With the intensification of population aging,
sarcopenia has emerged as a growing public health concern (1). Sarcopenia, an age-related decline in
muscle mass and function, is characterized by weight loss, slow
walking pace, limited mobility, reduced grip strength and frequent
falls (2). It affects the quality
of life of patients and is closely associated with the occurrence
and development of various chronic diseases, including chronic
kidney disease (3),
metabolic-associated fatty liver disease (4), inflammatory bowel disease (5,6),
Parkinson's disease (7,8), Alzheimer's disease and chronic
obstructive pulmonary disease (9).
Epidemiological data have reported an increasing incidence of
sarcopenia in the elderly population, leading to a significant
physical and economic burden on patients (1). Therefore, understanding the molecular
mechanisms underlying sarcopenia is important for developing new
therapeutic strategies (10).
In recent years, scientific research has revealed
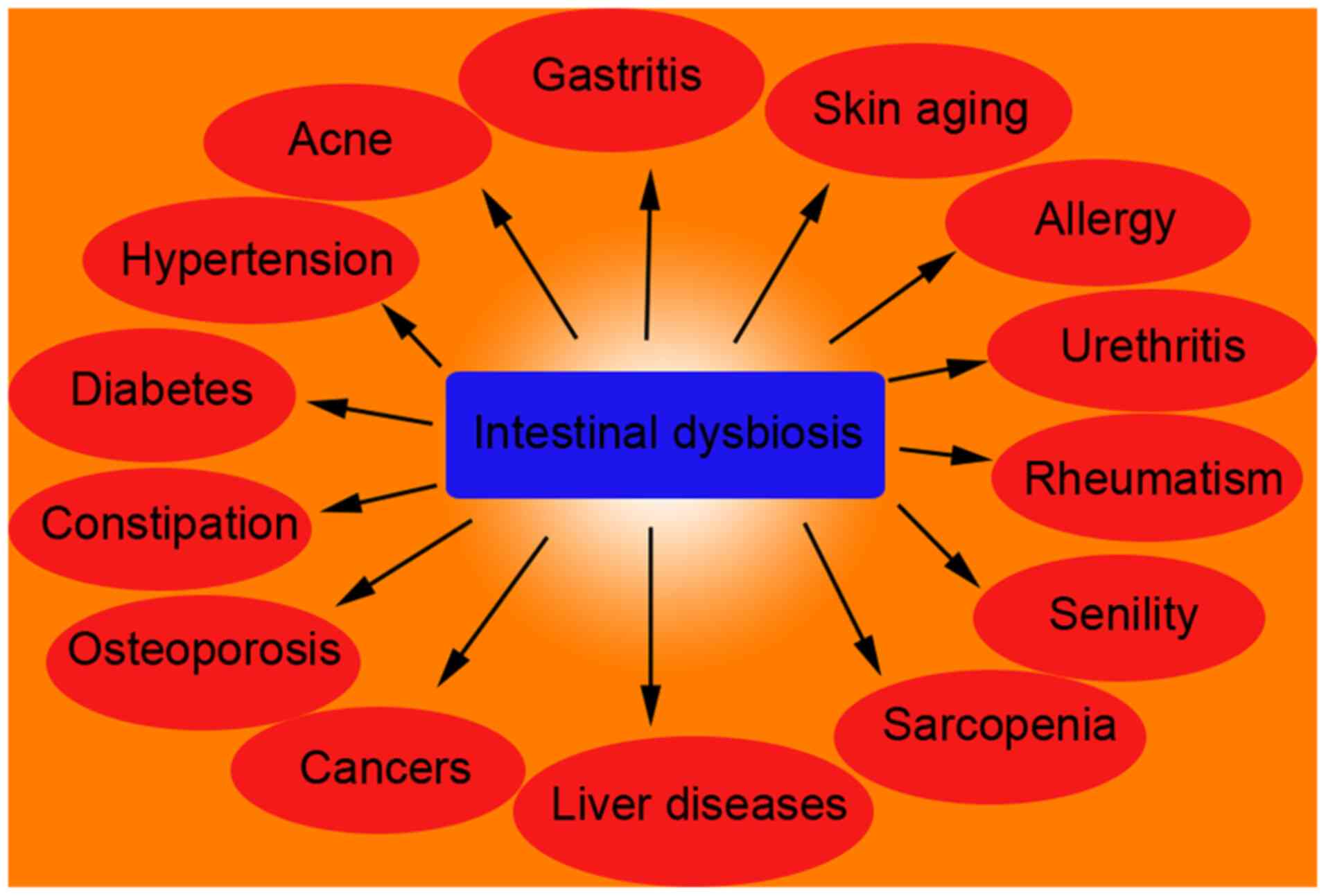
the pivotal role of the gut microbiota in human health and
diseases, particularly in the pathogenesis and progression of
sarcopenia (11). The gut
microbiota is a complex microbial community that actively
participates in several physiological and pathological processes
through its metabolites and interactions with the host (Fig. 1). Research on the association
between the gut microbiota and muscle health has revealed complex
interactions between the microbial community and the host, which
likely impact muscle metabolism, growth and atrophy through
multifaceted pathways, thereby influencing muscle quality and
function (12,13).
The present article aimed to provide a comprehensive
understanding of sarcopenia by introducing its definition and
epidemiological data, thereby offering readers valuable insights
into the disease background (14).
