Introduction
Low-temperature plasma is an ionized gas at
approximately room temperature that is composed of charged
particles, neutral particles and electrons (1). Over the past two decades,
low-temperature plasma medicine, as a newly developed discipline,
has made preliminary achievements in disinfection and
sterilization, wound healing, antitumor effects and other
applications (2–4). In in vitro experiments,
low-temperature plasma inhibits proliferation and induces apoptosis
in a variety of tumor cell lines (5–7), but
the mechanism has not been fully clarified. In vitro,
low-temperature plasma initially contacts the cell culture medium,
resulting in a series of complex physical and chemical reactions
(8). The active substances
(reactive nitrogen and oxygen species) in solution further interact
with cells to exert antitumor effects (1,6,8). This
finding confirms that the low-temperature plasma-activated solution
(PAS) also has good antitumor effects and eliminates the limitation
of the shallow penetration depth of direct treatment with
low-temperature plasma (9).
Therefore, low-temperature PAS treatment has the advantages of
eliminating the limitations of instruments and equipment and
convenient storage (10,11).
The skin is the largest and most superficial organ
of the human body. This characteristic gives low-temperature plasma
broad application prospects in skin diseases (12). Melanoma is a malignant tumor derived
from melanin. Epidemiological analysis revealed that its incidence
and mortality rates have been increasing in recent decades
(13). Malignant melanoma is more
common in the skin but can also occur in the mucosa, respiratory
tract, gastrointestinal tract, reproductive system and other parts
of the skin and is characterized by early metastasis and a high
recurrence rate (14,15). The incidence of melanoma is greater
in light-skinned individuals. Importantly, melanoma is one of the
most common malignant tumors in young individuals and the mortality
rate in young individuals is greater than that of most other
cancers (16,17). Surgical resection is generally used
for early localized lesions, whereas comprehensive treatments,
including cytotoxic drug chemotherapy, immunotherapy and molecular
targeted therapy, are used for patients with late-stage disease or
multiple organ metastases (14,18).
However, these treatments often cannot yield satisfactory results.
Therefore, more effective methods need to be developed for the
adjuvant treatment of melanoma (13).
The present study used the melanoma cell line A375
as the experimental object and used low-temperature
plasma-activated phosphate buffer solution (PBS) as the medium to
observe the imbalance in reactive oxygen species (ROS) levels and
mitochondrial and DNA damage in A375 cells treated with PAS. In
vivo, the antitumor effect of a PAS on subcutaneously
transplanted melanoma in nude mice was observed by intratumoral
injection. Moreover, the biological safety of PAS was evaluated by
monitoring the weight, blood parameters and liver and kidney
functions of the mice.
Materials and methods
Low-temperature plasma equipment and
preparation of low-temperature PAS
Low-temperature plasma equipment was provided by the
Institute of Plasma Physics, Chinese Academy of Sciences. The
equipment mainly consists of a high-voltage electrode, a grounding
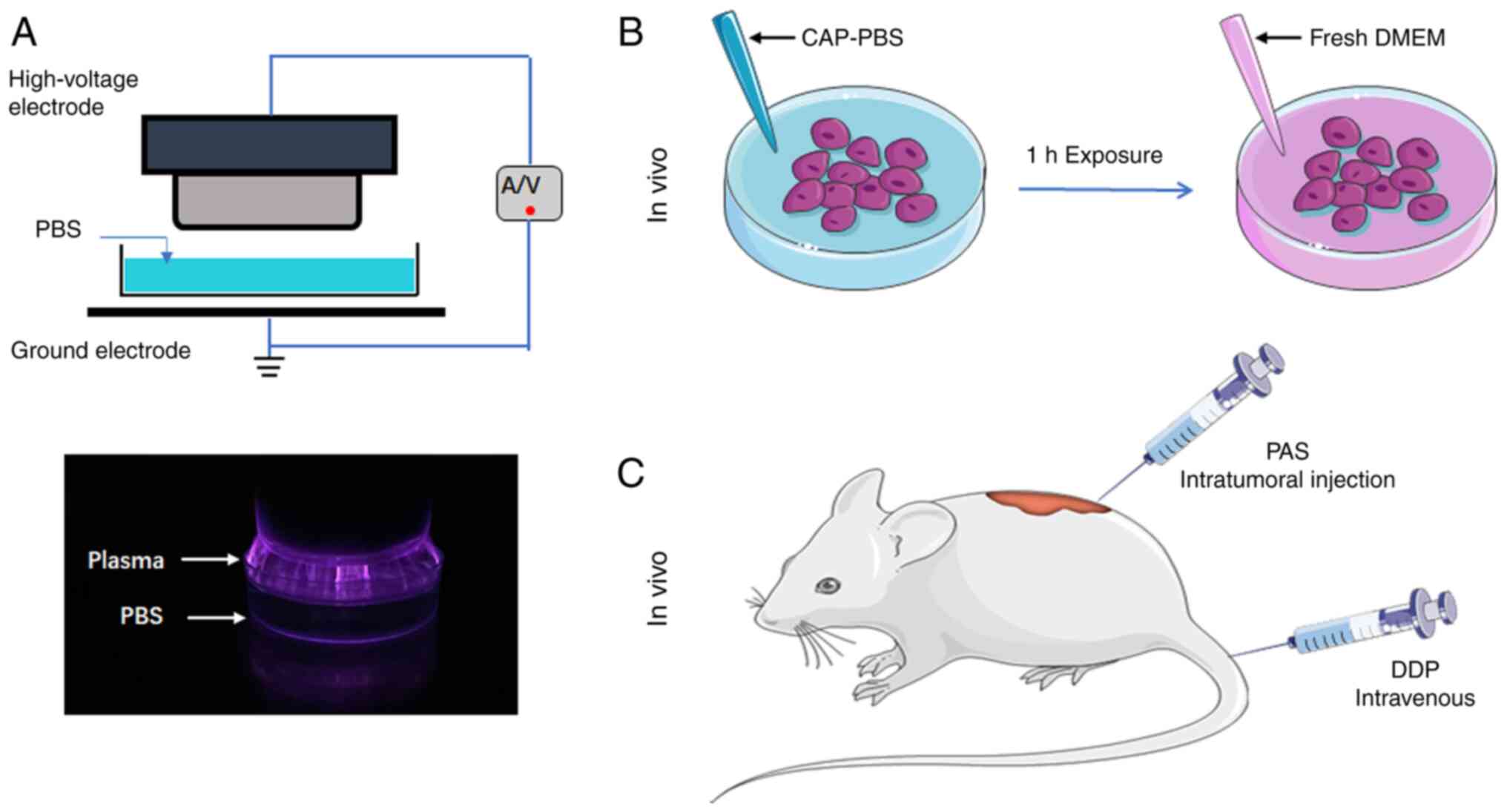
electrode and a power supply (Fig.
1A). Two electrodes were placed in the center of the PBS dish.
The spacing between the liquid surface and the high-voltage
electrode was fixed at 5 mm. The power supply was set to a constant
voltage of 40 V and a current of 2 A. The time of PBS exposure to
low-temperature plasma is defined as the dose of the PAS. The
prepared PAS was used to treat cells cultured in vitro and
subcutaneous tumors in nude mice. In the in vivo experiment,
50 µl of the PAS was injected intratumorally every two days. The
chemotherapy drug cisplatin (QlU Pharmaceutical Co. Ltd.; batch no.
aa2a0038b) was injected into the caudal vein to treat the tumors in
the positive control group. The drug concentration was 5 mg/kg and
the injection volume was 1 mg/ml (Fig.
1B and C).
Cell culture
A375 melanoma cell line (American Type Culture
Collection) was grown in RPMI-1640 medium (cat. no. G4531; Wuhan
Servicebio Technology Co., Ltd.) supplemented with 10% FBS (cat.
no. C04001; Shanghai VivaCell Biosciences, Ltd.) and 1% penicillin
and streptomycin (cat. no. C0222; Beyotime Institute of
Biotechnology). The cells were cultured in a culture flask (cat.
no. CCF-T25H; Wuhan Servicebio Technology Co., Ltd.) in a humid
environment at 37°C and 5% CO2 and passaged every 2–3
days.
Cell viability
Cell viability was determined using MTT assay. Cells
in the logarithmic growth stage were digested, counted and
inoculated into 96-well cell culture plates. Each well contained
10,000 cells and 100 µl of culture medium. After 24 h of culture,
the experiment was conducted once the cells had adhered completely
to the surface of the wells. After the cells were treated with PAS
for 1 h, fresh medium was added to the culture plate and the
culture was continued for 24 h. The antioxidant catalase (cat. no.
s0082; Beyotime Institute of Biotechnology) was added to each well
of the control group. MTT reagent was added to the culture plate
and the absorbance at 570 nm was detected using a SpectroMax i3×
(Molecular Devices, LLC).
