Introduction
Breast cancer (BRCA) has developed as a preeminent
life-threatening disease for women on a global scale as a
consequence of its elevated incidence and mortality rates. In 2020,
there were >2.3 million new cases and 685,000 deaths associated
with breast cancer. It is projected that the numbers will exceed 3
million new cases and 1 million deaths annually by 2040 (1). Although the 5-year survival rate of
triple-negative breast cancer (TNBC) has been bolstered by
advancements in comprehensive treatments, such as surgery,
chemotherapy, radiotherapy and molecular targeting therapy, its
prognosis remains suboptimal primarily owing to the absence of
discernible molecular targets or biomarkers (2,3). At
present, a combined approach involving chemotherapy and immune
checkpoint inhibitors has become the mainstream treatment for
locally advanced (4) and metastatic
TNBC (5). The combination of
atezolizumab with chemotherapy has demonstrated an increase in the
pathological complete response rate by ~17% (58 vs. 41%) compared
with chemotherapy alone (4).
Therefore, further research is crucial to achieve a comprehensive
understanding of the molecular mechanisms underlying TNBC
progression, facilitating the creation of more efficacious
treatment approaches for TNBC.
Aerobic glycolysis, a metabolic signature of tumor
cells (6), not only drives tumor
proliferation (7), metastasis
(8) and drug resistance (9), but also intricately mediates host
antitumor immunity within the tumor microenvironment (10–12).
Lactate can be produced by aerobic glycolysis and accumulated
within tumor tissues, the understanding of which has been extended
from its origins as a metabolic byproduct to its crucial role in
driving tumor progression (13).
Notably, intracellular lactate can induce lactylation modifications
on histone lysine residues, therefore affecting the transcription
of inflammation-related genes and advancing the transformation of
M1 macrophages to M2 macrophages (14).
Several studies have underscored the role of histone
lactylation in the epigenetic modulation of gene expression and its
prognostic significance in human cancer. For instance, higher
levels of pan-lysine lactylation (panKlac) and H3K18 lactylation
are linked to poorer overall survival outcomes in colon cancer and
foster bevacizumab resistance by hyper-activating rubicon-like
autophagy enhancer/Pacer transcription (15). In bladder cancer, H3K18 lactylation
boosts the transcription of the oncogenic transcription factors,
Y-box binding protein 1 and YY1, contributing to cisplatin
resistance (16). In ocular
melanoma, H3K18 lactylation is associated with elevations in YTH
N6-methyladenosine RNA-binding protein 2 (YTHDF2) transcription and
decreases in recurrence-free survival (RFS) (17). Therefore, nuclear protein
lactylation in TNBC merits comprehensive investigation.
Transfer RNA (tRNA) ligases refer to a class of
enzymes that facilitate the binding of specific amino acids to tRNA
molecules during intricate peptide chain synthesis. The notable
link between lactate and tRNA ligases remained elusive until recent
studies revealed the role of alanyl-tRNA synthetase 1 (AARS1) as a
potential lactyltransferase. Specifically, AARS1 was found to exert
dual effects of either fostering cell proliferation through the
lactylation of Yes-associated transcriptional regulator and TEA
domain transcription factor 1 or weakening the tumor-suppressive
function of p53 via p53 lactylation (18,19).
In this context, lactate can be directly recognized, bound and
transported to nuclear substrates by AARS1, catalyzing subsequent
lactylation reactions in the nucleus.
The current study aimed to investigate the
prognostic significance of protein lactylation in patients with
non-specific TNBC. Additionally, the oncogenic role and underlying
molecular mechanisms of glycolysis in TNBC cells were examined
through transcriptomic and bioinformatics analyses.
Materials and methods
Patients and tissue microarray
(TMA)
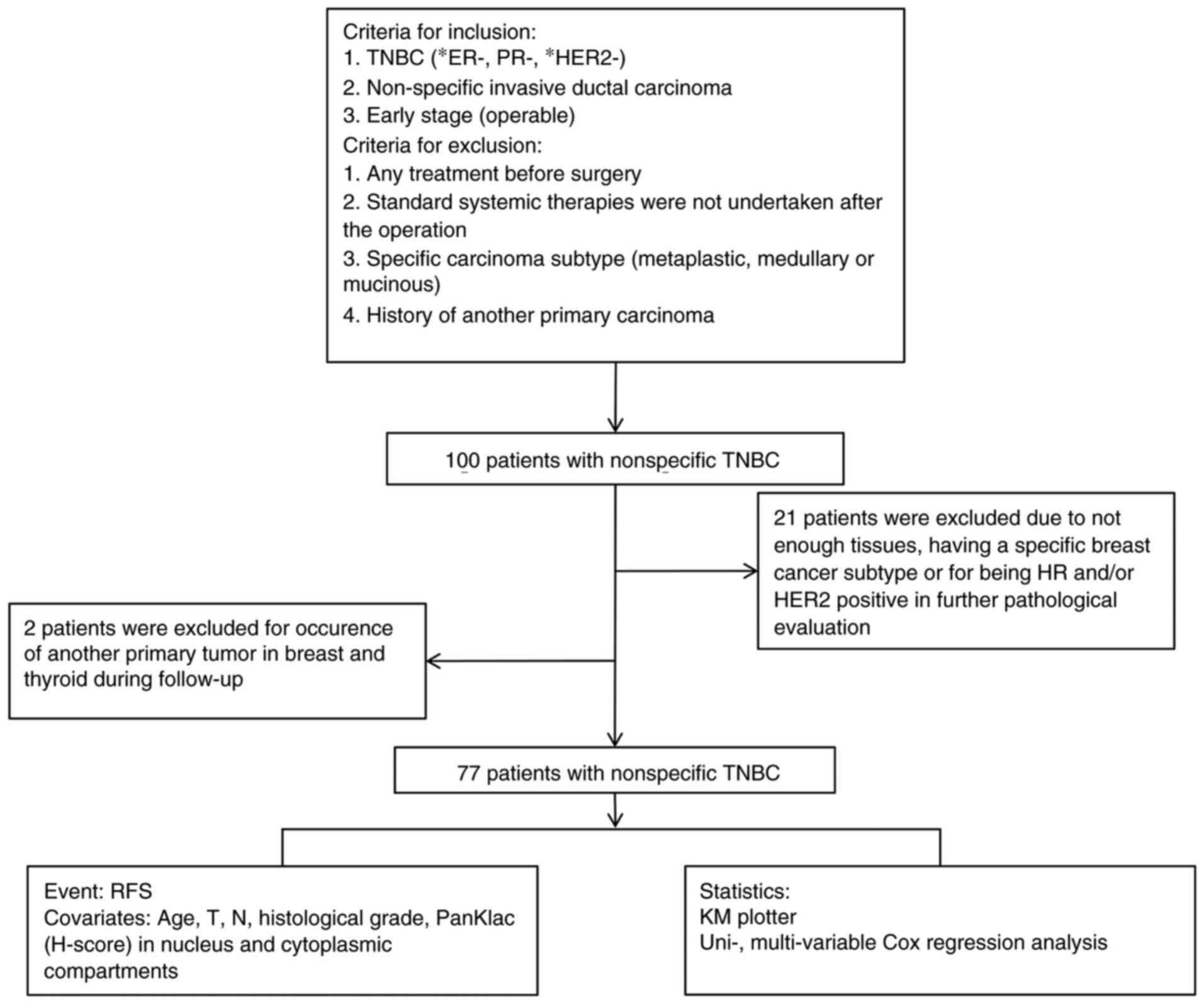
In the present study, samples from 100 patients
diagnosed with invasive ductal carcinoma (non-specific) type of
TNBC at the early stages (stage I–IIA) according to the eighth
edition of the primary tumor, lymph node, and metastasis (TNM)
classification of the American Joint Commission of Cancer for
breast cancer (20) were collected
in the Breast Center, People's Hospital of Zhongshan City
(Zhongshan, China) between January 2013 and December 2016. All
patients underwent surgery as the primary treatment modality
without any prior interventions. Adjuvant chemotherapy was
performed following radical resection, comprising 4 cycles of
epirubicin and cyclophosphamide followed by 4 cycles of paclitaxel.
Ipsilateral axillary lymph nodes were dissected if any lymph node
dissemination was indicated on imaging, such as ultrasound or
magnetic resonance imaging. Alternatively, sentinel lymph node
biopsy was performed to confirm no tumor invasion in the local
lymph nodes. If axillary lymph node metastasis was observed,
radiotherapy was performed.
Subsequent pathological analysis results further
excluded 21 patients from the present study due to insufficient
tissue samples, specific invasive types or positive hormone
receptor and/or human epidermal growth factor receptor 2 (HER2). To
reduce potential confounding factors from different biological
behaviors, specific invasive types, including metaplastic,
medullary and mucinous carcinomas, were excluded from the study.
Hormone receptor was considered positive when ≥1% of tumor cells
were stained, with intensity ranged from weak to strong (21). HER2 was considered positive when
scored 3+ via immunohistochemistry or 2+/+ via fluorescence in
situ hybridization amplification according to the 2018 American
Society of Clinical Oncology/College of American Pathologists
Clinical Practice guidelines (22).
