Introduction
Nasopharyngeal carcinoma (NPC) is a malignant
neoplasm originating from the parietal epithelial cells of the
nasopharynx (1). It is the most
prevalent type of cancer in otorhinolaryngology, with
age-standardized rates typically <1 per 100,000 person-years
(2). NPC is distinguished by its
localized distribution, complex etiology, subtle onset, extensive
metastasis and high invasiveness (1). The primary clinical strategy for the
management of NPC is radiotherapy, albeit with numerous adverse
effects (1,3). While the combination of
chemo-radiotherapy yields a satisfactory 5-year survival rate of
85–90%, a recurrence and tumor metastasis still occur in 8–10% of
patients (4). Hence, exploring its
pathogenesis and identifying novel drugs and therapeutic targets is
of utmost importance.
MicroRNAs (miRNAs/miRs) are a class of small RNA
molecules (19–24 nucleotides in length) that exert regulatory
control over gene expression by selectively binding and impeding
the translation of specific mRNA molecules (5–7). These
molecules occupy a pivotal position in a multitude of biological
processes, encompassing developmental events, cellular signaling
cascades and metabolic pathways (5,8). In
tumorigenesis, miRNAs can function as either oncogenes or tumor
suppressors, serves a crucial role in the regulation of cell
proliferation, apoptosis, invasion and metastasis (9). The dysregulation of miRNA expression
(such as miRNA-106a-5p, miRNA-9 and miRNA-194) has been observed in
NPC, contributing to aberrant cellular growth and the development
of NPC (10,11).
Among the numerous miRNAs identified, miRNA-22-3p
has emerged as a novel cancer-associated miRNA. A previous study
indicated that repression of miRNA-22-3p expression resulted in the
suppression of the proliferative ability and the arrest of cell
cycle progression, both of which were subsequently restored upon
the overexpression of cyclin dependent kinase inhibitor 2C (CDKN2C)
(12). In another study on patients
diagnosed with glioblastoma, a marked increased expression of
miRNA-22-3p was observed compared with that in healthy controls
(13). Additionally, miRNA-22-3p
has been reported to suppress human hepatocellular carcinoma cell
proliferation and metastasis by modulating the activity of
methylenetetrahydrofolate reductase (14). However, the role of miRNA-22-3p in
NPC remains largely unclear. Thus, the present study aimed to
elucidate the function and underlying molecular mechanisms of
miRNA-22-3p in NPC.
Forkhead box protein 1 (FOXP1), a forkhead box
transcription factor, has garnered significant attention due to its
association with cancer development and progression. The altered
expression of FOXP1 has been observed in several malignancies,
including lymphomas, breast cancer, prostate cancer and others
(15). Depending on the cancer type
and cellular context, FOXP1 can function as either a tumor
suppressor or oncogene, highlighting its complex role in
tumorigenesis (16). The aberrant
expression of FOXP1 is often associated with aggressive tumor
phenotypes, worse prognoses and resistance to therapy. Therefore,
elucidating the precise mechanisms through which FOXP1 regulates
cancer development and progression holds promise for identifying
novel therapeutic targets. The aim of the present study was to
explore the role and mechanisms of action of miRNA-22 and FOXP1 in
the occurrence and development of NPC.
Materials and methods
Bioinformatics analysis
StarBase (targetscan.org/vert_80/) and TargetScan
(rnasysu.com/encori/) was used to predict binding sites between
miRNAs and target genes.
Clinical specimens
The study protocols were approved by the Ethics
Committee of the Affiliated Hospital of Guizhou Medical University
(Guiyang, China; approval no. 2021-019). A total of 15 pairs of NPC
tissues and para-cancerous tissue samples were collected from
patients (10 male and 5 female patients; age, 54.60±11.01 years;
range, 36–72 years) who underwent surgery at the Affiliated
Hospital of Guizhou Medical University between February 2011 and
October 2022. Written informed consent was obtained from all
patients prior to the collection of samples. The tumor specimens
were obtained from surgical resections of the patients, and none of
the patients had undergone chemotherapy or radiotherapy prior to
tumor excision. Inclusion criteria were as follows: 1. All patients
must be pathologically diagnosed with NPC. 2. Include patients with
untreated, newly diagnosed NPC. Exclusion Criteria: 1. Exclude
patients with a history of other malignant tumors to avoid
confounding factors. 2. Exclude patients with severe complications
or systemic diseases, such as severe liver or kidney dysfunction,
heart disease, etc. 3. Exclude patients who have received
treatment.
Cells and cell culture
Human nasopharyngeal epithelial NP69SV40T cell lines
were purchased from Procell Life Science & Technology Co., Ltd.
Human NPC cell lines (C666-1 and HK-1) were purchased from iCell
Bioscience, Inc. The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) at a temperature of
37°C with 5% CO2 in a humidified incubator.
Transfection
Lipofectamine 3000® transfection reagent
(Thermo Fisher Scientific, Inc.) was used for the transient
transfection of pcDNA-negative control (NC), pcDNA-FOXP1 (100 nM),
NC mimic or miRNA-22-3p mimic (50 nM) into HK-1 cells
(1.0×105 cells/well). NC mimic (cat. no. B04002) and
miRNA-22-3p mimic (cat. no. B02001) were synthesized by Shanghai
GenePharma Co., Ltd. miRNA-22-3p mimics sequence were as follows:
Sense, 5′-AAGCUGCCAGUUGAAGAACUGU-3′; antisense,
5′-AGUUCUUCAACUGGCAGCUUUU-3′. NC mimics sequences were as follows:
Sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. Following transfection at 37°C for 20
min, the cells were cultured for 48 h prior to further
experiments.