Subsequently, a detailed exploration of the definition and
composition of the gut microbiota is presented. Furthermore, the
complex links between gut microbiota and sarcopenia will be
elucidated, unraveling the precise molecular mechanisms through
which the gut microbiota influences muscle quality and function.
Additionally, an overview of current research advancements in gut
microbiota interventions for sarcopenia is provided, along with
discussions on future research directions and potential therapeutic
strategies (15). By presenting
this progressive series of insights, the present study strived to
construct a robust theoretical framework that delves into the
specific mechanisms through which the gut microbiota affects muscle
metabolism and growth, while also laying the groundwork for
discussions on research progress, challenges and future prospects
regarding gut microbiota interventions for sarcopenia.
Gut microbiota refers to the microbial community
present in the human gastrointestinal tract, including bacteria,
fungi, viruses, and other microorganisms. Bacteria are the major
components of the gut microbiota (Table
I) (16). Commonly encountered
bacterial taxa in the gut microbiota include, but are not limited
to, the following major groups: Bacteroidetes (antagonistic group),
including genera such as Bacteroides and Prevotella,
which comprise antagonistic and tolerogenic bacteria, respectively;
Firmicutes (dominant group), including the Clostridia class and
Bacillus group, containing beneficial bacteria such as
Lactobacillus and Clostridium; Proteobacteria
(deformative group), encompassing the order Enterobacteriales and
the Vibrio cholerae species, including Escherichia
coli; Actinobacteria (actinobacteria group), including the
Actinobacteria phylum, where Bifidobacterium is commonly
found (17–19). In addition to these major groups,
various other microbial species from different phyla such as
anaerobic and Anaerococcus species may also inhabit the gut.
Furthermore, several types of viruses such as haloviruses and
bacteriophages are present in the intestinal tract. The composition
of the gut microbiota can be influenced by multiple factors,
including dietary habits, environmental factors, age, physiological
status and genetic factors (20).
Therefore, the composition of gut microbiota may vary among
individuals.
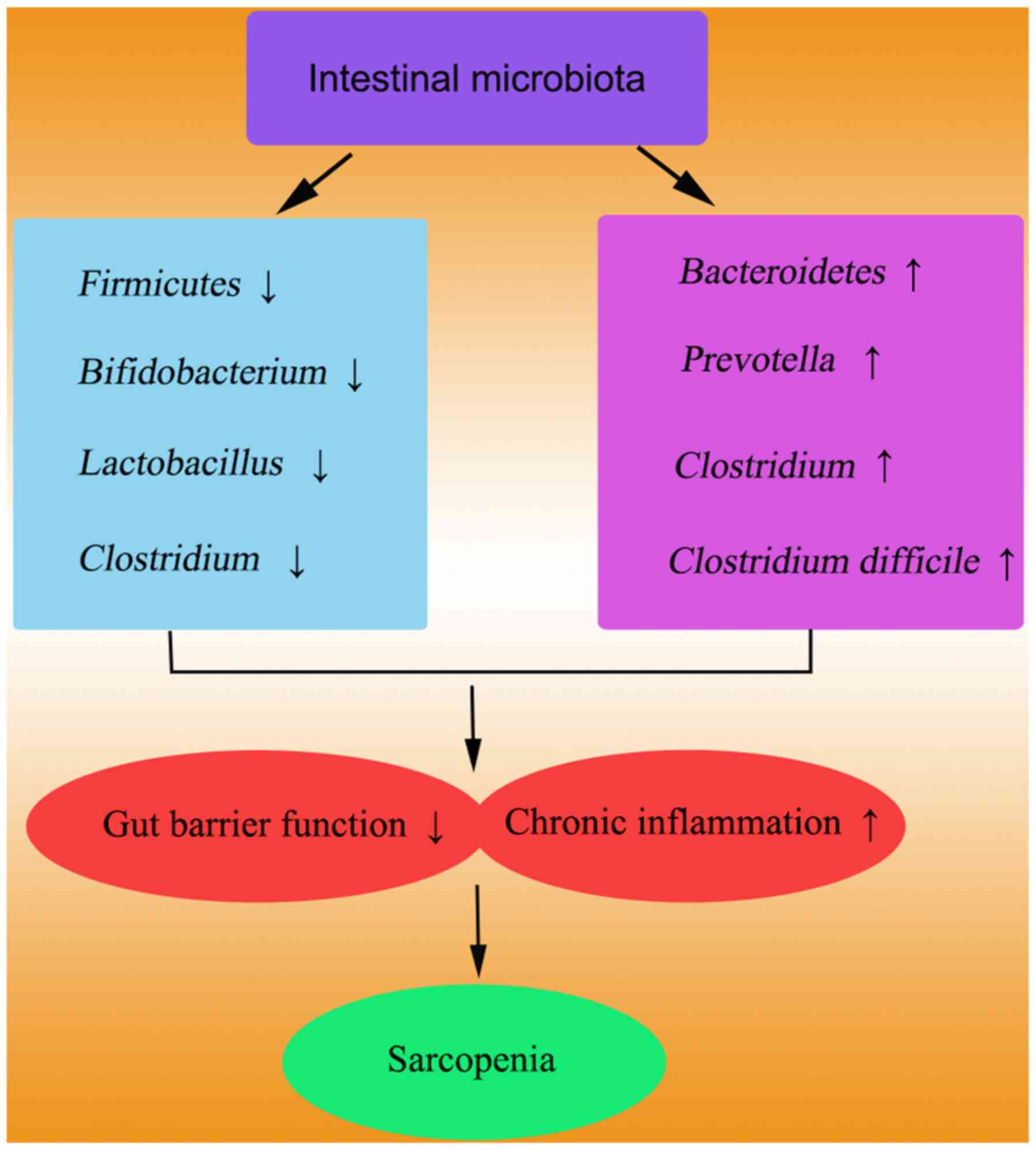
Previous studies have demonstrated significant
differences in gut microbiota composition between individuals with
sarcopenia and their healthy counterparts (21). Dysbiosis of the gut microbiota and
its metabolites may contribute to distinct clinical complexities
observed in frail elderly individuals (22). Specifically, the colonization of the
gut microbiota in sarcopenia patients undergoing maintenance
hemodialysis (MHD) has shown a diminished abundance of
Akkermansia in the intestines of mice, indicating a
potential role of altered gut microbiota in the development of
skeletal muscle disorders in MHD patients (23). Particularly, reduced diversity and
altered abundance of specific bacterial taxa have been observed in
the gut microbiota of patients with sarcopenia (24,25).