Intracellular reactive oxygen species
(ROS)
The ROS kit was purchased from Beyotime Institute of
Biotechnology Biological Company (cat. no. s0033s). The cell
inoculation procedure was consistent with that used for the MTT
assay. The fluorescent probe DCFH-DA was added to the cell culture
and the changes in cellular ROS levels were measured after cells
were treated with PAS for 1 h. Using a SpectroMax i3× (Molecular
Devices, LLC) fluorescence microplate reader, fluorescence was
determined at an excitation wavelength of 488 nm and an emission
wavelength of 525 nm and the level of ROS was detected.
Mitochondrial membrane potential
A JC-1 kit was used to detect the mitochondrial
membrane potential (cat. no. c2006; Beyotime Institute of
Biotechnology). Cells in the logarithmic growth phase were
harvested and inoculated into 10-mm diameter circular cell culture
dishes. When the cell density was ~70%, the cells were treated with
PAS for 1 h. The cells were stained and washed with JC-1 stain
followed by JC-1 buffer. The cells were observed with fluorescence
microscopy. When detecting the JC-1 monomer, the excitation
wavelength was set to 490 nm and the emission wavelength was set to
530 nm. When detecting the JC-1 polymer, the excitation wavelength
was set to 525 nm and the emission wavelength was set to 590
nm.
DNA damage marker
Intracellular DNA damage was detected using
immunofluorescence. The cells were inoculated and cultured in 10-mm
cell culture dishes and the experiment was performed when the cell
density reached 70%. Histone family 2A variant (H2AX) was used as a
marker of DNA damage. The H2AX antibody was purchased from Abcam
(cat no. ab81299) and the fluorescent secondary antibody FITC was
purchased from Beyotime Institute of Biotechnology (cat no. a0562).
After the cells were treated with PAS for 1 h, they were fixed with
methanol at −20°C overnight. After the cells were washed, primary
antibody diluent was added and the cells were incubated overnight
at 4°C. After the cells were rinsed again, they were incubated with
a FITC-conjugated secondary antibody in the dark at room
temperature for 30 min. Then, the cells were observed under a
fluorescence microscope.
Western blotting
For western blot analysis, cells were seeded in
6-well plates. When the cells were completely attached to the
plate, they were treated with PAS and cultivated for 8 h. The cells
were then washed three times with ice-cold PBS. RIPA solution (cat
no. p0013B; Beyotime Institute of Biotechnology), protease
inhibitor and phosphatase inhibitor (cat no. p1050; Beyotime
Institute of Biotechnology) were added to the wells at a ratio of
100:1:2. After 30 min of lysis on ice, the cell lysates were
scraped and collected in Eppendorf tubes. The protein
concentrations were determined using a BCA Protein Assay Kit
(Beyotime Institute of Biotechnology) after centrifuging the
lysates at 16,363 × g at 4°C for 25 min. Using a 7.5% gel, proteins
were separated electrophoretically after loading 10 ug of protein
per lane and then transferred to a PVDF membrane. Next, the PVDF
membranes were blocked for 30 min with protein-free rapid blocking
buffer (EpiZyme, Inc.) at 4°C and incubated overnight at 4°C with
the corresponding primary antibodies (H2AX, 1:10,00; Cell Signaling
Technology cat no. 7631T; γ-H2AX, 1:10,00; Cell Signaling
Technology cat no. 2577S; GAPDH: Dilution 1:50,00; Proteintech cat
no. 10494-1-AP). The membranes were incubated for 1 h at indoor
temperature with the corresponding secondary antibodies (Dilution
1:10,000; ProteinTech cat no. SA00001-2, SA00001-1) on the next day
and protein bands were visualized with visualization reagent
(Biosharp; cat no. BL520A) using a western blot imaging instrument
(Thermo Fisher Scientific, Inc.). Bands of interest were analyzed
using ImageJ (win-java8, National Institutes of Health).
Cell subunits
Changes in intracellular subunits in A375 cells
treated with PAS were observed using transmission electron
microscopy. A375 cells were exposed to PAS for 1 h and then fresh
medium was added to continue the cultures for 6 h for improved
observation of organelle morphology. The cells were then collected
by digestion and centrifugation. Glutaraldehyde (3%) was added and
the cells were fixed overnight at 4°C. Then, 1% osmium tetroxide
was added to the samples and the cells were collected following a
series of ethanol dehydrations. The samples were treated at room
temperature with epoxy resin (Sigma Aldrich; cat no. 31185) and
acetone (v/v=1/1) for 1 h, epoxy resin and acetone (V/V=3/1) for 3
h. The osmotically treated samples were embedded and heated at 70°C
overnight to obtain the embedded samples. The samples were dried
and cut into 80 nm sections on an ultrathin microtome, and then
stained with lead citrate solution and hydrogen peroxide acetic
acid 50% ethanol saturated solution for 5 min each at room
temperature and then the cell morphology was observed by
transmission electron microscopy. Photographs were analyzed and
processed using Digital Micrograph (Digital Micrograph 3.5, Gatan
USA, Inc.).
Subcutaneous tumor transplantation in
nude mice
A375 cells in logarithmic growth were inoculated
subcutaneously into BALB/c-nu mice at a density of 5×106
cells/100 µl/mouse. When the mean tumor volume reached ~90
mm3, 20 mice were randomly divided into 4 groups (G1-4)
of 5 mice each according to the tumor volume. Group G1 was the
negative control; 50 µl PBS was injected into the tumors. In
addition, 50 µl PAS was injected into the tumors in Groups G2 and
G3. Group G4 mice were subjected to cisplatin tail vein injection
as a positive control group. The day of grouping was defined as day
0, which was also the day of administration. The drugs were
administered every 2 days for 2 weeks. After initiation of drug
administration, tumor size was observed and mice were weighed on
days 0, 3, 6, 9, 12 and 15. The humane endpoints identified in the
present study were as follows: i) Tumor volume exceeding 3,000
mm3 in a single mouse; ii) persistent loose stools; iii)
retarded activity (unable to eat or drink); iv) arching of the back
and lying on the side; v) decreased activity and signs of muscle
wasting; vi) difficulty in breathing; vii) processive lowering of
body temperature; viii) paralysis and/or spasms; ix) persistent
bleeding; x) inability of the animal to move normally due to a
tumor that is too large or for other reasons; and xi) inability to
move normally due to severe ascites or increased abdominal
circumference. The endpoint of the experiment was day 15 post-dose.
Blood collection was performed through the submandibular vein of
mice before the endpoint of the experiment, collecting 50 µl per
mouse and then the samples were sent to Jiangsu GemPharmatech Co.,
Ltd. for the testing of blood indexes and liver and kidney
functions, no anesthetic injection was administered prior to
submandibular blood collection. Mice were sacrificed using cervical
dislocation and the complete absence of vital signs was confirmed
by observing the pinch reflex of the animals. Tumor was removed,
weighed, and the tumor tissue specimen was fixed in formalin for 12
h, dehydrated, embedded in paraffin, and cut into 4-micron
sections. Tissue sections were dewaxed, dehydrated and immersed in
methanol containing 0.3% hydrogen peroxide for 30 min. The sections
were heated in an autoclave containing 10 mM EDTA) buffer (pH 8.0)
for 2 min. Sections were incubated in 1% blocking serum for 30 min
to minimize non-specific binding. The sections are then incubated
with primary antibody (1:250) at 4°C overnight. Sections were then
incubated with biotinylated secondary antiserum, followed by
incubation with horseradish peroxidase-conjugated
streptavidin-biotin complex. Finally, sections were visualized with
DAB) and stained with hematoxylin. Tumor tissue sections were
deparaffinized and stained with hematoxylin for 4 min at room
temperature, rinsed under running water for 10 min and
differentiated with ethanol hydrochloride for 3 s. Sections were
again rinsed under running water until they returned blue, and then
stained at room temperature with eosin for 1 min. The cellular
experiments, mouse feeding, mouse modelling, blood sample
collection and tumor sample collection of the present study were
performed at Anhui Medical University (Anhui, China) and blood
sample analysis and tumor immunohistochemistry were performed at
Jiangsu GemPharmatech Co., Ltd. No mice in this experiment reached
the humane endpoints. The content related to animal use and the
experiments in this experimental scheme were reviewed and approved
by the IACUC Committee (Anhui, China).
Statistical analysis
The results of the experiments were expressed as
mean ± standard error of the mean. Comparisons between the two
groups of samples were made using the independent samples paired
Student's t-test, Kruskal-Wallis tests were used for datasets
containing ≥3 groups and the data were analyzed using SPSS (IBM
SPSS Statistics 25) P<0.05 was considered to indicate a
statistically significant difference.