Reverse transcription
(RT)-quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from the NP69SV40T, C666-1
and HK-1 cells or NPC tissues using TRIzol™ reagent
(Thermo Fisher Scientific, Inc.). cDNA was synthesized using a RT
kit (cat. no. 11904018; Invitrogen™; Thermo Fisher
Scientific, Inc.). The RT reaction conditions were as follows: 95°C
for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30
sec. The relative levels of target gene RNA transcriptome were
determined using qPCR using the SYBR Premix Ex Taq kit (cat. no.
RR820; Takara Bio Inc.). The thermocycling conditions were as
follows: Initial denaturation at 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 60 sec. The relative gene
expression level was calculated using the 2−ΔΔCq method
(17) using ABI software (Veriti
96-Well; Thermo Fisher Scientific, Inc.). The following primer
sequences were used for RT-qPCR: FOXP1 forward,
5′-TCCAGAAAAGCAGCTAACACTA-3′ and reverse,
5′-TTCTACTCGCACAAAACACTTG-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCTGTTGCTGTAGCCAAA-3′; miRNA-22-3p forward,
5′-AAGCTGCCAGTTGAAGAACTGTA-3′ and reverse,
5′-GCTGTCAACGATACGCTACGTA-3′; U6 forward,
5′-ACTTCAGCAGCACATATACTAAAAA-3′ and reverse,
5′-CGCTTCACGAATTTGCATGTCAT-3′. cDNA was synthesized using the Mir-X
miRNA FirstStrand Synthesis Kit (cat. no. 638315; Takara Bio Inc.)
The relative levels of target miRNA transcripts were determined by
RT-qPCR using the Mir-X miRNA qRT-PCR TB Green Kit (cat. no.
638316; Takara Bio Inc.).
Western blot analysis
The HK-1 cell line was used for western blot
analysis. The cell lysis solution was prepared using RIPA buffer
from Cell Signaling Technology, Inc. The protein concentration was
determined using a BCA assay. A total of 30 µg protein/lane was
separated using 10% SDS-PAGE and transferred onto nitrocellulose
membranes. The membranes were then blocked with 5% non-fat dried
milk overnight at 4°C and incubated with the following
corresponding protein antibodies: E-cadherin (1:2,000; cat. no.
A3044; ABclonal Biotech Co., Ltd.), N-cadherin (1:2,000; cat. no.
BS-1172R; BIOSS), vimentin (1:2,000; cat. no. A19607; ABclonal
Biotech Co., Ltd.), FOXP1 (1:2,000; cat. no. ab134055133595; Abcam)
and β-actin (1:50,000; cat. no. AC026; ABclonal Biotech Co., Ltd.)
overnight at 4°C.
Subsequently, the membranes underwent a washing
process with Tris-buffered saline/0.1% Tween (TBST) and were
subjected to a 1.5-h incubation period at room temperature with a
HRP goat anti-rabbit IgG (1:5,000; cat. no. S0001; Affinity
Biosciences, Ltd.). The bands were visualized using an ECL
detection system (ECL Plus; Cytiva), with β-actin serving as the
internal control. The net optical density was semi-quantified using
Quantity One software (V4.6.2; Bio-Rad Laboratories, Inc.).
Dual-luciferase reporter assay
Wild-type (Wt) and mutant (Mut) FOXP1-3′
untranslated region (3′UTR) sequences were cloned into the
luciferase reporter plasmid psiCHECK-2 vector (cat. no. C8021;
Promega Corporation). Subsequently, the luciferase reporter gene
plasmid and either miRNA-22-3p mimic or NC mimic were
co-transfected into 293T cells (Procell Life Science &
Technology Co., Ltd.; 4×104 cells/well) using
Lipofectamine 3000® (Thermo Fisher Scientific, Inc.).
The dual-luciferase activity was measured 48 h after transfection.
The dual-Luciferase reporter system (cat. no. E1910; Promega
Corporation) was used to quantify luciferase activities according
to manufacturer's protocol. Luciferase activity was standardized by
comparison with Renilla luciferase activity.
Cell counting kit-8 (CCK-8) assay
The viability of the HK-1 cells was assessed using
the CCK-8 assay (Thermo Fisher Scientific, Inc.) following the
manufacturer's guidelines. CCK-8 was added into each well and
incubated for 3 h. The absorbance was measured at 450 nm.
Wound-healing assay
HK-1 cells were cultured in 96-well plates until
they reached confluency in DMEM supplemented with 10% FBS at 37°C.
The cell monolayers were gently scratched using a 200-µl pipette
tip and the cells were incubated in serum-free DMEM for 24 h at
37°C. To remove any detached cells, the wells of the plate were
gently washed with fresh medium. The distance between edges of the
wound was measured under a light microscope (Olympus Corporation),
and multiple visual fields were selected for observing each well.
After 24 h, the wound channel distance was measured again for
analysis. The wound area was measured using Image J software
(Version 1.48; National Institutes of Health). Wound-healing assay
results were presented as migration rate (%)=(initial wound
area-wound area at 24 h)/initial wound area ×100.
Transwell assay
A concentration of 1×105 HK-1 cells/ml
was suspended in DMEM, and 200 µl of the cell suspension was plated
into the upper chambers of 24-well Transwell plate precoated with
Matrigel (BD Biosciences) at room temperature for 24 h. The lower
chambers were filled with 600 µl DMEM supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.). Subsequently, the cells
were incubated in a 5% CO2 and 37°C incubator for 48 h.