Additionally, 2 patients were excluded from the study due to the
occurrence of another primary breast or thyroid tumor during
follow-up. The workflow of experiments involving patients is
delineated in Fig. 1.
Core tissue samples (1.5 mm in diameter) were
obtained from paraffin-embedded blocks of 77 patients and 37
corresponding para-tumor tissues were also collected. These samples
were re-embedded into a TMA by Shanghai Zhuoli Biotechnology Co.,
Ltd. All tissues were acquired with the informed consent of the
patients. The study protocol was conducted with ethical approval
from the Clinical Practice and Experimental Research Ethics
Committee of the People's Hospital of Zhongshan City (approval no.
K2023-113).
Immunohistochemistry
The TMA was sliced into 0.4-µm slides. Following
deparaffinization and rehydration in a series of graded alcohols
(100, 95 and 80%), the slides were subjected to antigen retrieval
by boiling in 10 mM citrate buffer (pH 6.0) for 5 min in a
micro-oven. The slides were then immersed in 5% bovine serum
albumin (cat. no. A850222; Macklin Biochemical Technology Co.) in
Tris-buffered saline with 0.1% Tween 20 for 30 min at room
temperature to block non-specific protein binding, and the tissue
sections were incubated with 3% H2O2 in
methanol for 15 min to quench endogenous peroxidase. The slides
were then incubated with anti-L-Lactyllysine rabbit monoclonal
antibodies (cat. no. PTM-1401RM; 1:300; PTM Biolabs, Inc.)
overnight at 4°C and then with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibodies (cat. no.
A0208; 1:1,000; Beyotime Institute of Biotechnology) for 30 min at
room temperature. HRP signals were detected with
3,3′-diaminobenzidine. The stained slides were viewed by two
pathologists and any disagreement was addressed by a third senior
pathologist. A minimum of three fields (magnification, ×200) were
analyzed for each slide. The staining intensity was categorized
into four grades ranging from 0 to 3 (absent, weak, moderate and
strong), and the percentage of positively stained cancer cells was
calculated. H scores were calculated by multiplying the staining
intensity with the percentage of positively stained cancer cells
(23). The slides were scanned with
a light microscope (Hamamatsu Photonics K.K.) and images were
analyzed with K viewer software (v1.7.0.29; Konfoong Bioinformation
Tech Co., Ltd.).
Cell culture and pharmacological
interventions
The human TNBC cell line, MDA-MB-231, and the human
normal mammary ductal cell line, MCF-10A, were provided by Procell
Life Science & Technology Co., Ltd. The MDA-MB-231 cells were
maintained in Roswell Park Memorial Institute-1640 medium (cat. no.
11875500; Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS; cat. no. SV30208; Hyclone; Cytiva),
100 U/ml penicillin and 100 µg/ml streptomycin (cat. no. EH80010;
eLGbio). The MCF-10A cells were maintained in Dulbecco's Modified
Eagle Medium/F12 (1:1) supplemented with 10% FBS, 10 µg/ml insulin,
20 ng/ml epidermal growth factor, 100 U/ml penicillin and 100 µg/ml
streptomycin (cat. no. CL-0525; Procell Life Science &
Technology Co., Ltd.). All cells were cultured in a humidified
environment at 37°C with 5% CO2. For reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
cells were seeded into a 6-well plate (2×105 cells in 2
ml of medium per well) and treated with 10, 20 or 40 mM oxamate
(cat. no. S6871; Selleck Chemicals) in complete growth medium for
24 or 48 h, as indicated in figure legends, at 37°C. Since the drug
was dissolved in ddH2O, an equivalent volume of ddH2O was added to
cells in the control group.
Histone protein extraction
Histone proteins were isolated according to the
instructions of the EpiQuik Total Histone Extraction Kit (cat. no.
OP-0006-100; EpigenTek Group, Inc.) manual before further
quantitative analysis by western blotting (WB).
WB
After washing with ice-cold phosphate-buffered
saline, cells were lysed in ice-cold Radio-Immunoprecipitation
Assay buffers (cat. no. BL504A; Biosharp Life Sciences) containing
proteinase inhibitor cocktail (cat. no. 11836170001; Roche
Diagnostics) for 30 min. Cell lysates were later sonicated in an
ultrasonic cell disruptor (XM-650DT; Shanghai Jingxin Industrial
Development Co., Ltd.) at 65 W for 30 sec (2 sec on and 3 sec off)
on ice, followed by a 15-min centrifugation at 13,000 × g at 4°C.
Subsequently, sample concentrations were measured with the
bicinchoninic acid method (cat. no. A55864; Thermo Fisher
Scientific, Inc.). Thereafter, samples (20 or 30 µg of whole cell
lysate or 2 µg of extracted histone protein as indicated) were
loaded and separated using a 10 or 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis system and then
transferred onto a polyvinylidene fluoride membrane (cat. no.
ISEQ00010; MilliporeSigma). After blocking with 5% skim milk for 30
min at room temperature, the membrane was subjected to an overnight
incubation at 4°C with primary antibodies, including
anti-L-Lactyllysine rabbit mAb (cat. no. PTM-1401RM; 1:100; PTM
Biolabs, Inc.), anti-L-Lactyl-Histone H3 (Lys14) rabbit mAb (cat.
no. PTM-1414RM; 1:100; PTM Biolabs, Inc.), anti-L-Lactyl-Histone H3
(Lys18) Rabbit mAb-ChIP Grade (cat. no. PTM-1427RM; 1:100; PTM
Biolabs, Inc.), anti-Histone H3 Rabbit mAb (cat. no. EPR16987;
1:1,000; Abcam) and anti-β-actin mouse mAb (cat. no. 60008-1-Ig;
1:1,000; Proteintech Group, Inc.). The membrane was cut according
to the molecular weight markers prior to hybridization with
antibodies. Next, the membrane was washed with Tris-buffered saline
with 0.1% Tween 20 (pH 7.4) three times (10 min/time) before
incubation with HRP-labeled goat anti-mouse (cat. no. A0216) or
goat anti-rabbit (cat. no. A0208) secondary antibodies (both
1:1,000; Beyotime Institute of Biotechnology) for 45 min at room
temperature. HRP signals were visualized with electrogenerated
chemiluminescence reagents (cat. no. EBT002; eLGbio) on the Ephoto™
device [cat. no. L00797c; Mobao (Xiamen) Biotechnology, Co., Ltd.],
which was equipped with ePhoto software (v2).
Cell viability assay
For Cell Counting Kit (CCK)-8 assays, cells were
seeded into a 96-well plate (1×103 cells in 100 µl of
medium per well). CCK-8 reagents (10 µl; cat. no. CA1210; Beijing
Solarbio Science & Technology Co., Ltd.) were added to each
well. Following 2 h of cell culture at 37°C, optical density values
at 450 nm were measured with a spectrophotometer. In each
experimental setting, wells containing the same medium supplemented
with the specified drugs and no cells were utilized as blank
controls.
mRNA transcriptomic analysis
The mRNA transcriptomic analysis was conducted by
Igenecode Corporation (Beijing Boyun Huakang Gene Technology Co.,
Ltd.) on the DNBSEQ-T7 sequencing platform. RNA samples were
prepared as described in the RT-qPCR section. The clean reads were
compared with the human genome using HISAT2 software (v2.2.1;
http://daehwankimlab.github.io/hisat2/), followed by
quantification of gene expression with StringTie software (v2.1.5;
http://github.com/gpertea/stringtie)
and the ballgown package (v2.24.0; http://github.com/broadinstitute/ballgown) in R
software (v4.3.1; http://www.r-project.org). Principal component
analysis (PCA) was performed with the princomp function in R.
Differentially expressed genes (DEGs) were screened using the
DEseq2 method with the criteria of |log2(Fold Change)|≥1 and
adjusted P≤0.05. Subsequent to Gene ontology (GO) annotations with
QuickGO (http://www.ebi.ac.uk/QuickGO/), GO enrichment analysis
was conducted with the clusterProfiler package in R software. The
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis was also performed with the clusterProfiler package.
Additionally, protein-protein interaction analysis was carried out
using the STRING database (https://cn.string-db.org) and the stringDB package.
Gene Set Enrichment Analysis (GSEA) was conducted using the GSEA
software (http://www.broadinstitute.org/gsea/index.jsp) and the
MSigDB database (v7.4).