Results
Treatment with a PAS decreases cell
viability and increases intracellular ROS levels
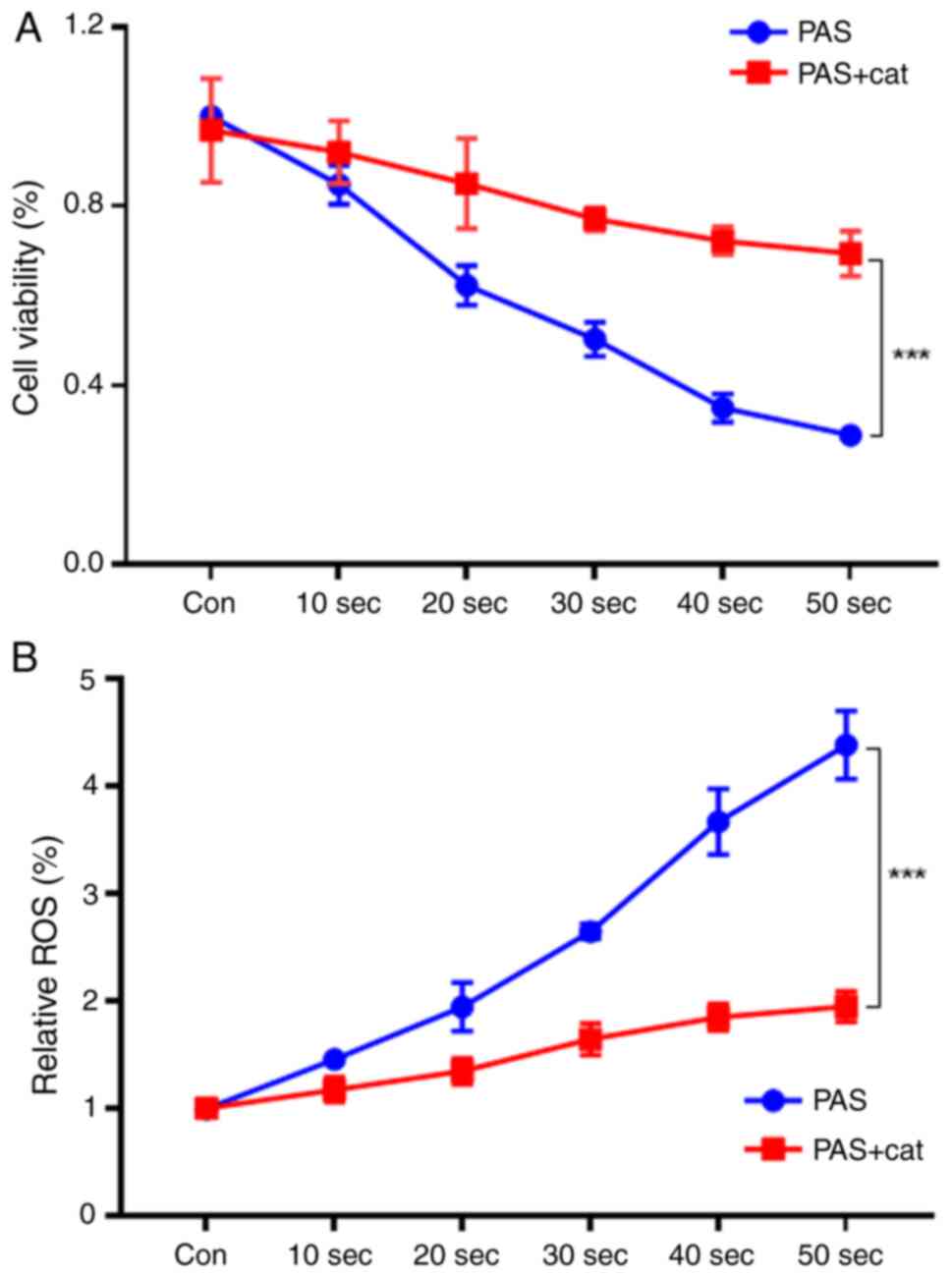
As shown in Fig. 2A,
after A375 cells were treated with different doses of the PAS for
10–50 sec, the cell viability tended to decrease. Treatment with a
50-sec dose of PAS for 1 h resulted in a cell survival rate of ~30%
after 24 h. Under the same conditions, the survival rate of cells
treated with the antioxidant catalase increased significantly.
Intracellular ROS in the PAS group increased significantly compared
with the control group. By contrast, the intracellular ROS level
increased only slightly after the cells were cotreated with the
antioxidant catalase (Fig. 2B).
Low-temperature plasma-activated
solution treatment induces mitochondrial and DNA damage
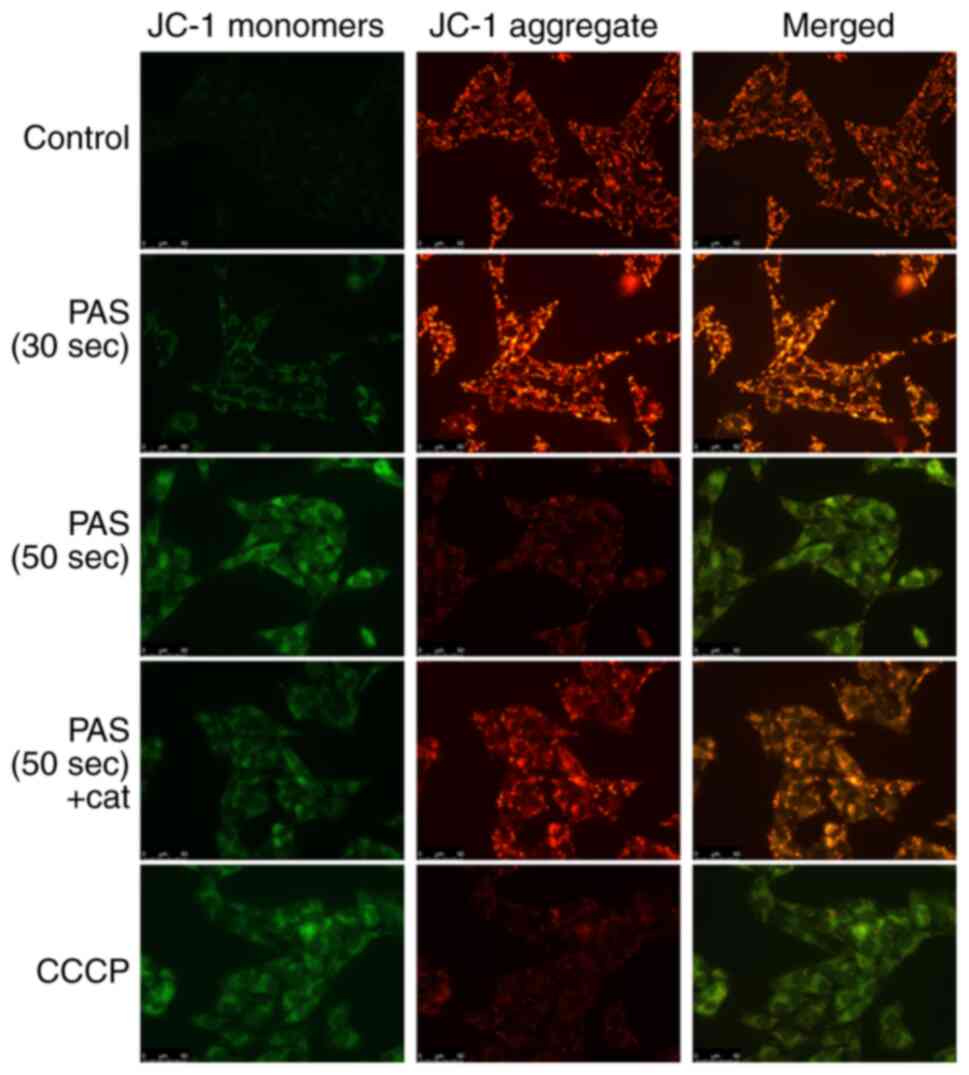
A decrease in the mitochondrial membrane potential
is a hallmark feature of apoptosis (19) JC-1 accumulates in the mitochondrial
matrix and forms polymers (J-aggregates) that produce red
fluorescence when the mitochondrial membrane potential is high and
the JC-1 monomer produces green fluorescence when the mitochondrial
membrane potential is low. As shown in Fig. 3, after the cells were subject to PAS
treatment, the mitochondrial membrane potential decreased. This
decreasing trend was dose dependent. The antioxidant catalase was
able to effectively alleviate this effect. Carbonyl cyanide
3-chlorophenylhydrazone (CCCP) was used as a positive control of
reduced mitochondrial membrane potential.
