Introduction
Colorectal cancer is ranked as the third most
prevalent malignancy globally and the second leading cause of
cancer-related mortality. Rectal cancer alone contributes to
approximately one-third of these cases, with adenocarcinoma
comprising >90% (1–3). Total mesorectal excision remains the
standard surgical procedure for resectable rectal cancer (4). Despite undergoing curative resection,
there is still a recurrence rate of 5–10%, with the distal
resection margin (DRM) serving a crucial role (5–7). It
has been reported that histologically normal tissues adjacent to
tumors undergo molecular alterations associated with tumorigenesis,
which can be attributed to field cancerization (8,9). If
the tissue exhibiting a precancerous state is not surgically
excised, it has the potential to progress into invasive cancer
(10). In rectal cancer surgery, an
insufficient length of DRM is often associated with a high
recurrence rate (5), whilst an
excessive length of DRM may result in inadequate remaining rectum
and increase the risk of postoperative complications (6). Therefore, it is important to
preoperatively determine the optimal DRM for formulating surgical
options.
The apparent diffusion coefficient (ADC), derived
from the signal intensity of diffusion-weighted imaging (DWI),
quantitatively evaluates the mobility of water molecules and
indirectly reflects the biological characteristics of tissues
(11–13). In general, the presence of low ADC
values indicates a restriction in the diffusion of water molecules,
which is commonly observed in several pathological conditions, such
as inflammatory, fibrotic or neoplastic processes (11,14).
It has been reported that an increase in both the mobility and
quantity of water molecules during the transition from normal cells
to cancerous cells leads to differences in water molecule diffusion
within tissues at different stages of tumorigenesis (15). In addition, stem cells derived from
adjacent tissues of the tumor have the potential to induce fibrosis
and an inflammatory response (16),
thereby affecting the ADC values of paracancerous tissues.
Chen et al (17) reported that ADC can be used to
differentiate between tumor tissues, tumor-adjacent tissues and
tumor-distant tissues in rectal adenocarcinoma (RA); however, the
investigation of distal paracancerous tissues only involved the
tissues located ~1 and 2 cm from the tumor margin. Currently, there
is no exact standard for the length of DRM (18–20),
to the best of our knowledge. Therefore, the aim of the present
study was to evaluate differences in ADC values of resectable
mid-high RA and distal paracancerous tissues located at several
distances from the tumor margin in detail, providing a potential
reference basis for preoperatively determining the optimal DRM.
Materials and methods
Patients
The study protocol was approved by the Institutional
Review Board of the Affiliated Hospital of North Sichuan Medical
College (Nanchong, China; approval no. 2024ER219-1), and the
requirement for informed consent was waived owing to the
retrospective nature of the present study.
From January 2017 to December 2022, clinical and
imaging data from 129 consecutive patients with resectable RA who
underwent preoperative pelvic magnetic resonance imaging (MRI)
scans were collected from The Affiliated Hospital of North Sichuan
Medical College (Nanchong, China). Inclusion criteria were as
follows: i) Multi-b-value DWI sequences performed within 2 weeks
prior to surgery; ii) no cancer-related treatment administered
before undergoing multiple b-values DWI; iii) adenocarcinoma
confirmed by pathology; and iv) the distal tumor margin (DTM) was
positioned ≥3 cm above the dentate line, as the dentate line
represents the transition between rectal columnar mucosa and anal
squamous mucosa (21), which can
result in significant variations in ADC values of the intestinal
wall below and above it. Exclusion criteria were as follows: i) No
visible tumors on MRI images (n=2); ii) tumors confirmed as
mucinous adenocarcinoma, attributing to their high ADC resulting
from the abundant mucinous content (n=5); iii) unsatisfactory image
quality due to susceptibility or movement artifacts (n=7); iv)
abnormal edema exhibited in the intestinal wall, leading to
elevated ADC values (n=3); and v) incomplete MRI images or clinical
records of patients (n=2). Finally, a total of 110 patients (62
male and 48 female patients; median age, 66 years; age range, 28–84
years) with RA confirmed by pathology were included in the present
study for analysis.
All patients with RA underwent pelvic MRI including
DWI 2 weeks before radical resection with regional lymph node
dissection. The tumor differentiation, pathological tumor stage
(pT) and pathological lymph node stage (pN) for rectal cancer were
determined based on the postoperative histopathological
examination. Tumors located within 15 cm from the DTM to the anal
verge were categorized as rectal neoplasms, further classified into
low (0–5 cm), middle (>5-10 cm) and high (>10-15 cm) rectal
tumors based on their respective distances from the DTM to the anal
verge (22). As the distance from
the dentate line to the anal verge was 2–3 cm (21), and DTM was required to be >3 cm
above the dentate line in the present study, patients with mid-high
RA were included.
MRI techniques
For each enrolled patient, imaging was performed
using a 3.0 Tesla scanner (Discovery™ MR750; GE
Healthcare) with a 32-channel phased-array torso coil in the pelvic
region. The imaging sequences included axial T2-weighted
(T2W) fast-recovery fast spin-echo with fat suppression
sequence, sagittal T2W Propeller with fat suppression
sequence, axial multi-b-value (0, 50, 100, 800, 1,000 and 1,500
sec/mm2) DWI based on echo-planar imaging sequence. The
parameters of all sequences are listed in Table I. The patients were positioned in a
supine posture during the scanning procedure. The scanning range
was from the level of the lumbar 4–5 intervertebral disc to 10 cm
below the pubic symphysis, ensuring coverage of the entire
rectum.
 | Table I.Scan parameters of the magnetic
resonance imaging sequences. |
Table I.
Scan parameters of the magnetic
resonance imaging sequences.
| Parameter | Axial
T2W imaging | Sagittal
T2W imaging | Axial multi-b-value
DWIa |
|---|
| Repetition time,
msec | 5,050 | 7,570 | 3,225 |
| Echo time,
msec | 120 | 60 | 57 |
| Flip angle, ° | 111 | 140 | 90 |
| Field of view,
cm | 36×36 | 36×36 | 38×30 |
| Slice thickness,
mm | 5 | 5 | 5 |
| Intersection gap,
mm | 1 | 1 | 1 |
| Matrix | 384×256 | 320×320 | 128×192 |
| Number of
excitations | 2 | 3 | 1/2/4 |
Image analysis
DWI data were transferred to the Advantage
Workstation (version 4.5; GE Healthcare), and ADC maps were
automatically generated using the post-processing Functool software
(version 10.4.04; GE Healthcare). All images were independently
evaluated by two abdominal radiologists (radiologist 1 and
radiologist 2, with 7 and 3 years of experience in this field,
respectively), and any disagreements were resolved by another
expert with 26 years of experience. The expert and two radiologists
were blinded to the clinical and histopathology information of
patients, except for the diagnosis of RA.
