The Global Cancer Observatory reported that the top
seven types of cancer according to incidence rates in 2022 were
breast (23.8%), lung (9.4%), colorectal (8.9%) cervix uteri (6.9%),
thyroid (6.4%), corpus uteri (4.3%) and stomach (3.5%) cancer for
females. For males, the top seven by incidence were lung (15.2%),
prostate (14.2%), colorectal (10.4%), stomach (6.1%), liver (5.8%),
bladder (4.6%) and esophagus (3.5%) cancers. Similarly, the top
seven types of cancer according to mortality rates in 2022 were
breast (15.4%), lung (13.5%), colorectal (9.4%), cervix uteri
(8.1%), liver (5.5%), stomach (5.4%) and pancreas (5.1%) cancers
for females. For males the top seven by mortality rate were llung
(22.7%), liver (9.6%), colorectal (9.2%), stomach (7.9%), prostate
(7.3%), esophagus (5.9%) and pancreas (4.6%) cancer (1). These statistics demonstrate sex
differences in cancer incidence and mortality rates that can be
attributed to varying expression levels of hormone receptors and
associated genes involved in cancer occurrence and malignancy
mechanisms (2,3).
Estrogen, commonly recognized as a female hormone,
also significantly impacts males. Among the four types of estrogen,
estrone (E1), estradiol (E2), estriol and estetrol, E2 is the most
predominant in both males and premenopausal females. While E2
levels in males are lower compared with in premenopausal females,
they are comparable to or exceed those in postmenopausal females
(4,5). In males, E2 is primarily produced in
extragonadal tissues, particularly increasing in individuals with a
higher BMI (4–7). Estrogen inhibits bone resorption and
provides cardiovascular protection, essential for maintaining
health in premenopausal women. However, after menopause, ovarian
estrogen production ceases, diminishing these protective effects
and increasing the risk of developing diseases, such as
cardiovascular disease and osteoporosis (8–12).
Additionally, in postmenopausal women, estrogen is mainly produced
in peripheral tissues, heightening the risk of developing breast
cancer (13,14).
Androgens, commonly known as male hormones, notably
impact females as well. In males, androgens are secreted by the
testes and adrenal glands; whereas in females, androgens are
produced by the ovaries and adrenal glands (15). Androgens bind to androgen receptors
(AR) in various tissues, including the prostate, seminal vesicles
and skeletal muscle, to regulate physiological activities, sperm
production and cancer growth in males (16,17).
In females, precursor substances of androgen, such as
androstenedione, are more likely to convert to estrogen compared
with androgens and act on estrogen receptors (ER) (18). Excessive androgen secretion in
females can worsen endocrine disorders such as polycystic ovary
syndrome and congenital adrenal hyperplasia (19,20).
The expression and activation of sex hormone
receptors serve a significant role in the progression and
malignancy of breast and prostate cancer, influencing tumor growth,
metastasis and response to treatment (21). While a direct linear correlation
between cancer stage and hormone receptor expression is not
consistently observed (22,23), sex hormone receptor status serves a
key role in determining treatment options and prognosis at various
stages of cancer (24). In the
early stages of cancer (stage 1 or 2, according to the AJCC Cancer
Staging Manual), cancer cell growth is mainly promoted in a sex
hormone-dependent manner, so that it may be treated with hormone
therapy. However, in the advanced stages of cancer (stage 3 or 4),
hormone receptor expression levels often diminish or become less
predictive of treatment efficacy, requiring more aggressive
therapies, such as chemotherapy or targeted therapy (25,26).
ER-low or ER-negative (−) patients with breast cancer have a higher
recurrence rate and show distinct clinicopathological findings
compared with ER-high patients. The 5-year recurrence rates are
5.1% for ER-high, 7.4% for ER-low and 9.7% for ER-negative
patients, with ER-negative cases showing significantly worse
outcomes (P<0.001). ER-high patients typically have lower tumor
grades, lower Ki-67 proliferation indices, and are associated with
the luminal A subtype, which responds well to hormone therapy. By
contrast, ER-negative patients present with higher tumor grades,
significantly elevated Ki-67 indices, and a higher prevalence of
triple-negative breast cancer, often leading to a poorer prognosis
(24). Furthermore, hormone
receptor-positive types of cancer, such as luminal A and B, respond
well to hormone therapies, while HER2-positive and triple-negative
breast cancer (TNBC) subtypes often require more aggressive
treatments, such as targeted therapy or chemotherapy (27–29).
Among these subtypes, both luminal subtypes typically show positive
ER and progesterone receptor (PR) expression, while luminal B
breast cancer generally displays increased HER2 expression levels
compared with luminal A. Luminal B breast cancer is also associated
with higher proliferation rates (e.g., Ki-67 index), increased HER2
expression and a poor prognosis, indicating more aggressive
clinical characteristics (27–30).
Prostate cancer is similarly categorized by its
dependence on AR signaling, with advanced cases evolving into
castration-resistant prostate cancer (CRPC), which requires more
intensive treatments. Hormone-sensitive prostate cancer, which
typically describes most early-stage cases, is commonly treated
with androgen deprivation therapy (ADT) (31). However, CRPC requires more
aggressive treatments, such as second-line hormonal therapies, such
as enzalutamide or chemotherapy (32). Thus, the expression levels of
hormone receptors in the early stages of cancer are a major factor
in treatment decisions. However, in advanced stages, their
predictive value for treatment decreases due to reduced dependence
on hormones.
Recent studies have reported that AR and ER
signaling pathways are closely associated, particularly in breast
cancer, where AR can either suppress or enhance estrogen receptor α
(ERα) activity depending on the context (33,34).
In ER-positive (+) breast cancer, ARs often act as a tumor
suppressor by inhibiting ERα-driven tumorigenesis, with AR
activation showing significant anti-tumor effects, even in cases of
resistance to ER and CDK4/6 inhibitors (35). AR redistributes ER and its
co-activators on chromatin, suppressing ER-regulated genes and
upregulating AR target genes, which correlates with improved
survival in patients with ER(+) breast cancer (36). However, AR can also promote
oncogenic functions in the context of androgen excess, where
androgens act as precursors to estrogen (37). This leads to overstimulation of
estrogen-regulated gene expression, driving tumor proliferation and
progression in ER-positive breast cancer (34). In CRPC, estrogen receptor β (ERβ)
activation reduces AR expression, inducing apoptosis and acting as
a tumor suppressor. These findings highlight the key role of AR and
ER interactions in cancer progression and present opportunities for
targeted therapies (38).
While cancer research spans numerous fields of
research, studies focusing on sex-specific differences are
currently limited. Addressing these differences may lead to
significant breakthroughs in cancer prevention and treatment.
Specifically, hormones can act on their receptors to influence the
expression of hormone-related transcription factors, which in turn
can affect the expression of oncogenes or tumor suppressors
(39). Understanding how sex
hormone receptors interact with common transcription factors in
different types of cancer may serve to identify novel therapeutic
targets, which could aid in the development of personalized
treatment strategies and thereby maximize the efficacy of cancer
therapies.
ERα is a pivotal molecule in the development and
progression of various types of cancer. In breast cancer, ERα is
associated with tumor progression and is upregulated in ~75% of
breast cancer tissues, in contrast to ~10% in healthy tissues
(50). ERα is more prevalent in the
luminal A type, compared with the basal type of breast cancer. ERα
interacts with estrogen to promote tumor growth (51). Given these properties, anti-hormone
therapies that target ERα, such as aromatase inhibitors, tamoxifen
and fulvestrant, have proven to be effective in breast cancer
(52,53).
Aromatase inhibitors reduce estrogen levels by
inhibiting the enzyme aromatase, which is responsible for
converting the androgen hormone androstenedione and androstenediol
into E1 and E2, respectively. As a result, these inhibitors are
used in the treatment of ER(+) breast cancer to lower estrogen
levels, thus suppressing cell proliferation and invasion (54). By contrast, tamoxifen is an
anti-estrogen drug that blocks the activity of estrogen in breast
cancer. Tamoxifen directly binds to ERα, which prevents estrogen
from exerting its effects, thereby inhibiting cell proliferation
and tumor growth (55). The
clinical study by Arpino et al (56) on patients with breast cancer
demonstrated that the ER(+)/PR(−) breast cancer group is less
sensitive to tamoxifen, which targets ER, compared with the
ER(+)/PR(−) breast cancer group. This is because tamoxifen inhibits
the estrogen effect, which influences the expression of the PR gene
(57). As a result, PR(−) patients
experience reduced efficacy from tamoxifen treatment. Moreover,
these patients tend to exhibit increased expression levels of other
receptors, such as HER-1 and HER-2, which contributes to more
aggressive tumor characteristics, including therapy resistance,
faster proliferation and a higher probability of metastasis.
Specifically, HER-2 positive tumors are known to exhibit resistance
to tamoxifen therapy, while HER-1 expression is predominantly
observed in ER-negative tumors, which are associated with poor
prognosis (58). Therefore,
tamoxifen and fulvestrant are specifically used in ER(+) breast
cancer, regardless of PR status, but their efficacy may vary
depending on the presence or absence of PR. Additionally, ERα is
comprised of 595 amino acids with a molecular weight of 66 kDa, and
alternative splicing results in several isoforms such as ERα46 and
ERα36, with the ERα46 isoform acting as a competitive inhibitor
when co-expressed with ERα66 (59–61).
In prostate cancer, ERα promotes cell proliferation
and inhibits apoptosis, thereby facilitating tumor growth (62). Notably, ERα expressed in stromal
tissue has been shown to stimulate the growth of prostatic
epithelium through growth factors (63). An in vivo study has
demonstrated that knocking down ERα suppresses tumor growth
(64), and research indicates that
patients with high ERα expression levels have poor prognoses
(65). In the majority of prostate
cancer subtypes, ERα activation is associated with tumorigenesis
and cancer progression (66–68).
ERα typically promotes cancer cell proliferation by activating
pathways such as IL-6 signaling, which supports cell survival and
resistance to ADT. This is particularly relevant in CRPC, where
androgen-independent mechanisms serve a pivotal role in sustaining
tumor growth (66,67). In aggressive prostate cancer
subtypes, including neuroendocrine prostate cancer and CRPC,
elevated ERα levels contribute to increased malignancy and
facilitate cancer cell survival and invasiveness, often through
interactions with AR-mediated pathways (68).
Non-small cell lung cancer (NSCLC), the most common
type of lung cancer, is characterized by ERα promoting tumor
progression by enhancing macrophage infiltration, which alters the
tumor microenvironment to favor cancer growth and increases cell
invasion (73). Clinical data also
demonstrates that within the same elderly cancer patient group,
women have a higher survival rate compared with men. Lung
adenocarcinoma, a common subtype of NSCLC, commonly occurs in
non-smokers, with a higher incidence rate in women (19.6%) compared
with men (11.8%) (74).
Premenopausal women with lung adenocarcinoma had a median survival
of 643 days compared with 735 days for postmenopausal women
(P=0.01). Additionally, premenopausal women presented with more
advanced stages of the disease, with 66% in stage IV compared with
53% in postmenopausal women. This highlights the significant impact
of menopausal status on disease progression and survival outcomes.
A study by Hsu et al (75)
indicated that E2 stimulates cancer cell migration, while ER
antagonists such as tamoxifen, targeting the estrogen signaling
pathway, inhibit lung cancer cell growth. Another study reported
that women >60 years have a survival advantage compared with
younger women, though this age effect is not observed in men
(76).
Meanwhile, research on gastric cancer suggests that
ERα may have a dual role. A study showed that transfection-induced
overexpression of ERα decreases β-catenin expression, thus
inhibiting cell proliferation and invasion (77). Additionally, ERα and ERβ mRNA levels
in tumors, compared with normal tissues, have been associated with
increased metastatic potential in gastric cancer (78). By contrast, it has also been
suggested that knockdown of ERα inhibits the proliferation,
migration and invasion of gastric cancer cells by regulating the
expression of factors such as p53 and CDK inhibitor 1A (CDKN1A),
associated with poor prognosis in patients (79).
Colon cancer is significantly influenced by estrogen
and exhibits varying effects depending on which estrogen receptor
it interacts with. ERα promotes the development and proliferation
of colon cancer cells (80,81). The type of ERs interacting with
estrogen varies with colon cancer stage, with ERα predominantly
driving tumor progression in late stages. Conversely, the isoform
ERα36 shows lower expression in tumor tissue compared with healthy
colorectal tissue and decreases with advancing Dukes' stage
(A+B>C+D, P=0.017) and lymph node metastasis stage (N0>N1/N2,
P=0.049), suggesting a function opposite to full-length ERα66
(82).
In hepatocellular carcinoma (HCC), the most common
type of liver cancer, ERα is expressed at lower levels compared
with adjacent normal tissue, and its promoter is hypermethylated
(83). Hou et al (84) reported that ERα acts as a tumor
suppressor by upregulating the expression of tyrosine phosphatase
receptor type O, which promotes apoptosis and inhibits cell
proliferation. However, in HCV-related HCC, ERα mRNA and protein
expression levels are elevated, and increased ERα expression is
associated with increased levels of inflammatory and oncogenic
genes, such as NF-κB and cyclin D1, suggesting a role in promoting
liver cancer progression (85).
In breast cancer, ERβ generally exhibits lower
expression levels and has a weak negative correlation with ERα
(Spearman R=−0.18, P=2.2×10−16) (91). ERβ is more abundantly expressed in
basal-like or normal-like breast cancer subtypes (91). In ERα(+) breast cancer, ERβ
suppresses ERα and thus inhibits tumor growth. Conversely, ERβ can
act as a carcinogen in ERα(−) breast cancer (89). Moreover, ERβ interacts with various
signaling pathway molecules including AR, p53, E-cadherin, cell
cycle arrest molecules, phosphatase and PTEN, PI3K and AKT, all of
which contribute to either inhibiting or promoting cancer growth
(92). Notably, stable expression
of ERβ in the ERα(+) cell line MCF7 results in decreased cell
proliferation. Of the 921 differentially expressed genes after E2
treatment in ERβ(+) compared with ERβ(−) breast cancer cells, 424
had ERβ binding sites within 10 kb. These target genes are crucial
in regulating cell proliferation, death, differentiation, motility,
adhesion, signal transduction and transcription (93).