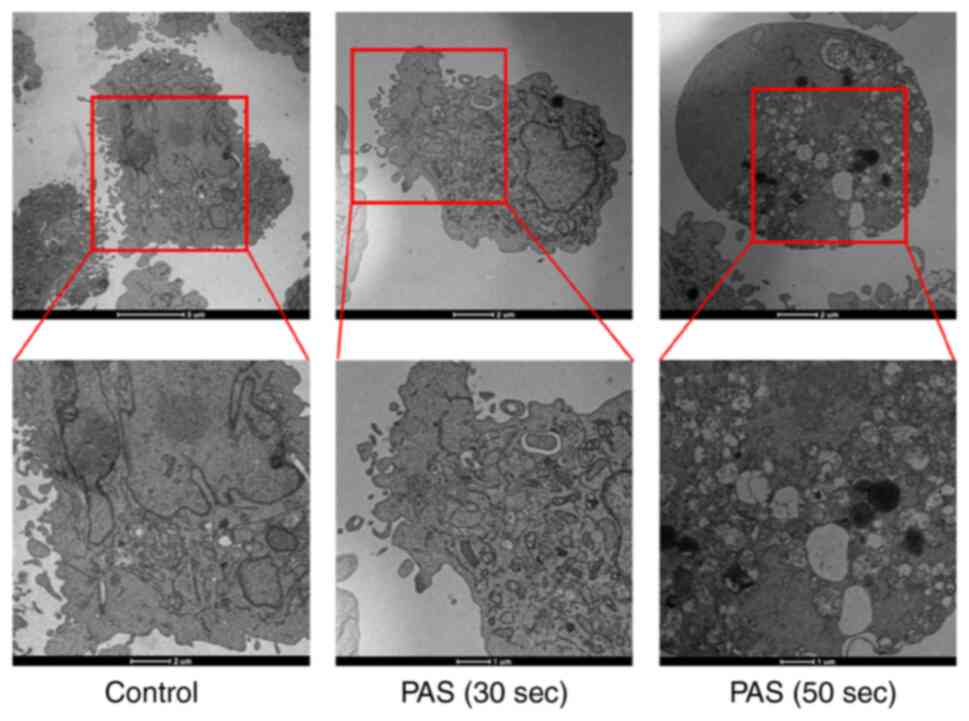
The cells subject to PAS treatment were observed
using transmission electron microscopy. Dense microvilli were
observed in the cell membranes of the normal control group. The
nuclei were large and centered and the organelle structures were
normal. After PAS treatment, the cell volume gradually decreased.
Moreover, the microvilli of the cell membranes were reduced and the
nuclei were smaller. Different sizes and diverse shapes of
mitochondria were observed. Most mitochondria were obviously
swollen and the cristae of the mitochondria were fuzzy, disordered
and even broken. Vacuole formation in the cell indicated inevitable
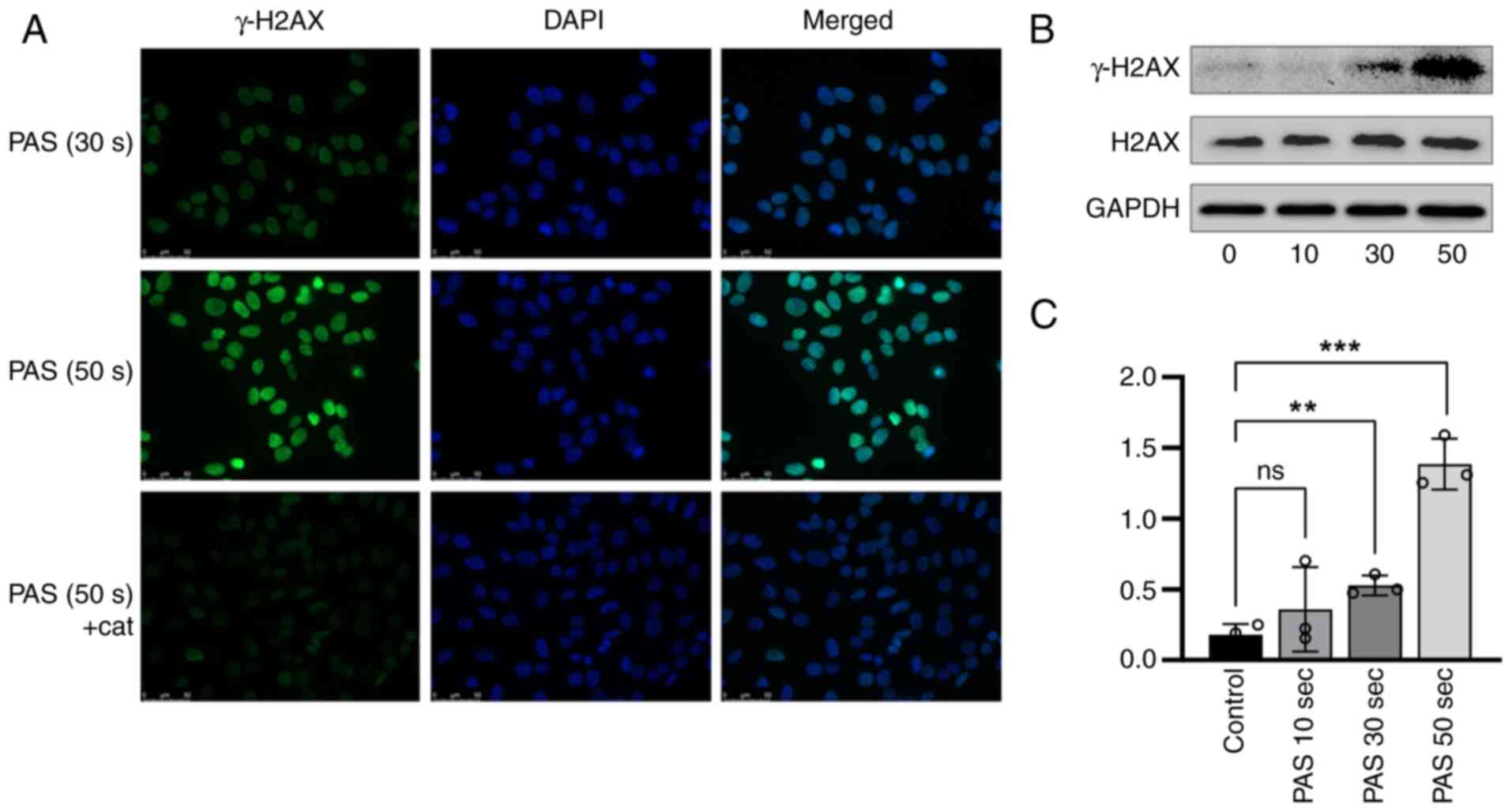
cell death (Fig. 4). A number of
DNA damage markers were also detected by immunofluorescence and
western blotting. γ-H2AX was overexpressed after A375 cells were
treated with PAS and the degree of injury remained dose dependent
(Fig. 5).
In vivo experiments confirm that
plasma-activated solution can effectively exert antitumor
effects
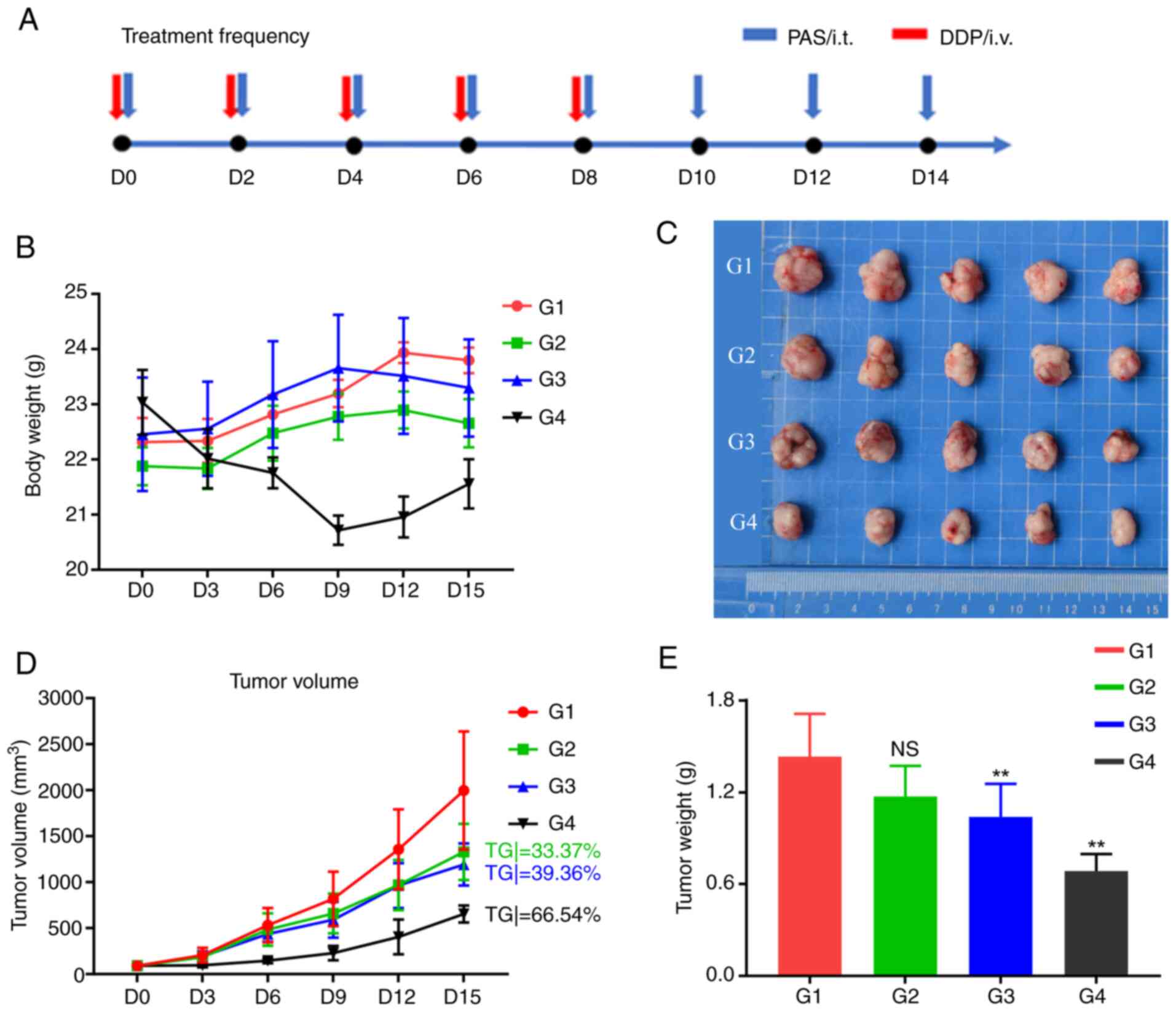
As shown in Fig. 6A,
mice in each group were administered the appropriate drugs every
two days and the experimental process lasted for 15 days. The G1-G3
groups received intratumoral injections and the chemotherapy drug
cisplatin was administered to the G4 group through caudal vein
injection. The mice in the cisplatin group experienced significant
weight loss and could not eat after the 8th day, so the treatment
was stopped (Fig. 6B). Fig. 6C shows the subcutaneous tumors of
the mice in each group that were removed after the experiment. As
shown in Fig. 6D, compared with the
control group, the G2 group (TGitv=33.73%) showed a tumor growth
inhibition effect, but the difference was not significant
(P>0.05; Fig. 6D). The G3 group
(TGitv=39.36%) showed significant tumor growth inhibition and the
G4 chemotherapy drug group (cisplatin; TGitv=66.54%) also showed
significant tumor growth inhibition. Following the experiment, the
tumors were removed from the mice. The size of the subcutaneous
tumors decreased significantly from the G1 group to the G4 group.