The ADC maps and values were derived from DWI using
five different b-value pairs (0 and 50; 0 and 100; 0 and 800; 0 and
1,000; and 0 and 1,500 sec/mm2) based on the
mono-exponential model formula:
ADC=ln(S0/S1)/(b1-b0),
where b represents the diffusion gradient value and S0
and S1 denote the signal intensity of tissue on DWI at
b0 and b1, respectively (12,17).
The Japanese guidelines recommend a DRM of ≥3 cm for rectal cancer
located above the peritoneal reflection and 2 cm for those below it
(19). Moreover, there have been
very few reports of distal tumor infiltration extending <3 cm
(5). Thus, a DRM of 3 cm is
generally considered a safe resection margin for patients with
rectal cancer. Subsequently, ADC measurements were performed on RA
and three distal paracancerous tissues located at ~1
(D1), ~2 (D2) and ~3 cm (D3) from
the DTM.
In the present study, the ADCs of RA, D1,
D2 and D3 were measured using the
single-slice region of interest (ROI) method. Firstly, location of
RA was determined based on the T2W and DWI images, where
the tumor presented as an irregular thickening of the intestinal
wall with isointense or hyperintense signal on the T2W
image and hyperintense signal on the DWI image (Fig. 1A-C). Secondly, the locations of
D1, D2 and D3 were determined
based on the sagittal T2W images (Fig. 1B), whilst the axial DWI images of
D1, D2 and D3 were captured at the
corresponding locations. Thirdly, on the axial high b-value DWI
image, the maximum cross-section of the tumor was selected and an
ROI was delineated along the tumor margin whilst carefully
excluding necrotic, fatty and vascular regions (Fig. 1C). In accordance with the method
described in a previously published report (17), ROIs of D1, D2
and D3 were delineated to cover more than a semicircle
of the rectal wall, and the surrounding adipose tissue, blood
vessels and luminal gas were excluded (Fig. 1D). After delineating the ROIs for
the tumor, D1, D2 and D3 on axial
high b-value DWI images, corresponding ROIs were automatically
generated on the ADC maps to obtain ADC values for each region
(Fig. 1E and F). The ADC
measurement was repeated three times for each tissue, and the final
value was determined by calculating the average of these three
measurements. Additionally, the ADCs of RA, D1,
D2 and D3 were independently measured by the
aforementioned two radiologists to evaluate the interobserver
agreement.
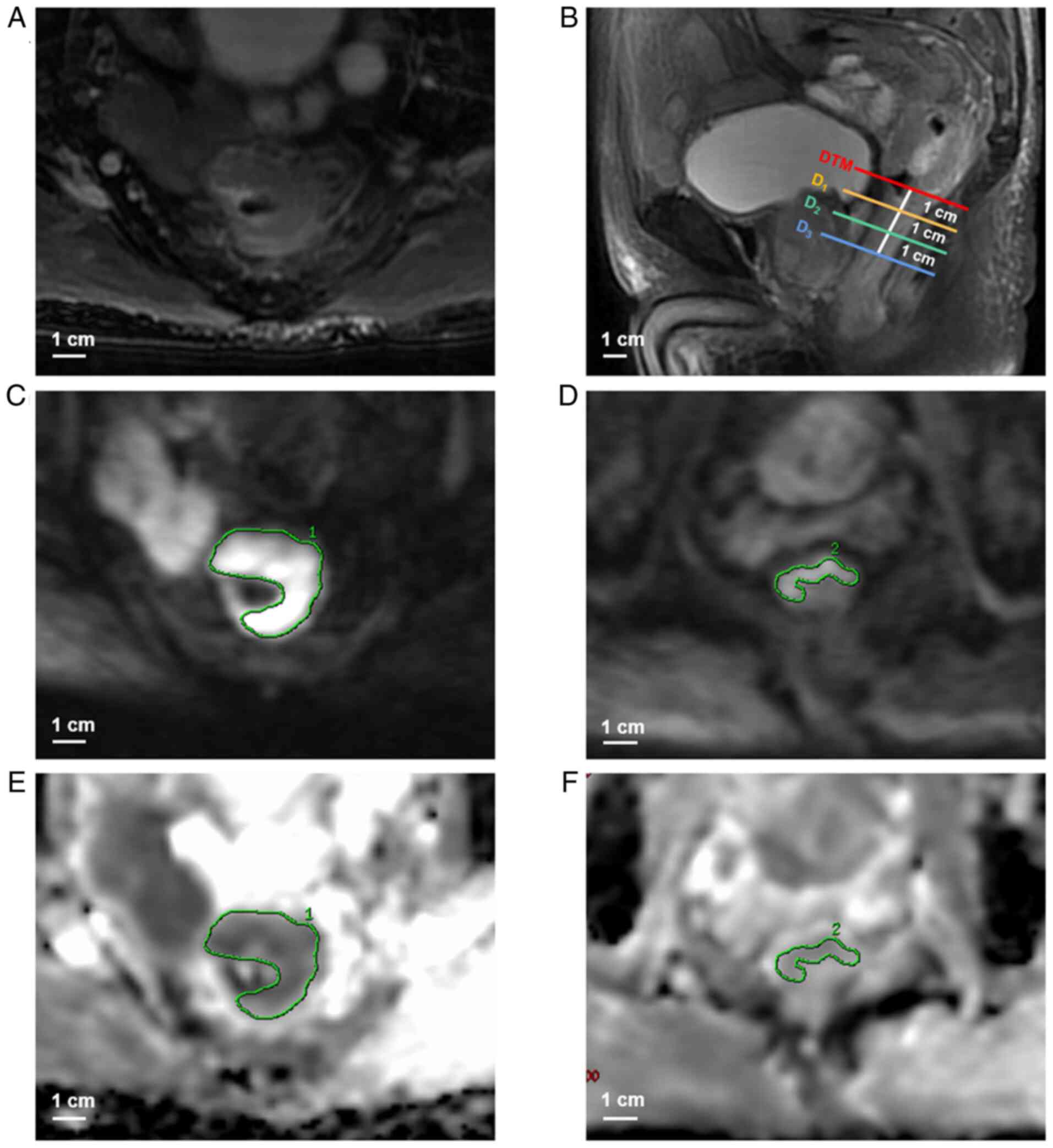 | Figure 1.Measurement of the ADC values of the
tumor and distal paracancerous tissues in a 73-year-old male
patient with pT3-staged adenocarcinoma of the middle rectum. (A)
Axial T2-weighted image shows the tumor is presented as
an irregular thickening of rectal wall. (B) Sagittal
T2-weighted image (red line, DTM; orange line,
D1; green line, D2; blue line,
D3). ROIs for the (C) tumor on the largest tumor
cross-section and (D) D1, which covers more than a
semicircle of the rectal wall, were manually delineated on axial
diffusion-weighted images (b=1,000 sec/mm2).