In the context of ovarian cancer, it has been
hypothesized that ERβ serves as a tumor suppressor. Indeed, studies
have demonstrated that in epithelial ovarian cancer (EOC),
comprising 90% of ovarian cancer, the expression of ERβ is
diminished in tumor tissues compared with normal tissues (102). Overexpression of ERβ has been
observed to suppress the expression and activity of ERα, and to
decrease levels of pAKT, cyclin D1 and cyclin A2. An in vivo
study employing orthotopic xenograft mouse models showed that
overexpression of ERβ curbs tumor growth (103). In EOC, ERβ interacts with an
indole derivative
(3-{[2-chloro-1-(4-chlorobenzyl)-5-methoxy-6-methyl-1H-indol-3-yl]methylene}-5-hydroxy-6-methyl-1,3-dihydro-2H-indol-2-one)
to inhibit ovarian cancer cell proliferation (104).
Lung cancer studies, particularly in NSCLC, suggest
that ERβ facilitates tumor progression and adversely affects
patient prognosis (95,105). ERβ expression positively
correlates with tumor size, lymph node metastasis, clinical stage
and differentiation. Silencing ERβ in vitro reduces cell
invasion and colony formation (105). Overexpression of ERβ in in
vivo mouse models has been shown to accelerate tumor
progression via the ERβ/circ-TMX4/miR-622/CXCR4 signaling pathway
(106).
In gastric cancer, ERβ operates as a tumor
suppressor and manifests at reduced levels in gastric cancer
tissues compared with normal gastric mucosa (107,108). Knockdown of ERβ in gastric cancer
cell lines AGS and MKN45 activates growth arrest and DNA damage
inducible α, leading to increased apoptosis and autophagy through
inhibition of the MAPK pathway. Furthermore, ERβ knockdown results
in fewer colonies formed (109).
Clinical studies have reported a negative correlation between ERβ
expression levels, tumor grade and Lauren type in gastric cancer
(110).
In liver cancer, ERβ also acts as a tumor
suppressor, notably in HCC, where ERβ interacts with E2 to exert
anti-proliferative and anti-inflammatory effects (116). It has been reported that ERβ
induces the expression of suppressor of cytokine signaling 1 and
inhibits the JAK1-STAT6 pathway, preventing the polarization of
tumor-associated macrophages to the M2 phenotype, thereby
inhibiting HCC growth (117).
Intrahepatic cholangiocarcinoma treatment with the ERβ antagonist
KB9520 has shown to increase apoptosis and reduce cell
proliferation in vitro using HuH-28 cells and in vivo
in a thioacetamide-induced experimental model of intrahepatic
cholangiocarcinoma (118). These
findings collectively affirm that ERβ functions as a tumor
suppressor in liver cancer.
AR was discovered in the 1960s, and since then, its
structural functions and mechanisms have been extensively studied
(119–122). AR consists of several domains:
NTD, DBD, hinge region, LBD and CTD. Similar to ERβ, AF-1 is
located in the NTD and AF-2 is in the LBD. The hinge region,
positioned between the DBD and LBD, contains the nuclear
localization signal, facilitating the translocation of AR from the
cytoplasm to the nucleus (120–122). Although the functions of AR are
well documented in prostate cancer, its roles in other cancer types
are less clearly understood.
In prostate cancer, AR promotes tumor progression. A
previous study reported that IL-6 activates the NTD of AR in LNCaP
cells, enhancing cell proliferation via MAPK and signal transducer
and activator of STAT3 signaling pathways (123). Consequently, primary cancer
therapy for prostate cancer frequently utilizes ADT, which aims to
inhibit androgens or block their binding to AR, thus suppressing
tumor growth (124). However, as
the cancer advances, it may evolve into CRPC, which resists both
ADT and drugs such as abiraterone and enzalutamide (125–127). Due to this resistance, extensive
research on ADT therapy continues to explore mechanisms of
resistance and develop novel therapeutic strategies (128,129). Shiota et al (130) investigated organelles generating
reactive oxygen species (ROS) following AR inhibition and reported
that ROS are primarily induced in peroxisomes through peroxisome
proliferator-activated receptor α (PPARα) activation. Additionally,
inhibiting PPARα reduced cell proliferation and restored
sensitivity to enzalutamide. Inhibition of enhancer of zeste
homolog 2 (EZH2) or achaete-scute homolog 1 (ASCL1) have been shown
to re-sensitize prostate cancer cells to enzalutamide. EZH2, a
component of the polycomb repressive complex 2, functions as a
histone methyltransferase. In CRPC, EZH2 can promote AR signaling
independent of its histone modification role, even in the absence
of androgens (100,131). ASCL1, a transcription factor
involved in neuroendocrine differentiation, is linked to resistance
against AR-targeted therapies, including enzalutamide, due to its
role in promoting neuroendocrine-like characteristics in prostate
cancer. Inhibiting either EZH2 or ASCL1 shifts the cancer cells
back to a phenotype more reliant on AR signaling, thereby restoring
sensitivity to enzalutamide (66).
In breast cancer, multiple studies have investigated
the antitumor activity of AR, highlighting its potential to
suppress estrogen-regulated tumorigenesis and improve clinical
outcomes, particularly in ER(+) breast cancer (36,132–135). AR activation displaces ER and key
co-activators such as p300 and SRC-3 from chromatin, leading to the
downregulation of ER-regulated genes and the upregulation of tumor
suppressor genes and AR target genes. This antitumor activity
remains effective even in ER(+) breast cancer resistant to CDK4/6
inhibitors (36,132). Conversely, in ER(−) breast cancer,
AR promotes tumor progression. Treatment with the AR agonist
dihydrotestosterone (DHT) has been shown to increase cell
proliferation, migration, invasion and metastasis, as confirmed
using in vivo mouse models (133). Additionally, AR is activated by
various signaling pathways, including the PI3K, MAPK and mTOR
pathways, further contributing to tumor progression (134,135).
Whether ARs act as a tumor suppressor or an oncogene
in lung cancer remains unresolved. Liu et al (142) reported that ARs impede cell
invasion in NSCLC and diminishes the expression of the oncogene
tumor protein D52 via the circular-SLCO1B7/microRNA (miR)-139-5p
axis, thereby impeding tumor progression. Additionally, miR-224-5p,
which hampers apoptosis and accelerates tumor growth, directly
targets ARs and patients with NSCLC with high AR expression levels
have a significantly longer overall survival rate [hazard ratio
(HR)=0.5, log rank P=8.9×10−16] (143). By contrast, Li et al
(144) demonstrated that treating
the NSCLC cell line A549 with luteolin suppressed AR expression and
subsequently reduced cell proliferation. Additionally, Recchia
et al (145) reported that
the interaction between AR and the EGFR-enhanced A549 cell
proliferation via the mTOR/CD1 pathway. Thus, further research into
the detailed mechanism of AR is essential.
In regards to gastric cancer, similar to lung
cancer, the precise role of AR is not well defined, but the
majority of studies suggest that AR promotes tumor progression. Liu
et al (146) reported that
AR upregulates the oncogenic miR-125b in gastric cancer, which
inhibits apoptosis and promotes proliferation. Conversely,
treatment with the AR antagonist bicalutamide induces apoptosis and
inhibits proliferation. Furthermore, Xia et al (147) found that the AR splice variant
AR-v12 is more highly expressed in tumor tissues compared with
normal tissues and upregulates myosin light chain kinase, enhancing
cell migration and invasion. Soleymani Fard et al (148) reported that >50% of the 60
patients with gastric cancer exhibited upregulated ARs, which were
significantly associated with the upregulation of EMT-related
genes, including Snail, β-catenin, Twist1 and STAT3. AR
upregulation is associated with poor survival outcomes (HR=3.478,
P=0.001), and treatment with enzalutamide has been found to inhibit
tumor progression.
The role of AR in colon cancer remains unclear.
Studies suggest that the activation of membrane-associated AR
inhibits the PI3K/AKT pathway, induces apoptosis and subsequently
suppresses tumor growth in colorectal cancer (149,150). Conversely, Rodríguez-Santiago
et al (151) reported that
ARs promote tumor progression, noting that their upregulation in
tumor tissues is associated with increased tumor size,
differentiation and metastasis. AR activation not only diminishes
antitumor immune activity but also increases the secretion of
tumor-promoting factors from the nervous system, thereby
facilitating tumor growth.
In liver cancer, similarly to colon cancer, the role
of AR is not well-defined. Acosta-Lopez et al (152) observed increased expression levels
of AR in tumor tissues compared with normal tissues in HCC,
associating increased AR activity with poorer prognosis in advanced
HCC. Furthermore, Ren et al (153) suggested that mTORC1 phosphorylates
AR at serine residue 96, which promotes tumor progression.
Meanwhile, Ren et al (154)
reported that treatment with DHT escalates cell proliferation,
invasion and migration in the HepG2 cell line, while Ouyang et
al (155) determined that AR
inhibits cell migration and invasion in HCC cell lines HA22T and
SK-HEP-1 via the miR-325/ACP5 signaling pathway.
The present review investigated the effects of three
hormone receptors across seven major types of cancer. The impact of
sex hormone receptors on each type of cancer is summarized in
Table I. Studies on the influence
of these receptors on tumor progression have advanced considerably
in hormone-responsive organs, although their effects in
non-responsive organs remain less understood.
The sex hormones examined in the present study
activate mechanisms of cancer malignancy or suppression through
their respective receptors. In this process, various transcription
factors are known to regulate the expression of key molecules
involved in these mechanisms via sex hormone signaling. Prominent
transcription factors include specificity protein 1 (SP1),
ETS-related gene (ERG), β-catenin, activator protein 1 (AP-1),
c-Myc, NF-κB and STAT3. Table II
provides a summary of how these key transcription factors influence
cancer progression through their interactions with sex hormone
receptors.
SP1 is a transcription factor that binds to specific
promoter regions containing GC-rich sequences and serves a key role
in activating the expression of various genes. SP1 is known to
function as an oncogene through the three sex hormone receptors
examined in this study. In breast cancer, the ER/SP1 complex binds
to DNA, promoting the expression of estrogen-induced genes such as
c-Myc, creatine kinase B-type (CKB), cathepsin D, retinoic acid
receptor α (RARα) and heat shock protein 27 (Hsp27), thereby
facilitating tumor progression (156). In ovarian cancer, estrogen
stimulates the expression of genes related to angiogenesis in the
endometrium and endothelial cells through the SP1/ERβ complex, with
this abnormal angiogenesis promoting tumor growth and invasion
(157). Furthermore, in prostate
cancer, the AR/SP1 complex binds to the VEGF core promoter in
chromatin, and androgen increases VEGF expression via the SP1
binding site, driving angiogenesis and tumor progression (158).
ERG is a key transcription factor belonging to the
ETS family that serves a key role in various biological processes
such as angiogenesis, cell differentiation, migration and
metastasis (159,160). In prostate cancer, ERG is notably
upregulated due to gene fusion with transmembrane serine protease 2
(TMPRSS2), and this upregulation has been associated with
aggressive prostate cancer (159).
Setlur et al (159)
demonstrated that TMPRSS2-ERG expression increased following ERα
agonist treatment, which also led to increased prostate cancer cell
viability. Conversely, ERβ agonist treatment resulted in a decrease
in both TMPRSS2-ERG expression and cancer cell viability,
indicating that the impact on cancer progression varies depending
on whether ERα or ERβ is activated by estrogen stimulation.
Moreover, an in vitro study by Kohvakka et al
(160) demonstrated that the
abnormal expression of prostate cancer-specific long non-coding
RNAs (lncRNAs) further promotes tumor development and
progression.
β-catenin, a key molecule in the Wnt signaling
pathway, exerts varying effects on cancer malignancy depending on
the type of sex hormone receptor involved. Experimental
overexpression of ERα via vector-based transfection inhibits
β-catenin, thereby suppressing the growth, proliferation and
invasion of gastric cancer cells, halting their entry into the
G1/G0 phase and promoting apoptosis (77). Meanwhile, in prostate cancer,
androgen interacts with AR to promote tumor progression, whereas
estrogen stimulates cell proliferation specifically in
androgen-responsive prostate cancer (63,126).
Increased expression levels of β-catenin via ERβ increases the
incorporation of [methyl-3H]thymidine and upregulates cyclin D2
expression, promoting cell cycle progression (161). Furthermore, β-catenin stimulates
AR transcriptional activity through transcriptional intermediary
factor 2 and glucocorticoid receptor-interacting protein 1, thereby
activating AR signaling. This activation increases androgen
affinity, reduces the efficacy of anti-androgen therapies and
accelerates tumor progression in prostate cancer (162).
The AP-1 family of transcription factors, including
c-Fos and c-Jun, increases cell proliferation in breast cancer via
E2-ERα signaling. However, through ERβ signaling, AP-1-mediated
transcription is suppressed by the recruitment of the
transcriptional repressor C-terminal binding protein, which
counteracts the proliferation driven by ERα (87,163).
In prostate cancer, AR not only mediates androgen-induced cancer
progression but also interacts with AP-1 to form a complex, wherein
they mutually inhibit each other's binding affinity to DNA-binding
sites (164,165).
Estrogen stimulation enhances the interaction
between c-Myc and ERα, with both binding closely to the VEGF
promoter. A study by Dadiani et al (166) demonstrated that estrogen activates
c-Myc expression via ERα in ERα(+) breast cancer cells, promoting
cell growth and proliferation while inhibiting differentiation.
Additionally, estrogen transiently induces the transcription of
VEGF, a key factor in angiogenesis, thereby facilitating cell
migration (166). By contrast, ERβ
signaling suppresses c-Myc transcription, modulating the expression
levels of proliferation-related genes. For instance, ERβ increases
the production of antiproliferative genes such as p21 and p27,
leading to G1 or G2 cell cycle arrest and
inhibiting the proliferation of breast and colorectal cancer cells
(112,167). Moreover, c-Myc is a major target
gene of AR signaling, with AR enhancing the transcription and
expression levels of c-Myc, thereby promoting prostate cancer cell
growth and progression. Consequently, c-Myc upregulation is
associated with the development and progression of prostate cancer
(168).