Tumor weight measurements revealed that tumor growth was
significantly inhibited in the G3 (P=0.037) and G4 (P<0.001)
groups (Fig. 6E).
Low-temperature plasma-activated
solution shows improved safety in in vivo experiments
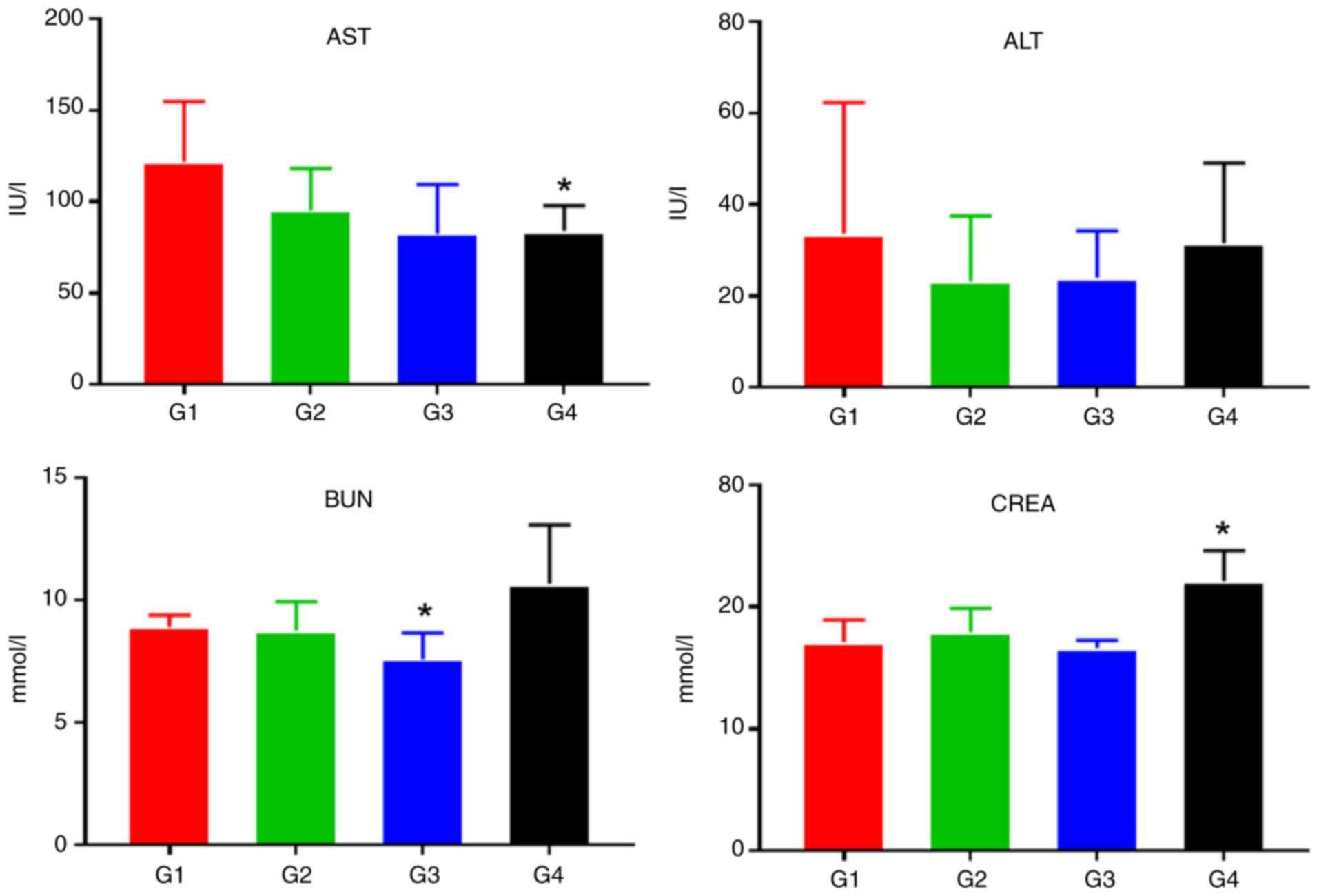
Liver and kidney function tests and routine blood
tests were performed on the mice before the end of the experiment.
As shown in Fig. 7, compared with
the G1 control group, cisplatin caused a significant decrease in
AST levels (0.01 ≤P<0.05*) and a significant increase in CREA
levels (0.05 ≤P<0.01**), indicating that acute renal injury had
occurred in the mice. However, no significant impairment of liver
or kidney function was observed in the G2 or G3 group.
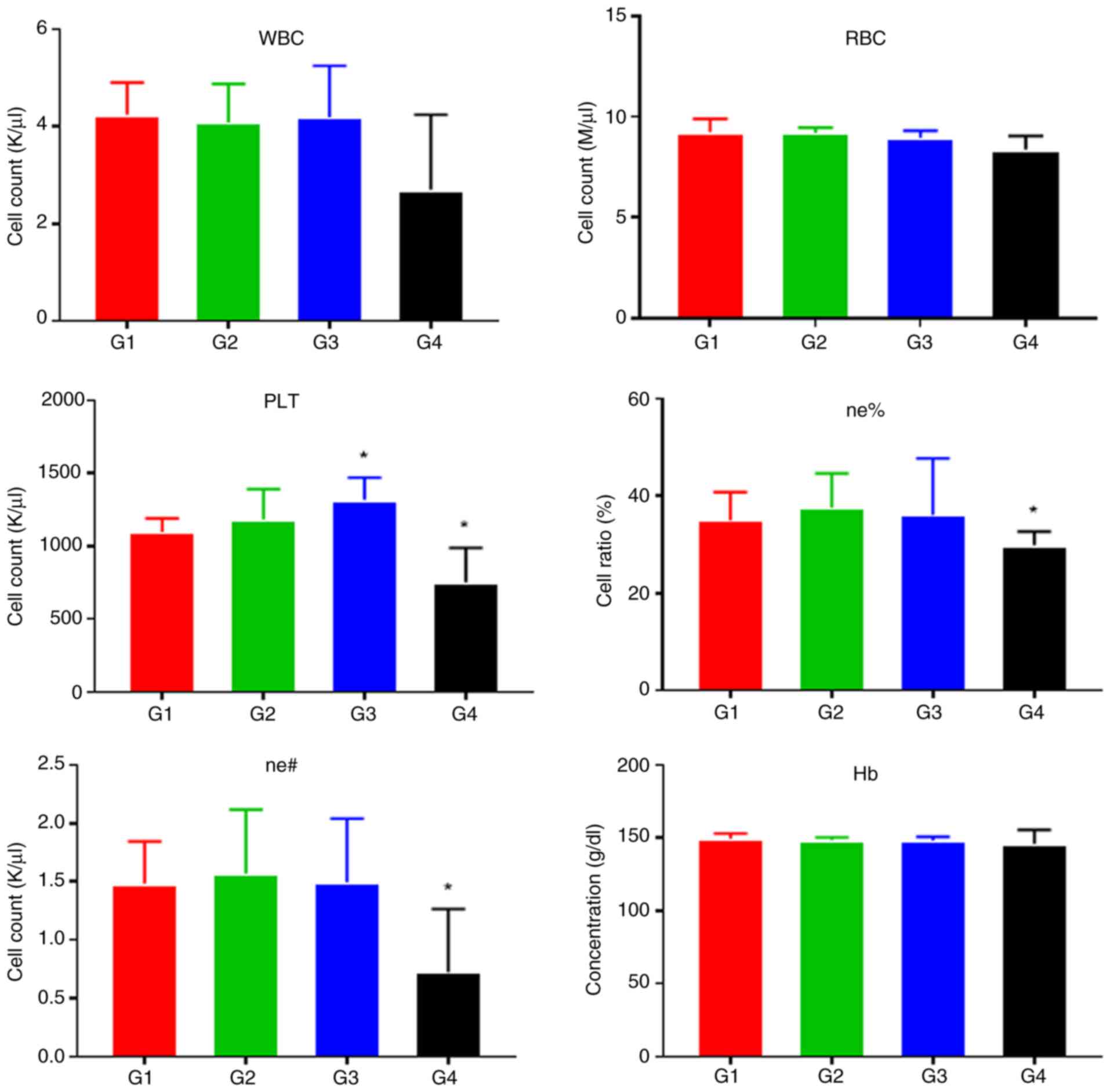
Fig. 8 shows the
statistical analysis of routine blood test results in mice.