These changes include a decrease in beneficial taxa, such as
Akkermansia and Lactobacillus, which play vital roles
in maintaining the gut barrier function and regulating immune
responses. Harmful bacteria, including Clostridium and
Proteobacteria, tend to be more prevalent in the gut of patients
(26–28). Metabolites produced by these
abnormal bacterial populations may negatively affect the muscle
health. These findings suggest that dysbiosis of the gut microbiota
significantly contributes to the onset and progression of
sarcopenia (Fig. 2). Thus, patients
with sarcopenia exhibit specific gut microbiota characteristics.
Reduced diversity, decreased abundance of beneficial taxa and
increased levels of harmful bacteria collectively suggest the
involvement of gut microbiota dysbiosis in the pathogenesis and
progression of sarcopenia.
Although the association between gut microbiota
imbalance and sarcopenia has been extensively reported, further
exploration is required to establish a causal relationship. Current
research has provided evidence from animal models and clinical
trials. For instance, transplanting gut microbiota from healthy
individuals into mice with muscle wasting demonstrated significant
improvements in the muscle mass and function, indicating the
therapeutic potential of gut microbiota restoration for treating
muscle wasting (29,30). Moreover, supplementation with
specific probiotics and prebiotics was reported to enhance the
muscle mass and function in patients with sarcopenia, further
supporting the role of gut microbiota in sarcopenia (31,32).
Nonetheless, additional longitudinal studies and randomized
controlled trials are necessary to elucidate the precise mechanisms
underlying the gut microbiota imbalance in sarcopenia.
An imbalance in gut microbiota, also known as
dysbiosis of gut microbiota, has been shown to be associated with
various muscle dysfunctions (33–57).
The gut microbiota influence the muscle growth and metabolic
processes by regulating the host energy balance and immune response
through several mechanisms (58,59).
Imbalances in gut microbiota may affect the muscle
health by triggering systemic inflammatory responses (21,60,61).
Research indicates that imbalanced gut microbiota can impair the
intestinal barrier function, increase the intestinal permeability
and facilitate the translocation of bacterial endotoxins such as
lipopolysaccharides, into the bloodstream, thus eliciting systemic
inflammation (62,63). This chronic low-grade inflammatory
state is considered a crucial pathological mechanism of sarcopenia,
as inflammatory factors like TNF-α and IL-6, activated via the
NF-κB signaling pathway, inhibit muscle protein synthesis and
promote muscle protein degradation (64,65).
Metabolites produced by the gut microbiota,
including short-chain fatty acids (SCFAs) and branched-chain amino
acids, play a pivotal role in regulating muscle metabolism and
function. SCFAs, such as butyrate and propionate, possess
anti-inflammatory and immunomodulatory effects, promoting the
muscle protein synthesis through the activation of the
AMP-activated protein kinase signaling pathway (66). Conversely, an imbalance in gut
microbiota can lead to a decline in the production of these
beneficial metabolites, thereby affecting muscle health.
Additionally, an increase in certain detrimental metabolites, such
as indole and p-cresol, has been associated with muscle atrophy
(67,68).
Genetic factors also contribute to the relationship
between gut microbiota imbalance and muscle atrophy (69). Certain genetic variations can
influence the composition and function of the gut microbiota,
indirectly affecting the muscle health. For instance, mutations in
FOXO3 are associated with gut microbiota diversity and muscle mass
(70,71). Moreover, gene-environment
interactions may regulate the muscle metabolism and function by
influencing the gut microbiota, offering new insights for future
personalized treatment strategies (72). Thus, dysbiosis of the gut microbiota
affects muscle health through various mechanisms, including
modulation of inflammatory responses, alterations in metabolite
production and interaction with genetic factors. Elucidating these
mechanisms is crucial for developing targeted interventions to
mitigate muscle dysfunction associated with gut microbiota
imbalance.
Gut microbiota can modulate the epigenetic status of
the host either directly or indirectly through the production of
various metabolites, including SCFAs (73,74).