RT-qPCR
RNA of the MDA-MB-231 cells was isolated as per the
protocols of the RNA easy fast animal tissue/cell total RNA
extraction kit (cat. no. DP451; Tiangen Biotech Co., Ltd.). The
concentration (>100 ng/µl) and purity (A260/280 >2.0) of RNA
samples were measured with a nanodrop photometer (NanoDrop 2000;
Thermo Fisher Scientific, Inc.). Subsequently, the RNA was kept on
ice before being reverse transcribed at 37°C for 15 min following
the manufacturer's instructions using HiScript III RT SuperMix for
qPCR (+gDNA wiper; cat. no. R323; Vazyme Biotech Co., Ltd.). The
obtained cDNA was quantified with PowerUp SYBR Green Master Mix
(cat. no. A25742; Applied Biosystems; Thermo Fisher Scientific,
Inc.) on an ABI 7500 Real-Time PCR system (7500; Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used: 50°C for 2 min and 40 cycles of
95°C for 2 min, 95°C for 15 sec and 60°C for 1 min. The primer
specificity was determined with melting curves. The cycling
threshold results were determined with the 2−ΔΔCq method
(24), with
glyceraldehyde-3-phosphate dehydrogenase as the normalization
control. Detailed primer sequences are listed in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene symbol | Primer sequences
(5′ to 3′) |
|---|
| GAPDH | F:
GCACCGTCAAGGCTGAGAAC |
|
| R:
TGGTGAAGACGCCAGTGGA |
| GCLM | F:
CGCACAGCGAGGAGGAGTTT |
|
| R:
AATCCAGCTGTGCAACTCCAA |
| CYP1B1 | F:
CCTCCTCTTCACCAGGTATCC |
|
| R:
TGGTAGCCCAAGACAGAGGT |
Database and web-based tool
A web-based tool (https://www.xiantaozi.com/) was used to compare the
RNA-sequencing data of paired or unpaired BRCA and normal tissues
from The Cancer Genome Atlas (TCGA)-BRCA (https://portal.gdc.cancer.gov). Additionally, the
correlation between the expression of YARS1 and other genes was
also evaluated using the xiantaozi web-based tool. Furthermore, the
disparity in RFS between YARS1-high and YARS1-low groups (split by
median value of mRNA expression level by gene chip) was analyzed
using the Kaplan-Meier Plotter database (https://kmplot.com/), in which the gene expression
data and survival information were downloaded from Gene Expression
Omnibus, European Genome-phenome Archive and TCGA (25).
Screening of hub genes
Cytoscape (v3.9.1), which combined public datasets
STRING, BioGRID and IntAct (https://cytoscape.org/release_notes_3_9_1.html), was
employed to screen the hub genes among the downregulated genes.
Betweenness values were acquired using the CytoNCA plugin
(https://apps.cytoscape.org/apps/cytonca).
Statistical analysis
The two-tailed Wilcoxon signed rank test was
utilized to assess the significance of differences in panKlac
levels between paired tumor and peri-tumor tissue. Unpaired
Student's t-test was used to compare the panKlac level in the
cytoplasm and nucleus of the tumor tissues, with each from
different patients. The association between age and panKlac
expression was tested using unpaired Student's t-test. The
association between tumor (T) and node (N) stage with panKlac
expression was tested using Fisher's exact test. The association
between Grade and panKlac expression was tested using the
χ2 test. The Gehan-Breslow-Wilcoxon test was employed
for comparing the survival differences between Kaplan-Meier plots.
Data from CCK-8 assays and WB were compared (between two groups)
with the unpaired Student's t-test. Covariates with significance
(P<0.1) in the univariate Cox regression analysis were
subsequently included in the multivariate Cox regression analysis
to screen the independent indicators of patient survival. YARS1
expression was compared between unpaired normal and tumor samples
using the Mann-Whitney U test and between paired normal and tumor
specimens with the paired Student's t-test. Spearman correlation
analyses were employed to determine expression correlations.
Statistical analyses were performed with GraphPad Prism 9.5.1
(Dotmatics), SPSS Statistics 27 (IBM Corp.) or the xiantaozi
web-based tool. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients
The clinicopathological information of the included
patients is listed in Table II.
Patients were all female, aged from 33 to 77, with a mean age of
50.44 years. Almost 90% of the patients had a tumor size of T1 or
T2 and 9.1% of the patients had a tumor size of T3 or T4.
Additionally, 85% of the patients were at N0 or N1, while the rest
of the patients were at N2 or N3. There were 61% of the patients at
G1 or G2 grades. The follow-up period, which was the interval from
the surgery date to disease relapse or loss to follow-up, ranged
from 0 to 10.3 years, with a median time of 4.2 years. The H scores
of panKlac levels in the cytoplasm and nucleus of tumor and
para-tumor mammary tissues are also listed in Table I. The median value was utilized to
stratify PanKlac levels in the cytoplasm and nucleus.
 | Table II.Clinicopathological characteristics
of the cohort of patients with non-specific triple-negative breast
cancer (n=77). |
Table II.
Clinicopathological characteristics
of the cohort of patients with non-specific triple-negative breast
cancer (n=77).
| Parameters | Value |
|---|
| Average age
(range), years | 50.44 (33–77) |
| T, n (%) |
|
| T1 | 21 (27.3) |
| T2 | 49 (63.6) |
| T3 or
4 | 7 (9.1) |
| N, n (%) |
|
| N0 | 45 (58.4) |
| N1 | 21 (27.3) |
| N2 | 6 (7.8) |
| N3 | 5 (6.5) |
| Histological grade,
n (%) |
|
| 1 or
2 | 47 (61.0) |
| 3 | 30 (39.0) |
| Median duration of
follow-up (range), years | 4.2 (0–10.3) |
| Median panKlac,
H-score (range) |
|
| Tumor
nucleus | 90 (0–280) |
| Tumor
cytosol | 100 (0–200) |
|
Peri-tumoral nucleus | 50 (0–180) |
|
Peri-tumoral cytosol | 50 (0–200) |
panKlac levels are upregulated in the
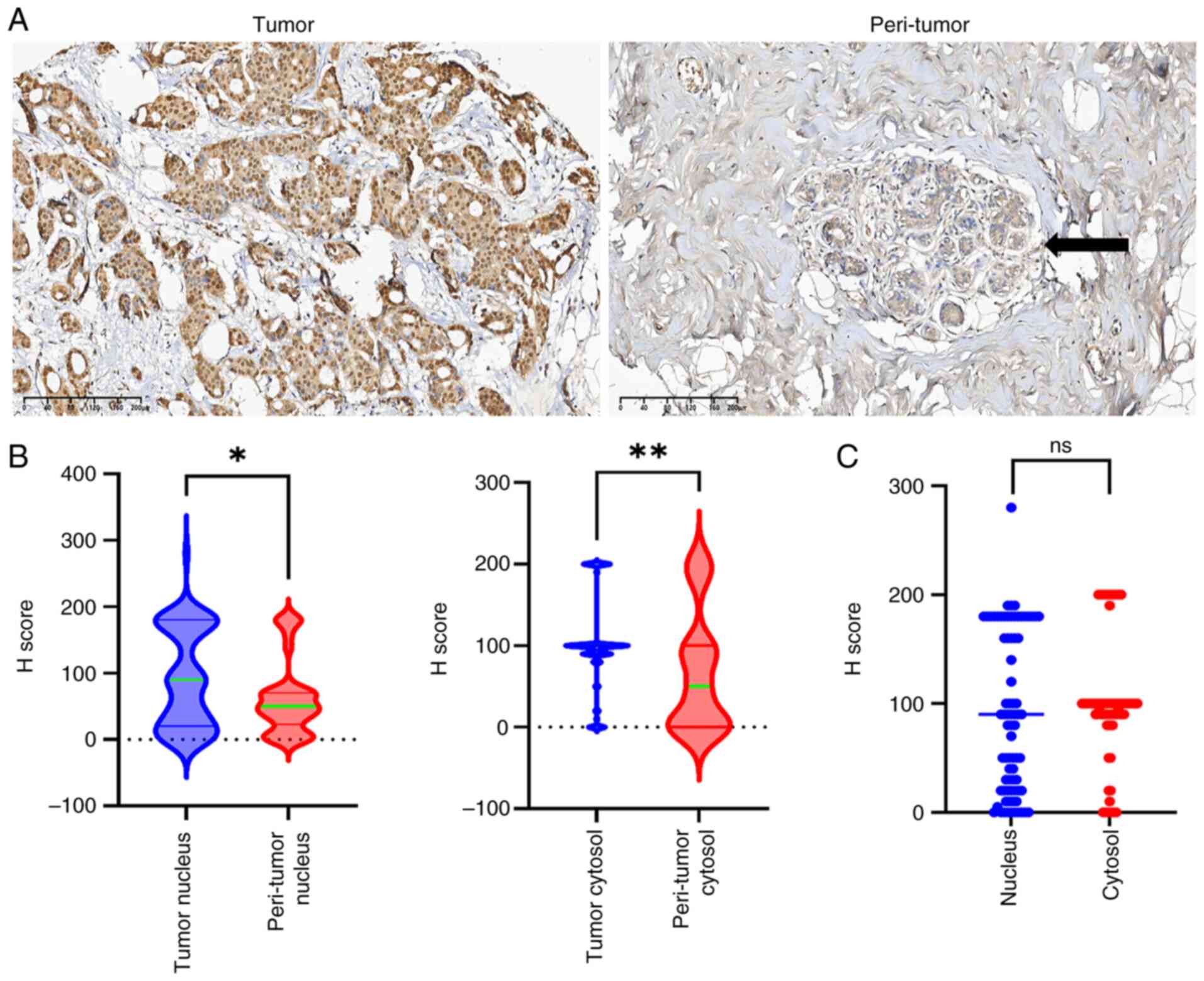
tumor tissues of patients with non-specific TNBC
The representative panKlac immunohistochemistry
images are presented in Fig. 2A.