Compared with those in the G1 control group, cisplatin treatment in
the G4 group caused leukopenia (P=0.074), neutropenia (0.01
≤P<0.05*) and thrombocytopenia (0.01 ≤P<0.05*). These results
indicated that cisplatin caused bone marrow suppression in mice. In
contrast, the G2 and G3 groups did not show any evidence of
significant inhibition of hematopoietic function, although a slight
increase in platelet count was observed in the G3 group (0.01
≤P<0.05*).
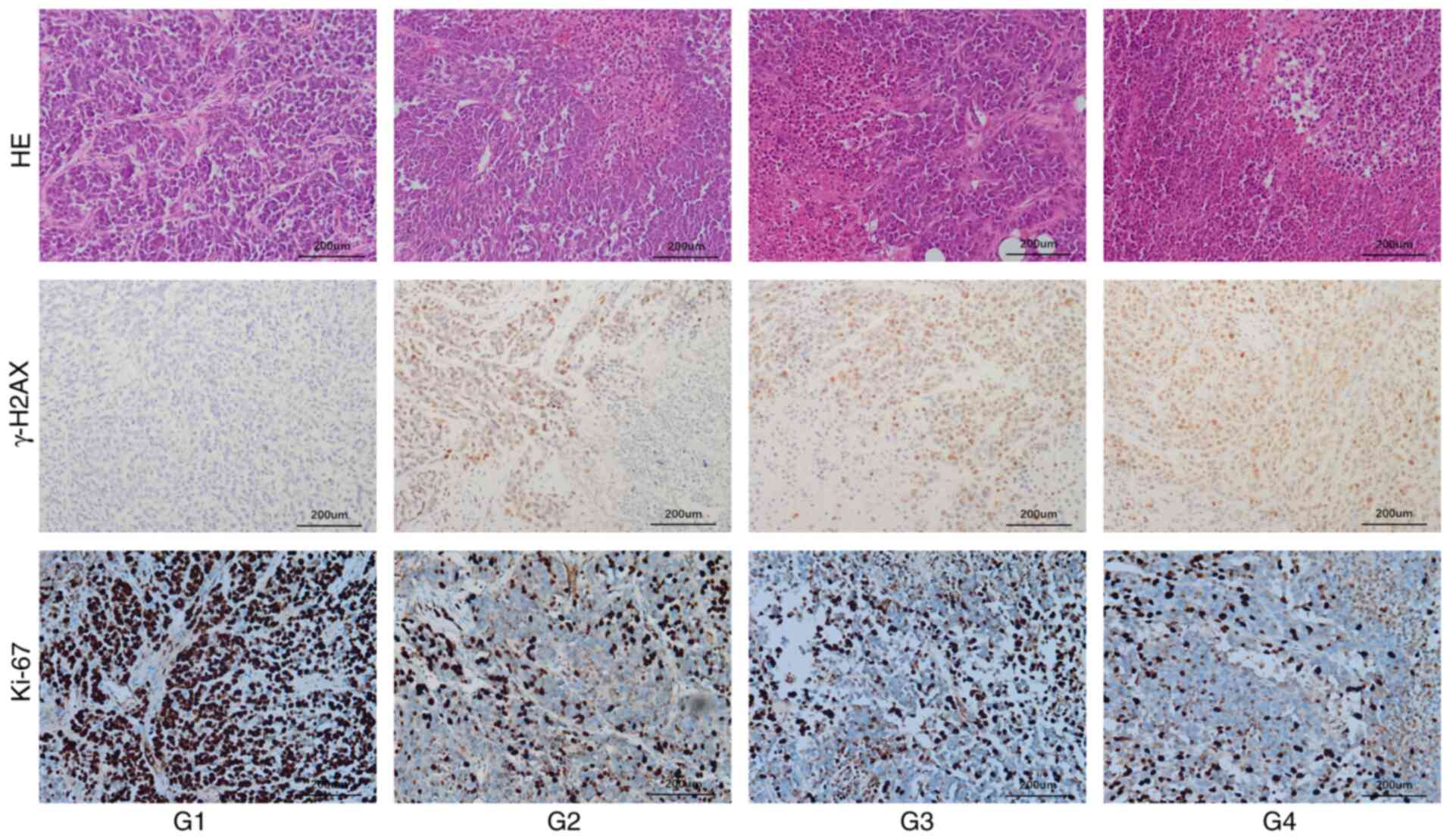
Histological effects in tumor
tissues
Hematoxylin/eosin staining and γ-H2AX and Ki-67
immunostaining were used to evaluate the histological effects in
mouse tumors following treatment (Fig.
9). Hematoxylin/eosin staining revealed that tumor necrosis
occurred in the G2-G4 groups. The PAS was injected into the tumors
in the G2 and G3 groups. These tumors clearly showed great regional
necrosis upon hematoxylin/eosin staining, whereas the
chemotherapeutic drug cisplatin exhibited more uniform effects in
the tumors. Similarly, in the tumor tissues of mice, γ-H2AX
expression was observed in the G2-G4 groups, but the expression
level in the G4 group was significantly greater than that in the
other groups. In G2 and G3 groups, γ-H2AX was expressed around
necrotic tumor tissues and displayed regional expression. Ki-67 is
a nuclear division- and proliferation-related protein and its
function is closely related to mitosis; it is often used as a
reliable marker of tumor cell proliferation. The greatest levels of
tumor proliferation were noted in the control group. The percentage
of Ki-67-positive cells was ~80% in control tumors, while the
percentage of Ki-67-positive cells in the G2-G4 groups decreased to
varying degrees.
Discussion
The present study described the antitumor effect of
PAS on the melanoma cell line A375 cultured in vitro and
subcutaneously transplanted tumors in mice. As a potential tumor
treatment, low-temperature plasma therapy has been evaluated in a
variety of tumor cell lines (5–7).
However, most of the existing studies have focused on the direct
effect of low-temperature plasma on cells cultured in vitro.
Ongoing studies have shown that low-temperature plasma-activated
DMEM has good antitumor effects (8,20,21).
These results suggested that PAS also exhibits great potential in
tumor treatment because the methodology eliminates the dependence
on instruments and equipment in the treatment process. The
effectiveness of direct treatment with low-temperature plasma may
depend on the size and location of the tumor, as some deep solid
tumors cannot be effectively treated (22,23).
PAS can be injected deep into tumors (24,25),
which overcomes the limitations of direct treatment and provides an
accurate and effective method for tumor treatment.
The transfer of active substances from
low-temperature plasma to a solution medium by physical or chemical
means is the theoretical basis for the biological effects of PAS
(26). It has been demonstrated
that the active oxygen components and active nitrogen components in
solution are the main active substances that induce biological
effects, among which H2O2 is the most
important (26,27). In our previous studies, changes in
H2O2 and NO3−
concentrations were also detected in DMEM and PBS exposed to a
low-temperature plasma environment at different time points
(28). The results showed that the
concentrations of these active substances increased with increasing
low-temperature plasma irradiation time (28). The results of the present study
suggested that exposure to low-temperature plasma-activated PBS for
a longer time will increase ROS levels in A375 cells. Catalase is
an important antioxidant enzyme that plays important roles in
scavenging ROS and maintaining the balance of the redox state
(29). Under the same experimental
conditions, catalase can significantly reduce the increase in
intracellular ROS caused by treatment with PAS and further prevent
cell death (5,30). These results also demonstrated that
ROS in PAS are the main active components that cause cell
damage.