NF-κB is known to mutually inhibit ERα, yet when
co-activated, NF-κB modifies ERα function, leading to endocrine
resistance and promoting breast cancer metastasis and recurrence,
making ER(+) tumors more aggressive (169). In prostate cancer, the effects of
NF-κB vary depending on the receptor involved. Estrogen-activated
ERβ mediates the proteasomal degradation of HIF-1α, which
suppresses NF-κB activation, thereby reducing inflammation and
potentially inhibiting the development of malignant tumors
(170). Conversely, Zhang et
al (171) reported that NF-κB
expression activates AR promoter transcription, increasing AR
expression levels and cell proliferation while inhibiting
apoptosis. This ultimately promotes metastasis and angiogenesis,
thereby accelerating tumor progression.
STAT3 functions as a key transcription factor
involved in various cancer progression pathways, including cellular
transformation, proliferation, survival and angiogenesis, often
through its interaction with sex hormone receptors (172–174). In breast cancer cells, leptin
signaling increases ERα expression, which in turn enhances STAT3
activity, improving ERα-dependent cell viability and promoting
tumor progression (172–174). In lung cancer cells, STAT3
activation upregulates ERβ signaling, leading to increased cell
proliferation (173). In prostate
cancer, AR directly interacts with STAT3, enhancing its activity.
Yamamoto et al (174),
reported that AR activation neutralizes the inhibitory effects of
the STAT3 protein inhibitor PIAS3, thus protecting STAT3 from
inhibition. Since STAT3 is an oncogene that mediates cellular
transformation and promotes prostate cancer, its interaction with
AR further accelerates tumor progression.
To identify sex-specific key transcription factors
involved in hormone receptor-driven cancer progression across seven
major types of cancer (breast, prostate, ovarian, colon, lung,
liver and gastric cancer), a comprehensive data analysis approach
using the SignaLink 3.0 database (http://signalink.org) was employed. The SignaLink
database integrates experimentally validated and curator-inferred
protein-protein interactions (PPIs) and regulatory mechanisms from
multiple sources, focusing on homo sapiens to ensure
human-specific relevance. The dataset includes curated data from
OmniPath (https://www.omnipathdb.org),
BioGRID (https://thebiogrid.org), Reactome
(https://reactome.org) and ComPPI (https://comppi.linkgroup.hu/) (175).
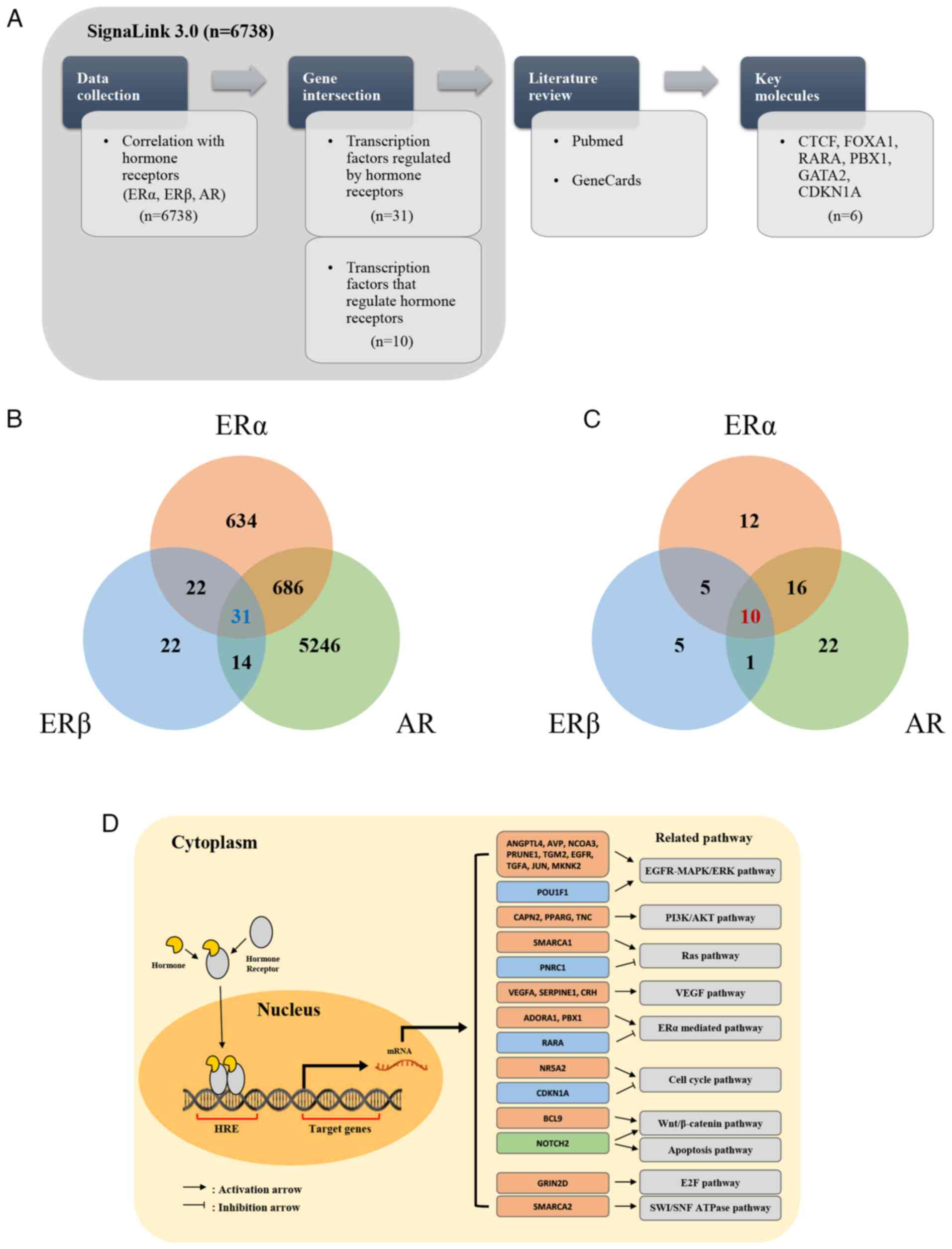
The initial analysis of SignaLink 3.0 identified
6,738 transcription factors associated with ERα, ERβ and AR. This
set was filtered to focus on transcription factors that either
regulate or are regulated by all three hormone receptors (ERα, ERβ
and AR). Through this process, 31 transcription factors were
identified that are regulated by all three hormone receptors and 10
transcription factors that regulate these receptors (Fig. 1; Table
III) (176–219). Notably, there was no overlap
between the two groups.
To further refine the selection, a text mining
approach was conducted by combining data from GeneCards (https://www.genecards.org/) and PubMed (https://pubmed.ncbi.nlm.nih.gov/). GeneCards is
a comprehensive database that provides detailed information on
human genes, including their functions, interactions and
involvement in diseases (220).
GeneCards was used to explore the biological roles of each
transcription factor and utilized PubMed to analyze how they
interact with hormone receptors. The selection criteria focused on
transcription factors that, as evidenced in the literature, both
regulate and are regulated by at least two of the three hormone
receptors and have demonstrated significant roles in cancer
progression. Through this process, studies were identified that
demonstrated the critical roles of these transcription factors in
modulating hormonal signaling pathways, which are implicated in
sex-specific tumor biology. These interactions were further
analyzed to assess their impact on tumor progression, with a focus
on understanding the mechanisms by which these transcription
factors regulate hormone receptor activity across various types of
cancer.
These transcription factors are primarily known to
influence hormone-related cancer progression through various
pathways, including the PI3K/AKT/mTOR signaling pathway and the
Hippo-YAP/TAZ pathway. PI3K/AKT/mTOR signaling is a common pathway
modulated by multiple transcription factors such as PPARG and AR,
leading to enhanced tumor growth and survival, especially in
prostate cancer (191,207,208). SMARCA4 and the Hippo-YAP/TAZ
signaling pathway are implicated in lung cancer, where they promote
tumorigenicity by regulating gene transcription involved in cell
proliferation and metastasis (213). Consequently, six key transcription
factors were identified: CCCTC-binding factor (CTCF), forkhead box
A1 (FOXA1), retinoic acid receptor α (RARA), PBX homeobox 1 (PBX1),
GATA binding protein 2 (GATA2) and CDKN1A, as candidate
hormone-related transcription factors. It was then investigated how
interactions between these transcription factors and hormone
receptors affect tumor progression, summarizing the pathways
influenced by these interactions (Fig.
2).
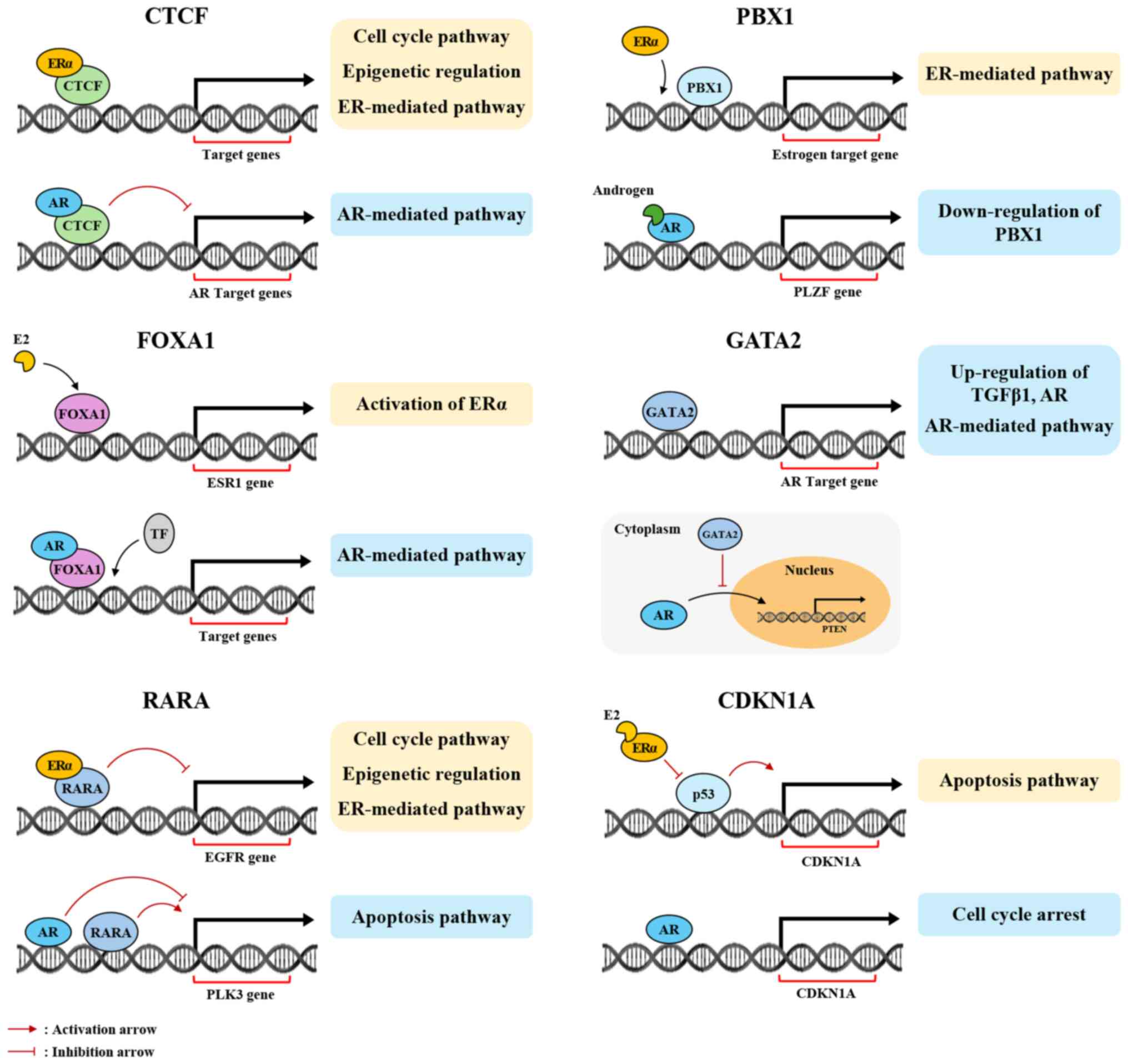
FOXA1 has been extensively researched in breast
cancer and is known to interact with ERα, regulating estrogen
responses and transcription activity and activating oncogene
expression (223–226). Additionally, FOXA1 has a positive
correlation with AR (r=0.8975, P<0.001) (227,228). Tsirigoti et al (229) reported that FOXA1 regulates AR
expression levels in TNBC and is inversely correlated with Snail
Family Transcriptional Repressor 1 (SNAI1) (Spearman's R=−0.377,
P<2.2×10−16), and suggested that in SNAI1-knockout
TNBC, FOXA1 induces AR expression, fostering basal-luminal
plasticity. In prostate cancer, FOXA1 is positively correlated with
ERβ (epithelial, ρ=0.41, P<0.001; stromal, ρ=0.354, P<0.001),
and FOXA1 knockdown via siRNA inhibits cell proliferation and
migration in LNCaP and PC-3 cell lines (230). FOXA1 is also implicated in
promoting tumor progression in prostate cancer and HCC through
AR-mediated signaling (231,232).
RARA has gained substantial attention in breast
cancer. It binds ERα to mediate transcription of ERα target genes
(233). Salvatori et al
(234) reported that, once
activated by retinoic acid, RARA suppresses EGFR expression, while
ERα, activated by E2, enhances EGFR expression. Nevertheless, in
the absence of ligands, ERα interacts with RARA to augment its
ability to suppress EGFR expression, functioning as a tumor
suppressor. RARA also participates in the AR-related transcription
network in prostate cancer, inversely regulating the expression of
target genes such as polo-like kinase 3 (PLK3). Specifically, RARA
increases PLK3 expression, while AR reduces it, contributing to
tumor progression (235). Notably,
in the HepG2 HCC cell line, ERβ does not interact with the RARA
promoter in the presence of estrogen, but upon 4-hydroxytamoxifen
(4-OHT) treatment, ERβ activates transcription of RARA (236).
