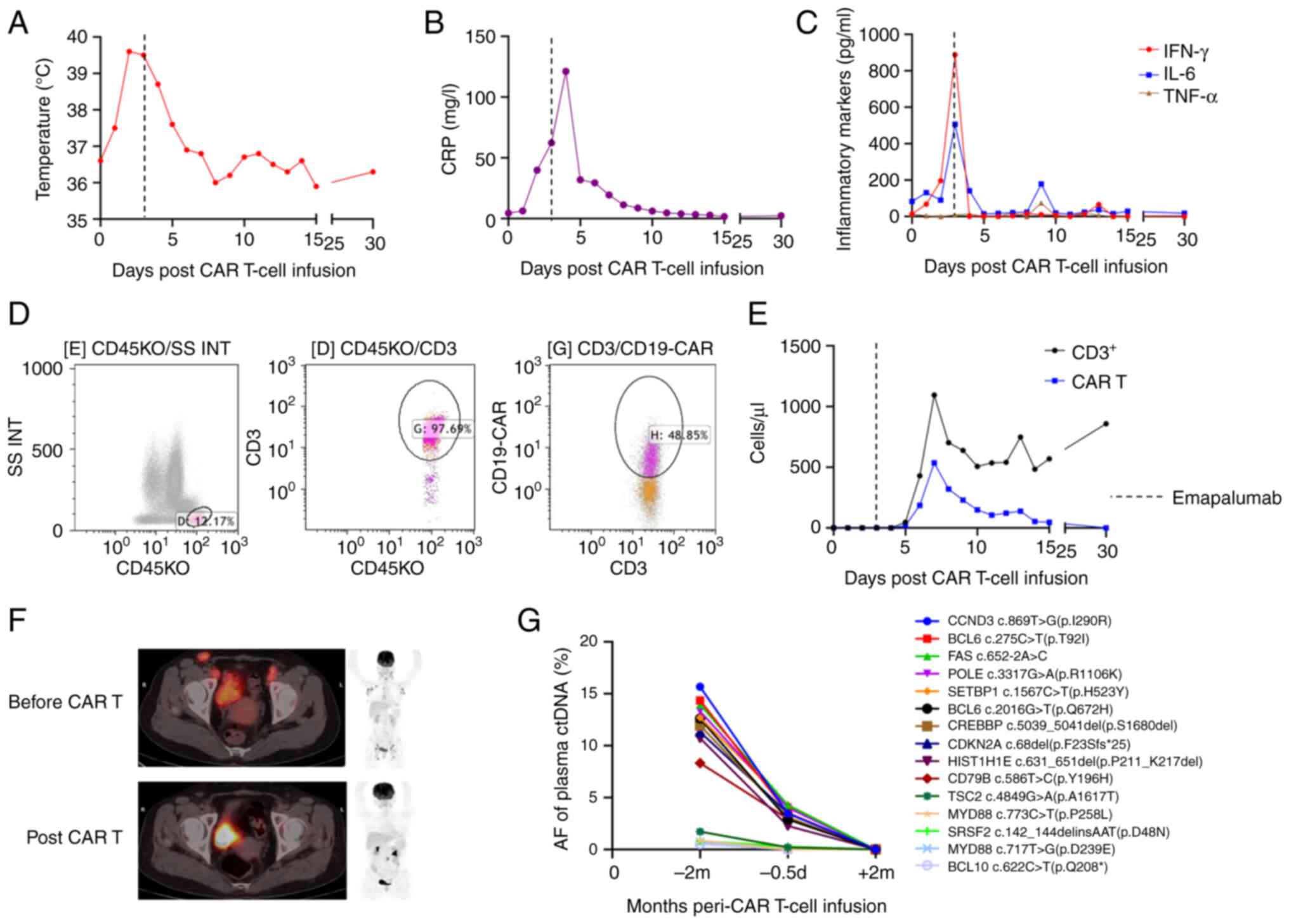 | Figure 2.Clinical and laboratory features
surrounding the use of emapalumab for CAR T-cell-induced cytokine
release syndrome in patient 2. (A) Temperature in °C. (B) CRP
levels in mg/l. (C) Serum cytokine levels in pg/ml of IFN-γ, IL-6
and TNF-α. (D) Representative flow cytometry of CAR T-cells. (E)
CAR T-cell expansion in cells/µl examined by flow cytometry. (F)
Efficacy of CAR T-cell therapy based on fluorodeoxyglucose positron
emission tomography scans at relapse, and at 1 month after CAR
T-cell therapy. (G) Efficacy of CAR T-cell therapy based on the
ctDNA test. AF, allele fraction; CAR T-cell, chimeric antigen
receptor T-cell; CRP, C-reactive protein; ctDNA, circulating tumor
DNA; d, days; IFN-γ, interferon-γ; IL-6, interleukin 6; m, months;
TNF-α, tumor necrosis factor-α. |
Discussion
The pathophysiology underpinning the development of
CRS stems from activation of immune cells of the tumor
microenvironment, directly resulting in excessive production of
inflammatory cytokines and chemokines (22). The most commonly affected cytokines
are IL-6, IFN-γ, tumor necrosis factor-α, CRP and ferritin
(23), which in turn lead to
endothelial injury and tissue damage. IL-6 serves a crucial role in
mediating CRS, and tocilizumab (anti-IL-6 receptor) has been
approved for the management of CRS (24). However, some patients exhibit a poor
response to tocilizumab and glucocorticoids (9,10),
necessitating adjustments to their treatment regimen based on
individual responses. Additionally, there is currently no
standardized treatment protocol for CRS.
Some patients with severe CRS exhibit
characteristics similar to those of hemophagocytic
lymphohistiocytosis (HLH) (10).
Notably, IFN-γ levels are elevated in both severe CRS and HLH, and
in HLH, IFN-γ is regarded as a key driver of inflammation (10). A maximum fold-change in IFN-γ of
>100 has been used to predict grade 3+ CRS, with a sensitivity
of 83% and a specificity of 100% (25). The knockdown of IFN-γ preserves the
benefits of anti-CD19 CAR T-cell therapy and inhibits the release
of multiple cytokines from CAR T-cells and peripheral blood
mononuclear cells, thereby reducing the effect of CRS and enhances
the safety profile of CAR T-cell therapy (26). In addition, IFN-γ blockade can
reduce macrophage activation and improve CAR T-cell function while