Mitochondria are important organelle structures in
cells and play leading roles in cell respiration and maintaining
redox balance. They also play a central role in the apoptotic
pathway (31,32). The present study showed that an
increase in intracellular ROS levels leads to mitochondrial damage.
When mitochondria are damaged, the permeability of the
mitochondrial membrane increases and mitochondrial lipids are
redistributed. The main feature of their structural change is
mitochondrial swelling, which can be accompanied by a reduction or
disappearance of the number of mitochondrial cristae. An increase
in mitochondrial membrane permeability further leads to
dysregulation of the distribution of protons and electrons in the
inner membrane of the mitochondria and a decrease in or loss of the
mitochondrial membrane potential. Maintenance of the mitochondrial
membrane potential is necessary for mitochondrial respiratory
function. Therefore, when the mitochondrial membrane potential
decreases, mitochondrial function is disrupted, which leads to
apoptosis (33–35).
DNA damage and mitochondrial damage are the most
common causes of damage to cancer cells treated with
low-temperature plasma. Previous studies have shown that increases
in intracellular ROS and DNA damage are observed after
low-temperature plasma treatment (28,36,37).
DNA double-strand breaks are the most important manifestation of
damage. H2AX is a subtype of histone H2A. When DNA damage occurs in
cells, H2AX is rapidly phosphorylated, producing γ-H2AX; therefore,
γ-H2AX is a good marker of DNA damage (38). Using immunofluorescence technology,
microscopic evaluation of γ-H2AX staining indicated regions of DNA
double-strand breaks, with each focus corresponding to one DSB.
When DNA damage cannot be repaired, cell cycle arrest occurs,
leading to apoptosis.
At present, strategies for the treatment of cancer
cells using low-temperature plasma are divided into direct and
indirect treatments. Direct treatment involves direct irradiation
of cancer cells or animal tumor models cultured in vitro
with low-temperature plasma. Indirect treatment uses media
activated by low-temperature plasma to further treat cancer cells
in vitro or tumors in vivo. In existing studies, it
has been confirmed that low-temperature plasma and PAS have clear
anticancer effects on dozens of cancer cell lines cultured in
vitro; however, for some non-superficial tumors, the use of
direct treatment with low-temperature plasma is limited (5–7,9). Chen
et al (2) developed a novel
miniature CAP device (µCAP) that is directly connected to
endoscopic devices and µCAP significantly inhibits the growth of
brain gliomas in mice. Vaquero et al (39) established a mouse subcutaneous
transplanted tumor model of cholangiocarcinoma and direct treatment
of subcutaneous tumors with PAS yielded good antitumor effects.
Notably, the jet of low-temperature plasma must contact the tumor
to demonstrate effective antitumor effects. Therefore, for the
treatment of solid tumors, local injection of PAS seems to
represent a promising treatment strategy. Tanaka et al
(40) used the cervical cell
carcinoma line SiHa to establish a mouse subcutaneous
transplantation tumor model and subcutaneously injected
low-temperature plasma-activated Ringer's solution to treat the
tumor, achieving good results. To demonstrate the safety of PAS,
Nastasa et al provided mice with low-temperature
plasma-activated water as a water source for 90 days.
Low-temperature plasma-activated water did not cause functional
damage or tissue damage to the heart, liver, kidney, brain,
digestive system or blood system of mice (41). The present study, proposed a new
treatment method. Specifically, PAS was injected into the tumor to
allow the effective components in the solution to reach the tumor
site more accurately. This treatment method can result in less
toxicity and fewer side effects. When cisplatin was used to treat
tumors, the mice experienced severe renal damage and bone marrow
loss and were depressed and unable to eat. However, injection of
the PAS into tumors did not significantly damage the liver, kidney
or hematopoietic functions of the mice. Notably, hematoxylin and
eosin and immunohistochemical staining of mouse tumor sections
revealed that the tumors exhibited focal necrosis following
injection of the PAS and that the DNA damage marker γ-H2AX was
expressed around necrotic tissue. These phenomena indicated that
the effective region of PAS is still limited even by intratumoral
injection. Cisplatin, a chemotherapeutic drug, is transported to
tumor sites through the blood circulation and can produce more
extensive antitumor effects (42).
However, the present study had several limitations.
First, only one fluid, namely, PBS, was used and no comparisons
were made with different media. Second, although a number of
researchers have conducted similar studies (5–7,8,28,37),
the present study used only one cell line to validate the effect of
PAS and errors due to chance could not be avoided. Finally, the
present study used BALB mice for allogeneic implant modeling. This
model lacks immune function and cannot reflect the immune
microenvironment of the normal human body. In addition, several
problems were exposed in this experiment. For example, the
parameters and specifications of low-temperature plasma-forming
devices have not yet been standardized and differences between
in vitro and in vivo environments made it difficult
to monitor the actual concentration of active substances. The
authors are working on exploring more possibilities, such as using
PAS to treat more melanoma cell lines of different origins as well
as normal melanocytes to explore the specific mechanism of its
killing effect on melanoma, using 3D tumor microenvironment models
to explore the modulation of the tumor immune microenvironment by
PAS and using mouse homologous melanoma cell implantation models to
preserve the immune function of the mice in order to explore the
PAS interacts with the immune microenvironment in an in vivo
setting. In the future, it is hoped to develop a treatment method
that combines PAS with novel materials to provide more durable,
precise and uniform antitumor effects.
Low-temperature plasma has shown good antitumor
effects in dozens of tumor cell lines. However, the method is
limited by currently available instruments and equipment and is
capable of only superficial penetration. PAS also showed good
antitumor effects in vitro. The present study not only
confirmed the effectiveness of local injection of PAS in the
treatment of melanoma A375 cells and mice with tumors but also
confirmed the safety of PAS in terms of hematopoietic, liver and
kidney functions by monitoring the blood biochemistry of mice.
Additional safety assessments need to be performed, such as an
evaluation of the treatment's reproductive toxicity. In conclusion,
the results of the present study confirmed that PAS represented a
promising new agent for the treatment of melanoma.
Acknowledgements
The authors would like to thank Dr Ruru Wang and Dr
Chen Bin (Anhui Province Key Laboratory of Environmental Toxicology
and Pollution Control Technology, Hefei Institutes of Physical
Science, Chinese Academy of Sciences, Anhui, China) for their help
in experiments.
Funding
The present study was supported by the 2020 Natural Science
Research Project of Anhui Universities, Anhui, China (Major
Project), grant no. KJ2020ZD19 to Chunjun Yang, Clinical
Cultivation Program of the Second Affiliated Hospital of Anhui
Medical University, Anhui, China (Key Project), grant no.
2020LCZD22 to Chunjun Yang, 2020 Provincial Quality Project of
Colleges and University, grant no. 2020mooc227 and Spark Plan,
Anhui Medical University, (grant no. 2015hhjh04).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The present study was conceived and designed by CY.
The main experiments were conducted by XY and CChen. SZ and MR also
conducted some experiments. As an expert in the field of cold
atmospheric plasma, CCheng proposed the concept of the present
study together with CY and participated in the drafting of the
manuscript. CZ contributed to the experimental design of the
present study and analyzed the experimental data. CY and CZ confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The Anhui Medical University Laboratory Animal
Ethics Committee (Anhui, China; approval no. LLSC20210406) approved
the present study and approved experiments conducted at Jiangsu
GemPharmatech Co., Ltd. (Jiangsu, China) as per the contract no.
GJS04202101026
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Motaln H, Recek N and Rogelj B:
Intracellular responses triggered by cold atmospheric plasma and
plasma-activated media in cancer cells. Molecules. 261:3362021.
|
|
2
|
Chen Z, Simonyan H, Cheng X, Gjika E, Lin
L, Canady J, Sherman JH, Young C and Keidar M: A novel micro cold
atmospheric plasma device for glioblastoma both in vitro and in
vivo. Cancers (Basel). 9:612017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arndt S, Unger P, Berneburg M, Bosserhoff
AK and Karrer S: Cold atmospheric plasma (CAP) activates
angiogenesis-related molecules in skin keratinocytes, fibroblasts
and endothelial cells and improves wound angiogenesis in an
autocrine and paracrine mode. J Dermatol Sci. 89:181–190. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dubuc A, Monsarrat P, Virard F, Merbahi N,
Sarrette JP, Laurencin-Dalicieux S and Cousty S: Use of
cold-atmospheric plasma in oncology: A concise systematic review.