Following this, the cells were fixed with 4% paraformaldehyde at
room temperature for 20 min and stained with 0.1% crystal violet at
room temperature for 15 min. The total number of cells in five
randomly selected fields of view was observed using an inverted
light microscope (Olympus Corporation) and the mean number of cells
was calculated.
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS 20.0
software (IBM Corp.). Multiple groups were compared using one-way
analysis of variance followed by Tukey's post hoc test, and two
groups were compared using unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miRNA-22-3p and FOXP1
differs between NPC tissues and para-cancerous tissues
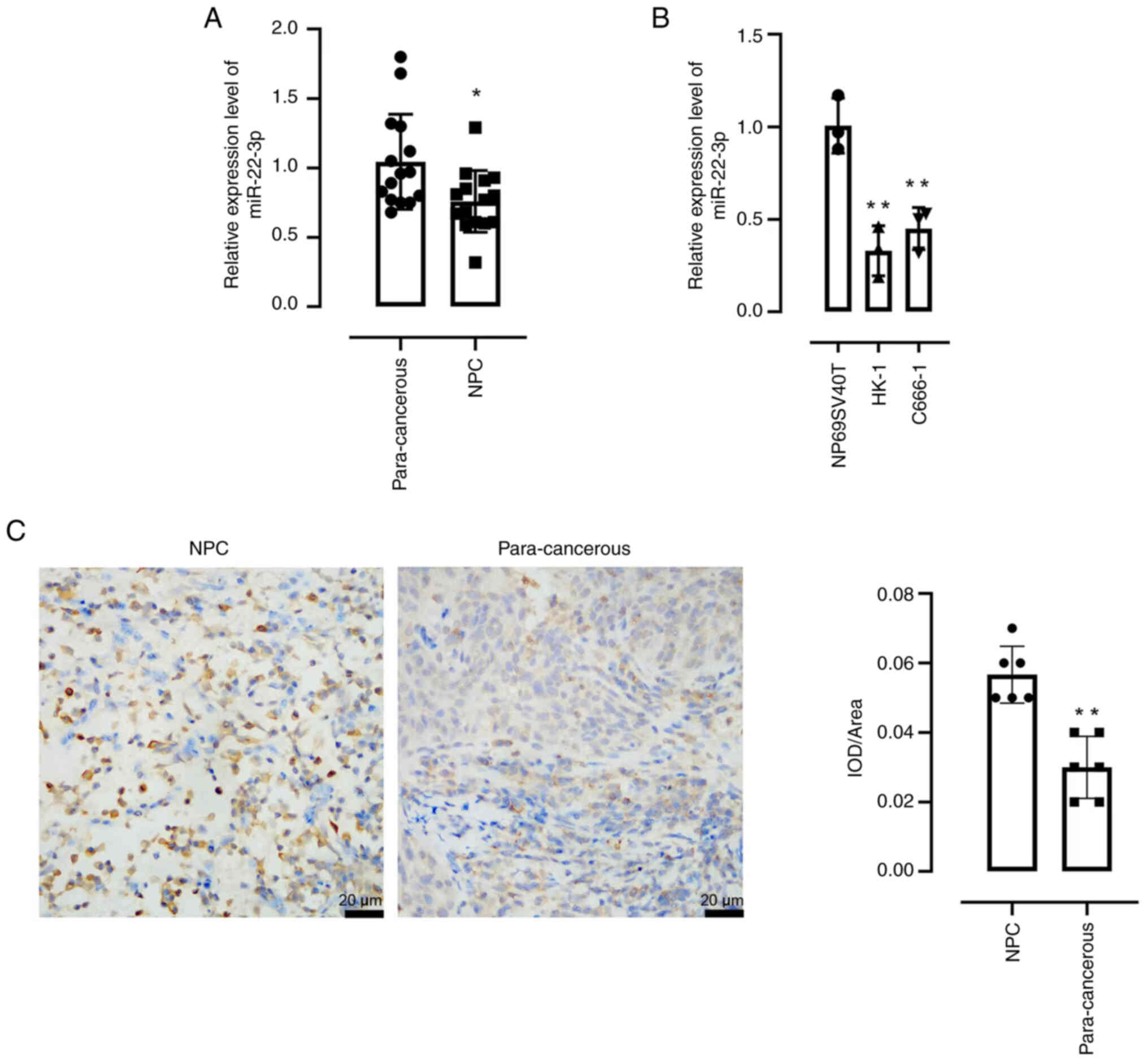
The expression of miRNA-22-3p was demonstrated to be
significantly reduced in NPC tissues compared with para-cancerous
tissues, as shown by RT-qPCR (Fig.
1A). Moreover, significantly decreased expression of
miRNA-22-3p also observed in the NPC cell lines, HK-1 and C666-1
compared with normal nasopharyngeal epithelial cells (Fig. 1B). In addition, the expression
levels of FOXP1 were significantly increased in NPC issues compared
with para-cancerous tissues (Fig.
1C).