Subsequently, ROIs for the (E) tumor and (F) D1 were
automatically generated on the corresponding ADC maps (b=0 and
1,000 sec/mm2) to obtain ADC values for each region. The
ADC values of the tumor and D1 were
1.160×10−3 mm2/sec and 1.530×10−3
mm2/sec, respectively. ADC measurements for
D2 and D3 were performed in a consistent
manner with that used for D1. ADC, apparent diffusion
coefficient; D1, distal paracancerous tissue located ~1
cm from the tumor margin; D2, distal paracancerous
tissue located ~2 cm from the tumor margin; D3, distal
paracancerous tissue located ~3 cm from the tumor margin; DTM,
distal tumor margin; ROI, region of interest; pT, pathological
tumor stage. |
Statistical analysis
Statistical analyses were performed using SPSS
software (version 25; IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference. The intraclass
correlation coefficient (ICC) was used to assess the interobserver
agreements for each ADC measurement. The ICC was classified into
poor (0–0.20), fair (0.21–0.40), moderate (0.41–0.60), good
(0.61–0.80) and excellent (0.81–1.00) agreements (23). If the agreement was good or
excellent, the measurements of radiologist 1 were used for
subsequent analysis. If the agreement was unsatisfactory, the
average of the measurements taken by two radiologists (radiologist
1 and radiologist 2) were used for subsequent analysis.
The ADCs of RA, D1, D2 and
D3 were compared using the Friedman test. In cases where
the P-values indicated statistically significant differences, post
hoc multiple pairwise comparisons between different tissues were
performed using the Bonferroni correction test. The results were
visually represented using boxplots. Subsequently, variables that
demonstrated statistically significant differences in pairwise
comparisons underwent receiver operating characteristic (ROC)
analysis to assess the efficacy of ADCs in distinguishing between
different tissues. In addition, considering that advanced-stage RA
may have more cell proliferation compared with early-stage RA, and
there may be differences in the length of safe DRM between the two
stages, the Mann-Whitney U test was used to analyze the ADCs of
different tissues between pT1-2 and pT3-4 stages.
Results
Patient characteristics
A total of 110 patients with RA were included in the
present study, comprising 62 (56.4%) male patients and 48 (43.6%)
female patients. The median age was 66 years (range, 28–84 years).
There were 31 (28.2%) tumors located in the high rectum and 79
(71.8%) tumors located in the middle rectum. The tumor
differentiation was observed as well-differentiated adenocarcinoma
in 39 (35.4%) patients, moderately differentiated adenocarcinoma in
65 (59.1%) patients and poorly differentiated adenocarcinoma in 6
(5.5%) patients. Regarding the pathological stage, there were 27
(24.5%) patients classified as pT1-2 and 83 (75.5%) patients
classified as pT3-4. Additionally, there were 65 (59.1%) patients
classified as pN0 and 45 (40.9%) patients classified as pN1-2.
Interobserver agreements of ADCs at
different b-value pairs
The interobserver agreements for the ADC values of
RA, D1, D2 and D3 are presented in
Table II. The interobserver
agreements for the ADC values at different b-value pairs were
excellent (all ICCs >0.80). Therefore, the measurements obtained
by radiologist 1 were used for subsequent analysis.
 | Table II.Evaluation of interobserver
agreements for apparent diffusion coefficient values of the tumor,
distal paracancerous tissue located ~1, 2 and 3 cm from the tumor
margin at different b-value pairs. |
Table II.
Evaluation of interobserver
agreements for apparent diffusion coefficient values of the tumor,
distal paracancerous tissue located ~1, 2 and 3 cm from the tumor
margin at different b-value pairs.
|
| Interobserver
intraclass correlation coefficient (95% CI) |
|---|
| b-value,
sec/mm2 |
|
|---|
| Tumor | D1 | D2 | D3 |
|---|
| 0 and 50 | 0.915
(0.875–0.942) | 0.876
(0.820–0.915) | 0.894
(0.845–0.928) | 0.867
(0.810–0.908) |
| 0 and 100 | 0.832
(0.764–0.882) | 0.904
(0.858–0.935) | 0.885
(0.827–0.923) | 0.878
(0.815–0.919) |
| 0 and 800 | 0.896
(0.844–0.930) | 0.924
(0.887–0.949) | 0.933
(0.900–0.955) | 0.913
(0.869–0.942) |
| 0 and 1,000 | 0.927
(0.865–0.957) | 0.929
(0.895–0.952) | 0.951
(0.928–0.967) | 0.943
(0916–0.961) |
| 0 and 1,500 | 0.900
(0.852–0.932) | 0.910
(0.870–0.937) | 0.916
(0.879–0.942) | 0.927
(0.895–0.950) |
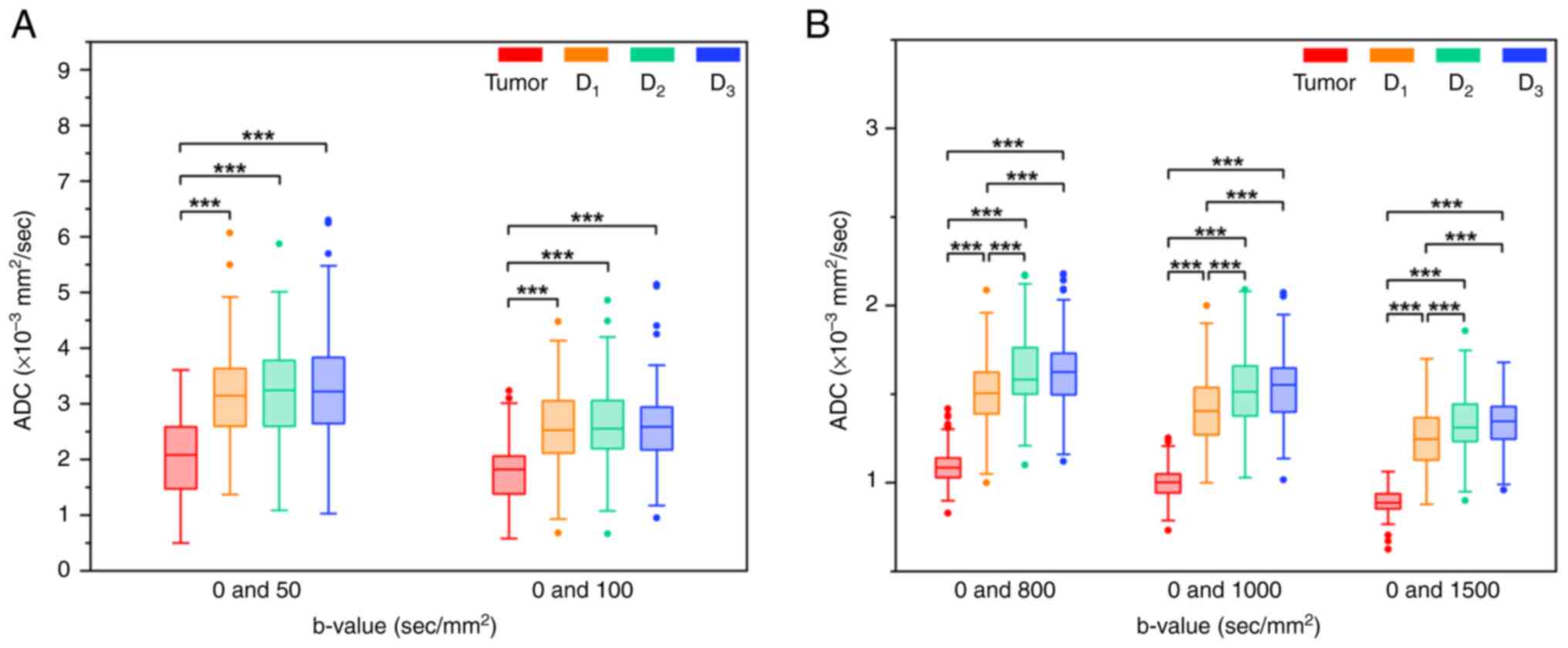
Comparisons of ADCs between RA,
D1, D2 and D3
The ADC values of RA, D1, D2
and D3 obtained from different b-value pairs are
summarized in Table III. The
Friedman test demonstrated significant differences in ADCs between
the four different tissues at all b-value pairs (all P<0.001).