These metabolites can enter the bloodstream and affect distant
tissues, including muscle cells (75). Fecal butyrate levels have been
reported in older individuals with low muscle mass, suggesting a
potential role for altered gut microbiota in the development of
sarcopenia. These findings highlight the potential of gut microbial
features and fecal butyrate as biomarkers for the early detection
of sarcopenia (76), making them
valuable diagnostic and intervention strategies. For instance,
butyric acid, an SCFA, inhibits histone deacetylases, leading to
increased histone acetylation and subsequent alterations in gene
expression (77). Epigenetic
regulation can influence the differentiation and regeneration
capacity of muscle cells, thereby affecting the muscle health and
function (78).
Furthermore, the gut microbiota modulates host gene
expression by regulating the expression of microRNAs (miRNAs).
miRNAs are a class of non-coding RNA molecules that regulate gene
expression by inhibiting translation or promoting the degradation
of specific mRNA targets (79).
Studies have indicated that changes in the gut microbiota
composition are associated with altered expression patterns of
specific miRNAs, which may be involved in regulating muscle
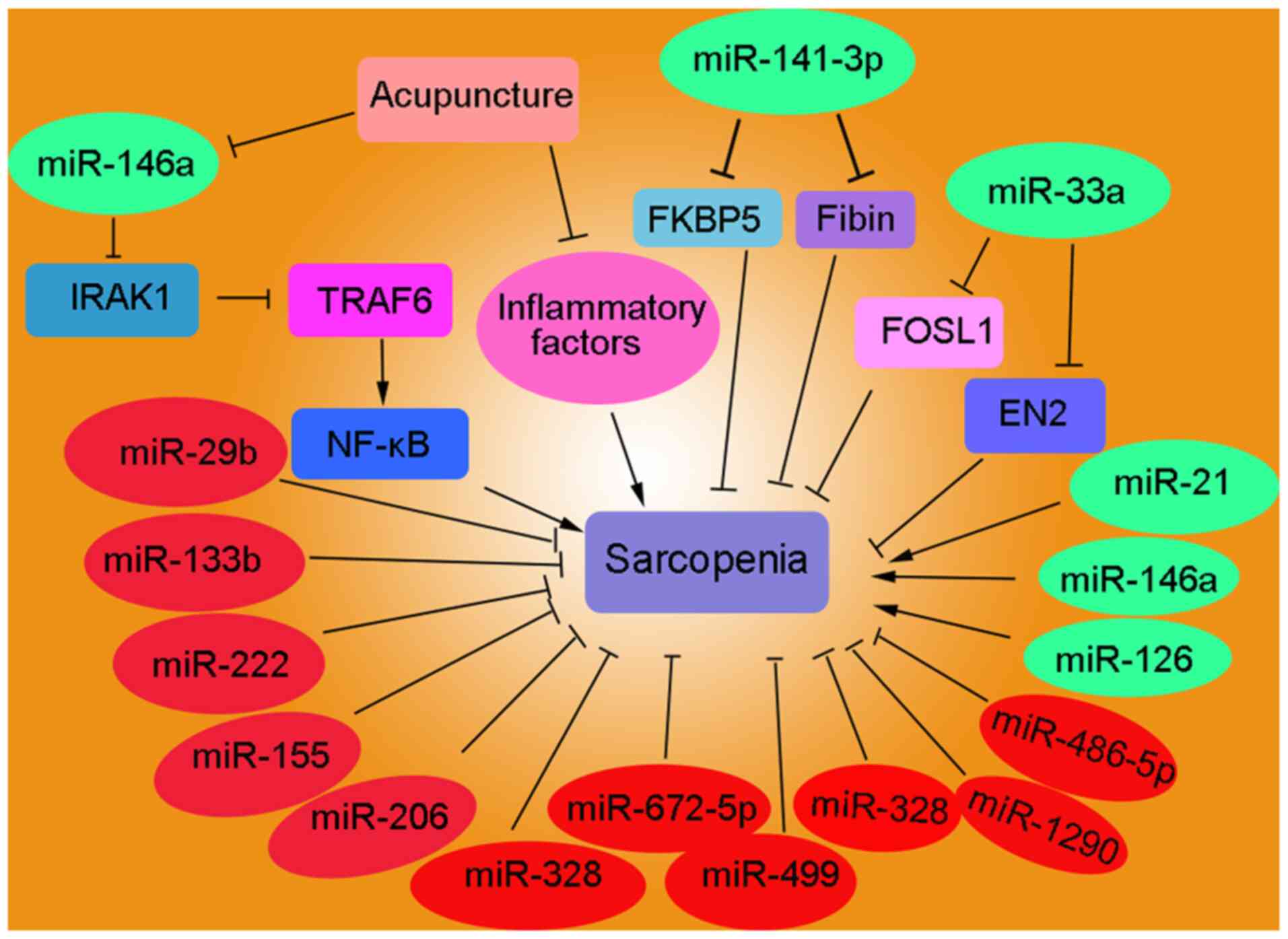
metabolism and processes associated with muscle atrophy (Fig. 3; Table
II) (80–95). Circulating miRNAs (c-miRNAs),
including miR-21, miR-126, miR-146a and miR-222, have been
identified as potential biomarkers for sarcopenia (81). The upregulation of miR-141-3p in
ovariectomized mice contributes to mitochondrial dysfunction by
inhibiting FKBP prolyl isomerase 5 and Fibin, indicating that
targeting miR-141-3p may be a promising therapeutic strategy for
mitigating obesogenic sarcopenia (82). The expression profiles of miR-1,
miR-133a/b, miR-206, miR-208b and miR-499 in 109 non-sarcopenic and
109 sarcopenic individuals were analyzed. These results revealed
that sarcopenia and malnutrition frequently coexist in elderly
individuals, suggesting that lower levels of miR-133b and miR-206
are associated with sarcopenia. The relationship between miR-133b
and sarcopenia is mediated by the nutritional status, indicating
the potential role of nutrition in modulating age-related muscle
decline (83). The downregulation
of miR-532-3p, which is associated with inflammation, regulates the
apoptotic pathway during the development of sarcopenia by targeting
BCL2 antagonist/killer 1 (84).