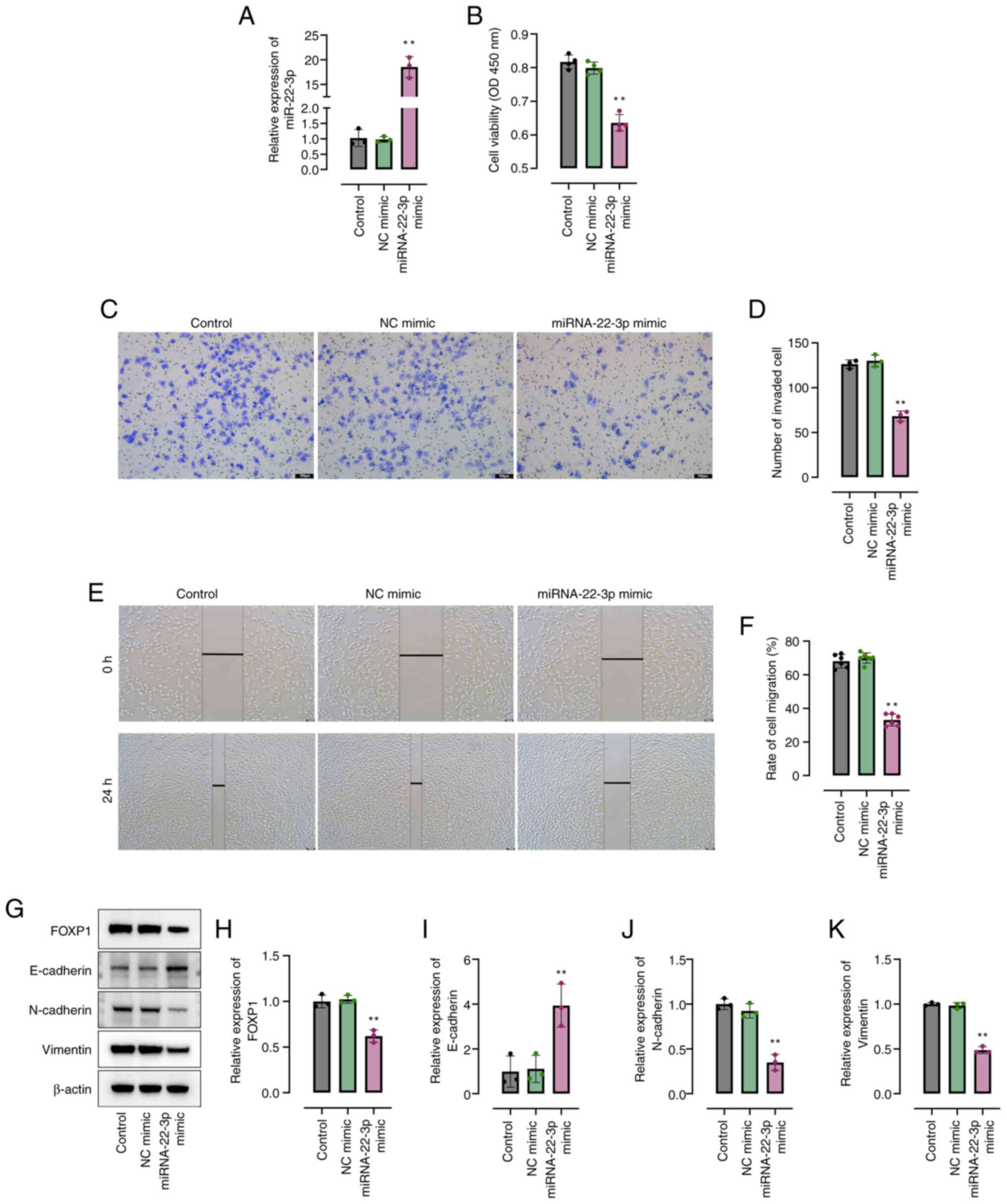
Overexpression of miRNA-22-3p reduces
the malignant behaviors of NPC cells
miRNA-22-3p mimic was constructed to enhance
miRNA-22-3p expression. Compared with the control group, the
miRNA-22-3p levels significantly increased effectively by
transfection with miRNA-22-3p mimic in HK-1 cells (Fig. 2A). Furthermore, the results revealed
that compared to the control group, miRNA-22-3p mimic significantly
inhibited the viability of HK-1 cells (Fig. 2B). Moreover, compared to the control
group, the overexpression of miRNA-22-3p significantly suppressed
the migration of HK-1 cells (Fig.
2C-F). Compared to the control group, the expression of FOXP1
and the epithelial-mesenchymal transition-related proteins,
vimentin and N-cadherin, was significantly reduced, and the
expression of E-cadherin was significantly induced in miRNA-22-3p
mimic-transfected HK-1 cells (Fig.
2G-K).
Binding association between
miRNA-22-3p and FOXP1
Using the bioinformatics databases, StarBase and
TargetScan, miRNA-22-3p was predicted to bind to the 3′UTR of FOXP1
(Fig. 3A). The miRNA-22-3p mimic
significantly suppressed the luciferase activity of the FOXP1
wild-type (Wt) reporter compared with the NC mimic, but not that of
the mutant (Mut) reporter in 293T cells (Fig. 3B). Moreover, specific primers were
designed to perform RT-qPCR analysis of the expression of FOXP1 in
15 pairs of NPC tissues and para-cancerous tissues, and it was
demonstrated that the mRNA level of FOXP1 was significantly
increased in NPC tissues compared with the para-cancerous tissues
(Fig. 3C).