Post hoc multiple pairwise comparisons using the Bonferroni
correction test were performed to further assess the differences in
ADCs between different tissues (Fig.
2). The tumor exhibited lower ADC values compared with
D1, D2 and D3 at all b-value pairs
(all P<0.001). Furthermore, at b-value pairs with the maximum
b-value of ≥800 sec/mm2 (0 and 800; 0 and 1,000; and 0
and 1,500 sec/mm2), the ADC of D1 was
significantly lower compared with those of both D2 and
D3 (P<0.001). However, no significant differences in
ADCs were observed between D1, D2 and
D3 at b-value pairs with the maximum b-value of ≤100
sec/mm2 (0 and 50, and 0 and 100 sec/mm2;
P>0.05). There were no significant differences in ADCs between
D2 and D3 at all b-value pairs (all
P>0.05).
 | Table III.Comparison of apparent diffusion
coefficients between the tumor, distal paracancerous tissue located
~1, 2 and 3 cm from the tumor margin at different b-value
pairs. |
Table III.
Comparison of apparent diffusion
coefficients between the tumor, distal paracancerous tissue located
~1, 2 and 3 cm from the tumor margin at different b-value
pairs.
|
| Median ADC (25%
quantile, 75% quantile), ×10−3 mm2/sec |
|---|
| b-value,
sec/mm2 |
|
|---|
| Tumor | D1 | D2 | D3 | P-value |
|---|
| 0 and 50 | 2.080 (1.473,
2.589) | 3.148 (2.598,
3.645) | 3.244 (2.598,
3.783) | 3.224 (2.645,
3.840) | <0.001 |
| 0 and 100 | 1.825 (1.382,
2.064) | 2.527 (2.113,
3.059) | 2.557 (2.187,
3.070) | 2.587 (2.173,
2.965) | <0.001 |
| 0 and 800 | 1.083 (1.028,
1.141) | 1.505 (1.389,
1.623) | 1.584 (1.500,
1.764) | 1.625 (1.495,
1.730) | <0.001 |
| 0 and 1,000 | 1.003 (0.942,
1.051) | 1.405 (1.269,
1.538) | 1.514 (1.377,
1.661) | 1.552 (1.400,
1.647) | <0.001 |
| 0 and 1,500 | 0.887 (0.852,
0.937) | 1.247 (1.126,
1.368) | 1.312 (1.230,
1.443) | 1.347 (1.246,
1.430) | <0.001 |
ADCs of RA, D1,
D2 and D3 between different pT stages
The ADC values of RA, D1, D2
and D3 obtained from different b-value pairs at pT1-2
and pT3-4 stages are summarized in Table IV. Only when b=0 and 1,000
sec/mm2, and b=0 and 1,500 sec/mm2, the ADC
values of pT1-2 staged tumors were significantly higher compared
with those of pT3-4 staged tumors (P<0.05). However, no
statistically significant differences in ADC values between tumors
with different pathological stages were demonstrated at the other
b-value pairs (P>0.05). Furthermore, there were no statistically
significant differences in ADC values of D1,
D2 and D3 between tumors with different
pathological stages at all b-value pairs (all P>0.05).
Therefore, further subgroup analysis was not performed.
 | Table IV.Comparison of apparent diffusion
coefficients of the tumor, distal paracancerous tissue located ~1,
2 and 3 cm from the tumor margin between different pathological
tumor stages. |
Table IV.
Comparison of apparent diffusion