Acupuncture has the potential to alleviate sarcopenia by regulating
mitochondrial function and suppressing chronic inflammation through
the miR-146a/interleukin 1 receptor associated kinase 1/TNF
receptor associated factor 6/NF-kB signaling pathway, thus
potentially decreasing the muscle wastage (85). Severe malnutrition and sarcopenia
are strongly associated with poor surgical and oncological outcomes
in patients with cancer. Decreased psoas muscle mass index (PMI) is
an independent prognostic factor for overall survival, disease-free
survival and metastasis in patients with colorectal cancer (CRC).
Serum miR-21 expression, which is associated with PMI, may serve as
a potential biomarker of sarcopenia in patients with CRC (86). miR-33a serves as a clinical
prognostic marker for sarcopenia and glioma by targeting FOS-like
1, AP-1 transcription factor subunit and engrailed homeobox 2
(87). The plasma levels of
miR-29b, miR-181a and miR-494 were detected in a cohort of 93
individuals with sarcopenia. The results revealed a significant
downregulation of plasma miR-29b in elderly individuals with
sarcopenia and cardiovascular risk factors, including diabetes,
hypertension and dyslipidemia (88).
Epigenetics serve a pivotal role in the development
of sarcopenia, with DNA methylation, histone modifications and
non-coding RNA regulation being the most extensively studied.
Alterations in DNA methylation patterns in patients with sarcopenia
can lead to the dysregulation of gene expression associated with
muscle growth and repair (96). For
instance, genes such as myogenic differentiation 1 and myocyte
enhancer factor 2, which are crucial for muscle development, have
promoter regions susceptible to changes in the methylation status
that can affect their activity (97). Histone modifications also serve a
critical role in sarcopenia, particularly in the imbalance between
histone acetylation and deacetylation, which affects the muscle
fiber-type conversion and energy metabolism (98). Non-coding RNAs, particularly miRNAs
and long non-coding RNAs (lncRNAs), have emerged as key regulators
of muscle atrophy and regeneration. These molecules modulate the
muscle mass and function by targeting multiple signaling pathways,
such as insulin-like growth factor 1/AKT/mTOR and TGF-β/SMAD
pathways (99,100).
Currently, the diagnosis of gut microbiota relies
heavily on high-throughput sequencing technologies, such as 16S
ribosomal RNA gene sequencing and metagenomic analysis (101). Although these methods offer
detailed information about the diversity and abundance of the gut
microbiota, they have few limitations. Firstly, they often require
expensive equipment and specialized knowledge, which limits their
widespread application in clinical practice (102). Secondly, data interpretation can
be complex and influenced by sample processing and analysis
platforms (103). Furthermore,
these techniques do not provide information on the microbial
activity and function, which is crucial for understanding the
relationship between gut microbiota dysbiosis and sarcopenia.
To overcome the limitations of the current
diagnostic techniques, researchers are exploring new approaches for
monitoring and diagnosing gut microbiota dysbiosis. One promising
method is metabolomic analysis, which assesses microbial activity
by detecting small molecular metabolites in blood, urine or fecal
samples (102,104). Metabolomics not only reflects the
functional state of the microbial community, but also reveals
interactions between the host and microbiota (105). Moreover, the development of
bioinformatics tools in recent years has enabled improved
interpretation of complex datasets and the identification of
disease-related biomarkers (106).
Another emerging field is microbiome editing technologies, such as
the CRISPR-Cas system, which provide a potential means of
modulating specific microbial members to correct dysbiosis
(107,108). Finally, portable devices and rapid
testing platforms are under development, potentially allowing gut
microbiota monitoring in the home or primary healthcare settings in
the future (109).
The application of probiotics and prebiotics has
emerged as an important strategy for modulating gut microbiota
balance and impacting host health. In the context of sarcopenia
treatment, probiotics can positively influence muscle metabolism by
improving gut microbiota composition, enhancing intestinal barrier
function and attenuating inflammatory responses (110). For instance, specific strains of
lactic acid bacteria have been shown to increase the production of
SCFAs in the gut, which are vital for maintaining the muscle
function and promoting muscle synthesis (111). Furthermore, prebiotics, as
non-digestible food ingredients, promote the growth of beneficial
bacterial communities such as Bifidobacteria and
Lactobacilli, which indirectly affect muscle health through
the production of metabolites such as SCFAs (112). However, the clinical application
of probiotics and prebiotics requires further randomized controlled
trials to validate their efficacy and safety.