reducing treatment-related toxicity in hematologic malignancies
(27).
Emapalumab is a human anti-IFN-γ antibody that binds
to both free and receptor-bound IFN-γ, inhibiting receptor
dimerization and the transduction of IFN-γ signaling, thereby
neutralizing its biological activity (28). A prospective clinical study has
demonstrated its effectiveness as a targeted therapy for primary
HLH (28). Additionally, it has
shown efficacy in treating CRS following CAR T-cell therapy in
patients with relapsed B-cell acute lymphoblastic leukemia
(29). McNerney and DiNofia
reported the case of a patient with B-cell acute lymphoblastic
leukemia who developed grade 4 CRS that was refractory to both
tocilizumab and glucocorticoids. This patient was treated with
tocilizumab, methylprednisolone, siltuximab and the IFN-γ inhibitor
emapalumab, achieving complete remission for 12 months (10). Rainone et al (13) described a case of CAR T-cell
therapy-associated macrophage activation syndrome/HLH that was
successfully treated with emapalumab in combination with anakinra
and corticosteroids.
In the present study, patient 1 developed severe CRS
and received tocilizumab, but did not respond adequately,
ultimately achieving a PR with emapalumab. In case 2, the IFN-γ
levels were elevated, up to 888 pg/ml. A study has indicated that
elevated IFN-γ levels are associated with severe CRS (30). Therefore, the patient was directly
treated with emapalumab and achieved a favorable response. These
findings are consistent with the limited previously reported cases
(10,13), suggesting that the clinical use of
emapalumab can effectively mitigate severe CRS in CAR
T-cell-treated patients without compromising antitumor efficacy.
Common AEs associated with emapalumab include infection,
hypertension and infusion-related reactions (31). However, the 2 patients in the
present report had no obvious AEs and the treatment was well
tolerated. The patients felt no discomfort or dissatisfaction
during the entire treatment. The cost of the medication was
acceptable to the patients and they remained proactive and
cooperative throughout the treatment. However, the present study
also had certain limitations. It only reported two patients and
lacked large-scale statistical data, and further prospective
clinical trials are necessary to confirm the long-term efficacy and
safety of emapalumab.