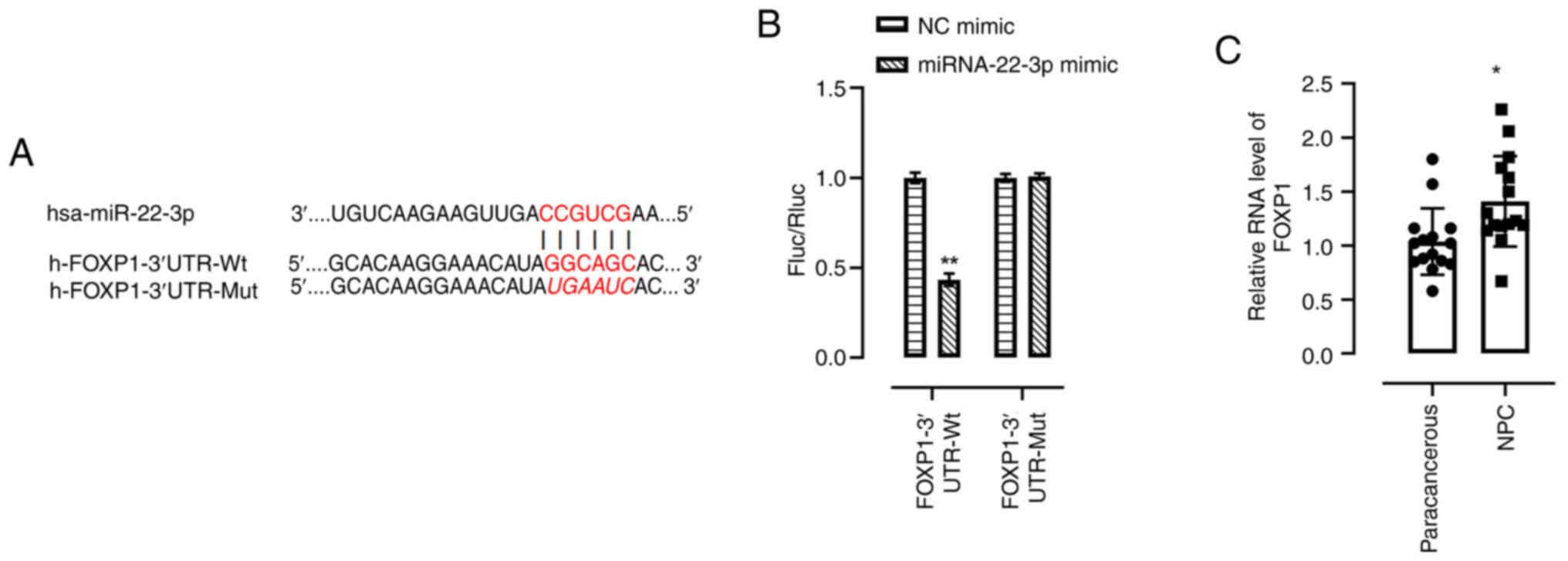 | Figure 3.Binding association between
miRNA-22-3p and FOXP1. (A) The predicted miRNA-22-3p binding site
in the 3′UTR sequences of FOXP1. (B) miRNA-22-3p negatively
regulated the luciferase activity of FOXP1-3′UTR-Wt, but not
FOXP1-3′UTR-Mut in 293T cells. (C) FOXP1 expression in NPC tissues
was analyzed using reverse transcription-quantitative polymerase
chain reaction. *P<0.05; **P<0.01 vs. NC mimic. miRNA/miR,
microRNA; NC, negative control; Wt, wild type; Mut, mutant; UTR,
untranslated region; Rluc, Renilla luciferase; fluc, firefly
luciferase; FOXP1, forkhead box protein 1; NPC, nasopharyngeal
carcinoma. |
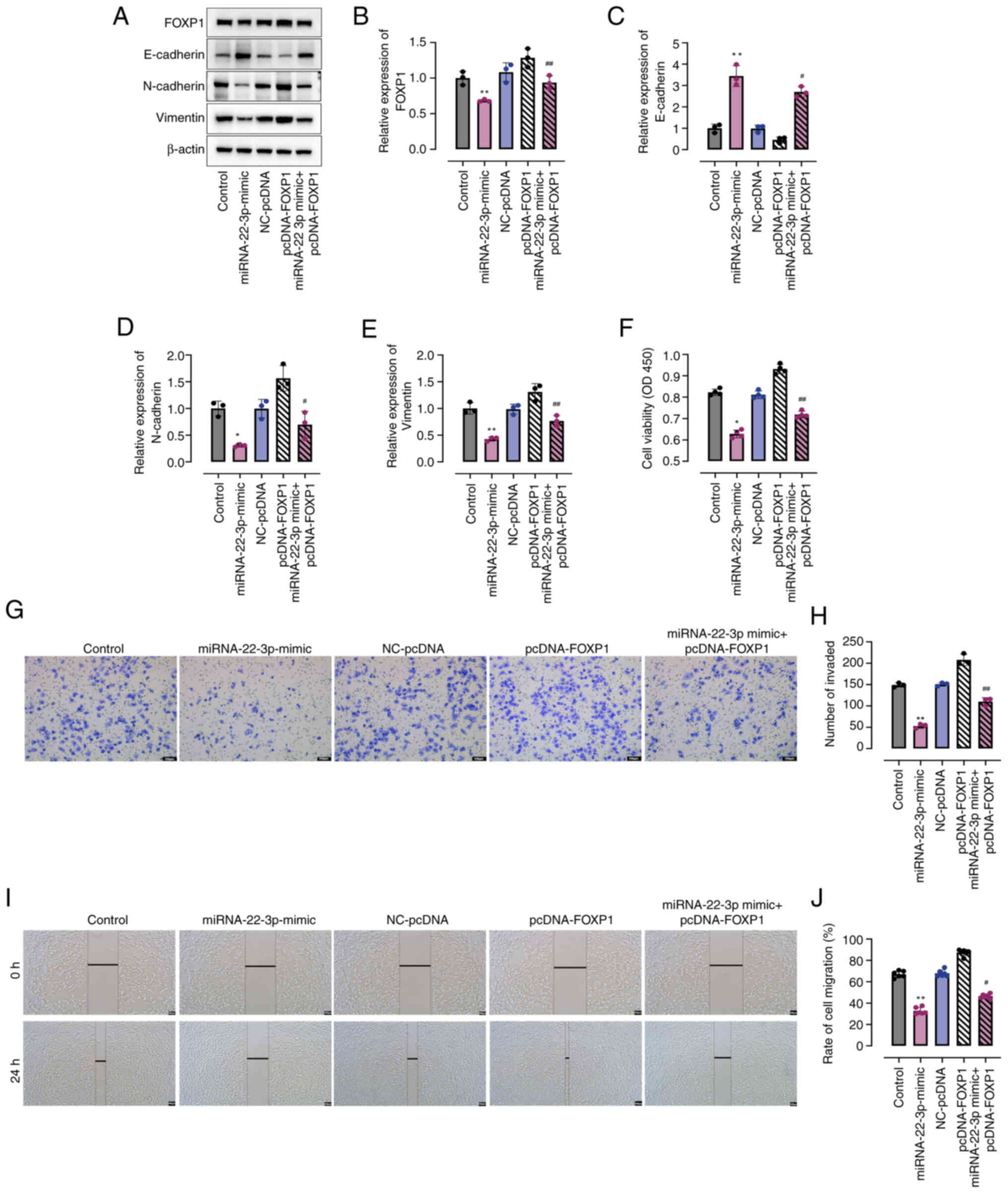
FOXP1 overexpression inhibits
miRNA-22-3p mimic-mediated NPC progression
Subsequently, the present study aimed to investigate
the effects of FOXP1 on the miRNA-22-3p-mediated progression of
NPC. Notably, compared with the control, transfection with
miRNA-22-3p mimic resulted in significant inhibition of FOXP1
protein expression, which was subsequently reversed upon
transfection with pcDNA-FOXP1 (Fig. 4A
and B). Furthermore, similar trends were observed in the
protein expression levels of vimentin and N-cadherin, as well as
E-cadherin (Fig. 4C-E). Notably,
the overexpression of FOXP1 in HK-1 cells in miRNA-22-3p + FOXP1
significantly counteracted the suppressive effects of miRNA-22-3p
mimic on cell viability compared to the miRNA-22-3p group (Fig. 4F). Consistently, co-treatment with
pcDNA-FOXP1 in the miRNA-22-3p + FOXP1 group markedly abrogated the
inhibitory effects exerted by miRNA-22-3p mimic on NPC cell
migration, as compared to the miRNA-22-3p group (Fig. 4G-I). Collectively, these findings
indicate that miRNA-22-3p mimics suppress NPC cell viability and
migration through the negative regulation of FOXP1 expression.
Discussion
The regulation of target gene expression by miRNAs
involves the specific binding of miRNAs to their complementary
target mRNAs, resulting in the inhibition of target gene
translation or degradation of the target mRNAs (18). In cancer cells, the aberrant
expression of miRNAs can lead to dysregulated target gene
expression, thereby affecting the development and progression of
cancer (19,20). Numerous investigations have been
performed to explore the regulatory role of miRNAs in NPC. Notably,
a previous study reported that the expression of miRNA-194 was
markedly decreased in both NPC tissue and cells, resulting in the