coefficients of the tumor, distal paracancerous tissue located ~1,
2 and 3 cm from the tumor margin between different pathological
tumor stages.
|
|
| Median ADC (25%
quantile, 75% quantile), ×10−3 mm2/sec |
|---|
| b-value,
sec/mm2 | Tissue |
|
|---|
| pT1-2 (n=27) | pT3-4 (n=83) | P-value |
|---|
| 0 and 50 | Tumor | 2.110 (1.533,
2.683) | 2.053 (1.440,
2.550) | 0.518 |
|
| D1 | 3.210 (2.717,
3.667) | 3.080 (2.553,
3.637) | 0.451 |
|
| D2 | 3.277 (2.710,
3.793) | 3.220 (2.563,
3.780) | 0.654 |
|
| D3 | 3.123 (2.677,
3.953) | 3.293 (2.540,
3.837) | 0.870 |
| 0 and 100 | Tumor | 1.900 (1.427,
2.257) | 1.783 (1.377,
2.033) | 0.202 |
|
| D1 | 2.633 (2.220,
3.097) | 2.497 (2.047,
3.055) | 0.485 |
|
| D2 | 2.737 (2.317,
2.923) | 2.517 (2.023,
3.297) | 0.534 |
|
| D3 | 2.630 (2.373,
2.933) | 2.560 (2.083,
3.100) | 0.710 |
| 0 and 800 | Tumor | 1.107 (1.050,
1.193) | 1.080 (1.017,
1.133) | 0.090 |
|
| D1 | 1.540 (1.410,
1.723) | 1.490 (1.373,
1.600) | 0.198 |
|
| D2 | 1.523 (1.420,
1.780) | 1.600 (1.520,
1.763) | 0.406 |
|
| D3 | 1.630 (1.440,
1.717) | 1.623 (1.500,
1.73) | 0.797 |
| 0 and 1,000 | Tumor | 1.023 (0.980,
1.113) | 0.989 (0.929,
1.040) | 0.006 |
|
| D1 | 1.493 (1.223,
1.580) | 1.397 (1.270,
1.533) | 0.380 |
|
| D2 | 1.510 (1.377,
1.677) | 1.523 (1.383,
1.653) | 0.800 |
|
| D3 | 1.500 (1.397,
1.637) | 1.553 (1.413,
1.663) | 0.555 |
| 0 and 1,500 | Tumor | 0.909 (0.864,
0.974) | 0.879 (0.847,
0.929) | 0.041 |
|
| D1 | 1.300 (1.097,
1.400) | 1.243 (1.127,
1.340) | 0.240 |
|
| D2 | 1.390 (1.190,
1.480) | 1.300 (1.233,
1.410) | 0.397 |
|
| D3 | 1.343 (1.250,
1.423) | 1.347 (1.243,
1.430) | 0.947 |
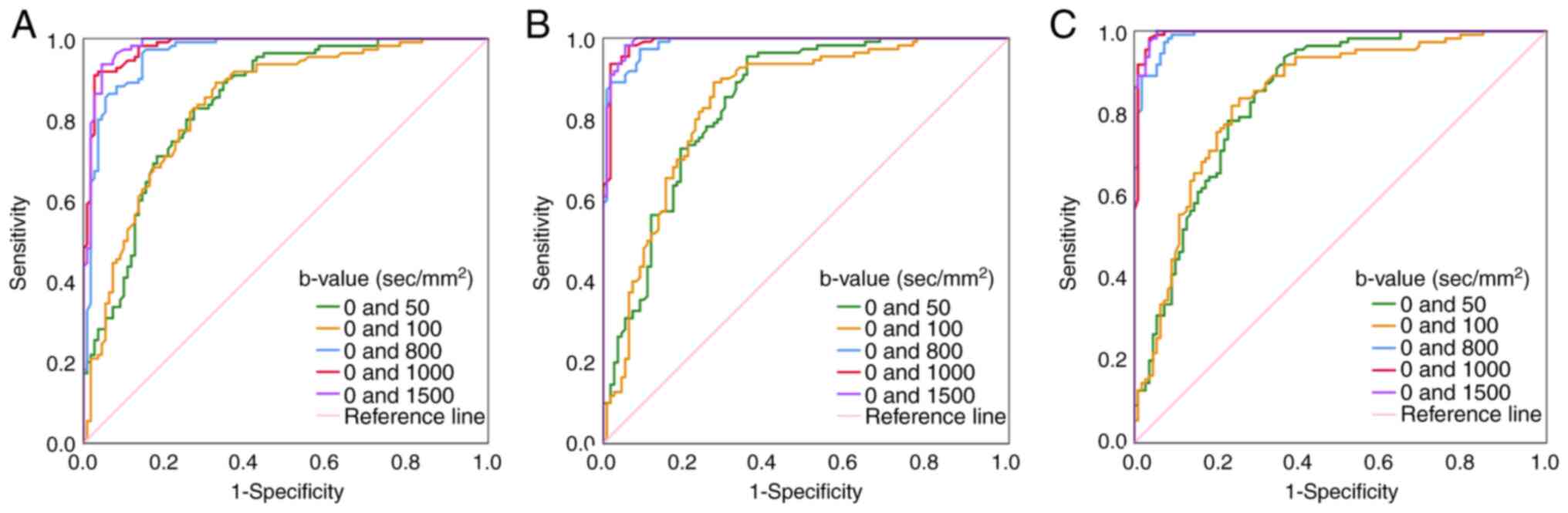
ROC analyses of ADCs for
differentiation between RA, D1, D2 and
D3
The efficacy of ADCs in distinguishing between
different tissues was assessed using ROC analysis for variables
that showed statistically significant differences, based on the
aforementioned results. This analysis yielded several metrics,
including the cut-off value, sensitivity, specificity, accuracy and
area under the ROC curve (AUC), which are presented in Table V. ADCs at the maximum b-value of
≥800 sec/mm2 exhibited superior discriminatory
capability in distinguishing RA from D1 (Fig. 3A), RA from D2 (Fig. 3B) and RA from D3
(Fig. 3C) compared with those at
the maximum b-value of ≤100 sec/mm2. Particularly at
b-values of 0 and 1,500 sec/mm2, ADC cut-off values of
1.009×10−3 mm2/sec, 1.050×10−3
mm2/sec and 1.070×10−3 mm2/sec
demonstrated superior ability in distinguishing RA from
D1, from D2 and from D3 (AUCs:
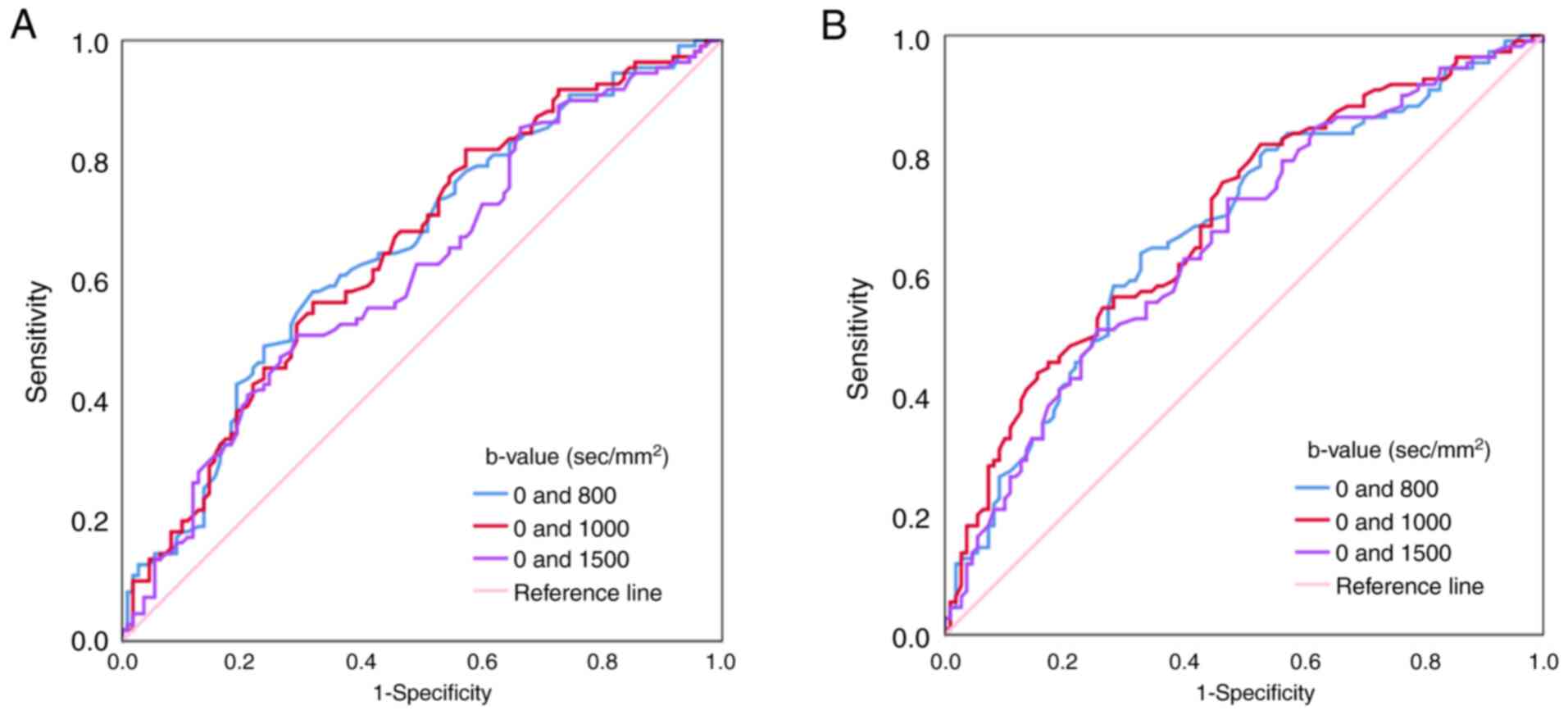
0.982, 0.992 and 0.996, respectively). Additionally, ADC cut-off
values of 1.522×10−3 mm2/sec (b=0 and 800
sec/mm2) and 1.539×10−3 mm2/sec
(b=0 and 1000 sec/mm2) exhibited optimal diagnostic
performance in differentiating D1 from D2
(AUC, 0.652; Fig. 4A) and
D1 from D3 (AUC, 0.692; Fig. 4B).