Notably, the panKlac levels were significantly higher in tumor
tissues than in para-tumor mammary tissues, both in the nucleus
(P=0.0175) and cytoplasm (P=0.0038) (Fig. 2B). In the tumor samples, the
distribution of the panKlac levels was similar between the
cytoplasm and nucleus (P>0.05; Fig.
2C). Notably, the protein lactylation levels within tumors were
not associated with various clinicopathological parameters such as
age, tumor size, lymph node status or histological grade (Table III).
 | Table III.Association between the Klac level
and other clinicopathological factors. |
Table III.
Association between the Klac level
and other clinicopathological factors.
|
| panKlac nuclear
localization | panKlac cytosolic
localization |
|---|
|
|
|
|
|---|
| Parameters | High (n=39) | Low (n=38) | P-value | High (n=44) | Low (n=33) | P-value |
|---|
| Mean age (SD),
years | 51.28 (9.73) | 49.58 (8.689) | 0.421 | 49.41 (9.751) | 51.82 (8.383) | 0.259 |
| T, n |
|
|
|
|
|
|
| T1 | 14 | 7 |
| 12 | 9 |
|
| T2 | 21 | 28 |
| 29 | 20 |
|
|
T3/4 | 4 | 3 | 0.211 | 3 | 4 | 0.776 |
| N, n |
|
|
|
|
|
|
| N0 | 22 | 23 |
| 27 | 18 |
|
| N1 | 12 | 9 |
| 12 | 9 |
|
| N2 | 2 | 4 |
| 4 | 2 |
|
| N3 | 3 | 2 | 0.744 | 1 | 4 | 0.406 |
| Grade, n |
|
|
|
|
|
|
|
1/2 | 24 | 23 |
| 26 | 21 |
|
| 3 | 15 | 15 | 0.927 | 18 | 12 | 0.686 |
Association of high panKlac levels in
the nucleus with the survival of the cohort of patients with
non-specific TNBC
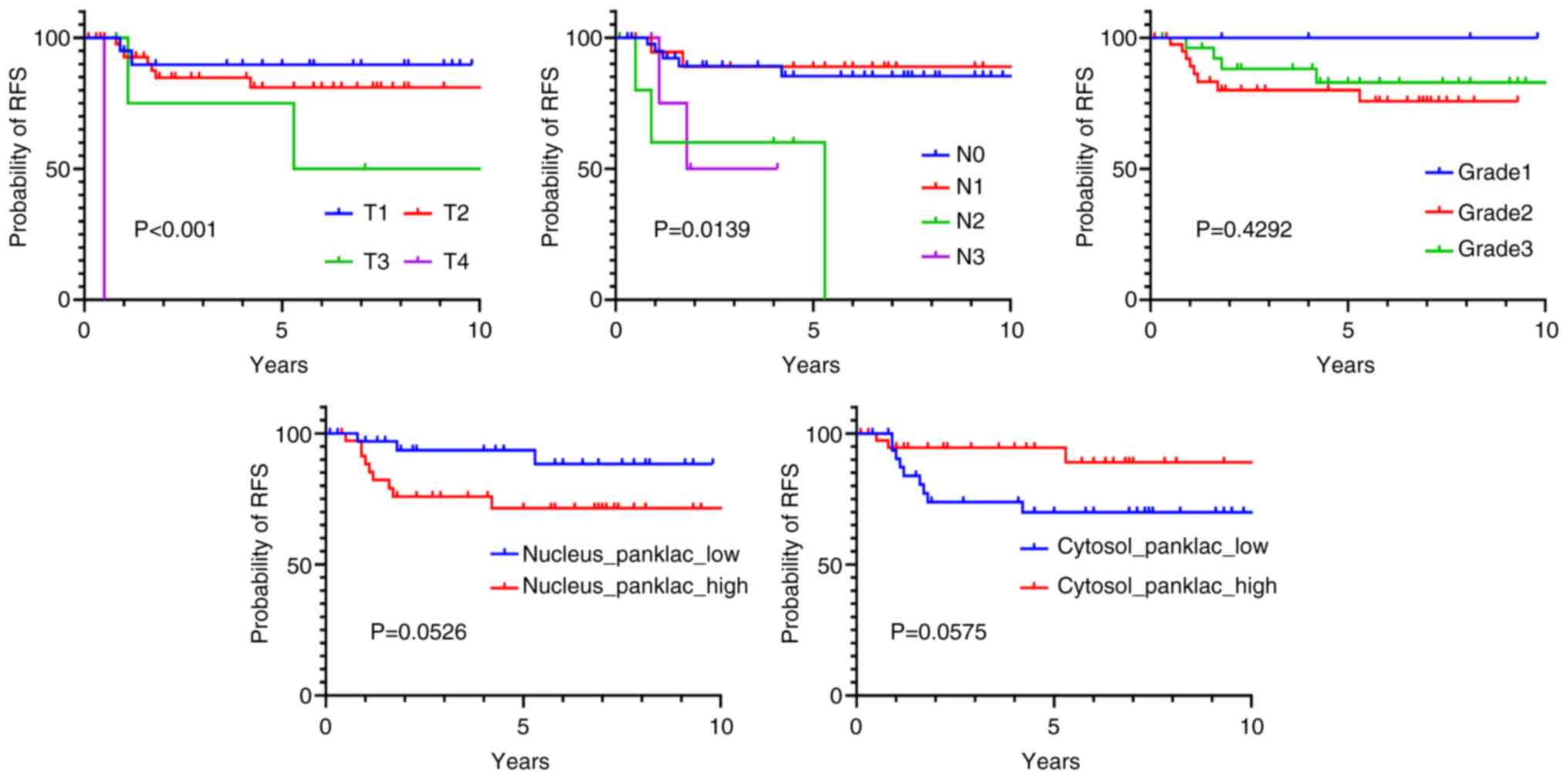
RFS was chosen as the evaluation metric as RFS may
be influenced by fewer confounding factors compared with overall
survival. All patients with operable TNBC were treated with uniform
regimens. However, patients with recurrent disease might have
received varying treatments influenced by factors such as economic
considerations and the availability of new drugs. RFS displayed
associations with clinicopathological features including tumor size
(P<0.0001) and lymph node status (P=0.0139) and showed a trend
towards an association with panKlac levels in both the nucleus
(P=0.0526) and cytoplasm (P=0.0575), but these results were not
statistically significant (Fig. 3).
In the univariate Cox regression analysis, RFS was linked to T3 or
T4 [hazard ratio (HR), 6.918; P=0.034] and N2 (HR, 6.529; P=0.01),
and together with N3 (HR, 4.528; P=0.077) and panKlac levels in the
nucleus (HR, 3.182; P=0.083) and cytoplasm (HR, 0.297; P=0.069),
these variables were passed through to multivariate analysis
(Table IV). Through multivariate
Cox regression analysis, N2 (HR, 11.171; P=0.010) and elevated
panKlac levels in the nucleus (HR, 5.682; P=0.034) were identified
as independent prognostic determinants (Table IV).
 | Table IV.Univariate and multivariate Cox
regression analysis of recurrence-free survival. |
Table IV.