Ther Adv Med Oncol. 10:4335657812018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aggelopoulos CA, Christodoulou AM,
Tachliabouri M, Meropoulis S, Christopoulou ME, Karalis TT,
Chatzopoulos A and Skandalis SS: Cold atmospheric plasma attenuates
breast cancer cell growth through regulation of cell
microenvironment effectors. Front Oncol. 11:8268652021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Loenhout J, Flieswasser T, Freire
Boullosa L, De Waele J, Van Audenaerde J, Marcq E, Jacobs J, Lin A,
Lion E, Dewitte H, et al: Cold atmospheric plasma-treated pbs
eliminates immunosuppressive pancreatic stellate cells and induces
immunogenic cell death of pancreatic cancer cells. Cancers (Basel).
11:15972019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soni V, Adhikari M, Simonyan H, Lin L,
Sherman JH, Young CN and Keidar M: In vitro and in vivo enhancement
of temozolomide effect in human glioblastoma by non-invasive
application of cold atmospheric plasma. Cancers (Basel).
13:44852021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zahedian S, Hekmat A, Tackallou SH and
Ghoranneviss M: The impacts of prepared plasma-Activated medium
(PAM) combined with doxorubicin on the viability of MCF-7 breast
cancer cells: A new cancer treatment strategy. Rep Biochem Mol
Biol. 10:640–652. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan D, Sherman JH and Keidar M: Cold
atmospheric plasma, a novel promising anti-cancer treatment
modality. Oncotarget. 8:15977–15995. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan D, Cui H, Zhu W, Nourmohammadi N,
Milberg J, Zhang LG, Sherman JH and Keidar M: The specific
vulnerabilities of cancer cells to the cold atmospheric
plasma-stimulated solutions. Sci Rep. 7:4412–4479. 2017.PubMed/NCBI
|
|
11
|
Privat-Maldonado A, Schmidt A, Lin A,
Weltmann KD, Wende K, Bogaerts A and Bekeschus S: ROS from physical
plasmas: Redox chemistry for biomedical therapy. Oxid Med Cell
Longev. 2019:90620982019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gan L, Zhang S, Poorun D, Liu D, Lu X, He
M, Duan X and Chen H: Medical applications of nonthermal
atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges.
16:7–13. 2018. View Article : Google Scholar
|
|
13
|
Long GV, Swetter SM, Menzies AM,
Gershenwald JE and Scolyer RA: Cutaneous melanoma. Lancet.
402:485–502. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leonardi GC, Falzone L, Salemi R, Zanghì
A, Spandidos DA, Mccubrey JA, Candido S and Libra M: Cutaneous
melanoma: From pathogenesis to therapy (Review). Int J Oncol.
52:1071–1080. 2018.PubMed/NCBI
|
|
15
|
Strashilov S and Yordanov A: Aetiology and
pathogenesis of cutaneous melanoma: Current concepts and advances.
Int J Mol Sci. 22:63952021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coelho SG and Hearing VJ: UVA tanning is
involved in the increased incidence of skin cancers in fair-skinned
young women. Pigment Cell Melanoma Res. 23:57–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Newton-Bishop J, Bishop DT and Harland M:
Melanoma genomics. Acta Derm Venereol. 100:adv1382020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rozeman EA, Dekker TJA, Haanen JBAG and
Blank CU: Advanced melanoma: Current treatment options, biomarkers,
and future perspectives. Am J Clin Dermatol. 19:303–317. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaib S, Hayyat A, Ali N, Gul A, Naveed M
and Khan I: Role of mitochondrial membrane potential and lactate
dehydrogenase a in apoptosis. Anticancer Agents Med Chem.
22:2048–2062. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jo A, Bae JH, Yoon YJ, Chung TH, Lee EW,
Kim YH, Joh HM and Chung JW: Plasma-activated medium induces
ferroptosis by depleting FSP1 in human lung cancer cells. Cell
Death Dis. 13:2122022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ikeda JI, Tanaka H, Ishikawa K, Sakakita
H, Ikehara Y and Hori M: Plasma-activated medium (PAM) kills human
cancer-initiating cells. Pathol In. 68:23–30. 2018.
|
|
22
|
Ishikawa K, Hosoi Y, Tanaka H, Jiang L,
Toyokuni S, Nakamura K, Kajiyama H, Kikkawa F, Mizuno M and Hori M:
Non-thermal plasma-activated lactate solution kills U251SP
glioblastoma cells in an innate reductive manner with altered
metabolism. Arch Biochem Biophys. 688:1084142020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Griseti E, Merbahi N and Golzio M:
Anti-cancer potential of two plasma-activated liquids: Implication
of Long-lived reactive oxygen and nitrogen species. Cancers
(Basel). 12:7212020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang T, Cao X, Shen B, Chen Z and Chen G:
Injectable cold atmospheric plasma-activated immunotherapeutic
hydrogel for enhanced cancer treatment. Biomaterials.
300:1221892023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Solé-Martí X, Espona-Noguera A, Ginebra MP
and Canal C: Plasma-conditioned liquids as anticancer therapies in
vivo: Current state and future directions. Cancers (Basel).
13:4522021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan D, Talbot A, Nourmohammadi N, Cheng X,
Canady J, Sherman J and Keidar M: Principles of using cold
atmospheric plasma stimulated media for cancer treatment. Sci Rep.
5:183392015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uchiyama H, Zhao QL, Hassan MA, Andocs G,
Nojima N, Takeda K, Ishikawa K, Hori M and Kondo T: EPR-Spin
trapping and flow cytometric studies of free radicals generated
using cold atmospheric argon plasma and X-Ray irradiation in
aqueous solutions and intracellular milieu. PLoS One.
10:e1369562015. View Article : Google Scholar
|
|
28
|
Yang X, Yang C, Wang L, Cao Z, Wang Y,
Cheng C, Zhao G and Zhao Y: Inhibition of basal cell carcinoma
cells by cold atmospheric plasma-activated solution and
differential gene expression analysis. Int J Oncol. 56:1262–1273.
2020.PubMed/NCBI
|
|
29
|
Glorieux C and Calderon PB: Catalase, a
remarkable enzyme: Targeting the oldest antioxidant enzyme to find
a new cancer treatment approach. Biol Chem. 398:1095–1108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahn HJ, Kim KI, Hoan NN, Kim CH, Moon E,
Choi KS, Yang SS and Lee JS: Targeting cancer cells with reactive
oxygen and nitrogen species generated by atmospheric-pressure air
plasma. PLoS One. 9:e861732014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Annesley SJ and Fisher PR: Mitochondria in
health and disease. Cells. 8:6802019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong L and Neuzil J: ‘Chapter
eight-mitochondria in cancer: Why mitochondria are a good target
for cancer therapy’ in Progress in Molecular Biology and
Translational Science. Osiewacz HD: (Academic Press); pp. 211–227.
2014
|
|
33
|
Vafai SB and Mootha VK: Mitochondrial
disorders as windows into an ancient organelle. Nature.
491:374–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rickard BP, Overchuk M, Chappell VA, Kemal
Ruhi M, Sinawang PD, Nguyen Hoang TT, Akin D, Demirci U, Franco W,
Fenton SE, et al: Methods to evaluate changes in mitochondrial
structure and function in cancer. Cancers (Basel). 15:25642023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu S, Zhou F, Zhang Z and Xing D:
Mitochondrial oxidative stress causes mitochondrial fragmentation
via differential modulation of mitochondrial fission-fusion
proteins. FEBS J. 278:941–954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Xia C, Guo Y, Yang C, Cheng C,
Zhao J, Yang X and Cao Z: Bactericidal efficacy of cold atmospheric
plasma treatment against multidrug-resistant Pseudomonas
aeruginosa. Future Microbiol. 15:115–125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Yang X, Yang C, Gao J, Zhao Y,
Cheng C, Zhao G and Liu S: The inhibition effect of cold
atmospheric plasma-activated media in cutaneous squamous carcinoma
cells. Future Oncol. 15:495–505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kuo LJ and Yang LX: Gamma-H2AX-a novel
biomarker for DNA double-strand breaks. In Vivo. 22:305–309.
2008.PubMed/NCBI
|
|
39
|
Vaquero J, Judée F, Vallette M, Decauchy
H, Arbelaiz A, Aoudjehane L, Scatton O, Gonzalez-Sanchez E,
Merabtene F, Augustin J, et al: Cold-atmospheric plasma induces
tumor cell death in preclinical in vivo and in vitro models of
human cholangiocarcinoma. Cancers (Basel). 12:12802020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka H, Nakamura K, Mizuno M, Ishikawa
K, Takeda K, Kajiyama H, Utsumi F, Kikkawa F and Hori M:
Non-thermal atmospheric pressure plasma activates lactate in
Ringer's solution for anti-tumor effects. Sci Rep. 6:362822016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nastasa V, Pasca AS, Malancus RN,
Bostanaru AC, Ailincai LI, Ursu EL, Vasiliu AL, Minea B, Hnatiuc E
and Mares M: Toxicity assessment of long-term exposure to
non-thermal plasma activated water in mice. Int J Mol Sci.
22:115342021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|