PBX1 is predominantly studied in breast cancer,
specifically for its interaction with ERα compared with other
hormone receptors. PBX1 serves as both a transcription and pioneer
factor in the estrogen signaling pathway, binding to chromatin
before ERα to enhance its accessibility and elevate the expression
of estrogen-responsive genes linked to aggressive tumor behavior.
This mechanism supports PBX1 as a poor prognostic biomarker for
breast cancer (237,238). Although direct interactions
between PBX1 and AR are not documented, evidence suggests an
indirect regulatory pathway. Kikugawa et al (239) demonstrated that promyelocytic
leukemia zinc finger (PLZF), an AR-regulated tumor suppressor gene,
inhibits PBX1 expression. Thus, when androgen interacts with AR,
PLZF expression increases, which subsequently suppresses PBX1
expression and inhibits tumor progression.
GATA2 has been extensively studied in relation to
AR, particularly in prostate cancer, where it significantly affects
AR. GATA2 activates AR and the AR signaling pathway, which promotes
tumor progression. GATA2 also enhances the expression of TGFβ1,
further driving tumor progression through interaction with the AR
signaling pathway (240–242). In breast cancer, GATA2 inhibits AR
translocation from the cytoplasm to the nucleus, thereby
suppressing the expression of the tumor suppressor PTEN (243). Treeck et al (244) showed that in the ovarian cancer
cell line HEC-1A, a threefold increase in GATA2 expression occurred
following ERβ knockdown. Additionally, GATA2 closely interacts with
TP53.
CDKN1A, also known as p21, is a key transcription
factor in various types of cancer. CDKN1A acts as a tumor
suppressor in breast cancer. The presence of estrogen leads to ERα
inhibiting p53 transcriptional activity, which reduces CDKN1A
expression and promotes tumor progression (245,246). Conversely, treatment with
tamoxifen or ERα inhibitors elevates CDKN1A expression and
decreases cell proliferation (245–247). ERβ also regulates CDKN1A
indirectly; in breast cancer, ERβ suppresses CDKN1A expression in
the presence of wild-type p53, but increases CDKN1A expression
levels in cases with mutant p53, as demonstrated in vitro
(248). The effects of ERβ have
been explored in ovarian cancer; He et al (249) concluded that LY500307, an ERβ
agonist, increases CDKN1A levels and apoptosis in ovarian cancer
stem cells. Kim et al (201) showed that the interplay between AR
and CDKN1A in prostate cancer demonstrates AR inhibiting cyclin
D1/2 and CDK4/6 transcription while increasing CDKN1A
transcription, leading to cell cycle arrest and reduced cell
proliferation.
Studies on the interaction between hormone
receptors and their transcription factors in cancer are limited and
mainly focused on breast cancer. This focus is due to the high
hormone dependency of breast cancer, with the majority of cases
expressing hormone receptors, especially estrogen receptors,
crucial for cancer cell growth and progression (250). Prostate cancer, also sensitive to
hormones, shows significant influence from androgen receptors in
its development and progression (251,252). By contrast, cancers such as
gastric and lung cancer are less dependent on hormone signaling,
resulting in fewer studies and less evidence on the impact of
hormone receptor interactions. The established role of hormone
therapy in treating breast and prostate cancer further stimulates
research in these areas, while the absence of similar therapeutic
approaches in other types of cancer restricts research on hormone
receptor interactions.
In hormone-dependent types of cancer such as breast
and prostate cancer, the interplay between AR, ER and other hormone
receptors serves a key role in tumor progression and therapy
resistance. In ER(+) breast cancer, when ER signaling is inhibited,
AR can compensate by becoming more active, potentially driving
tumor progression or resistance to treatment (253). Similarly, ER may assume a more
prominent role when AR activity is diminished. This compensatory
relationship also extends to other types of cancer such as prostate
cancer, where AR is the main driver of tumor growth, but ER can
contribute to cancer progression under certain conditions (254). The compensatory dynamics between
AR and ER underscore the need for therapies that target both
receptors simultaneously to prevent one from compensating for the
inhibition of the other (255).
The interactions between these hormone receptors are important in
understanding cancer malignancy and developing more effective,
comprehensive therapeutic strategies.
The present study underscores the roles of
sex-specific hormone receptors ERα, ERβ and AR across seven types
of pan-cancer, highlighting their interactions with key
transcription factors such as CTCF, FOXA1, RARA, PBX1, GATA2 and
CDKN1A, and their impact on tumor progression. In conclusion, sex
hormone receptors can either function as oncogenes or tumor
suppressors depending on the type of cancer, and may exhibit both
roles within a single tumor. Moreover, key transcription factors
that interact with these hormone receptors serve crucial roles in
regulating cancer prognosis and tumor progression. In certain types
of cancer closely associated with sex hormones, such as breast and
prostate cancer, hormone receptors significantly influence cancer
prognosis and progression. Utilizing these sex-specific
characteristics in cancer treatments enables precision medicine
tailored to the unique characteristics of each patient, and
transcription factors that interact with sex hormone receptors in
pan-cancer may serve as novel anticancer therapeutic targets. To
advance therapeutic strategies, further in-depth studies are
essential in several areas: The molecular mechanisms that underlie
the dual roles of sex hormone receptors as oncogenes and tumor
suppressors, the specific interactions between hormone receptors
and transcription factors in various types of cancer, and the
development of targeted therapies that exploit these interactions.
Additionally, further research is required to explore the
sex-specific differences in cancer biology and their implications
for treatment, as well as the potential for personalized medicine
approaches based on hormone receptor status and transcription
factor profiles.
Not applicable.
The present study was supported by the National Research
Foundation of Korea (grant nos. 2022R1F1A1076029 and
2022R1A2C1093335).
Not applicable.
Conceptualization was conducted by JK and SYK.
Formal analysis and data interpretation were conducted by JK, HB,
CS, EK and SYK. Literature analysis was conducted by JK, HB and CS.
Writing of the original draft was conducted by JK, HB, CS and SYK.
Reviewing and editing of the manuscript was conducted by JK, HB,
CS, EK and SYK. Visualization was conducted by JK and SYK.
Supervision was conducted by JK, EK and SYK. Project administration
was conducted by EK and SYK. All authors read and approved the
final version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen X, Jain A, Aladelokun O, Yan H,
Gilbride A, Ferrucci LM, Lu L, Khan SA and Johnson CH: Asparagine,
colorectal cancer, and the role of sex, genes, microbes, and diet:
A narrative review. Front Mol Biosci. 9:9586662022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuentes N, Silva Rodriguez M and Silveyra
P: Role of sex hormones in lung cancer. Exp Biol Med (Maywood).
246:2098–2110. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coelingh Bennink HJT, Prowse A, Egberts
JFM, Debruyne FMJ, Huhtaniemi IT and Tombal B: The loss of
estradiol by androgen deprivation in prostate cancer patients shows
the importance of estrogens in males. J Endocr Soc. 8:bvae1072024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frederiksen H, Johannsen TH, Andersen SE,
Albrethsen J, Landersoe SK, Petersen JH, Andersen AN, Vestergaard
ET, Schorring ME, Linneberg A, et al: Sex-specific estrogen levels
and reference intervals from infancy to late adulthood determined
by LC-MS/MS. J Clin Endocrinol Metab. 105:754–768. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gates MA, Mekary RA, Chiu GR, Ding EL,
Wittert GA and Araujo AB: Sex steroid hormone levels and body
composition in men. J Clin Endocrinol Metab. 98:2442–2450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marriott RJ, Murray K, Adams RJ, Antonio
L, Ballantyne CM, Bauer DC, Bhasin S, Biggs ML, Cawthon PM, Couper
DJ, et al: Factors associated with circulating sex hormones in men:
Individual participant data meta-analyses. Ann Intern Med.
176:1221–1234. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simpson ER, Misso M, Hewitt KN, Hill RA,
Boon WC, Jones ME, Kovacic A, Zhou J and Clyne CD: Estrogen-the
good, the bad, and the unexpected. Endocr Rev. 26:322–330. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gandhi N, Omer S and Harrison RE: In vitro
cell culture model for osteoclast activation during estrogen
withdrawal. Int J Mol Sci. 25:61342024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hughes DE, Dai A, Tiffee JC, Li HH, Mundy
GR and Boyce BF: Estrogen promotes apoptosis of murine osteoclasts
mediated by TGF-beta. Nat Med. 2:1132–1136. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Srivastava S, Toraldo G, Weitzmann MN,
Cenci S, Ross FP and Pacifici R: Estrogen decreases osteoclast
formation by down-regulating receptor activator of NF-kappa B
ligand (RANKL)-induced JNK activation. J Biol Chem. 276:8836–8840.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gavali S, Gupta MK, Daswani B, Wani MR,
Sirdeshmukh R and Khatkhatay MI: LYN, a key mediator in
estrogen-dependent suppression of osteoclast differentiation,
survival, and function. Biochim Biophys Acta Mol Basis Dis.
1865:547–557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kharb R, Haider K, Neha K and Yar MS:
Aromatase inhibitors: Role in postmenopausal breast cancer. Arch
Pharm (Weinheim). 353:e20000812020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arumugam A, Lissner EA and Lakshmanaswamy
R: The role of hormones and aromatase inhibitors on breast tumor
growth and general health in a postmenopausal mouse model. Reprod
Biol Endocrinol. 12:662014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parish SJ, Simon JA, Davis SR, Giraldi A,
Goldstein I, Goldstein SW, Kim NN, Kingsberg SA, Morgentaler A,
Nappi RE, et al: International society for the study of women's
sexual health clinical practice guideline for the use of systemic
testosterone for hypoactive sexual desire disorder in women. J Sex
Med. 18:849–867. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van-Duyne G, Blair IA, Sprenger C,
Moiseenkova-Bell V, Plymate S and Penning TM: The androgen
receptor. Vitam Horm. 123:439–481. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai CC, Yang YSH, Chen YF, Huang LY, Yang
YN, Lee SY, Wang WL, Lee HL, Whang-Peng J, Lin HY, et al: Integrins
and actions of androgen in breast cancer. Cells. 12:21262023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Naamneh Elzenaty R, du Toit T and Flück
CE: Basics of androgen synthesis and action. Best Pract Res Clin
Endocrinol Metab. 36:1016652022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bienenfeld A, Azarchi S, Lo Sicco K,
Marchbein S, Shapiro J and Nagler AR: Androgens in women:
Androgen-mediated skin disease and patient evaluation. J Am Acad
Dermatol. 80:1497–1506. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang R, Hu K, Bai H, Liu H, Pu Y, Yang C,
Liu Q and Fan P: Increased oxidative stress is associated with
hyperandrogenemia in polycystic ovary syndrome evidenced by
oxidized lipoproteins stimulating rat ovarian androgen synthesis in
vitro. Endocrine. 84:1238–1249. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paakinaho V and Palvimo JJ: Genome-wide
crosstalk between steroid receptors in breast and prostate cancers.
Endocr Relat Cancer. 28:R231–R250. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amirghofran Z, Monabati A and Gholijani N:
Androgen receptor expression in relation to apoptosis and the
expression of cell cycle related proteins in prostate cancer.
Pathol Oncol Res. 10:37–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bodner K, Laubichler P, Kimberger O,
Czerwenka K, Zeillinger R and Bodner-Adler B: Oestrogen and
progesterone receptor expression in patients with adenocarcinoma of
the uterine cervix and correlation with various clinicopathological
parameters. Anticancer Res. 30:1341–1345. 2010.PubMed/NCBI
|
|
24
|
Yoon K, Park Y, Kang E, Kim E, Kim JH, Kim
SH, Suh KJ, Kim SM, Jang M, Yun BR, et al: Effect of estrogen
receptor expression level and hormonal therapy on prognosis of
early breast cancer. Cancer Res Treat. 54:1081–1090. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giuliano AE, Edge SB and Hortobagyi GN:
Eighth edition of the AJCC cancer staging manual: Breast cancer.
Ann Surg Oncol. 25:1783–1785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheang MCU, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huggins C and Hodges CV: Studies on
prostatic cancer. I. The effect of castration, of estrogen and of
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate. 1941. J Urol. 167:948–952. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scher HI and Sawyers CL: Biology of
progressive, castration-resistant prostate cancer: Directed
therapies targeting the androgen-receptor signaling axis. J Clin
Oncol. 23:8253–8261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu G, Tian L, Herzog SK, Rechoum Y,
Gelsomino L, Gao M, Du L, Kim JA, Dustin D, Lo HC, et al: Hormonal
modulation of ESR1 mutant metastasis. Oncogene. 40:997–1011. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kolyvas EA, Caldas C, Kelly K and Ahmad
SS: Androgen receptor function and targeted therapeutics across
breast cancer subtypes. Breast Cancer Res. 24:792022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peters AA, Buchanan G, Ricciardelli C,
Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia
L, Moore NL, et al: Androgen receptor inhibits estrogen
receptor-alpha activity and is prognostic in breast cancer. Cancer
Res. 69:6131–6140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hickey TE, Selth LA, Chia KM, Laven-Law G,
Milioli HH, Roden D, Jindal S, Hui M, Finlay-Schultz J, Ebrahimie
E, et al: The androgen receptor is a tumor suppressor in estrogen
receptor-positive breast cancer. Nat Med. 27:310–320. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Secreto G, Girombelli A and Krogh V:
Androgen excess in breast cancer development: Implications for
prevention and treatment. Endocr Relat Cancer. 26:R81–R94. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gehrig J, Kaulfuß S, Jarry H, Bremmer F,
Stettner M, Burfeind P and Thelen P: Prospects of estrogen receptor
β activation in the treatment of castration-resistant prostate
cancer. Oncotarget. 8:34971–34979. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lung DK, Reese RM and Alarid ET: Intrinsic
and extrinsic factors governing the transcriptional regulation of
ESR1. Horm Cancer. 11:129–147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jensen EV, Desombre ER, Kawashima T,
Suzuki T, Kyser K and Jungblut PW: Estrogen-binding substances of
target tissues. Science. 158:529–530. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kato S, Endoh H, Masuhiro Y, Kitamoto T,
Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H,
et al: Activation of the estrogen receptor through phosphorylation
by mitogen-activated protein kinase. Science. 270:1491–1494. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bunone G, Briand PA, Miksicek RJ and
Picard D: Activation of the unliganded estrogen receptor by EGF
involves the MAP kinase pathway and direct phosphorylation. EMBO J.