Univariate and multivariate Cox
regression analysis of recurrence-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Parameters | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Age | 0.097 | 1.048 | 0.991–1.109 | 0.134 | 1.058 | 0.983–1.139 |
| T |
|
|
|
|
|
|
| T1 |
|
|
|
|
|
|
| T2 | 0.492 | 1.736 | 0.361–8.362 | 0.688 | 1.429 | 0.249–8.191 |
|
T3/4 | 0.034 | 6.918 | 1.153–41.508 | 0.218 | 3.527 | 0.476–26.157 |
| N |
|
|
|
|
|
|
| N0 |
|
|
|
|
|
|
| N1 | 0.754 | 0.769 | 0.149–3.969 | 0.466 | 0.522 | 0.091–2.993 |
| N2 | 0.010 | 6.529 | 1.552–27.470 | 0.010 | 11.171 | 1.788–69.813 |
| N3 | 0.077 | 4.528 | 0.849–24.162 | 0.759 | 1.386 | 0.173–11.104 |
| Grade |
|
|
|
|
|
|
|
Grade1/2 |
|
|
|
|
|
|
|
Grade3 | 0.574 | 0.709 | 0.213–2.356 | 0.729 | 0.774 | 0.182–3.298 |
| panKlac level |
|
|
|
|
|
|
|
Nucleus-panKlac high | 0.083 | 3.182 | 0.86–11.769 | 0.034 | 5.682 | 1.137–28.394 |
|
Cytosol-panKlac high | 0.069 | 0.297 | 0.08–1.097 | 0.159 | 0.356 | 0.085–1.500 |
High levels of lactylation and the
proliferation-inhibitory impact of oxamate on BRCA cells
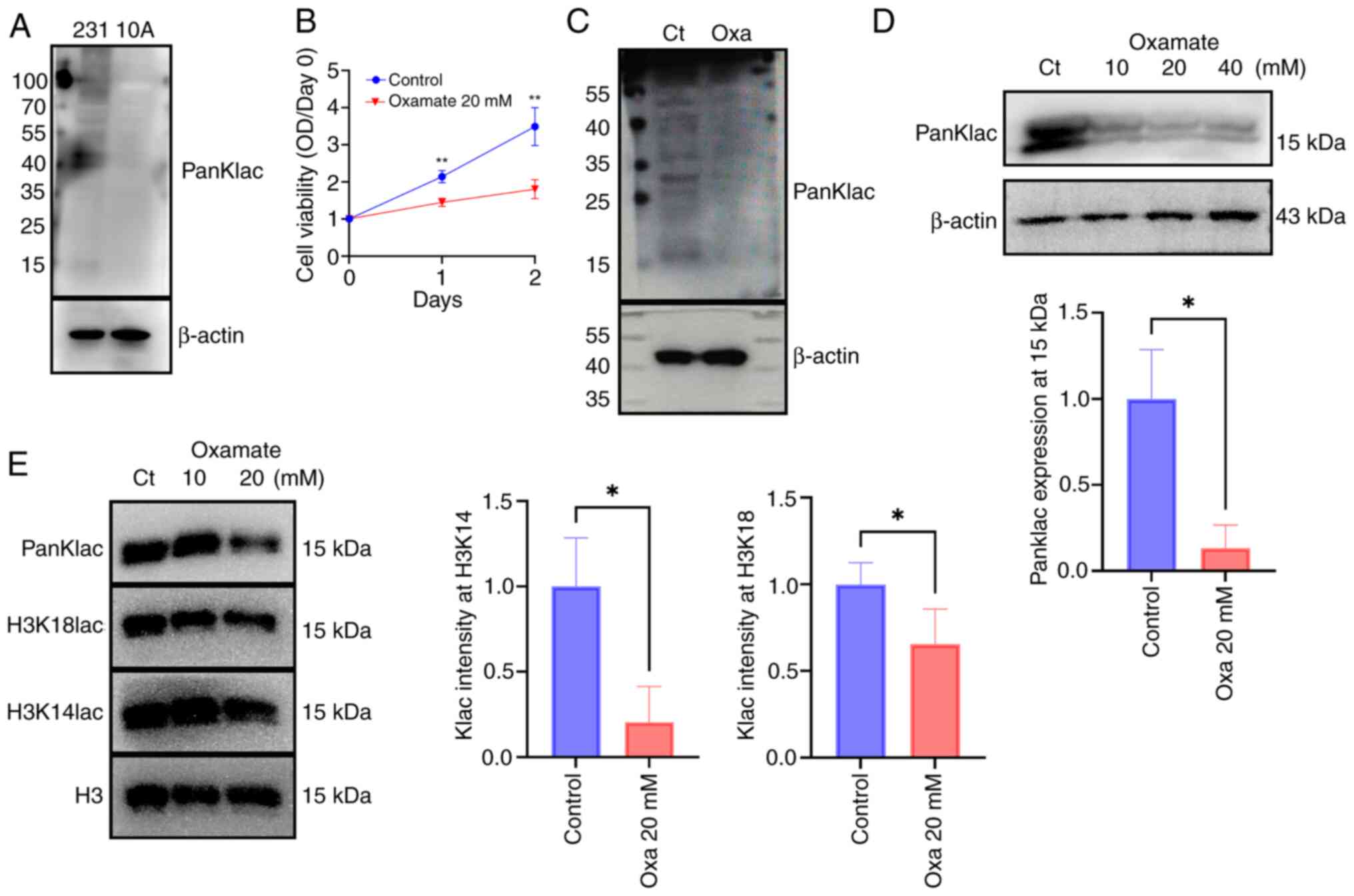
Global lactylation levels were markedly higher in
MDA-MB-231 cells compared with the benign mammary epithelial cell
line, MCF-10A (Fig. 4A). Moreover,
treatment with the lactate dehydrogenase A (LDHA) inhibitor,
oxamate, notably diminished the viability (Fig. 4B) and global lactylation levels
(Fig. 4C) in MDA-MB-231 cells.
Additionally, the lactylation levels of histones (molecular weight
of ~15 kDa) were downregulated following oxamate treatment
(Fig. 4D). While no decrease in
lactylation levels was noted at a concentration of 10 mM, treatment
with 20 mM Oxamate significantly induced dose-dependent reductions
in lactylation levels of H3K18 and H3K14 residues (Fig. 4E). Overall, the global lactylation
status and/or histone lactylation may play a pivotal role in BRCA
cell proliferation.
DEGs in the transcriptome
A transcriptomic analysis was conducted to obtain
the DEGs between oxamate-treated and untreated cell populations.
The PCA results revealed a distinct transcriptomic signature in the
oxamate group compared with the control group (Fig. 5A). Following 24 h of oxamate
exposure, 265 genes were notably upregulated, while 71 genes were
downregulated (Fig. 5B). The
fragments per kilobase of transcript per million fragments mapped
values of these DEGs were subjected to hierarchical clustering
analyses (Fig. 5C). Notably, GO
enrichment analysis results demonstrated the involvement of these
DEGs in biological processes (BPs) such as ‘extracellular matrix
organization’, ‘external encapsulating structure organization’ and
‘secondary metabolic processes’ (Fig.
5D). Furthermore, the KEGG pathway enrichment analysis results
indicated the enrichment of these DEGs in pathways including
‘Steroid hormone biosynthesis’, ‘Folate biosynthesis’ and
‘Metabolism of xenobiotics by cytochrome P450’ (Fig. 5E). For validation, RT-qPCR with the
RNA samples from the control and oxamate treatment groups was
performed, which confirmed the upregulation of cytochrome P450
family 1 subfamily B member 1 and glutamate-cysteine ligase
modifier subunit, genes enriched in steroid hormone biosynthesis
and ferroptosis pathways, respectively (Fig. 5F).
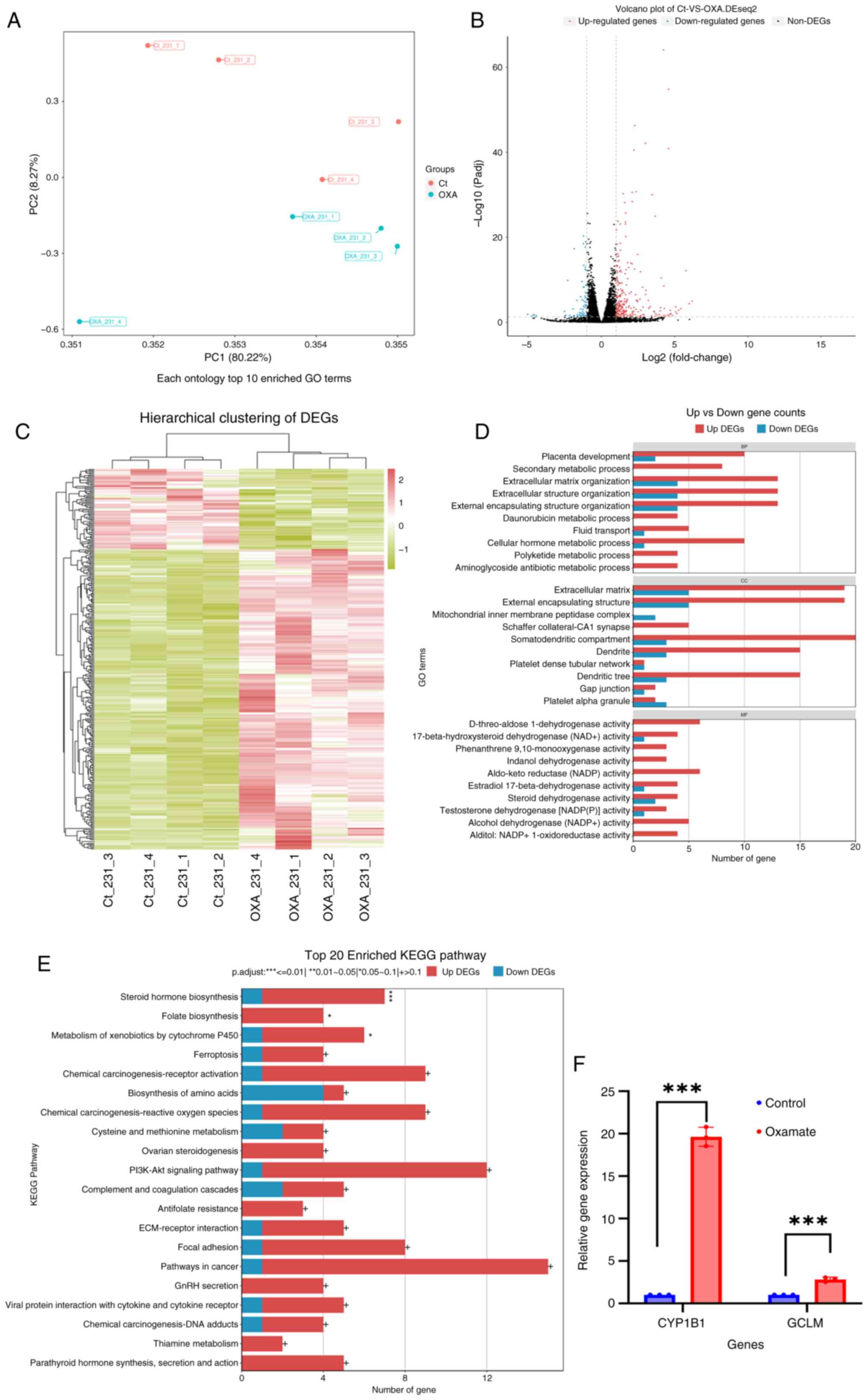 | Figure 5.DEG screening and GO/KEGG enrichment
between the oxamate and control groups in the transcriptomic
analysis. (A) PC analysis of the transcriptome profiles of the
oxamate (blue dots) and control (red dots) groups consisting of
four biological replicates. (B) Visualization of the DEGs through a
volcano plot, where upregulated genes are represented by red dots
and downregulated genes are marked by blue dots. Genes with
|log2(fold change)|>1 and False Discovery Rate <0.05 were
considered DEGs. (C) Hierarchical clustering of the DEGs, with the
horizontal axis showing the different samples and the vertical axis
representing log10 (fragments per kilobase of transcript per
million fragments mapped +1) of gene expression counts, followed by
Z-score normalization. (D) GO enrichment analysis of the DEGs, with
red bars indicating the upregulated DEGs and blue bars indicating
the downregulated DEGs. (E) KEGG enrichment analysis of the DEGs.