suppression of proliferation and invasion in NPC cells through the
direct targeting of MAP3K3 (11).
Furthermore, another study reported that miRNA-146a enhanced NPC
progression by modulating Epstein-Barr virus latent membrane
protein 1 (21). In addition, the
decreased expression of miRNA-506 has been observed in NPC, and it
functions as a potent tumor suppressor by facilitating apoptosis
and suppressing invasion and migration of NPC cells through the
direct targeting of EZH2 (22).
Thus, miRNAs are of utmost importance in the progression of NPC,
rendering them promising targets for both fundamental and applied
research into this ailment.
In the present study, the data revealed that the
expression of miRNA-22-3p was significantly decreased in NPC
tissues and cells, and the overexpression of miRNA-22-3p inhibited
the cell viability and migration of NPC cells in vitro by
directly targeting FOXP1. It has been reported that miRNA-22-3p
exhibits diverse biological functions by regulating several target
genes. By targeting MAPK14, miRNA-22-3p has been reported to
suppress the proliferation and differentiation, while enhancing the
apoptosis, of CD14+ peripheral blood mononuclear cells
(23). Targeting of high mobility
group box 1 by miRNA-22-3p in arteriosclerosis obliterans leads to
suppression of arterial smooth muscle cell proliferation and
migration, as well as a reduction in neointimal hyperplasia
(24). The long non-coding RNA
metastasis-associated lung adenocarcinoma transcript 1 safeguards
endothelial function against oxidized low-density
lipoprotein-induced dysfunction by enhancing the expression of
miRNA-22-3p target genes, C-X-C motif chemokine receptor 2 and AKT
(25). Notably, miRNA-22-3p
promotes the occurrence and development of hepatocellular carcinoma
by targeting CDKN2C (14) and
methylenetetrahydrofolate reductase proteins (16). In triple-negative breast cancer,
miRNA-22-3p exerts tumor suppressive effects by selectively
targeting clinically relevant oncogenic signaling pathways,
including the eEF2K/PI3K/Akt and Src signaling cascades (26). In the present study, another target
gene of miRNA-22-3p, FOXP1, was identified, and the role of
miRNA-22-3p in inhibiting the progression of NPC was reported for
the first time. In addition, the bidirectional regulatory effect of
miRNA-22-3p on the progression of different cancers may be due to
the fact that, in different types of cancer cells, miRNA-22-3p
targets different genes and thus performs different functions.