In summary, the present study trialed the use of the
drug emapalumab to neutralize IFN-γ, in order to mitigate CRS while
maintaining the efficacy of CAR T-cell proliferation. Prospective
clinical trials are warranted to determine its role in CAR T-cell
therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the National
Natural Science Foundation of China (grant no. 82000158).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available to protect patient privacy
but are available from the corresponding author on reasonable
request.
Authors' contributions
CL and DW were responsible for conception and
design. WC and YL were responsible for providing study materials or
patients. Collection and assembly of data was performed by HG, QY
and LZ. Data analysis and interpretation was performed by WC and
YL. HH, JL and SL advised on patient treatment, drafted the
discussion and confirmed the authenticity of all the raw data. All
authors helped to write the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Soochow University
(Suzhou, China; approval number 2017-053-2).
Patient consent for publication
Written informed consent was obtained from the
patients for the publication of any potentially identifiable images
or data included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crump M, Neelapu SS, Farooq U, Van Den
Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L,
et al: Outcomes in refractory diffuse large B-cell lymphoma:
Results from the international SCHOLAR-1 study. Blood.
130:1800–1808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Locke FL, Ghobadi A, Jacobson CA, Miklos
DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT,
Timmerman JM, et al: Long-term safety and activity of axicabtagene
ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A
single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 20:31–42.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abramson JS, Palomba ML, Gordon LI,
Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG,
Andreadis C, et al: Lisocabtagene maraleucel for patients with
relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001):
A multicentre seamless design study. Lancet. 396:839–852. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ying Z, Yang H, Guo Y, Li W, Zou D, Zhou
D, Wang Z, Zhang M, Wu J, Liu H, et al: Long-term outcomes of
relmacabtagene autoleucel in Chinese patients with
relapsed/refractory large B-cell lymphoma: Updated results of the
RELIANCE study. Cytotherapy. 25:521–529. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neelapu SS, Jacobson CA, Ghobadi A, Miklos
DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT,
Timmerman JM, et al: Five-year follow-up of ZUMA-1 supports the
curative potential of axicabtagene ciloleucel in refractory large
B-cell lymphoma. Blood. 141:2307–2315. 2023.PubMed/NCBI
|
|
6
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Topp MS, van Meerten T, Houot R, Minnema
MC, Bouabdallah K, Lugtenburg PJ, Thieblemont C, Wermke M, Song KW,
Avivi I, et al: Earlier corticosteroid use for adverse event
management in patients receiving axicabtagene ciloleucel for large
B-cell lymphoma. Br J Haematol. 195:388–398. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li P, Liu Y, Liang Y, Bo J, Gao S, Hu Y,
Hu Y, Huang H, Huang X, Jing H, et al: 2022 Chinese expert
consensus and guidelines on clinical management of toxicity in
anti-CD19 chimeric antigen receptor T-cell therapy for B-cell
non-Hodgkin lymphoma. Cancer Biol Med. 20:129–146. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hughes AD, Teachey DT and Diorio C: Riding
the storm: Managing cytokine-related toxicities in CAR-T cell
therapy. Semin Immunopathol. 46:52024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McNerney KO, DiNofia AM, Teachey DT, Grupp
SA and Maude SL: Potential Role of IFNγ inhibition in refractory
cytokine release syndrome associated with CAR T-cell therapy. Blood
Cancer Discov. 3:90–94. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strati P, Ahmed S, Furqan F, Fayad LE, Lee
HJ, Iyer SP, Nair R, Nastoupil LJ, Parmar S, Rodriguez MA, et al:
Prognostic impact of corticosteroids on efficacy of chimeric
antigen receptor T-cell therapy in large B-cell lymphoma. Blood.
137:3272–3276. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19-28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra2252014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rainone M, Ngo D, Baird JH, Budde LE, Htut
M, Aldoss I and Pullarkat V: Interferon-γ blockade in CAR T-cell
therapy-associated macrophage activation syndrome/hemophagocytic
lymphohistiocytosis. Blood Adv. 7:533–536. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sehn LH and Salles G: Diffuse large B-cell
lymphoma. N Engl J Med. 384:842–858. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meignan M, Gallamini A, Meignan M,
Gallamini A and Haioun C: Report on the first international
workshop on interim-PET-scan in lymphoma. Leuk Lymphoma.