15:2174–2183. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tremblay A, Tremblay GB, Labrie F and
Giguère V: Ligand-independent recruitment of SRC-1 to estrogen
receptor beta through phosphorylation of activation function AF-1.
Mol Cell. 3:513–519. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yi P, Wang Z, Feng Q, Pintilie GD, Foulds
CE, Lanz RB, Ludtke SJ, Schmid MF, Chiu W and O'Malley BW:
Structure of a biologically active estrogen receptor-coactivator
complex on DNA. Mol Cell. 57:1047–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brzozowski AM, Pike AC, Dauter Z, Hubbard
RE, Bonn T, Engström O, Ohman L, Greene GL, Gustafsson JA and
Carlquist M: Molecular basis of agonism and antagonism in the
oestrogen receptor. Nature. 389:753–758. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Heery DM, Kalkhoven E, Hoare S and Parker
MG: A signature motif in transcriptional co-activators mediates
binding to nuclear receptors. Nature. 387:733–736. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Torchia J, Rose DW, Inostroza J, Kamei Y,
Westin S, Glass CK and Rosenfeld MG: The transcriptional
co-activator p/CIP binds CBP and mediates nuclear-receptor
function. Nature. 387:677–684. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kumar R, Betney R, Li J, Thompson EB and
McEwan IJ: Induced alpha-helix structure in AF1 of the androgen
receptor upon binding transcription factor TFIIF. Biochemistry.
43:3008–3013. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bevan CL, Hoare S, Claessens F, Heery DM
and Parker MG: The AF1 and AF2 domains of the androgen receptor
interact with distinct regions of SRC1. Mol Cell Biol.
19:8383–8392. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang B, Omoto Y, Iwase H, Yamashita H,
Toyama T, Coombes RC, Filipovic A, Warner M and Gustafsson JÅ:
Differential expression of estrogen receptor alpha, beta1, and
beta2 in lobular and ductal breast cancer. Proc Natl Acad Sci USA.
111:1933–1938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Couse JF and Korach KS: Estrogen receptor
null mice: What have we learned and where will they lead us? Endocr
Rev. 20:358–417. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jia M, Dahlman-Wright K and Gustafsson JÅ:
Estrogen receptor alpha and beta in health and disease. Best Pract
Res Clin Endocrinol Metab. 29:557–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Martin EC, Conger AK, Yan TJ, Hoang VT,
Miller DF, Buechlein A, Rusch DB, Nephew KP, Collins-Burow BM and
Burow ME: MicroRNA-335-5p and −3p synergize to inhibit estrogen
receptor alpha expression and promote tamoxifen resistance. FEBS
Lett. 591:382–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Santner SJ, Feil PD and Santen RJ: In situ
estrogen production via the estrone sulfatase pathway in breast
tumors: Relative importance versus the aromatase pathway. J Clin
Endocrinol Metab. 59:29–33. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jordan VC: Antiestrogenic action of
raloxifene and tamoxifen: Today and tomorrow. J Natl Cancer Inst.
90:967–971. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Arpino G, Weiss H, Lee AV, Schiff R, De
Placido S, Osborne CK and Elledge RM: Estrogen receptor-positive,
progesterone receptor-negative breast cancer: Association with
growth factor receptor expression and tamoxifen resistance. J Natl
Cancer Inst. 97:1254–1261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Horwitz KB, Koseki Y and McGuire WL:
Estrogen control of progesterone receptor in human breast cancer:
Role of estradiol and antiestrogen. Endocrinology. 103:1742–1751.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dowsett M, Houghton J, Iden C, Salter J,
Farndon J, A'Hern R and Baum M: Estrogen receptor status,
progesterone receptor status, and HER2 status as biomarkers for
predicting response to endocrine therapy. J Clin Oncol.
26:1814–1820. 2008.
|
|
59
|
Kumar R, Zakharov MN, Khan SH, Miki R,
Jang H, Toraldo G, Singh R, Bhasin S and Jasuja R: The dynamic
structure of the estrogen receptor. J Amino Acids. 2011:8125402011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Weihua Z, Andersson S, Cheng G, Simpson
ER, Warner M and Gustafsson JA: Update on estrogen signaling. FEBS
Lett. 546:17–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Flouriot G, Brand H, Denger S, Metivier R,
Kos M, Reid G, Sonntag-Buck V and Gannon F: Identification of a new
isoform of the human estrogen receptor-alpha (hER-alpha) that is
encoded by distinct transcripts and that is able to repress
hER-alpha activation function 1. EMBO J. 19:4688–4700. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Langley RE, Godsland IF, Kynaston H,
Clarke NW, Rosen SD, Morgan RC, Pollock P, Kockelbergh R, Lalani
EN, Dearnaley D, et al: Early hormonal data from a multicentre
phase II trial using transdermal oestrogen patches as first-line
hormonal therapy in patients with locally advanced or metastatic
prostate cancer. BJU Int. 102:442–445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lau KM and To KF: Importance of estrogenic
signaling and its mediated receptors in prostate cancer. Int J Mol
Sci. 17:14342016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ricke WA, McPherson SJ, Bianco JJ, Cunha
GR, Wang Y and Risbridger GP: Prostatic hormonal carcinogenesis is
mediated by in situ estrogen production and estrogen receptor alpha
signaling. FASEB J. 22:1512–1520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Olczak M, Orzechowska MJ, Bednarek AK and
Lipiński M: The transcriptomic profiles of ESR1 and MMP3 stratify
the risk of biochemical recurrence in primary prostate cancer
beyond clinical features. Int J Mol Sci. 24:83992023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin Q, Cao J, Du X, Yang K, Yang X, Liang
Z, Shi J and Zhang J: CYP1B1-catalyzed 4-OHE2 promotes the
castration resistance of prostate cancer stem cells by estrogen
receptor α-mediated IL6 activation. Cell Commun Signal. 20:312022.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Reis LO, Zani EL and García-Perdomo HA:
Estrogen therapy in patients with prostate cancer: A contemporary
systematic review. Int Urol Nephrol. 50:993–1003. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Christoforou P, Christopoulos PF and
Koutsilieris M: The role of estrogen receptor β in prostate cancer.
Mol Med. 20:427–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sieh W, Köbel M, Longacre TA, Bowtell DD,
deFazio A, Goodman MT, Høgdall E, Deen S, Wentzensen N, Moysich KB,
et al: Hormone-receptor expression and ovarian cancer survival: An
ovarian tumor tissue analysis consortium study. Lancet Oncol.
14:853–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Park SH, Cheung LWT, Wong AST and Leung
PCK: Estrogen regulates Snail and Slug in the down-regulation of
E-cadherin and induces metastatic potential of ovarian cancer cells
through estrogen receptor alpha. Mol Endocrinol. 22:2085–2098.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chan KK, Leung TH, Chan DW, Wei N, Lau GT,
Liu SS, Siu MK and Ngan HY: Targeting estrogen receptor subtypes
(ERα and ERβ) with selective ER modulators in ovarian cancer. J
Endocrinol. 221:325–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Langdon SP, Herrington CS, Hollis RL and
Gourley C: Estrogen signaling and its potential as a target for
therapy in ovarian cancer. Cancers (Basel). 12:16472020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
He M, Yu W, Chang C, Miyamoto H, Liu X,
Jiang K and Yeh S: Estrogen receptor α promotes lung cancer cell
invasion via increase of and cross-talk with infiltrated
macrophages through the CCL2/CCR2/MMP9 and CXCL12/CXCR4 signaling
pathways. Mol Oncol. 14:1779–1799. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Siegel DA, Fedewa SA, Henley SJ, Pollack
LA and Jemal A: Proportion of never smokers among men and women
with lung cancer in 7 US States. JAMA Oncol. 7:302–304. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hsu LH, Liu KJ, Tsai MF, Wu CR, Feng AC,
Chu NM and Kao SH: Estrogen adversely affects the prognosis of
patients with lung adenocarcinoma. Cancer Sci. 106:51–59. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chlebowski RT, Schwartz AG, Wakelee H,
Anderson GL, Stefanick ML, Manson JE, Rodabough RJ, Chien JW,
Wactawski-Wende J, Gass M, et al: Oestrogen plus progestin and lung
cancer in postmenopausal women (Women's Health Initiative trial): A
post-hoc analysis of a randomised controlled trial. Lancet.
374:1243–1251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhou J, Teng R, Xu C, Wang Q, Guo J, Xu C,
Li Z, Xie S, Shen J and Wang L: Overexpression of ERα inhibits
proliferation and invasion of MKN28 gastric cancer cells by
suppressing β-catenin. Oncol Rep. 30:1622–1630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Takano N, Iizuka N, Hazama S, Yoshino S,
Tangoku A and Oka M: Expression of estrogen receptor-alpha and
-beta mRNAs in human gastric cancer. Cancer Lett. 176:129–135.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tang W, Liu R, Yan Y, Pan X, Wang M, Han
X, Ren H and Zhang Z: Expression of estrogen receptors and androgen
receptor and their clinical significance in gastric cancer.
Oncotarget. 8:40765–40777. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen P, Li B and Ou-Yang L: Role of
estrogen receptors in health and disease. Front Endocrinol
(Lausanne). 13:8390052022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen J and Iverson D: Estrogen in
obesity-associated colon cancer: Friend or foe? Protecting
postmenopausal women but promoting late-stage colon cancer. Cancer
Causes Control. 23:1767–1773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jiang H, Teng R, Wang Q, Zhang X, Wang H,
Wang Z, Cao J and Teng L: Transcriptional analysis of estrogen
receptor alpha variant mRNAs in colorectal cancers and their
matched normal colorectal tissues. J Steroid Biochem Mol Biol.
112:20–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Dai B, Geng L, Yu Y, Sui C, Xie F, Shen W,
Zheng T and Yang J: Methylation patterns of estrogen receptor α
promoter correlate with estrogen receptor α expression and
clinicopathological factors in hepatocellular carcinoma. Exp Biol
Med (Maywood). 239:883–890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hou J, Xu J, Jiang R, Wang Y, Chen C, Deng
L, Huang X, Wang X and Sun B: Estrogen-sensitive PTPRO expression
represses hepatocellular carcinoma progression by control of STAT3.
Hepatology. 57:678–688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Iyer JK, Kalra M, Kaul A, Payton ME and
Kaul R: Estrogen receptor expression in chronic hepatitis C and
hepatocellular carcinoma pathogenesis. World J Gastroenterol.
23:6802–6816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kuiper GG, Enmark E, Pelto-Huikko M,
Nilsson S and Gustafsson JA: Cloning of a novel receptor expressed
in rat prostate and ovary. Proc Natl Acad Sci USA. 93:5925–5930.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Mal R, Magner A, David J, Datta J,
Vallabhaneni M, Kassem M, Manouchehri J, Willingham N, Stover D,
Vandeusen J, et al: Estrogen receptor beta (ERβ): A ligand
activated tumor suppressor. Front Oncol. 10:5873862020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lewandowski S, Kalita K and Kaczmarek L:
Estrogen receptor beta. Potential functional significance of a
variety of mRNA isoforms. FEBS Lett. 524:1–5. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hua H, Zhang H, Kong Q and Jiang Y:
Mechanisms for estrogen receptor expression in human cancer. Exp
Hematol Oncol. 7:242018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Haldosén LA, Zhao C and Dahlman-Wright K:
Estrogen receptor beta in breast cancer. Mol Cell Endocrinol.
382:665–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dalal H, Dahlgren M, Gladchuk S, Brueffer
C, Gruvberger-Saal SK and Saal LH: Clinical associations of ESR2
(estrogen receptor beta) expression across thousands of primary
breast tumors. Sci Rep. 12:46962022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang J, Zhang C, Chen K, Tang H, Tang J,
Song C and Xie X: ERβ1 inversely correlates with PTEN/PI3K/AKT
pathway and predicts a favorable prognosis in triple-negative
breast cancer. Breast Cancer Res Treat. 152:255–269. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Grober OM, Mutarelli M, Giurato G, Ravo M,
Cicatiello L, De Filippo MR, Ferraro L, Nassa G, Papa MF, Paris O,
et al: Global analysis of estrogen receptor beta binding to breast
cancer cell genome reveals an extensive interplay with estrogen
receptor alpha for target gene regulation. BMC Genomics. 12:362011.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hurtado A, Pinós T, Barbosa-Desongles A,
López-Avilés S, Barquinero J, Petriz J, Santamaria-Martínez A,
Morote J, de Torres I, Bellmunt J, et al: Estrogen receptor beta
displays cell cycle-dependent expression and regulates the G1 phase
through a non-genomic mechanism in prostate carcinoma cells. Cell
Oncol. 30:349–365. 2008.PubMed/NCBI
|
|
95
|
Hwang NM and Stabile LP: Estrogen receptor
ß in cancer: To ß(e) or not to ß(e)? Endocrinology.
162:bqab1622021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Mak P, Leav I, Pursell B, Bae D, Yang X,
Taglienti CA, Gouvin LM, Sharma VM and Mercurio AM: ERbeta impedes
prostate cancer EMT by destabilizing HIF-1alpha and inhibiting
VEGF-mediated snail nuclear localization: Implications for Gleason
grading. Cancer Cell. 17:319–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mak P, Chang C, Pursell B and Mercurio AM:
Estrogen receptor β sustains epithelial differentiation by
regulating prolyl hydroxylase 2 transcription. Proc Natl Acad Sci
USA. 110:4708–4713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lim W, Cho J, Kwon HY, Park Y, Rhyu MR and
Lee Y: Hypoxia-inducible factor 1 alpha activates and is inhibited
by unoccupied estrogen receptor beta. FEBS Lett. 583:1314–1318.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chaurasiya S, Widmann S, Botero C, Lin CY,
Gustafsson JÅ and Strom AM: Estrogen receptor β exerts tumor
suppressive effects in prostate cancer through repression of
androgen receptor activity. PLoS One. 15:e02260572020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jefferi NES, Shamhari AA, 'Azhar NKZN,
Shin JGY, Kharir NAM, Azhar MA, Hamid ZA, Budin SB and Taib IS: The
role of ERα and ERβ in castration-resistant prostate cancer and
current therapeutic approaches. Biomedicines. 11:8262023.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Leung YK, Lam HM, Wu S, Song D, Levin L,
Cheng L, Wu CL and Ho SM: Estrogen receptor beta2 and beta5 are
associated with poor prognosis in prostate cancer, and promote
cancer cell migration and invasion. Endocr Relat Cancer.