Furthermore, the influence of the cellular environment and
signaling pathways may also lead to changes in the action of
miRNA-22-3p. Therefore, further studies are required to explore the
possibility that miRNA-22-3p modulates changes in signaling
pathways in NPC cells.
The biological function of the FOX transcription
factor family proteins lies in their ability to modulate gene
expression through specific DNA binding, thereby exerting
regulatory control over target genes and ultimately influencing
cellular growth, differentiation and development (27,28).
Among these, FOXP1 exhibits a diverse array of biological
functions, encompassing the regulation of B-cell development and
the multifaceted differentiation of monocytes (29,30).
Furthermore, FOXP1 has been implicated as either an oncogenic or
tumor suppressor gene in several malignancies. The 3p14.1 position
of FOXP1 was identified as a potential tumor suppressor binding
site due to the loss of heterozygosity at the 3p position of
chromosomes in a variety of human tumors (31). The decreased expression of FOXP1 has
been reported in several solid tumors, such as bowel cancer and
lung cancer (32). In addition, it
has been reported that a high expression of FOXP1 is predictive of
a good prognosis in patients with non-small cell lung cancer,
suggesting a tumor-suppressive effect of FOXP1 (33,34).
By contrast, other studies have reported that the increased
expression of FOXP1 in patients with hepatocellular carcinoma,
gastric mucosa-associated lymphoma and B-cell lymphoma is
associated with a poor prognosis (35–37).
As an oncogene, FOXP1 can widely inhibit the expression of numerous
pro-apoptotic genes in B-cell lymphoma, such as tumor protein 63,
Ras association domain family member 6 and tumor protein P53
inducible nuclear protein 1 (36).
A previous study has substantiated the role of FOXP1 in enhancing
the activity of the Wnt/β-catenin signaling pathway in B-cell
lymphoma (38). Consequently,
activation of the Wnt signaling pathway has been implicated in
facilitating tumor growth (38).
However, little is known about the expression level and role of
FOXP1 in NPC. In the present study, it was found that FOXP1
reversed the suppressive effects of miRNA-22-3p mimic on NPC cell
viability and migration. The results presented in the present study
indirectly demonstrate the oncogenic role of FOXP1 in NPC.
In conclusion, the present study demonstrates that
miRNA-22-3p directly inhibits the expression of FOXP1, thereby
inhibiting the cell viability and migration of NPC cells. The
results confirmed that miRNA-22-3p serves a role as a tumor
suppressor in NPC, suggesting that miRNA-22-3p may be a novel
therapeutic target for NPC. However, the present study has
limitations. Firstly, the clinical sample size was relatively small
and more patients need to be included in subsequent studies.
Secondly, the present study has not been validated in animal
models. Further in vivo studies are required to clarify the
anti-NPC effects of miRNA-22-3p and its mechanisms with the
intention of clinical applications in detail. Finally, further
studies are required to determine other downstream mechanisms of
miRNA-22-3p in NPC, such as some signaling pathways.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guiyang Science and
Technology Plan Projects [grant no. ZKH (2022)-4-2-7], the National
Natural Science Foundation Cultivation Project of Affiliated
Hospital of Guizhou Medical University (grant no.
gyfynsfc-2021-31), the Guizhou Provincial Science and Technology
Projects [grant no. QKHJC-ZK (2023) YB365] and the Science and
Technology Fund project of Guizhou Provincial Health Commission
(grant no. gzwkj2022-157).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YJin, ZW, YL and YJia wrote the manuscript and
performed experiments. FY and TZ analyzed and interpretation of
data. All authors have read and approved the final manuscript. YJin
and TZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study protocols were approved by the Ethics
Committee of the Affiliated Hospital of Guizhou Medical University
(Guiyang, China; approval no. 2021-019). Written informed consent
was obtained from all patients prior to the collection of
samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun
Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang ET, Ye W, Zeng YX and Adami HO: The
evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 30:1035–1047. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chua MLK, Lee VHF and Lee AWM:
Hyperfractionation for reirradiation of recurrent nasopharyngeal
carcinoma. Lancet. 401:878–879. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guan S, Wei J, Huang L and Wu L:
Chemotherapy and chemo-resistance in nasopharyngeal carcinoma. Eur
J Med Chem. 207:1127582020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chekulaeva M: First demonstration of
miRNA-dependent mRNA decay. Nat Rev Mol Cell Biol. 24:1642023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pozniak T, Shcharbin D and Bryszewska M:
Circulating microRNAs in medicine. Int J Mol Sci. 23:39962022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruggieri F, Jonas K, Ferracin M, Dengler
M, Jӓger V and Pichler M: MicroRNAs as regulators of tumor
metabolism. Endocr Relat Cancer. 30:e2202672023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Q, Zhang Q, Gu M, Zhang K, Xia T,
Zhang S, Chen W, Yin H, Yao H, Fan Y, et al: MIR106A-5p
upregulation suppresses autophagy and accelerates malignant
phenotype in nasopharyngeal carcinoma. Autophagy. 17:1667–1683.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin W, Shi L and Mao Y: MiR-194 regulates
nasopharyngeal carcinoma progression by modulating MAP3K3
expression. FEBS Open Bio. 9:43–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong D, Wang X, Wang X, Wang Z and Wang F:
Downregulated miRNA-22-3p promotes the progression and leads to
poor prognosis of hepatocellular carcinoma through targeting
CDKN2C. J BUON. 26:409–417. 2021.PubMed/NCBI
|
|
13
|
Barut Z and Akdeniz FT: Evaluation of the
relationship between miRNA-22-3p and Gal-9 levels in glioblastoma.