(F) Upregulation of CYP1B1 and GCLM (two upregulated DEGs) were
confirmed using reverse transcription-quantitative PCR.
***P.adjust<0.01, **P.adjust=0.01–0.05, *P.adjust=0.05–0.1; +
>0.1.; The experiments were repeated three times. PC, principal
component; DEGs, differentially expressed genes; GO, Gene Ontology;
KEGG, Kyoto Encyclopedia of Genes and Genomes; Ct, control group;
OXA, oxamate group; GCLM, glutamate-cysteine ligase modifier
subunit; CYP1B1, cytochrome P450 family 1 subfamily B member 1. |
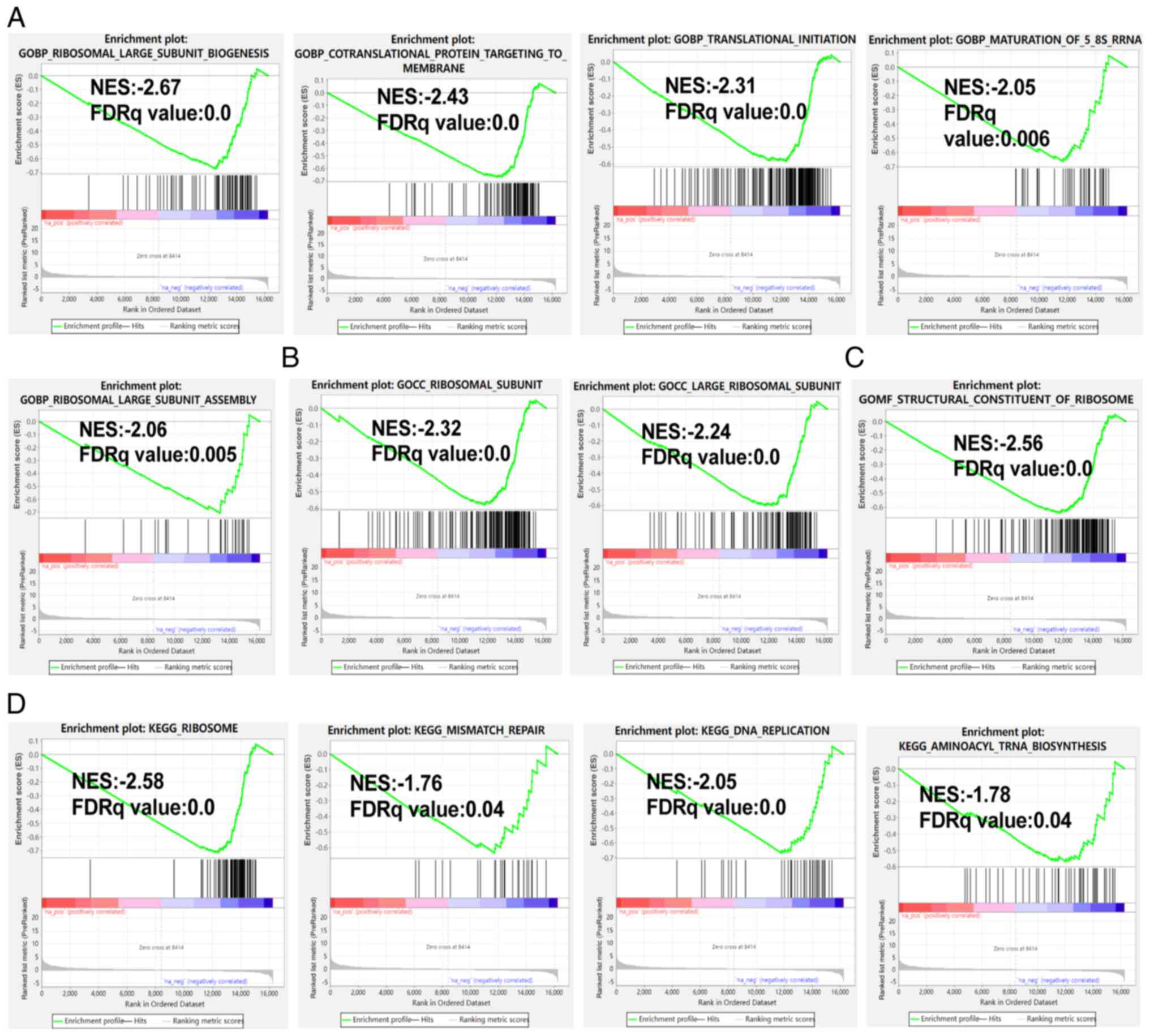
GSEA results
Compared with the control group, the oxamate-treated
group showed reductions in the levels of BP gene sets including
ribosomal large subunit biogenesis, translational initiation and
ribosomal large subunit assembly (Fig.
6A), cellular compartment gene sets such as ribosomal subunit
and large ribosomal subunit (Fig.
6B) and molecular function gene sets associated with the
structural constitution of the ribosome (Fig. 6C). Furthermore, the activity of KEGG
gene sets encompassing ribosome, DNA replication and aminoacyl-tRNA
biosynthesis was lower in the treatment group than in the control
group (Fig. 6D). Hallmark gene sets
such as Myc, E2F, G2M checkpoint, oxidative phosphorylation, DNA
repair and MTORC signaling were also downregulated in the treatment
group vs. the control group (data not shown).
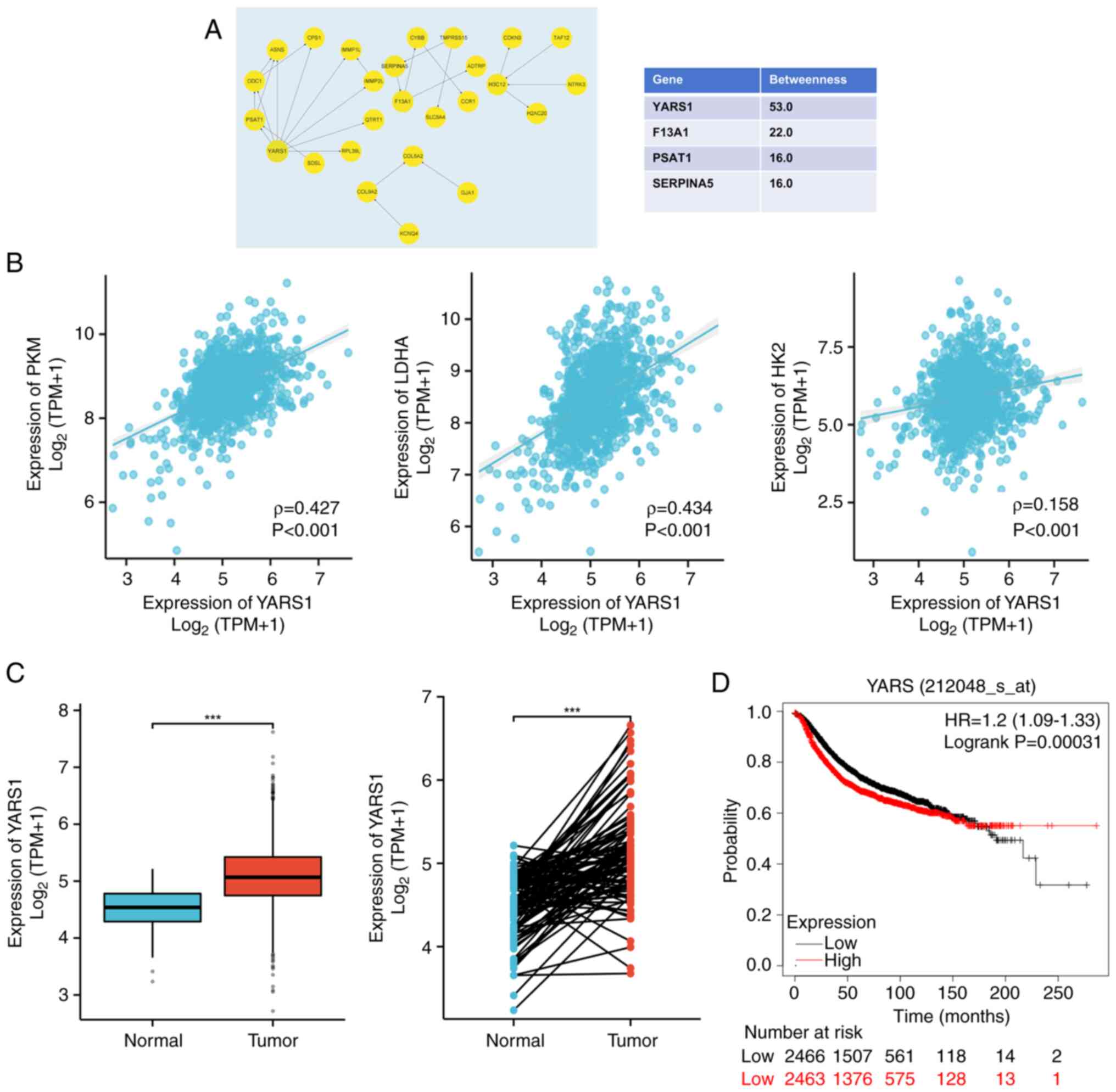
YARS1 as the hub genes
The present study next identified the hub genes
among the downregulated DEGs. The top 3 genes, YARS1, coagulation
factor XIII A chain and serpina5 were singled out for further
analysis due to the high betweenness values (Fig. 7A). Furthermore, the expression
correlations of these 3 genes with 3 key glycolysis-related enzymes
[LDHA, hexokinase 2 (HK2) and pyruvate kinase M1/2 (PKM)] were
analyzed to ascertain their association with glycolysis and protein
lactylation. YARS1 exhibited marked correlations with LDHA
(ρ=0.434; P<0.001), PKM (ρ=0.427; P<0.001) and HK2 (ρ=0.158;
P<0.001) (Fig. 7B), illustrating
that YARS1 expression was closely associated with lactate and
lactylation. According to TCGA-BRCA data, the RNA expression of
YARS1 was significantly higher in BRCA tissues than in normal
tissues (Fig. 7C). Moreover, mRNA
gene chip data from the Kaplan-Meier Plotter database revealed a
significant association between high YARS1 expression and reduced
RFS (P=0.00031; HR, 1.2; 95% confidence interval, 1.09–1.33;
Fig. 7D).