17:675–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lazennec G: Estrogen receptor beta, a
possible tumor suppressor involved in ovarian carcinogenesis.
Cancer Lett. 231:151–157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bossard C, Busson M, Vindrieux D, Gaudin
F, Machelon V, Brigitte M, Jacquard C, Pillon A, Balaguer P,
Balabanian K and Lazennec G: Potential role of estrogen receptor
beta as a tumor suppressor of epithelial ovarian cancer. PLoS One.
7:e447872012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Verardi L, Fiori J, Andrisano V, Locatelli
A, Morigi R, Naldi M, Bertucci C, Strocchi E, Boga C, Micheletti G
and Calonghi N: Indole derivative interacts with estrogen receptor
beta and inhibits human ovarian cancer cell growth. Molecules.
25:44382020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chen W, Xin B, Pang H, Han L, Shen W, Zhao
Z, Duan L, Cao P, Liu L and Zhang H: Downregulation of estrogen
receptor β inhibits lung adenocarcinoma cell growth. Oncol Rep.
41:2967–2974. 2019.PubMed/NCBI
|
|
106
|
Liu S, Hu C, Li M, An J, Zhou W, Guo J and
Xiao Y: Estrogen receptor beta promotes lung cancer invasion via
increasing CXCR4 expression. Cell Death Dis. 13:702022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Peri S and Niv Y: Estrogen receptor beta
(ERß) in gastric cancer-A systematic review and meta-analysis.
Microb Health Dis. 6:e9842024.
|
|
108
|
Zhou F, Xu Y, Shi J, Lan X, Zou X, Wang L
and Huang Q: Expression profile of E-cadherin, estrogen receptors,
and P53 in early-onset gastric cancers. Cancer Med. 5:3403–3411.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhou F, Jin J, Zhou L, Wu L, Cao Y, Yan H,
Huang Q, Wang L and Zou X: Suppression of estrogen receptor-beta
promotes gastric cancer cell apoptosis with induction of autophagy.
Am J Transl Res. 12:4397–4409. 2020.PubMed/NCBI
|
|
110
|
Gan L, He J, Zhang X, Zhang YJ, Yu GZ,
Chen Y, Pan J, Wang JJ and Wang X: Expression profile and
prognostic role of sex hormone receptors in gastric cancer. BMC
Cancer. 12:5662012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Caiazza F, Ryan EJ, Doherty G, Winter DC
and Sheahan K: Estrogen receptors and their implications in
colorectal carcinogenesis. Front Oncol. 5:192015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hartman J, Edvardsson K, Lindberg K, Zhao
C, Williams C, Ström A and Gustafsson JA: Tumor repressive
functions of estrogen receptor beta in SW480 colon cancer cells.
Cancer Res. 69:6100–6106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Topi G, Satapathy SR, Dash P, Fred Mehrabi
S, Ehrnström R, Olsson R, Lydrup ML and Sjölander A:
Tumour-suppressive effect of oestrogen receptor β in colorectal
cancer patients, colon cancer cells, and a zebrafish model. J
Pathol. 251:297–309. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Konstantinopoulos PA, Kominea A, Vandoros
G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G
and Papavassiliou AG: Oestrogen receptor beta (ERbeta) is
abundantly expressed in normal colonic mucosa, but declines in
colon adenocarcinoma paralleling the tumour's dedifferentiation.
Eur J Cancer. 39:1251–1258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rudolph A, Toth C, Hoffmeister M, Roth W,
Herpel E, Jansen L, Marx A, Brenner H and Chang-Claude J:
Expression of oestrogen receptor β and prognosis of colorectal
cancer. Br J Cancer. 107:831–839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Iavarone M, Lampertico P, Seletti C,
Donato MF, Ronchi G, Del Ninno E and Colombo M: The clinical and
pathogenetic significance of estrogen receptor-beta expression in
chronic liver diseases and liver carcinoma. Cancer. 98:529–534.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yang W, Lu Y, Xu Y, Xu L, Zheng W, Wu Y,
Li L and Shen P: Estrogen represses hepatocellular carcinoma (HCC)
growth via inhibiting alternative activation of tumor-associated
macrophages (TAMs). J Biol Chem. 287:40140–40149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Marzioni M, Torrice A, Saccomanno S,
Rychlicki C, Agostinelli L, Pierantonelli I, Rhönnstad P, Trozzi L,
Apelqvist T, Gentile R, et al: An oestrogen receptor β-selective
agonist exerts anti-neoplastic effects in experimental intrahepatic
cholangiocarcinoma. Dig Liver Dis. 44:134–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Handelsman DJ: History of androgens and
androgen action. Best Pract Res Clin Endocrinol Metab.
36:1016292022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Brinkmann AO, Faber PW, van Rooij HC,
Kuiper GG, Ris C, Klaassen P, van der Korput JA, Voorhorst MM, van
Laar JH, Mulder E, et al: The human androgen receptor: Domain
structure, genomic organization and regulation of expression. J
Steroid Biochem. 34:307–310. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Davey RA and Grossmann M: Androgen
receptor structure, function and biology: From bench to bedside.
Clin Biochem Rev. 37:3–15. 2016.PubMed/NCBI
|
|
122
|
Moilanen A, Rouleau N, Ikonen T, Palvimo
JJ and Jänne OA: The presence of a transcription activation
function in the hormone-binding domain of androgen receptor is
revealed by studies in yeast cells. FEBS Lett. 412:355–358. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ueda T, Bruchovsky N and Sadar MD:
Activation of the androgen receptor N-terminal domain by
interleukin-6 via MAPK and STAT3 signal transduction pathways. J
Biol Chem. 277:7076–7085. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Asai S, Goto Y, Tanigawa K, Tomioka Y,
Kato M, Mizuno K, Sakamoto S and Seki N: MiR-15b-5p inhibits
castration-resistant growth of prostate cancer cells by targeting
the muscarinic cholinergic receptor CHRM3. FEBS Lett.
597:1164–1175. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Martin-Caraballo M: Regulation of
molecular biomarkers associated with the progression of prostate
cancer. Int J Mol Sci. 25:41712024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Muralidhar A, Gamat-Huber M, Vakkalanka S
and McNeel DG: Sequence of androgen receptor-targeted vaccination
with androgen deprivation therapy affects anti-prostate tumor
efficacy. J Immunother Cancer. 12:e0088482024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhong M, Xu W, Tian P, Zhang Q, Wang Z,
Liang L, Zhang Q, Yang Y, Lu Y and Wei GH: An inherited allele
confers prostate cancer progression and drug resistance via
RFX6/HOXA10-orchestrated TGFβ signaling. Adv Sci (Weinh).
11:e24014922024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liu C, Chen B, Xu P, Yang J, Nip C, Wang
L, Shen Y, Ning S, Shang Y, Corey E, et al: Plexin D1 emerges as a
novel target in the development of neural lineage plasticity in
treatment-resistant prostate cancer. Res Sq [Preprint].
rs.3.rs-4095949. 2024.
|
|
129
|
Matsumoto K, Kosaka T, Takeda T, Fukumoto
K, Yasumizu Y, Tanaka N, Morita S, Mizuno R, Asanuma H and Oya M:
Appropriate definition of non-metastatic castration-resistant
prostate cancer (nmCRPC) and optimal timing of androgen receptor
signaling inhibitor (ARSI). Int J Clin Oncol. 29:1198–1203. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Shiota M, Ushijima M, Tsukahara S,
Nagakawa S, Okada T, Tanegashima T, Kobayashi S, Matsumoto T and
Eto M: Oxidative stress in peroxisomes induced by androgen receptor
inhibition through peroxisome proliferator-activated receptor
promotes enzalutamide resistance in prostate cancer. Free Radic
Biol Med. 221:81–88. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ramírez-de-Arellano A, Pereira-Suárez AL,
Rico-Fuentes C, López-Pulido EI, Villegas-Pineda JC and Sierra-Diaz
E: Distribution and effects of estrogen receptors in prostate
cancer: Associated molecular mechanisms. Front Endocrinol
(Lausanne). 12:8115782022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Palmieri C, Linden H, Birrell SN,
Wheelwright S, Lim E, Schwartzberg LS, Dwyer AR, Hickey TE, Rugo
HS, Cobb P, et al: Activity and safety of enobosarm, a novel, oral,
selective androgen receptor modulator, in androgen
receptor-positive, oestrogen receptor-positive, and HER2-negative
advanced breast cancer (Study G200802): A randomised, open-label,
multicentre, multinational, parallel design, phase 2 trial. Lancet
Oncol. 25:317–325. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Xu F, Xu K, Fan L, Li X, Liu Y, Yang F,
Zhu C and Guan X: Estrogen receptor beta suppresses the androgen
receptor oncogenic effects in triple-negative breast cancer. Chin
Med J (Engl). 137:338–349. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cuenca-López MD, Montero JC, Morales JC,
Prat A, Pandiella A and Ocana A: Phospho-kinase profile of triple
negative breast cancer and androgen receptor signaling. BMC Cancer.
14:3022014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Garay JP, Karakas B, Abukhdeir AM,
Cosgrove DP, Gustin JP, Higgins MJ, Konishi H, Konishi Y, Lauring
J, Mohseni M, et al: The growth response to androgen receptor
signaling in ERα-negative human breast cells is dependent on p21
and mediated by MAPK activation. Breast Cancer Res. 14:R272012.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chang C, Lee SO, Yeh S and Chang TM:
Androgen receptor (AR) differential roles in hormone-related tumors
including prostate, bladder, kidney, lung, breast and liver.
Oncogene. 33:3225–3234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chadha S, Rao BR, Slotman BJ, van
Vroonhoven CC and van der Kwast TH: An immunohistochemical
evaluation of androgen and progesterone receptors in ovarian
tumors. Hum Pathol. 24:90–95. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Cardillo MR, Petrangeli E, Aliotta N,
Salvatori L, Ravenna L, Chang C and Castagna G: Androgen receptors
in ovarian tumors: Correlation with oestrogen and progesterone
receptors in an immunohistochemical and semiquantitative image
analysis study. J Exp Clin Cancer Res. 17:231–237. 1998.PubMed/NCBI
|
|
139
|
Kohan-Ivani K, Gabler F, Selman A, Vega M
and Romero C: Role of dihydrotestosterone (DHT) on TGF-β1 signaling
pathway in epithelial ovarian cancer cells. J Cancer Res Clin
Oncol. 142:47–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ligr M, Patwa RR, Daniels G, Pan L, Wu X,
Li Y, Tian L, Wang Z, Xu R, Wu J, et al: Expression and function of
androgen receptor coactivator p44/Mep50/WDR77 in ovarian cancer.
PLoS One. 6:e262502011. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Martins FC, Couturier DL, Paterson A,
Karnezis AN, Chow C, Nazeran TM, Odunsi A, Gentry-Maharaj A, Vrvilo
A, Hein A, et al: Clinical and pathological associations of PTEN
expression in ovarian cancer: A multicentre study from the ovarian
tumour tissue analysis consortium. Br J Cancer. 123:793–802. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Liu S, Hu C, Li M, Zhou W, Wang R and Xiao
Y: Androgen receptor suppresses lung cancer invasion and increases
cisplatin response via decreasing TPD52 expression. Int J Biol Sci.
19:3709–3725. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhou J, Wang H, Sun Q, Liu X, Wu Z, Wang
X, Fang W and Ma Z: miR-224-5p-enriched exosomes promote
tumorigenesis by directly targeting androgen receptor in non-small
cell lung cancer. Mol Ther Nucleic Acids. 23:1217–1228. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Li X, Tang Y, Liang P, Sun M, Li T, Shen Z
and Sha S: Luteolin inhibits A549 cells proliferation and migration
by down-regulating androgen receptors. Eur J Med Res. 28:3532023.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Recchia AG, Musti AM, Lanzino M, Panno ML,
Turano E, Zumpano R, Belfiore A, Andò S and Maggiolini M: A
cross-talk between the androgen receptor and the epidermal growth
factor receptor leads to p38MAPK-dependent activation of mTOR and
cyclinD1 expression in prostate and lung cancer cells. Int J
Biochem Cell Biol. 41:603–614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Liu B, Zhou M, Li X, Zhang X, Wang Q, Liu
L, Yang M, Yang D, Guo Y, Zhang Q, et al: Interrogation of gender
disparity uncovers androgen receptor as the transcriptional
activator for oncogenic miR-125b in gastric cancer. Cell Death Dis.
12:4412021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Xia N, Cui J, Zhu M, Xing R and Lu Y:
Androgen receptor variant 12 promotes migration and invasion by
regulating MYLK in gastric cancer. J Pathol. 248:304–315. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Soleymani Fard S, Yazdanbod M, Sotoudeh M,
Bashash D, Mahmoodzadeh H, Saliminejad K, Mousavi SA, Ghaffari SH
and Alimoghaddam K: Prognostic and therapeutic significance of
androgen receptor in patients with gastric cancer. Onco Targets
Ther. 13:9821–9837. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Gu S, Honisch S, Kounenidakis M, Alkahtani
S, Alarifi S, Alevizopoulos K, Stournaras C and Lang F: Membrane
androgen receptor down-regulates c-src-activity and beta-catenin
transcription and triggers GSK-3beta-phosphorylation in colon tumor
cells. Cell Physiol Biochem. 34:1402–1412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Alkahtani S: Testosterone induced
apoptosis in colon cancer cells is regulated by PI3K/Rac1
signaling. Asian J Androl. 15:831–834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Rodríguez-Santiago Y, Garay-Canales CA,
Nava-Castro KE and Morales-Montor J: Sexual dimorphism in
colorectal cancer: Molecular mechanisms and treatment strategies.
Biol Sex Differ. 15:482024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Acosta-Lopez S, Diaz-Bethencourt D,
Concepción-Massip T, Martin-Fernandez de Basoa MC, Plata-Bello A,
Gonzalez-Rodriguez A, Perez-Hernandez F and Plata-Bello J: The
androgen receptor expression and its activity have different
relationships with prognosis in hepatocellular carcinoma. Sci Rep.