The development of drugs targeting gut microbiota
represents a promising therapeutic strategy to address the
connection between gut microbiota dysbiosis and sarcopenia. These
drugs regulate specific microbial communities or their metabolites
to restore the gut microbiota balance and improve muscle function
(113). For example, the
administration of antibiotics or specific antimicrobial peptides
can inhibit detrimental bacterial communities and alleviate their
detrimental effects on host health (114). Additionally, research is exploring
the utilization of prebiotics, invertase inhibitors and other
approaches to modulate the activity and metabolic pathways of
specific bacterial communities. Although these methods have
potential, precise targeting and dose control are required to avoid
adverse effects on microbial communities.
FMT is a method for restoring the gut microbiota
balance in the recipient's intestines by transplanting the gut
microbiota of healthy donors. Although research on the use of FMT
for the treatment of sarcopenia is still in its early stages, some
encouraging findings have been reported. An animal study
demonstrated that FMT transplantation of the gut microbiota of a
healthy donor significantly improved the muscle atrophy caused by
gut microbiota dysbiosis (115).
Additionally, FMT positively affects muscle health by restoring gut
microbiota diversity, enhancing intestinal barrier function and
reducing inflammation (116).
Despite the potential of FMT, further verification of its safety,
efficacy and long-term effects in clinical applications is
required.
In addition to direct interventions using
probiotics, prebiotics or medications, lifestyle modifications are
effective approaches for modulating the gut microbiota and treating
sarcopenia. Dietary habits have a significant impact on gut
microbial diversity and function. Consumption of a high-fiber diet
promotes the growth of beneficial bacterial communities and
increases the production of SCFAs, which are advantageous for
maintaining muscle health. Moreover, moderate exercise has been
shown to improve gut microbiota composition and enhance beneficial
functions for overall health (117). Therefore, combining dietary
adjustments with appropriate physical activity may represent a
comprehensive and sustainable strategy for improving gut health and
preventing or treating sarcopenia.
The present review article discusses the complex
relationship between the gut microbiota and sarcopenia, emphasizing
the important role of the gut microbiota in muscle health. By
analyzing the mechanisms by which the gut microbiota influence
muscle metabolism and growth, the present study provides a novel
perspective for the prevention and treatment of sarcopenia. Until
now, to the best of our knowledge, there have been no existing
reports directly associating the specific types of bacteria
discussed in the present study with the regulation of specific
miRNAs in the context of sarcopenia, such as the Akkermansia,
Lactobacillus, Faecalibacterium, Prevotella, Proteobacteria.
This highlights miRNAs as a novel research direction and starting
points in the current understanding of the molecular mechanisms
involved.
In terms of treatment, the research progress on gut
microbiota interventions for sarcopenia is promising. Intervention
strategies such as probiotics, prebiotics and FMT have the
potential to improve gut microbiota balance and muscle health.
However, the safety and efficacy of these interventions requires
further validation. Additionally, the development of personalized
treatment strategies is essential, as there may be significant
variations in the gut microbiota composition and response among
individuals. Future research should also investigate the specific
mechanisms through which the gut microbiota influences sarcopenia
and address the limitations of existing technologies and methods.
Firstly, an in-depth exploration of the complex relationship
between gut microbiota and muscle metabolism, inflammatory
responses and immune regulation is crucial for understanding the
underlying mechanisms. Secondly, the development of more precise
and efficient gut microbiota modulation technologies, such as gene
editing technologies based on CRISPR-Cas9, will enable the precise
control of specific microbial communities. Additionally,
large-scale, long-term clinical studies evaluating the long-term
effects and safety of different intervention methods are essential
for establishing the clinical value of these interventions
(118). Finally, interdisciplinary
collaborations combining bioinformatics, systems biology and
artificial intelligence technologies will help uncover the complex
network relationships between gut microbiota and sarcopenia,
providing a theoretical basis and technical support for the
development of new treatment strategies.
There were several studies on gut microbiota and
sarcopenia; however, each study focused on different aspects. For
example, Li et al (120) primarily
focused on exercise as an intervention method, exploring how
physical activity can impact muscle health by altering the gut
microbiota and how it can prevent sarcopenia through modifications
to the gut microbial community. Moreover, the study by Liu et al
(16) included a total of 26
preclinical studies and 10 clinical studies, systematically
reviewing the association between the gut microbiota and sarcopenia
and investigating the relationship between changes in the gut
microbiota and muscle/physical performance. Zhang et al (121) investigated the correlation between
the gut microbiota and sarcopenia by analyzing data from human and
animal studies, as well as the potential biological mechanisms
through which the gut microbiota may affect muscle health,
including protein synthesis, mitochondrial function, chronic
inflammation and immune response. The novelty of this review lies
in elucidating the molecular mechanisms between the gut microbiota
and muscle atrophy, as well as mediating the progression of
sarcopenia. Moreover, the present study also explored the potential
strategies for treating muscle atrophy by regulating the gut
microbiota.
In conclusion, this review discusses the association
between the gut microbiota and sarcopenia and elucidates the
molecular mechanisms through which the gut microbial community
affects the muscle metabolism and function. Furthermore, it
summarizes the current research progress on the relationship
between gut microbiota imbalance and sarcopenia, and proposes
potential therapeutic strategies based on signaling pathways.
Overall, gut microbiota plays an important role in the onset and
development of sarcopenia. By investigating the mechanisms and
intervention strategies in depth, the present study hopes to
provide novel solutions for the prevention and treatment of
sarcopenia, thereby improving the quality of life of patients.
Not applicable.
Funding: No funding was received.
Not applicable.