 | Table V.Receiver operating characteristic
curve analyses of apparent diffusion coefficients for the
differentiation between the tumor and distal paracancerous tissue
located ~1, 2 and 3 cm from the tumor margin at different b-value
pairs. |
Table V.
Receiver operating characteristic
curve analyses of apparent diffusion coefficients for the
differentiation between the tumor and distal paracancerous tissue
located ~1, 2 and 3 cm from the tumor margin at different b-value
pairs.
| A, Tumor vs.
D1 |
|---|
|
|---|
| b-value,
sec/mm2 | Cut-off value,
×10−3 mm2/sec | Sensitivity | Specificity | Accuracy | AUC (95% CI) |
|---|
| 0 and 50 | 2.714 | 0.827 | 0.727 | 0.777 | 0.842
(0.790–0.894) |
| 0 and 100 | 2.277 | 0.891 | 0.673 | 0.782 | 0.838
(0.784–0.891) |
| 0 and 800 | 1.329 | 0.964 | 0.855 | 0.909 | 0.963
(0.939–0.987) |
| 0 and 1,000 | 1.117 | 0.909 | 0.973 | 0.941 | 0.981
(0.966–0.995) |
| 0 and 1,500 | 1.009 | 0.936 | 0.955 | 0.945 | 0.982
(0.967–0.997) |
|
| B, Tumor vs.
D2 |
|
| b-value,
sec/mm2 | Cut-off value,
×10−3 mm2/sec |
Sensitivity |
Specificity |
Accuracy | AUC (95%
CI) |
|
| 0 and 50 | 2.960 | 0.955 | 0.645 | 0.800 | 0.843
(0.790–0.895) |
| 0 and 100 | 2.275 | 0.891 | 0.727 | 0.809 | 0.840
(0.786–0.894) |
| 0 and 800 | 1.339 | 0.973 | 0.909 | 0.941 | 0.987
(0.976–0.997) |
| 0 and 1,000 | 1.167 | 0.936 | 0.982 | 0.959 | 0.990
(0.980–1.000) |
| 0 and 1,500 | 1.050 | 0.982 | 0.945 | 0.964 | 0.992
(0.983–1.000) |
|
| C, Tumor vs.
D3 |
|
| b-value,
sec/mm2 | Cut-off value,
×10−3 mm2/sec |
Sensitivity |
Specificity |
Accuracy | AUC (95%
CI) |
|
| 0 and 50 | 2.932 | 0.936 | 0.636 | 0.786 | 0.842
(0.790–0.895) |
| 0 and 100 | 2.158 | 0.818 | 0.764 | 0.791 | 0.839
(0.785–0.893) |
| 0 and 800 | 1.340 | 0.973 | 0.927 | 0.950 | 0.989
(0.980–0.998) |
| 0 and 1,000 | 1.220 | 0.982 | 0.964 | 0.973 | 0.994
(0.986–1.000) |
| 0 and 1,500 | 1.070 | 1.000 | 0.945 | 0.973 | 0.996
(0.991–1.000) |
|
| D, D1
vs. D2 |
|
| b-value,
sec/mm2 | Cut-off value,
×10−3 mm2/sec |
Sensitivity |
Specificity |
Accuracy | AUC (95%
CI) |
|
| 0 and 800 | 1.522 | 0.582 | 0.682 | 0.632 | 0.652
(0.580–0.724) |
| 0 and 1,000 | 1.425 | 0.564 | 0.682 | 0.623 | 0.651
(0.578–0.723) |
| 0 and 1,500 | 1.249 | 0.509 | 0.709 | 0.609 | 0.617
(0.543–0.691) |
|
| E, D1
vs. D3 |
|
| b-value,
sec/mm2 | Cut-off value,
×10−3 mm2/sec |
Sensitivity |
Specificity |
Accuracy | AUC (95%
CI) |
|
| 0 and 800 | 1.559 | 0.636 | 0.673 | 0.655 | 0.674
(0.603–0.745) |
| 0 and 1,000 | 1.539 | 0.755 | 0.536 | 0.645 | 0.692
(0.623–0.761) |
| 0 and 1,500 | 1.249 | 0.509 | 0.745 | 0.627 | 0.658
(0.586–0.729) |
Discussion
In the present study, the disparities in ADCs
between RA and distal paracancerous tissues (~1, 2 and 3 cm from
the tumor margin) were evaluated, and the diagnostic performance of
ADCs in distinguishing between these tissues was assessed.
As demonstrated in the present study, the tumor
exhibited lower ADC values compared with distal paracancerous
tissues at all b-value pairs, which was consistent with the results
of a previous study (17). A
possible explanation for this finding is the higher cellular
density and irregular cell morphology within the tumor, which
results in narrower and distorted intercellular spaces that
restrict the diffusion of water molecules (24,25).
Consequently, the tumor exhibits lower ADC values compared with
D1, D2 and D3. Furthermore, the
present study demonstrated that ADCs at the maximum b-values of
≥800 sec/mm2 (AUCs, 0.963 to 0.996) exhibited superior
discriminatory capability in distinguishing the tumor from distal
paracancerous tissues compared with those at the maximum b-values
of ≤100 sec/mm2 (AUCs, 0.838 to 0.843). Particularly at
b-values of 0 and 1,500 sec/mm2, ADC demonstrated
optimal diagnostic efficacy in distinguishing RA from
D1, D2 and D3 (AUCs, 0.982 to
0.996). The results may be due to the fact that when calculating
ADC at the higher maximum b-values, it predominantly reflects the
diffusion of water molecules; conversely, when computing ADC at the
lower maximum b-value, it primarily reflects microcapillary
perfusion (26).