50:1257–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene ciloleucel CAR T-cell therapy in
refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ying Z, Yang H, Guo Y, Li W, Zou D, Zhou
D, Wang Z, Zhang M, Wu J, Liu H, et al: Relmacabtagene autoleucel
(relma-cel) CD19 CAR-T therapy for adults with heavily pretreated
relapsed/refractory large B-cell lymphoma in China. Cancer Med.
10:999–1011. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayden PJ, Roddie C, Bader P, Basak GW,
Bonig H, Bonini C, Chabannon C, Ciceri F, Corbacioglu S, Ellard R,
et al: Management of adults and children receiving CAR T-cell
therapy: 2021 Best practice recommendations of the European society
for blood and marrow transplantation (EBMT) and the Joint
accreditation committee of ISCT and EBMT (JACIE) and the European
haematology association (EHA). Ann Oncol. 33:259–275. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manni S, Del Bufalo F, Merli P, Silvestris
DA, Guercio M, Caruso S, Reddel S, Iaffaldano L, Pezzella M, Di
Cecca S, et al: Neutralizing IFNγ improves safety without
compromising efficacy of CAR-T cell therapy in B-cell malignancies.
Nat Commun. 14:34232023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carbone A, Roulland S, Gloghini A, Younes
A, von Keudell G, López-Guillermo A and Fitzgibbon J: Follicular
lymphoma. Nat Rev Dis Primers. 5:832019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmitz R, Wright GW, Huang DW, Johnson
CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer
AL, et al: Genetics and pathogenesis of diffuse large B-cell
lymphoma. N Engl J Med. 378:1396–1407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Norelli M, Camisa B, Barbiera G, Falcone
L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C,
Cristofori P, et al: Monocyte-derived IL-1 and IL-6 are
differentially required for cytokine-release syndrome and
neurotoxicity due to CAR T cells. Nat Med. 24:739–748. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maude SL, Barrett D, Teachey DT and Grupp
SA: Managing cytokine release syndrome associated with novel T
cell-engaging therapies. Cancer J. 20:119–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kotch C, Barrett D and Teachey DT:
Tocilizumab for the treatment of chimeric antigen receptor T
cell-induced cytokine release syndrome. Expert Rev Clin Immunol.
15:813–822. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Lv X, Kong Q and Tan Y:
IL-6/IFN-γ double knockdown CAR-T cells reduce the release of
multiple cytokines from PBMCs in vitro. Hum Vaccin Immunother.
18:1–14. 2022. View Article : Google Scholar
|
|
27
|
Bailey SR, Vatsa S, Larson RC, Bouffard
AA, Scarfò I, Kann MC, Berger TR, Leick MB, Wehrli M, Schmidts A,
et al: Blockade or deletion of IFNγ reduces macrophage activation
without compromising CAR T-cell Function in hematologic
malignancies. Blood Cancer Discov. 3:136–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Locatelli F, Jordan MB, Allen C, Cesaro S,
Rizzari C, Rao A, Degar B, Garrington TP, Sevilla J, Putti MC, et
al: Emapalumab in children with primary hemophagocytic
lymphohistiocytosis. N Engl J Med. 382:1811–1822. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schuelke MR, Bassiri H, Behrens EM, Canna
S, Croy C, DiNofia A, Gollomp K, Grupp S, Lambert M, Lambrix A, et
al: Emapalumab for the treatment of refractory cytokine release
syndrome in pediatric patients. Blood Adv. 7:5603–5607. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Teachey DT, Lacey SF, Shaw PA, Melenhorst
JJ, Maude SL, Frey N, Pequignot E, Gonzalez VE, Chen F, Finklestein
J, et al: Identification of predictive biomarkers for cytokine
release syndrome after chimeric antigen receptor T-cell therapy for
acute lymphoblastic leukemia. Cancer Discov. 6:664–679. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Salama ZT: Emapalumab: First global
approval. Drugs. 79:99–103. 2019. View Article : Google Scholar : PubMed/NCBI
|