CY designed the concept of the study, wrote and
reviewed the manuscript and read and confirmed the final version of
the manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declares that they have no competing
interests.
|
1
|
Hussain H: Effectiveness of exercise
interventions on body composition and functional outcomes in
sarcopenia: A systematic review. Clin Med (Lond). 23 (Suppl
6):S762023. View Article : Google Scholar
|
|
2
|
Gay-As MU, Lee SC and Lai FC: Sarcopenia
among older people in the philippines: A scoping review. Creat
Nurs. 30:133–144. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong Y, Jiang X, Zhong Q, Zhang Y, Zhang
H, Liu Z and Wang X: Possible sarcopenia and risk of chronic kidney
disease: A four-year follow-up study and Mendelian randomization
analysis. Endocr Res. 49:165–178. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bali T, Chrysavgis L and Cholongitas E:
Metabolic-Associated fatty liver disease and sarcopenia. Endocrinol
Metab Clin North Am. 52:497–508. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blagec P, Sara S, Tripalo Batos A, Trivic
Mazuranic I, Mocic Pavic A, Misak Z and Hojsak I: Magnetic
resonance imaging can be used to assess sarcopenia in children with
newly diagnosed crohn's disease. Nutrients. 15:38382023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y and Tian L: Research progress on the
predictive role of sarcopenia in the course and prognosis of
inflammatory bowel disease. PeerJ. 11:e164212023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu QW, Mao CJ, Lu ZH, Shi RF, Zhang YC,
Zhao P and Liu CF: Sarcopenia is associated with non-motor symptoms
in Han Chinese patients with Parkinson's Disease: A cross-sectional
study. BMC Geriatr. 23:4942023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim M, Kim D, Kang H, Park S, Kim S and
Yoo JI: A machine learning model for prediction of sarcopenia in
patients with Parkinson's Disease. PLoS One. 19:e02962822024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nan Y, Zhou Y, Dai Z, Yan T, Zhong P,
Zhang F, Chen Q and Peng L: Role of nutrition in patients with
coexisting chronic obstructive pulmonary disease and sarcopenia.
Front Nutr. 10:12146842023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pedauye-Rueda B, Garcia-Fernandez P,
Maicas-Perez L, Mate-Munoz JL and Hernandez-Lougedo J: Different
diagnostic criteria for determining the prevalence of sarcopenia in
older adults: A systematic review. J Clin Med. 13:25202024.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He Y, Cui W, Fang T, Zhang Z and Zeng M:
Metabolites of the gut microbiota may serve as precise diagnostic
markers for sarcopenia in the elderly. Front Microbiol.
14:13018052023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lahiri S, Kim H, Garcia-Perez I, Reza MM,
Martin KA, Kundu P, Cox LM, Selkrig J, Posma JM, Zhang H, et al:
The gut microbiota influences skeletal muscle mass and function in
mice. Sci Transl Med. 11:eaan56622019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan X, Li H, Xie R, Lin L, Ding L, Cheng
X, Xu J, Bai L and Qiao Y: Relationships between sarcopenia,
nutrient intake, and gut microbiota in Chinese community-dwelling
older women. Arch Gerontol Geriatr. 113:1050632023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q, Li X, Huang T, Zhang S, Teng K,
Rousitemu N, Lan T and Wen Y: Alterations in the diversity,
composition and function of the gut microbiota in Uyghur
individuals with sarcopenia. Exp Gerontol. 187:1123762024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Picca A, Fanelli F, Calvani R, Mule G,
Pesce V, Sisto A, Pantanelli C, Bernabei R, Landi F and Marzetti E:
Gut dysbiosis and muscle aging: Searching for Novel Targets against
Sarcopenia. Mediators Inflamm. 2018:70261982018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu C, Cheung WH, Li J, Chow SK, Yu J,
Wong SH, Ip M, Sung JJY and Wong RMY: Understanding the gut
microbiota and sarcopenia: A systematic review. J Cachexia
Sarcopenia Muscle. 12:1393–1407. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aboshady HM, Gavriilidou A, Ghanem N,
Radwan MA, Elnahas A, Agamy R, Fahim NH, Elsawy MH, Shaarawy ABM,
Abdel-Hafeez AM, et al: Gut microbiota diversity of local egyptian
cattle managed in different ecosystems. Animals (Basel).
14:27522024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim X, Ooi L, Ding U, Wu HHL and
Chinnadurai R: Gut microbiota in patients receiving dialysis: A
review. Pathogens. 13:8012024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu S, Yin J, Wan D and Yin Y: The role of
iron in intestinal mucus: Perspectives from both the host and gut
microbiota. Adv Nutr. 15:1003072024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao H, Nepovimova E, Adam V, Heger Z,
Valko M, Wu Q and Kuca K: Age-associated changes in innate and
adaptive immunity: Role of the gut microbiota. Front Immunol.
15:14210622024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Zhu Y, Guo Q, Wang W and Zhang L:
High-throughput sequencing analysis of the characteristics of the
gut microbiota in aged patients with sarcopenia. Exp Gerontol.
182:1122872023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Casati M, Ferri E, Azzolino D, Cesari M
and Arosio B: Gut microbiota and physical frailty through the
mediation of sarcopenia. Exp Gerontol. 124:1106392019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang J and Zhang H, Yin L, Zhou Q and
Zhang H: The gut microbiota from maintenance hemodialysis patients
with sarcopenia influences muscle function in mice. Front Cell
Infect Microbiol. 13:12259912023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Toole PW and Jeffery IB:
Microbiome-health interactions in older people. Cell Mol Life Sci.