Diffusion and perfusion are distinct physical and
biological phenomena, both serving as indicators for several
physiological or pathological processes (25). The diffusion coefficient has been
reported to be a more effective diagnostic parameter than the
perfusion coefficient in distinguishing the tumor from
paracancerous tissue (27).
Additionally, the findings of the present study indicated that the
ADC values of pT1-2 staged tumors were significantly higher
compared with those of pT3-4 staged tumors at certain b-value pairs
(b=0 and 1,000 sec/mm2, and b=0 and 1,500
sec/mm2), which is consistent with previous studies
(28,29). This may be due to the increased
density of tumor cells and reduced extracellular space as the tumor
progresses, leading to greater restriction of water molecule
diffusion.
In the present study, the ADC of D1 was
lower compared with those of D2 and D3 at the
maximum b-values of ≥800 sec/mm2. Chen et al
(17) also reported a decrease in
ADC values of D1 compared with D2 in their
assessment of distal paracancerous tissues, but the ADC values
between D1/D2 and D3 were not
compared and cases of lower rectal cancer were not excluded. The
result of the present study may be attributed to molecular
alterations during tumorigenesis that can lead to abnormal
increases in water molecule mobility and quantity (15). In addition, the molecular
alterations associated with tumorigenesis are more pronounced in
paracancerous tissues located closer to the tumor compared with
tumor-distant tissues (8,9). Furthermore, previous research has
demonstrated that the more severe the intestinal inflammation and
fibrosis, the lower the ADC value (30). Stem cells derived from the adjacent
tissues of the tumor have the potential to induce fibrosis and an
inflammatory response (16),
indicating that inflammation and fibrosis are more pronounced in
paracancerous tissues located closer to the tumor compared with
tumor-distant tissues. Hence, the diffusion of water molecules is
more restricted in D1 compared with D2 and
D3. However, there were no significant differences in
ADCs at the maximum b-value of ≤100 sec/mm2 between
D1, D2 and D3 in the present
study. This may be because microcapillary perfusion cannot
accurately discern the subtle differences within these tissues.
Currently, there is no exact standard on DWI for the
definition of the length of DRM. The present study demonstrated
that there were no significant differences in ADCs between
D2 and D3 at all b-value pairs, suggesting
that D2 and D3 may possess similar biological
characteristics, such as microstructure, cell sequencing and tissue
composition. Therefore, we hypothesize that the safe DRM in
mid-high RA surgery may be reduced to 2 cm. According to the
National Comprehensive Cancer Network guidelines, patients with
mid-high rectal cancer are recommended to have a DRM length of 4–5
cm (20). Japanese guidelines
recommend a minimum distance of 3 cm for DRM in cases of rectal
cancer located above the peritoneal reflection and 2 cm for those
below it (19). Furthermore, there
have been very few reports of distal tumor infiltration extending
<3 cm (5). From a clinical
perspective, a DRM of 3 cm is generally considered a safe resection
margin for patients with rectal cancer. A previous metabolomic
study by Zhang et al (18)
may support the finding of the present study, which also suggests
that a DRM of 2 cm may be considered as a safe resection margin.
With the advancement of medical technology, the length of DRM in
rectal cancer surgery has been progressively reduced. Manegold
et al (31) reported that R0
resection of rectal cancer following preoperative chemoradiotherapy
achieved excellent outcomes, even with DRM <1 cm, without
impacting recurrence-free survival of patients. However, a
meta-analysis reported that for patients with rectal cancer
undergoing surgery alone, DRM <1 cm may not be deemed safe
(32).
In addition, the results of the present further
indicated that the ADCs at the maximum b-values of ≥800
sec/mm2 exhibited a certain diagnostic value in
discriminating between D1 and
D2/D3 (AUCs, 0.617 to 0.692). The ADCs at
b-values of 0 and 800 sec/mm2 or b-values of 0 and 1,000
sec/mm2 demonstrated improved diagnostic performance in
differentiating between D1 and D2 (AUC, 0.652
or 0.651), and the ADC at b-values of 0 and 1,000
sec/mm2 exhibited optimal diagnostic performance in
differentiating between D1 and D3 (AUC,
0.692). Although ADC could not achieve an excellent diagnostic
performance in distinguishing between D1 and
D2/D3, it may still have clinical importance
for preoperative surgical decision-making in resectable mid-high
RA.
The present study has several limitations. Firstly,
it was a single-center retrospective study, and a prospective study
across multiple institutions and scanners is needed to validate the
findings. Secondly, patients with RA who had not undergone
preoperative chemoradiotherapy were assessed, and further studies
are needed for patients who have undergone this therapy. Thirdly,
the results of the present investigation on distal paracancerous
tissues located at several distances from the tumor margin has not
been verified by comprehensive comparison with the corresponding
histopathology and molecular alterations of these tissues. Relevant
research should be performed in the future to support the findings
of the present study.
In conclusion, the results of the present study
demonstrated that there were differences in ADCs between RA,
D1 and D2/D3, and ADC could
distinguish RA from D1, D2 and D3,
and differentiate D1 from D2/D3 to
a certain degree. However, no significant differences were
demonstrated in ADCs between D2 and D3,
indicating that they may possess similar biological characteristics
and that the safe DRM in RA surgery may be reduced to 2 cm. The
findings suggest that ADC may potentially serve as a valuable tool
for evaluating the optimal distal resection range, contributing to
the development of surgical strategies to reduce the risk of local
recurrence and postoperative complications.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TWC, HYZ, XMZ participated in the design of the
study. HL, YQG, YSW and HLQ contributed to data analysis. TWC, HL
and YQG drafted and revised the article, gave final approval of the
version to be published, agreed to the submitted journal and agreed
to be accountable for all aspects of the work. TWC, HL and YQG
proofread the manuscript. TWC submitted the manuscript. All authors
have read and approved the final version of the manuscript. TWC and
HL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the Affiliated Hospital of North Sichuan Medical
College (Nanchong, China; approval no. 2024ER219-1). The ethics
committee waived the need for informed consent due to the
retrospective nature of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADC
|
apparent diffusion coefficient
|
|
DWI
|
diffusion-weighted imaging
|
|
MRI
|
magnetic resonance imaging
|
|
T2W
|
T2-weighted
|
|
RA
|
rectal adenocarcinoma
|
|
D1
|
distal paracancerous tissue located
~1 cm from the tumor margin
|
|
D2
|
distal paracancerous tissue located
~2 cm from the tumor margin
|
|
D3
|
distal paracancerous tissue located
~3 cm from the tumor margin
|
|
DRM
|
distal resection margin
|
|
DTM
|
distal tumor margin
|
|
ROI
|
region of interest
|
|
ICC
|
intraclass correlation
coefficient
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the receiver operating
characteristic curve
|
|
pT
|
pathological tumor stage
|
|
pN
|
pathological lymph node stage
|
References
|
1
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Islam SMA, Patel R, Bommareddy RR, Khalid
KM and Acevedo-Duncan M: The modulation of actin dynamics via
atypical protein kinase-C activated cofilin regulates metastasis of
colorectal cancer cells. Cell Adh Migr. 13:106–120. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kosuge M, Eto K, Sasaki S, Sugano H,
Yatabe S, Takeda Y, Ito D, Ohkuma M and Yanaga K: Clinical factors
affecting the distal margin in rectal cancer surgery. Surg Today.