10:220462020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ren QN, Zhang H, Sun CY, Zhou YF, Yang XF,
Long JW, Li XX, Mai SJ, Zhang MY, Zhang HZ, et al: Phosphorylation
of androgen receptor by mTORC1 promotes liver steatosis and
tumorigenesis. Hepatology. 75:1123–1138. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ren H, Ren B, Zhang J, Zhang X, Li L, Meng
L, Li Z, Li J, Gao Y and Ma X: Androgen enhances the activity of
ETS-1 and promotes the proliferation of HCC cells. Oncotarget.
8:109271–109288. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ouyang X, Feng L, Liu G, Yao L, Wang Z,
Liu S, Xiao Y and Zhang G: Androgen receptor (AR) decreases HCC
cells migration and invasion via miR-325/ACP5 signaling. J Cancer.
12:1915–1925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Porter W, Saville B, Hoivik D and Safe S:
Functional synergy between the transcription factor Sp1 and the
estrogen receptor. Mol Endocrinol. 11:1569–1580. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Greaves E, Collins F, Critchley HOD and
Saunders PTK: ERβ-dependent effects on uterine endothelial cells
are cell specific and mediated via Sp1. Hum Reprod. 28:2490–2501.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Eisermann K, Broderick CJ, Bazarov A,
Moazam MM and Fraizer GC: Androgen up-regulates vascular
endothelial growth factor expression in prostate cancer cells via
an Sp1 binding site. Mol Cancer. 12:72013. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Setlur SR, Mertz KD, Hoshida Y, Demichelis
F, Lupien M, Perner S, Sboner A, Pawitan Y, Andrén O, Johnson LA,
et al: Estrogen-dependent signaling in a molecularly distinct
subclass of aggressive prostate cancer. J Natl Cancer Inst.
100:815–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kohvakka A, Sattari M, Shcherban A, Annala
M, Urbanucci A, Kesseli J, Tammela TLJ, Kivinummi K, Latonen L,
Nykter M and Visakorpi T: AR and ERG drive the expression of
prostate cancer specific long noncoding RNAs. Oncogene.
39:5241–5251. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lombardi AP, Pisolato R, Vicente CM,
Lazari MF, Lucas TF and Porto CS: Estrogen receptor beta (ERβ)
mediates expression of β-catenin and proliferation in prostate
cancer cell line PC-3. Mol Cell Endocrinol. 430:12–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Song LN and Gelmann EP: Interaction of
beta-catenin and TIF2/GRIP1 in transcriptional activation by the
androgen receptor. J Biol Chem. 280:37853–37867. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Dahlman-Wright K, Qiao Y, Jonsson P,
Gustafsson JÅ, Williams C and Zhao C: Interplay between AP-1 and
estrogen receptor α in regulating gene expression and proliferation
networks in breast cancer cells. Carcinogenesis. 33:1684–1691.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Frønsdal K, Engedal N, Slagsvold T and
Saatcioglu F: CREB binding protein is a coactivator for the
androgen receptor and mediates cross-talk with AP-1. J Biol Chem.
273:31853–31859. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Sato N, Sadar MD, Bruchovsky N, Saatcioglu
F, Rennie PS, Sato S, Lange PH and Gleave ME: Androgenic induction
of prostate-specific antigen gene is repressed by protein-protein
interaction between the androgen receptor and AP-1/c-Jun in the
human prostate cancer cell line LNCaP. J Biol Chem.
272:17485–17494. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Dadiani M, Seger D, Kreizman T, Badikhi D,
Margalit R, Eilam R and Degani H: Estrogen regulation of vascular
endothelial growth factor in breast cancer in vitro and in vivo:
The role of estrogen receptor alpha and c-Myc. Endocr Relat Cancer.
16:819–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Paruthiyil S, Parmar H, Kerekatte V, Cunha
GR, Firestone GL and Leitman DC: Estrogen receptor beta inhibits
human breast cancer cell proliferation and tumor formation by
causing a G2 cell cycle arrest. Cancer Res. 64:423–428. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Gao L, Schwartzman J, Gibbs A, Lisac R,
Kleinschmidt R, Wilmot B, Bottomly D, Coleman I, Nelson P, McWeeney
S and Alumkal J: Androgen receptor promotes ligand-independent
prostate cancer progression through c-Myc upregulation. PLoS One.
8:e635632013. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Smart E, Semina SE and Frasor J: Update on
the role of NFκB in promoting aggressive phenotypes of estrogen
receptor-positive breast cancer. Endocrinology. 161:bqaa1522020.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Mak P, Li J, Samanta S and Mercurio AM:
ERβ regulation of NF-kB activation in prostate cancer is mediated
by HIF-1. Oncotarget. 6:40247–40254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Zhang L, Altuwaijri S, Deng F, Chen L, Lal
P, Bhanot UK, Korets R, Wenske S, Lilja HG, Chang C, et al:
NF-kappaB regulates androgen receptor expression and prostate
cancer growth. Am J Pathol. 175:489–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Binai NA, Damert A, Carra G, Steckelbroeck
S, Löwer J, Löwer R and Wessler S: Expression of estrogen receptor
alpha increases leptin-induced STAT3 activity in breast cancer
cells. Int J Cancer. 127:55–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wang HC, Yeh HH, Huang WL, Lin CC, Su WP,
Chen HHW, Lai WW and Su WC: Activation of the signal transducer and
activator of transcription 3 pathway up-regulates estrogen
receptor-beta expression in lung adenocarcinoma cells. Mol
Endocrinol. 25:1145–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Yamamoto T, Sato N, Sekine Y, Yumioka T,
Imoto S, Junicho A, Fuse H and Matsuda T: Molecular interactions
between STAT3 and protein inhibitor of activated STAT3, and
androgen receptor. Biochem Biophys Res Commun. 306:610–615. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Csabai L, Fazekas D, Kadlecsik T,
Szalay-Bekő M, Bohár B, Madgwick M, Módos D, Ölbei M, Gul L,
Sudhakar P, et al: SignaLink3: A multi-layered resource to uncover
tissue-specific signaling networks. Nucleic Acids Res. 50(D1):
D701–D709. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Lin Z, Yin P, Reierstad S, O'Halloran M,
Coon VJS, Pearson EK, Mutlu GM and Bulun SE: Adenosine A1 receptor,
a target and regulator of estrogen receptoralpha action, mediates
the proliferative effects of estradiol in breast cancer. Oncogene.
29:1114–1122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Xu J, Wu F, Zhu Y, Wu T, Cao T, Gao W, Liu
M, Qian W, Feng G, Xi X and Hou S: ANGPTL4 regulates ovarian cancer
progression by activating the ERK1/2 pathway. Cancer Cell Int.
24:542024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Zhao N, Peacock SO, Lo CH, Heidman LM,
Rice MA, Fahrenholtz CD, Greene AM, Magani F, Copello VA, Martinez
MJ, et al: Arginine vasopressin receptor 1a is a therapeutic target
for castration-resistant prostate cancer. Sci Transl Med.
11:eaaw46362019. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Guan B, Ma J, Yang Z, Yu F and Yao J:
LncRNA NCK1-AS1 exerts oncogenic property in gastric cancer by
targeting the miR-22-3p/BCL9 axis to activate the Wnt/β-catenin
signaling. Environ Toxicol. 36:1640–1653. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Wang X, Feng M, Xiao T, Guo B, Liu D, Liu
C, Pei J, Liu Q, Xiao Y, Rosin-Arbesfeld R, et al: BCL9/BCL9L
promotes tumorigenicity through immune-dependent and independent
mechanisms in triple negative breast cancer. Oncogene.
40:2982–2997. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Li P, Miao C, Liang C, Shao P, Wang Z and
Li J: Silencing CAPN2 expression inhibited castration-resistant
prostate cancer cells proliferation and invasion via AKT/mTOR
signal pathway. Biomed Res Int. 2017:25936742017.PubMed/NCBI
|
|
182
|
Fang X, Hong Y, Dai L, Qian Y, Zhu C, Wu B
and Li S: CRH promotes human colon cancer cell proliferation via
IL-6/JAK2/STAT3 signaling pathway and VEGF-induced tumor
angiogenesis. Mol Carcinog. 56:2434–2445. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Jin C, Han-Hua D, Qiu-Meng L, Deng N,
Peng-Chen D, Jie M, Lei X, Xue-Wu Z, Hui-Fang L, Yan C, et al:
MTDH-stabilized DDX17 promotes tumor initiation and progression
through interacting with YB1 to induce EGFR transcription in
hepatocellular carcinoma. Oncogene. 42:169–183. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Chen Z, Song Y, Li P and Gao W: GRIN2D
knockdown suppresses the progression of lung adenocarcinoma by
regulating the E2F signalling pathway. Cell Signal. 107:1106852023.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Yan T, Huang L, Yan Y, Zhong Y, Xie H and
Wang X: MAPK/AP-1 signaling pathway is involved in the protection
mechanism of bone marrow mesenchymal stem cells-derived exosomes
against ultraviolet-induced photoaging in human dermal fibroblasts.
Skin Pharmacol Physiol. 36:98–106. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Guo Z, Peng G, Li E, Xi S, Zhang Y, Li Y,
Lin X, Li G, Wu Q and He J: MAP kinase-interacting serine/threonine
kinase 2 promotes proliferation, metastasis, and predicts poor
prognosis in non-small cell lung cancer. Sci Rep. 7:106122017.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Gupta A, Hossain MM, Miller N, Kerin M,
Callagy G and Gupta S: NCOA3 coactivator is a transcriptional
target of XBP1 and regulates PERK-eIF2α-ATF4 signalling in breast
cancer. Oncogene. 35:5860–5871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Baquié M, St-Onge L, Kerr-Conte J,
Cobo-Vuilleumier N, Lorenzo PI, Jimenez Moreno CM, Cederroth CR,
Nef S, Borot S, Bosco D, et al: The liver receptor homolog-1
(LRH-1) is expressed in human islets and protects {beta}-cells
against stress-induced apoptosis. Hum Mol Genet. 20:2823–2833.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zhu J, Zou Z, Nie P, Kou X, Wu B, Wang S,
Song Z and He J: Downregulation of microRNA-27b-3p enhances
tamoxifen resistance in breast cancer by increasing NR5A2 and CREB1
expression. Cell Death Dis. 7:e24542016. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Kao TW, Chen HH, Lin J, Wang TL and Shen
YA: PBX1 as a novel master regulator in cancer: Its regulation,
molecular biology, and therapeutic applications. Biochim Biophys
Acta Rev Cancer. 1879:1890852024. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Elix C, Pal SK and Jones JO: The role of
peroxisome proliferator-activated receptor gamma in prostate
cancer. Asian J Androl. 20:238–243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Carotenuto M, De Antonellis P, Liguori L,
Benvenuto G, Magliulo D, Alonzi A, Turino C, Attanasio C, Damiani
V, Bello AM, et al: H-Prune through GSK-3β interaction sustains
canonical WNT/β-catenin signaling enhancing cancer progression in
NSCLC. Oncotarget. 5:5736–5749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Teng F, Zhang JX, Chen Y, Shen XD, Su C,
Guo YJ, Wang PH, Shi CC, Lei M, Cao YO and Liu SQ: LncRNA
NKX2-1-AS1 promotes tumor progression and angiogenesis via
upregulation of SERPINE1 expression and activation of the VEGFR-2
signaling pathway in gastric cancer. Mol Oncol. 15:1234–1255. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Sun G, Wei Y, Zhou B, Wang M, Luan R, Bai
Y, Li H, Wang S, Zheng D, Wang C, et al: BAP18 facilitates
CTCF-mediated chromatin accessible to regulate enhancer activity in
breast cancer. Cell Death Differ. 30:1260–1278. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Pospiech K, Orzechowska M, Nowakowska M,
Anusewicz D, Płuciennik E, Kośla K and Bednarek AK: TGFα-EGFR
pathway in breast carcinogenesis, association with WWOX expression
and estrogen activation. J Appl Genet. 63:339–359. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Maeda A, Nishino T, Matsunaga R, Yokoyama
A, Suga H, Yagi T and Konishi H: Transglutaminase-mediated
cross-linking of WDR54 regulates EGF receptor-signaling. Biochim
Biophys Acta Mol Cell Res. 1866:285–295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Cao L, Petrusca DN, Satpathy M, Nakshatri
H, Petrache I and Matei D: Tissue transglutaminase protects
epithelial ovarian cancer cells from cisplatin-induced apoptosis by
promoting cell survival signaling. Carcinogenesis. 29:1893–1900.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Qian Y, Liu X, Feng Y, Li X and Xuan Y:
Tenascin C regulates cancer cell glycolysis and tumor progression
in prostate cancer. Int J Urol. 29:578–585. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wei L, Sun C, Zhang Y, Han N and Sun S:
miR-503-5p inhibits colon cancer tumorigenesis, angiogenesis, and
lymphangiogenesis by directly downregulating VEGF-A. Gene Ther.
29:28–40. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Xiao L, Parolia A, Qiao Y, Bawa P, Eyunni
S, Mannan R, Carson SE, Chang Y, Wang X, Zhang Y, et al: Targeting
SWI/SNF ATPases in enhancer-addicted prostate cancer. Nature.
601:434–439. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Kim YC, Chen C and Bolton EC: Androgen
receptor-mediated growth suppression of HPr-1AR and PC3-Lenti-AR
prostate epithelial cells. PLoS One. 10:e01382862015. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zhou D, Chen B, Ye JJ and Chen S: A novel
crosstalk mechanism between nuclear receptor-mediated and growth
factor/Ras-mediated pathways through PNRC-Grb2 interaction.