In Vivo. 37:2577–2584. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Li X, Wang H, Guo X, Xue J, Wang X
and Ni J: MicroRNA-22-3p and MicroRNA-149-5p inhibit human
hepatocellular carcinoma cell growth and metastasis properties by
regulating methylenetetrahydrofolate reductase. Curr Issues Mol
Biol. 44:952–962. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koon HB, Ippolito GC, Banham AH and Tucker
PW: FOXP1: A potential therapeutic target in cancer. Expert Opin
Ther Targets. 11:955–965. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ijichi N, Ikeda K, Horie-Inoue K and Inoue
S: FOXP1 and estrogen signaling in breast cancer. Vitam Horm.
93:203–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Heikkinen L, Wang C, Yang Y, Sun H
and Wong G: Trends in the development of miRNA bioinformatics
tools. Brief Bioinform. 20:1836–1852. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi
S, Xie H, Peng X, Yin W, Tao Y and Wang X: miRNA-based biomarkers,
therapies, and resistance in cancer. Int J Biol Sci. 16:2628–2647.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Chen X, Jing M, Du H and Zeng Y:
Expression of miRNA-146a in nasopharyngeal carcinoma is upregulated
by Epstein-Barr virus latent membrane protein 1. Oncol Rep.
28:1237–1242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan DC, Zhao YR, Qi H, Hou JX and Zhang
TH: MiRNA-506 presents multiple tumor suppressor activities by
targeting EZH2 in nasopharyngeal carcinoma. Auris Nasus Larynx.
47:632–642. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia X, Yang M, Hu W and Cai S:
Overexpression of miRNA-22-3p attenuates osteoporosis by targeting
MAPK14. Exp Ther Med. 22:6922021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang SC, Wang M, Wu WB, Wang R, Cui J, Li
W, Li ZL, Li W and Wang SM: Mir-22-3p inhibits arterial smooth
muscle cell proliferation and migration and neointimal hyperplasia
by targeting HMGB1 in arteriosclerosis obliterans. Cell Physiol
Biochem. 42:2492–2506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang Y, Jin X, Xiang Y, Chen Y, Shen CX,
Zhang YC and Li YG: The lncRNA MALAT1 protects the endothelium
against ox-LDL-induced dysfunction via upregulating the expression
of the miR-22-3p target genes CXCR2 and AKT. FEBS Lett.
589:3189–3196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gorur A, Bayraktar R, Ivan C, Mokhlis HA,
Bayraktar E, Kahraman N, Karakas D, Karamil S, Kabil NN,
Kanlikilicer P, et al: ncRNA therapy with miRNA-22-3p suppresses
the growth of triple-negative breast cancer. Mol Ther Nucleic
Acids. 23:930–943. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katoh M and Katoh M: Human FOX gene family
(Review). Int J Oncol. 25:1495–1500. 2004.PubMed/NCBI
|
|
28
|
Golson ML and Kaestner KH: Fox
transcription factors: From development to disease. Development.
143:4558–4570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patzelt T, Keppler SJ, Gorka O, Thoene S,
Wartewig T, Reth M, Förster I, Lang R, Buchner M and Ruland J:
Foxp1 controls mature B cell survival and the development of
follicular and B-1 B cells. Proc Natl Acad Sci USA. 115:3120–3125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi C, Sakuma M, Mooroka T, Liscoe A, Gao
H, Croce KJ, Sharma A, Kaplan D, Greaves DR, Wang Y and Simon DI:
Down-regulation of the forkhead transcription factor Foxp1 is
required for monocyte differentiation and macrophage function.
Blood. 112:4699–4711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fox SB, Brown P, Han C, Ashe S, Leek RD,
Harris AL and Banham AH: Expression of the forkhead transcription
factor FOXP1 is associated with estrogen receptor alpha and
improved survival in primary human breast carcinomas. Clin Cancer
Res. 10:3521–3527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng J, Zhang X, Zhu H, Wang X, Ni S and
Huang J: High expression of FoxP1 is associated with improved
survival in patients with non-small cell lung cancer. Am J Clin
Pathol. 138:230–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Zhang S, Wang X, Liu J, Yang L,
He S, Chen L and Huang J: Prognostic significance of FOXP1 as an
oncogene in hepatocellular carcinoma. J Clin Pathol. 65:528–533.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han SL, Wu XL, Wan L, Zeng QQ, Li JL and
Liu Z: FOXP1 expression predicts polymorphic histology and poor
prognosis in gastric mucosa-associated lymphoid tissue lymphomas.
Dig Surg. 26:156–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barrans SL, Fenton JAL, Banham A, Owen RG
and Jack AS: Strong expression of FOXP1 identifies a distinct
subset of diffuse large B-cell lymphoma (DLBCL) patients with poor
outcome. Blood. 104:2933–2935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van Keimpema M, Grüneberg LJ, Mokry M, van
Boxtel R, Koster J, Coffer PJ, Pals ST and Spaargaren M: FOXP1
directly represses transcription of proapoptotic genes and
cooperates with NF-κB to promote survival of human B cells. Blood.
124:3431–3440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gascoyne DM and Banham AH: The
significance of FOXP1 in diffuse large B-cell lymphoma. Leuk
Lymphoma. 58:1037–1051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walker MP, Stopford CM, Cederlund M, Fang
F, Jahn C, Rabinowitz AD, Goldfarb D, Graham DM, Yan F, Deal AM, et
al: FOXP1 potentiates Wnt/β-catenin signaling in diffuse large B
cell lymphoma. Sci Signal. 8:ra122015. View Article : Google Scholar : PubMed/NCBI
|