50:743–748. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ekkarat P, Boonpipattanapong T,
Tantiphlachiva K and Sangkhathat S: Factors determining low
anterior resection syndrome after rectal cancer resection: A study
in Thai patients. Asian J Surg. 39:225–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song SH, Park JS, Choi GS, Seo AN, Park
SY, Kim HJ, Lee SM and Yoon G: Impact of the distal resection
margin on local recurrence after neoadjuvant chemoradiation and
rectal excision for locally advanced rectal cancer. Sci Rep.
11:229432021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trujillo KA, Hines WC, Vargas KM, Jones
AC, Joste NE, Bisoffi M and Griffith JK: Breast field
cancerization: Isolation and comparison of telomerase-expressing
cells in tumor and tumor adjacent, histologically normal breast
tissue. Mol Cancer Res. 9:1209–1221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo H, Zeng W, Feng L, Yu X, Li P, Zhang
K, Zhou Z and Cheng S: Integrated transcriptomic analysis of
distance-related field cancerization in rectal cancer patients.
Oncotarget. 8:61107–61117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Braakhuis BJ, Tabor MP, Kummer JA, Leemans
CR and Brakenhoff RH: A genetic explanation of Slaughter's concept
of field cancerization: Evidence and clinical implications. Cancer
Res. 63:1727–1730. 2003.PubMed/NCBI
|
|
11
|
Chavhan GB and Caro-Dominguez P:
Diffusion-weighted imaging in pediatric body magnetic resonance
imaging. Pediatr Radiol. 46:847–857. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Subhawong TK, Jacobs MA and Fayad LM:
Diffusion-weighted MR imaging for characterizing musculoskeletal
lesions. Radiographics. 34:1163–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ko CC, Yeh LR, Kuo YT and Chen JH: Imaging
biomarkers for evaluating tumor response: RECIST and beyond.
Biomark Res. 9:522021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harold KM, MacCuaig WM, Holter-Charkabarty
J, Williams K, Hill K, Arreola AX, Sekhri M, Carter S,
Gomez-Gutierrez J, Salem G, et al: Advances in imaging of
inflammation, fibrosis, and cancer in the gastrointestinal tract.
Int J Mol Sci. 23:161092022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marques MPM, Batista de Carvalho ALM,
Mamede AP, Dopplapudi A, García Sakai V and Batista de Carvalho
LAE: Role of intracellular water in the normal-to-cancer transition
in human cells-insights from quasi-elastic neutron scattering.
Struct Dyn. 7:054702020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Guo M, Zhao F, Liu Q and Wang X:
Colonic stem cells from normal tissues adjacent to tumor drive
inflammation and fibrosis in colorectal cancer. Cell Commun Signal.
21:1862023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen XQ, Tan BG, Xu M, Zhou HY, Ou J,
Zhang XM, Yu ZY and Chen TW: Apparent diffusion coefficient derived
from diffusion-weighted imaging to differentiate between tumor,
tumor-adjacent and tumor-distant tissues in resectable rectal
adenocarcinoma. Eur J Radiol. 155:1105062022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Pan G, Liu Z, Kong Y and Wang D:
Association of levels of metabolites with the safe margin of rectal
cancer surgery: A metabolomics study. BMC Cancer. 22:10432022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese society for cancer of the colon and rectum.
Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benson AB, Venook AP, Al-Hawary MM, Azad
N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Garrido-Laguna I, et al: Rectal cancer, version 2.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:1139–1167. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Wang Z, Ren H, Wang Z and Li J:
Accuracy of magnetic resonance imaging in defining dentate line in
anal fistula. BMC Med Imaging. 22:2012022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalisz KR, Enzerra MD and Paspulati RM:
MRI evaluation of the response of rectal cancer to neoadjuvant
chemoradiation therapy. Radiographics. 39:538–556. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye Z, Yao S, Yang T, Li Q, Li Z and Song
B: Abdominal diffusion-weighted MRI with simultaneous multi-slice
acquisition: Agreement and reproducibility of apparent diffusion
coefficients measurements. J Magn Reson Imaging. 59:1170–1178.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Shen F, Li Z, Lu H, Chen Y, Wang Z
and Lu J: Diffusion-weighted imaging of rectal cancer on
repeatability and cancer characterization: An effect of b-value
distribution study. Cancer Imaging. 18:432018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sumi M, Van Cauteren M, Sumi T, Obara M,
Ichikawa Y and Nakamura T: Salivary gland tumors: Use of intravoxel
incoherent motion MR imaging for assessment of diffusion and
perfusion for the differentiation of benign from malignant tumors.
Radiology. 263:770–777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Palmucci S, Cappello G, Attinà G, Fuccio
Sanzà G, Foti PV, Ettorre GC and Milone P: Diffusion-weighted MRI
for the assessment of liver fibrosis: principles and applications.
Biomed Res Int. 2015:8742012015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang G, Wang S, Wen D and Zhang J, Wei X,
Ma W, Zhao W, Wang M, Wu G and Zhang J: Comparison of non-Gaussian
and Gaussian diffusion models of diffusion weighted imaging of
rectal cancer at 3.0 T MRI. Sci Rep. 6:387822016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou M, Gong T, Chen M and Wang Y:
High-resolution integrated dynamic shimming diffusion-weighted
imaging (DWI) in the assessment of rectal cancer. Eur Radiol.
33:5769–5778. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Li Q, Tang L, Huang Z and Lin Q:
Correlations of mean and mimimum apparent diffusion coefficient
values with the clinicopathological features in rectal cancer. Acad
Radiol. 28:S105–S111. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du JF, Lu BL, Huang SY, Mao R, Zhang ZW,
Cao QH, Chen ZH, Li SY, Qin QL, Sun CH, et al: A novel
identification system combining diffusion kurtosis imaging with
conventional magnetic resonance imaging to assess intestinal
strictures in patients with Crohn's disease. Abdom Radiol (NY).
46:936–947. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manegold P, Taukert J, Neeff H,
Fichtner-Feigl S and Thomusch O: The minimum distal resection
margin in rectal cancer surgery and its impact on local
recurrence–A retrospective cohort analysis. Int J Surg. 70:1022019.
View Article : Google Scholar
|
|
32
|
Yan H, Wang PY, Wu YC and Liu YC: Is a
distal resection margin of ≤ 1 cm safe in patients with
intermediate- to low-lying rectal cancer? A systematic review and
meta-analysis. J Gastrointest Surg. 26:1791–1803. 2022. View Article : Google Scholar : PubMed/NCBI
|