Oncogene. 23:5394–5404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Huang YL, Chou WC, Hsiung CN, Hu LY, Chu
HW and Shen CY: FGFR2 regulates Mre11 expression and double-strand
break repair via the MEK-ERK-POU1F1 pathway in breast
tumorigenesis. Hum Mol Genet. 24:3506–3517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Hua S, Kittler R and White KP: Genomic
antagonism between retinoic acid and estrogen signaling in breast
cancer. Cell. 137:1259–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
O'Neill CF, Urs S, Cinelli C, Lincoln A,
Nadeau RJ, León R, Toher J, Mouta-Bellum C, Friesel RE and Liaw L:
Notch2 signaling induces apoptosis and inhibits human MDA-MB-231
×enograft growth. Am J Pathol. 171:1023–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Xiu MX and Liu YM: The role of oncogenic
Notch2 signaling in cancer: A novel therapeutic target. Am J Cancer
Res. 9:837–854. 2019.PubMed/NCBI
|
|
207
|
Shen T, Wang W, Zhou W, Coleman I, Cai Q,
Dong B, Ittmann MM, Creighton CJ, Bian Y, Meng Y, et al: MAPK4
promotes prostate cancer by concerted activation of androgen
receptor and AKT. J Clin Invest. 131:e1354652021. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Ross-Innes CS, Brown GD and Carroll JS: A
co-ordinated interaction between CTCF and ER in breast cancer
cells. BMC Genomics. 12:5932011. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Yang SZ and Abdulkadir SA: Early growth
response gene 1 modulates androgen receptor signaling in prostate
carcinoma cells. J Biol Chem. 278:39906–39911. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Grinshpun A, Chen V, Sandusky ZM, Fanning
SW and Jeselsohn R: ESR1 activating mutations: From structure to
clinical application. Biochim Biophys Acta Rev Cancer.
1878:1888302023. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Barker R, Biernacka K, Kingshott G, Sewell
A, Gwiti P, Martin RM, Lane JA, McGeagh L, Koupparis A, Rowe E, et
al: Associations of CTCF and FOXA1 with androgen and IGF pathways
in men with localized prostate cancer. Growth Horm IGF Res. 69–70.
1015332023.
|
|
212
|
Pu J, Zhang D, Wang B, Zhu P, Yang W, Wang
K, Yang Z and Song Q: FOXA1/UBE2T inhibits CD8+T cell
activity by inducing mediates glycolysis in lung adenocarcinoma.
Front Biosci (Landmark Ed). 29:1342024. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Vaclavicek A, Bermejo JL, Schmutzler RK,
Sutter C, Wappenschmidt B, Meindl A, Kiechle M, Arnold N, Weber
BHF, Niederacher D, et al: Polymorphisms in the Janus kinase 2
(JAK)/signal transducer and activator of transcription (STAT)
genes: Putative association of the STAT gene region with familial
breast cancer. Endocr Relat Cancer. 14:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Xu WF, Ma YC, Ma HS, Shi L, Mu H, Ou WB,
Peng J, Li TT, Qin T, Zhou HM, et al: Co-targeting CK2α and YBX1
suppresses tumor progression by coordinated inhibition of the
PI3K/AKT signaling pathway. Cell Cycle. 18:3472–3490. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Liu X, Xu M, Jia W, Duan Y, Ma J and Tai
W: PU.1 negatively regulates tumorigenesis in non-small-cell lung
cancer. Med Oncol. 40:792023. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Tortorella E, Giantulli S, Sciarra A and
Silvestri I: AR and PI3K/AKT in prostate cancer: A tale of two
interconnected pathways. Int J Mol Sci. 24:20462023. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Hua S, Kallen CB, Dhar R, Baquero MT,
Mason CE, Russell BA, Shah PK, Liu J, Khramtsov A, Tretiakova MS,
et al: Genomic analysis of estrogen cascade reveals histone variant
H2A.Z associated with breast cancer progression. Mol Syst Biol.
4:1882008. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Taslim C, Chen Z, Huang K, Huang TH, Wang
Q and Lin S: Integrated analysis identifies a class of
androgen-responsive genes regulated by short combinatorial
long-range mechanism facilitated by CTCF. Nucleic Acids Res.
40:4754–4764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Zhang H, Li S, Zhou R, Dong T, Zhang X, Yu
M, Lin J, Shi M, Geng E, Li J, et al: SRCAP complex promotes lung
cancer progression by reprograming the oncogenic transcription of
Hippo-YAP/TAZ signaling pathway. Cancer Lett. 585:2166672024.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Stelzer G, Rosen R, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The GeneCards suite: From gene data mining to disease genome
sequence analyses. Curr Protoc Bioinformatics. 54:1.30.1–1.30.33.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
221
|
Montes-de-Oca-Fuentes EV, Jácome-López K,
Zarco-Mendoza A, Guerrero G, Ventura-Gallegos JL, Juárez-Méndez S,
Cabrera-Quintero AJ, Recillas-Targa F and Zentella-Dehesa A:
Differential DNA methylation and CTCF binding between the ESR1
promoter a of MCF-7 and MDA-MB-231 breast cancer cells. Mol Biol
Rep. 51:1482024. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Rossi EL, Dunlap SM, Bowers LW, Khatib SA,
Doerstling SS, Smith LA, Ford NA, Holley D, Brown PH, Estecio MR,
et al: Energy balance modulation impacts epigenetic reprogramming,
ERα and ERβ expression, and mammary tumor development in MMTV-neu
transgenic mice. Cancer Res. 77:2500–2511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Chaudhary S, Krishna BM and Mishra SK: A
novel FOXA1/ESR1 interacting pathway: A study of
Oncomine™ breast cancer microarrays. Oncol Lett.
14:1247–1264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Zhang Y, Huang YX, Wang DL, Yang B, Yan
HY, Lin LH, Li Y, Chen J, Xie LM, Huang YS, et al: LncRNA DSCAM-AS1
interacts with YBX1 to promote cancer progression by forming a
positive feedback loop that activates FOXA1 transcription network.
Theranostics. 10:10823–10837. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Wu Y, Li Z, Wedn AM, Casey AN, Brown D,
Rao SV, Omarjee S, Hooda J, Carroll JS, Gertz J, et al: FOXA1
reprogramming dictates retinoid X receptor response in ESR1-mutant
breast cancer. Mol Cancer Res. 21:591–604. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Lupien M, Eeckhoute J, Meyer CA, Wang Q,
Zhang Y, Li W, Carroll JS, Liu XS and Brown M: FoxA1 translates
epigenetic signatures into enhancer-driven lineage-specific
transcription. Cell. 132:958–970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Rangel N, Fortunati N, Osella-Abate S,
Annaratone L, Isella C, Catalano MG, Rinella L, Metovic J,
Boldorini R, Balmativola D, et al: FOXA1 and AR in invasive breast
cancer: New findings on their co-expression and impact on prognosis
in ER-positive patients. BMC Cancer. 18:7032018. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Seachrist DD, Anstine LJ and Keri RA:
FOXA1: A pioneer of nuclear receptor action in breast cancer.
Cancers (Basel). 13:52052021. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Tsirigoti C, Ali MM, Maturi V, Heldin CH
and Moustakas A: Loss of SNAI1 induces cellular plasticity in
invasive triple-negative breast cancer cells. Cell Death Dis.
13:8322022. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Gerhardt J, Montani M, Wild P, Beer M,
Huber F, Hermanns T, Müntener M and Kristiansen G: FOXA1 promotes
tumor progression in prostate cancer and represents a novel
hallmark of castration-resistant prostate cancer. Am J Pathol.
180:848–861. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Jin HJ, Zhao JC, Ogden I, Bergan RC and Yu
J: Androgen receptor-independent function of FoxA1 in prostate
cancer metastasis. Cancer Res. 73:3725–3736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Li Z, Tuteja G, Schug J and Kaestner KH:
Foxa1 and Foxa2 are essential for sexual dimorphism in liver
cancer. Cell. 148:72–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Ross-Innes CS, Stark R, Holmes KA, Schmidt
D, Spyrou C, Russell R, Massie CE, Vowler SL, Eldridge M and
Carroll JS: Cooperative interaction between retinoic acid
receptor-alpha and estrogen receptor in breast cancer. Genes Dev.
24:171–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Salvatori L, Ravenna L, Caporuscio F,
Principessa L, Coroniti G, Frati L, Russo MA and Petrangeli E:
Action of retinoic acid receptor on EGFR gene transactivation and
breast cancer cell proliferation: Interplay with the estrogen
receptor. Biomed Pharmacother. 65:307–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Gorodetska I, Offermann A, Püschel J,
Lukiyanchuk V, Gaete D, Kurzyukova A, Freytag V, Haider MT, Fjeldbo
CS, Di Gaetano S, et al: ALDH1A1 drives prostate cancer metastases
and radioresistance by interplay with AR- and RAR-dependent
transcription. Theranostics. 14:714–737. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Zou A, Marschke KB, Arnold KE, Berger EM,
Fitzgerald P, Mais DE and Allegretto EA: Estrogen receptor beta
activates the human retinoic acid receptor alpha-1 promoter in
response to tamoxifen and other estrogen receptor antagonists, but
not in response to estrogen. Mol Endocrinol. 13:418–430. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Magnani L, Patten DK, Nguyen VT, Hong SP,
Steel JH, Patel N, Lombardo Y, Faronato M, Gomes AR, Woodley L, et
al: The pioneer factor PBX1 is a novel driver of metastatic
progression in ERα-positive breast cancer. Oncotarget.
6:21878–21891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Magnani L, Ballantyne EB, Zhang X and
Lupien M: PBX1 genomic pioneer function drives ERα signaling
underlying progression in breast cancer. PLoS Genet.
7:e10023682011. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Kikugawa T, Kinugasa Y, Shiraishi K, Nanba
D, Nakashiro KI, Tanji N, Yokoyama M and Higashiyama S: PLZF
regulates Pbx1 transcription and Pbx1-HoxC8 complex leads to
androgen-independent prostate cancer proliferation. Prostate.
66:1092–1099. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Shen T, Dong B, Meng Y, Moore DD and Yang
F: A COP1-GATA2 axis suppresses AR signaling and prostate cancer.
Proc Natl Acad Sci USA. 119:e22053501192022. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Yang X, Zhang Q, Li S, Devarajan R, Luo B,
Tan Z, Wang Z, Giannareas N, Wenta T, Ma W, et al: GATA2 co-opts
TGFβ1/SMAD4 oncogenic signaling and inherited variants at 6q22 to
modulate prostate cancer progression. J Exp Clin Cancer Res.
42:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Chaytor L, Simcock M, Nakjang S, Heath R,
Walker L, Robson C, Jones D and Gaughan L: The pioneering role of
GATA2 in androgen receptor variant regulation is controlled by
bromodomain and extraterminal proteins in castrate-resistant
prostate cancer. Mol Cancer Res. 17:1264–1278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Wang Y, He X, Ngeow J and Eng C: GATA2
negatively regulates PTEN by preventing nuclear translocation of
androgen receptor and by androgen-independent suppression of PTEN
transcription in breast cancer. Hum Mol Genet. 21:569–576. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Treeck O, Diepolder E, Skrzypczak M,
Schüler-Toprak S and Ortmann O: Knockdown of estrogen receptor β
increases proliferation and affects the transcriptome of
endometrial adenocarcinoma cells. BMC Cancer. 19:7452019.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Konduri SD, Medisetty R, Liu W,
Kaipparettu BA, Srivastava P, Brauch H, Fritz P, Swetzig WM,
Gardner AE, Khan SA and Das GM: Mechanisms of estrogen receptor
antagonism toward p53 and its implications in breast cancer
therapeutic response and stem cell regulation. Proc Natl Acad Sci
USA. 107:15081–15086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Fritah A, Saucier C, Mester J, Redeuilh G
and Sabbah M: p21WAF1/CIP1 selectively controls the transcriptional
activity of estrogen receptor alpha. Mol Cell Biol. 25:2419–2430.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Nishimura FG, Sampaio BB, Komoto TT, da
Silva WJ, da Costa MMG, Haddad GI, Peronni KC, Evangelista AF,
Hossain M, Dimmock JR, et al: Exploring CDKN1A upregulation
mechanisms: Insights into cell cycle arrest induced by NC2603
curcumin analog in MCF-7 breast cancer cells. Int J Mol Sci.
25:49892024. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Mukhopadhyay UK, Oturkar CC, Adams C,
Wickramasekera N, Bansal S, Medisetty R, Miller A, Swetzig WM,
Silwal-Pandit L, Børresen-Dale AL, et al: TP53 status as a
determinant of pro-vs anti-tumorigenic effects of estrogen
receptor-beta in breast cancer. J Natl Cancer Inst. 111:1202–1215.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
He Y, Alejo S, Venkata PP, Johnson JD,
Loeffel I, Pratap UP, Zou Y, Lai Z, Tekmal RR, Kost ER and Sareddy
GR: Therapeutic targeting of ovarian cancer stem cells using
estrogen receptor beta agonist. Int J Mol Sci. 23:71592022.
View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Lee CI, Goodwin A and Wilcken N:
Fulvestrant for hormone-sensitive metastatic breast cancer.
Cochrane Database Syst Rev. CD0110932017.PubMed/NCBI
|
|
251
|
Augusto TV, Amaral C, Varela CL, Bernardo
F, da Silva ET, Roleira FFM, Costa S, Teixeira N and
Correia-da-Silva G: Effects of new C6-substituted steroidal
aromatase inhibitors in hormone-sensitive breast cancer cells: Cell
death mechanisms and modulation of estrogen and androgen receptors.
J Steroid Biochem Mol Biol. 195:1054862019. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Jacobson EM, Hugo ER, Tuttle TR, Papoian R
and Ben-Jonathan N: Unexploited therapies in breast and prostate
cancer: Blockade of the prolactin receptor. Trends Endocrinol
Metab. 21:691–698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Basile D, Cinausero M, Iacono D, Pelizzari
G, Bonotto M, Vitale MG, Gerratana L and Puglisi F: Androgen
receptor in estrogen receptor positive breast cancer: Beyond
expression. Cancer Treat Rev. 61:15–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Bonkhoff H, Fixemer T, Hunsicker I and
Remberger K: Estrogen receptor expression in prostate cancer and
premalignant prostatic lesions. Am J Pathol. 155:641–647. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Robinson JLL, Macarthur S, Ross-Innes CS,
Tilley WD, Neal DE, Mills IG and Carroll JS: Androgen receptor
driven transcription in molecular apocrine breast cancer is
mediated by FoxA1. EMBO J. 30:3019–3027. 2011. View Article : Google Scholar : PubMed/NCBI
|