Discussion
Based on immunohistochemistry and survival analyses,
no significant association was found between the panKlac expression
level and patient survival using a TMA consisting of various BRCA
subtypes (luminal, HER2 amplification and TNBC; cat. no.
ZL-Brcsur1801; Shanghai Zhuoli Biotechnology Co., Ltd.) (data not
shown). Given that immune surveillance evasion could be a crucial
mechanism in TNBC progression (26)
and lactylation has been linked to the BRCA immune microenvironment
and immunotherapy (27), TNBC was
chosen as the focus of the present study. The present study
dissected the prognostic significance of global lactylation in
non-specific TNBC. To the best of our knowledge, the findings of
the present study unveiled for the first time that lactylation
levels within the nucleus could independently predict the prognosis
of TNBC. However, lactylation levels were not associated with
clinicopathological factors such as tumor size, lymph node status
and grade. In the in vitro experiments, BRCA cells presented
with heightened global pan-lactylation levels compared with benign
mammary epithelial cells. Additionally, the repression of lactate
production mediated by LDHA decreased BRCA cell proliferation. The
subsequent transcriptomic analysis results disclosed a potential
close correlation between lactylation and the perturbation of
ribosomal subunit synthesis and reassembly processes in the
nucleus. Furthermore, the results of the present study highlighted
the pivotal implication of a tyrosine-tRNA synthetase, YARS1, in
both lactylation and BRCA progression.
Until now, limited studies have assessed the
prognostic significance of lactylation in BRCA. In a prior
lactylome analysis involving 8 paired TNBC samples (tumor and
adjacent tissues), upregulation of H4K12 lactylation was determined
as an independent prognostic biomarker for TNBC (28). Similarly, the findings of the
present study revealed that various lysine residues on proteins and
histones were universally lactylated in BRCA cells and clinical
specimens and that global lactylation within the nucleus was a
predictive factor for the prognosis of TNBC. Accordingly, it is of
great value to investigate the oncogenic implication of nuclear
protein lactylation in TNBC. Prior comprehensive lactylproteomic
studies have unveiled numerous lactylation sites in liver carcinoma
specimens and oral squamous cell carcinoma cells, with only a
handful of sites identified on histones (29,30),
signifying that lactylation is a widespread protein modification
that extends beyond histones. Certain studies have demonstrated
that lactylation may contribute to protein stabilization, therefore
potentially exerting oncogenic effects within the nucleus, which
may be achieved by boosting oncogene transcription or DNA repair
mechanisms in human cancer (31,32).
Specifically, in the context of prostate cancer, lactylation
stabilizes hypoxia inducible factor 1 subunit α, activating
KIAA1199 transcription and ultimately facilitating vasculogenic
mimicry in tumor cells (31).
Additionally, lactylation enhances the function of MRE11, a pivotal
protein participating in homologous recombination that recognizes
and repairs damaged DNA in human cancer, and inhibition of MRE11
lactylation impedes DNA repair, thereby inducing the sensitivity of
tumor cells to drugs such as cisplatin and poly (ADP-ribose)
polymerase 1 inhibitors (32).
These findings corroborate the results of the present study and
demonstrate that lactylation levels within the nucleus could serve
as a predictor for the prognosis of patients with TNBC.
In the present study, for mechanistic analyses, a
comparative transcriptomic analysis was conducted on MDA-MB-231
cells treated with or without a glycolysis inhibitor, which
elucidated that DEGs were enriched in gene sets related to
ribosomal subunit synthesis/assembly and aminoacyl-tRNA
biosynthesis. Hence, further research is warranted to understand
the involvement of lactylation in ribosome synthesis and assembly.
Additionally, the present study identified YARS1 as a hub gene
among the downregulated DEGs following glycolysis inhibition.
Further results showed that YARS1 was positively correlated with
key enzymes in glycolysis and lactate production and markedly
associated with the prognosis of patients with BRCA. YARS1 has been
newly discovered as an oncogene. A previous study revealed the
promotional role of YARS1 in the progression of gastric cancer
through the PI3K-Akt pathway (33).
Furthermore, a more recent study demonstrated that YARS1 is an
independent prognostic marker for bladder cancer and potentially
affects immune infiltration dynamics and various cancer phenotypes,
including senescence, ferroptosis and stemness (34). The present study identified YARS1 as
a prognostic marker for TNBC that was closely linked to lactate
production and lactylation. Nevertheless, further studies are
needed to unveil the intricate mechanisms involved in the
regulation of YARS1 expression by lactate and lactylation and the
oncogenic mechanism of YARS1 in BRCA.
A limitation of the present study lies in the small
sample size of patients with non-specific TNBC. Although RFS
displayed a tendency towards correlation with panKlac levels in the
nucleus, no statistical significance was observed in the
Kaplan-Meier analysis. This lack of significance may be attributed
to the small sample size. Nonetheless, it was revealed that
increased nuclear panKlac served as an independent marker
predictive of an unfavorable prognosis in the multivariate Cox
analysis, a comprehensive evaluation that considers various
variables. These findings underscore the need for more extensive
studies involving a larger sample size to confirm the oncogenic
implications of nuclear protein lactylation in TNBC. Furthermore,
additional functional experiments and detailed mechanistic
investigations are warranted to validate the association between
lactylation and YARS1, as the association was only suggested based
on bioinformatic analysis in the present study.
In conclusion, it was demonstrated in the present
study that lactylation levels in TNBC tissues were higher compared
with those in normal tissues, and elevated lactylation levels
within the nucleus could be predictive of RFS in patients with
TNBC. GSEA and hub gene screening indicated that nuclear
lactylation potentially assumes an oncogenic role in TNBC via
ribosomal subunit synthesis/assembly and aminoacyl-tRNA
biosynthesis pathways. Furthermore, an association was observed
between YARS1 and lactylation, highlighting the need for further
in-depth mechanistic studies to delve into the intricate
relationship among these factors.
Acknowledgements
The authors would like to thank Dr Lan Ting
(Department of Advanced Diagnosis Center, People's Hospital of
Zhongshan City) for providing technical support throughout the
study. The authors would also like to thank Dr Chu Bing (Senior
Pathologist, Department of Pathology, People's Hospital of
Zhongshan City), for their help when there was a disagreement
between our two pathologists. The authors also thank Dr Huang
Chensheng (Breast Center, People's Hospital of Zhongshan City) and
Dr Zhang Jinhua (Breast Center, People's Hospital of Zhongshan
City) for their contributions to the collection of paraffin
blocks.
Funding
This study was supported by the Funding of the Key Department of
General Surgery (grant no. T2019009) and the Funding of the
Graduate Advisor in 2022 (grant no. SG2022YJS0040).
Availability of data and materials
The transcriptome data generated in the present
study may be found in the Sequence Read Archive under accession
number PRJNA1174689 or at the following URL: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1174689.
Otherwise, the data generated in the present study may be requested
from the corresponding author.
Authors' contributions
SM conceptualized the study, while AG devised the
research methodology and data collection protocols. AG performed
the in vitro experimentation and data acquisition. XC
performed the immunohistochemistry assays. FM and YC examined the
slides and assigned H scores. AG analyzed and elucidated all data
to obtain conclusions, construct figures and compose tables. The
initial draft of the manuscript was written by AG. SM meticulously
evaluated and refined the intellectual depth of the manuscript. HC
and SM procured the essential funding for the research endeavor,
with HC also involved in conceptualizing the study, and providing
supervision and guidance throughout this study. AG, FM and SM
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Tissues were routinely collected from patients
during surgery with prior written informed consent from the
patients for the use of their tissues and data in research. The
study protocol received ethical approval from the Clinical Practice
and Experimental Research Ethics Committee of the People's Hospital
of Zhongshan City (Zhongshan, China; approval no. K2023-113),
followed international and national regulations, and obeyed the
Declaration of Helsinki.
Patient consent for publication
Patients provided written informed consent for the
publication of their clinical data and images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Klac
|
lysine lactylation
|
|
TNBC
|
triple-negative breast cancer
|
|
RFS
|
recurrence-free survival
|
|
TMA
|
tissue microarray
|
|
HER2
|
human epidermal growth factor receptor
2
|
References
|
1
|
Arnold M, Morgan E, Rumgay H, Mafra A,
Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S
and Soerjomataram I: Current and future burden of breast cancer:
Global statistics for 2020 and 2040. Breast. 66:15–23. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Derakhshan F and Reis-Filho JS:
Pathogenesis of triple-negative breast cancer. Annu Rev Pathol.
17:181–204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bianchini G, De Angelis C, Licata L and
Gianni L: Treatment landscape of triple-negative breast
cancer-expanded options, evolving needs. Nat Rev Clin Oncol.
19:91–113. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mittendorf EA, Zhang H, Barrios CH, Saji
S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, et al:
Neoadjuvant atezolizumab in combination with sequential
nab-paclitaxel and anthracycline-based chemotherapy versus placebo
and chemotherapy in patients with early-stage triple-negative
breast cancer (IMpassion031): A randomised, double-blind, phase 3
trial. Lancet. 396:1090–1100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winer EP, Lipatov O, Im SA, Goncalves A,
Muñoz-Couselo E, Lee KS, Schmid P, Tamura K, Testa L, Witzel I, et
al: Pembrolizumab versus investigator-choice chemotherapy for
metastatic triple-negative breast cancer (KEYNOTE-119): A
randomised, open-label, phase 3 trial. Lancet Oncol. 22:499–511.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JH, Pyun WY and Park HW: Cancer
metabolism: Phenotype, signaling and therapeutic targets. Cells.
9:23082020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lunt SY and Vander Heiden MG: Aerobic
glycolysis: Meeting the metabolic requirements of cell
proliferation. Annu Rev Cell Dev Biol. 27:441–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Ren B, Yang G, Wang H, Chen G, You
L, Zhang T and Zhao Y: The enhancement of glycolysis regulates
pancreatic cancer metastasis. Cell Mol Life Sci. 77:305–321. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Zhang J, Zhang M, Song Y, Zhang Y,
Fan S, Ren S, Fu L, Zhang N, Hui H and Shen X: Baicalein
resensitizes tamoxifen-resistant breast cancer cells by reducing
aerobic glycolysis and reversing mitochondrial dysfunction via
inhibition of hypoxia-inducible factor-1α. Clin Transl Med.
11:e5772021. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao J, Jin D, Huang M, Ji J, Xu X, Wang
F, Zhou L, Bao B, Jiang F, Xu W, et al: Glycolysis in the tumor
microenvironment: A driver of cancer progression and a promising
therapeutic target. Front Cell Dev Biol. 12:14164722024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang H, Zou X, Yang S, Zhang A, Li N and
Ma Z: Identification of lactylation related model to predict
prognostic, tumor infiltrating immunocytes and response of
immunotherapy in gastric cancer. Front Immunol. 14:11499892023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiao Y, Ji F, Hou L, Lv Y and Zhang J:
Lactylation-related gene signature for prognostic prediction and
immune infiltration analysis in breast cancer. Heliyon.
10:e247772024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang T, Ye Z, Li Z, Jing DS, Fan GX, Liu
MQ, Zhuo QF, Ji SR, Yu XJ, Xu XW and Qin Y: Lactate-induced protein
lactylation: A bridge between epigenetics and metabolic
reprogramming in cancer. Cell Prolif. 56:e134782023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L,
Xu Y, He J, Lan J, Ou Q, et al: Tumor-derived lactate promotes
resistance to bevacizumab treatment by facilitating autophagy
enhancer protein RUBCNL expression through histone H3 lysine 18
lactylation (H3K18la) in colorectal cancer. Autophagy. 20:114–130.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li F, Zhang H, Huang Y, Li D, Zheng Z, Xie
K, Cao C, Wang Q, Zhao X, Huang Z, et al: Single-cell transcriptome
analysis reveals the association between histone lactylation and
cisplatin resistance in bladder cancer. Drug Resist Updat.
73:1010592024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X
and Jia R: Histone lactylation drives oncogenesis by facilitating
m6A reader protein YTHDF2 expression in ocular melanoma.
Genome Biol. 22:852021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ju J, Zhang H, Lin M, Yan Z, An L, Cao Z,
Geng D, Yue J, Tang Y, Tian L, et al: The alanyl-tRNA synthetase
AARS1 moonlights as a lactyltransferase to promote YAP signaling in
gastric cancer. J Clin Invest. 134:e1745872024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zong Z, Xie F, Wang S, Wu X, Zhang Z, Yang
B and Zhou F: Alanyl-tRNA synthetase, AARS1, is a lactate sensor
and lactyltransferase that lactylates p53 and contributes to
tumorigenesis. Cell. 187:2375–2392.e33. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giuliano AE, Connolly JL, Edge SB,
Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ and
Hortobagyi GN: Breast cancer-major changes in the American joint
committee on cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:290–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American society of clinical oncology/college of
American pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update.
Arch Pathol Lab Med. 142:1364–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bertozzi S, Londero AP, Viola L, Orsaria
M, Bulfoni M, Marzinotto S, Corradetti B, Baccarani U, Cesselli D,
Cedolini C and Mariuzzi L: TFEB, SIRT1, CARM1, beclin-1 expression
and PITX2 methylation in breast cancer chemoresistance: A
retrospective study. BMC Cancer. 21:11182021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Győrffy B: Survival analysis across the
entire transcriptome identifies biomarkers with the highest
prognostic power in breast cancer. Comput Struct Biotechnol J.
19:4101–4109. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Knab VM, Gotthardt D, Klein K,
Grausenburger R, Heller G, Menzl I, Prinz D, Trifinopoulos J, List
J, Fux D, et al: Triple-negative breast cancer cells rely on
kinase-independent functions of CDK8 to evade NK-cell-mediated
tumor surveillance. Cell Death Dis. 12:9912021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng J and Liao X: Lysine lactylation
(Kla) might be a novel therapeutic target for breast cancer. BMC
Med Genomics. 16:2832023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui Z, Li Y, Lin Y, Zheng C, Luo L, Hu D,
Chen Y, Xiao Z and Sun Y: Lactylproteome analysis indicates histone
H4K12 lactylation as a novel biomarker in triple-negative breast
cancer. Front Endocrinol (Lausanne). 15:13286792024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S,
Shen X, Wu Y, Zhang S, Wang X, et al: Lactylome analysis suggests
lactylation-dependent mechanisms of metabolic adaptation in
hepatocellular carcinoma. Nat Metab. 5:61–79. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song F, Hou C, Huang Y, Liang J, Cai H,
Tian G, Jiang Y, Wang Z and Hou J: Lactylome analyses suggest
systematic lysine-lactylated substrates in oral squamous cell
carcinoma under normoxia and hypoxia. Cell Signal. 120:1112282024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo Y, Yang Z, Yu Y and Zhang P: HIF1α
lactylation enhances KIAA1199 transcription to promote angiogenesis
and vasculogenic mimicry in prostate cancer. Int J Biol Macromol.
222:2225–2243. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Wu J, Zhai L, Zhang T, Yin H, Gao
H, Zhao F, Wang Z, Yang X, Jin M, et al: Metabolic regulation of
homologous recombination repair by MRE11 lactylation. Cell.
187:294–311.e21. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Lin X, Zhao Q, Wang Y, Jiang F,
Ji C, Li Y, Gao J, Li J and Shen L: YARS as an oncogenic protein
that promotes gastric cancer progression through activating
PI3K-Akt signaling. J Cancer Res Clin Oncol. 146:329–342. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Wang J, Zhang L, He J, Ji B, Wang
J, Ding B and Ren M: Unveiling the role of YARS1 in bladder cancer:
A prognostic biomarker and therapeutic target. J Cell Mol Med.
28:1–20. 2024. View Article : Google Scholar
|