75:119–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Claesson MJ, Jeffery IB, Conde S, Power
SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan
B, O'Sullivan O, et al: Gut microbiota composition correlates with
diet and health in the elderly. Nature. 488:178–184. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biagi E, Candela M, Turroni S, Garagnani
P, Franceschi C and Brigidi P: Ageing and gut microbes:
Perspectives for health maintenance and longevity. Pharmacol Res.
69:11–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ning M, An L, Dong L, Zhu R, Hao J, Liu X
and Zhang Y: Causal associations between gut microbiota, gut
microbiota-derived metabolites, and Alzheimer's Disease: A
Multivariable Mendelian Randomization Study. J Alzheimers Dis.
100:229–237. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu J, Gong X, Zhang C, Yang T and Pei D: A
multi-omics approach to investigate characteristics of gut
microbiota and metabolites in hypertension and diabetic nephropathy
SPF rat models. Front Microbiol. 15:13561762024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SY, Kim JH, Lee DY and Hur SJ:
Characterization of gut microbiota in mouse models of aging and
sarcopenia. Microbiol Res. 275:1274622023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan ZX, Gao XJ, Li T, Wei B, Wang PP, Yang
Y and Yan R: Fecal microbiota transplantation in experimental
ulcerative colitis reveals associated gut microbial and host
metabolic reprogramming. Appl Environ Microbiol. 84:e00434–18.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qaisar R, Burki A, Karim A, Iqbal MS and
Ahmad F: Probiotics supplements improve the sarcopenia-related
quality of life in older adults with age-related muscle decline.
Calcif Tissue Int. 114:583–591. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nistor-Cseppento CD, Moga TD, Bungau AF,
Tit DM, Negrut N, Pasca B, Bochis CF, Ghitea TC, Jurcau A, Purza AL
and Uivarosan D: The contribution of diet therapy and probiotics in
the treatment of sarcopenia induced by prolonged immobilization
caused by the COVID-19 Pandemic. Nutrients. 14:47012022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu X, Li P, Li B, Yu F, Zhao W, Wang X,
Wang X, Wang Y, Gao H, Cheng M and Li X: d-pinitol improves
diabetic sarcopenia by regulation of the gut microbiome,
metabolome, and proteome in STZ-Induced SAMP8 Mice. J Agric Food
Chem. 72:14466–14478. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mo X, Shen L, Cheng R, Wang P, Wen L, Sun
Y, Wang Q, Chen J, Lin S, Liao Y, et al: Faecal microbiota
transplantation from young rats attenuates age-related sarcopenia
revealed by multiomics analysis. J Cachexia Sarcopenia Muscle.
14:2168–2183. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baek JS, Shin YJ, Ma X, Park HS, Hwang YH
and Kim DH: Bifidobacterium bifidum and Lactobacillus paracasei
alleviate sarcopenia and cognitive impairment in aged mice by
regulating gut microbiota-mediated AKT, NF-ĸB, and FOXO3a signaling
pathways. Immun Ageing. 20:562023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lou J, Wang Q, Wan X and Cheng J: Changes
and correlation analysis of intestinal microflora composition,
inflammatory index, and skeletal muscle mass in elderly patients
with sarcopenia. Geriatr Gerontol Int. 24:140–146. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee J, Kang M, Yoo J, Lee S, Kang M, Yun
B, Kim JN, Moon H, Chung Y and Oh S: Lactobacillus rhamnosus JY02
ameliorates sarcopenia by anti-atrophic effects in a
dexamethasone-induced cellular and murine model. J Microbiol
Biotechnol. 33:915–925. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karimian S, Farahmandzad N and
Mohammadipanah F: Manipulation and epigenetic control of silent
biosynthetic pathways in actinobacteria. World J Microbiol
Biotechnol. 40:652024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zahr R, Zahr S, El Hajj R and Khalil M:
Characterization of Actinobacteria strains in Lebanese soil with an
emphasis on investigating their antibacterial activity. Braz J
Microbiol. 55:255–267. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Z, Xu X, Deji Y, Gao S, Wu C, Song Q,
Shi Z, Xiang X, Zang J and Su J: Bifidobacterium as a potential
biomarker of Sarcopenia in elderly women. Nutrients. 15:12662023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lv WQ, Lin X, Shen H, Liu HM, Qiu X, Li
BY, Shen WD, Ge CL, Lv FY, Shen J, et al: Human gut microbiome
impacts skeletal muscle mass via gut microbial synthesis of the
short-chain fatty acid butyrate among healthy menopausal women. J
Cachexia Sarcopenia Muscle. 12:1860–1870. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sugimura Y, Yang Y, Kanda A, Mawatari A,
Tamada Y, Mikami T, Nakaji S and Ihara K: Association between Gut
Microbiota and Muscle Strength in Japanese General Population of
the Iwaki Health Promotion Project. Microorganisms. 12:6222024.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang M, Ren F, Zhou Y, He Y, Du T and Tan
Y: Age-related sarcopenia and altered gut microbiota: A systematic
review. Microb Pathog. 195:1068502024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ticinesi A, Nouvenne A, Cerundolo N,
Catania P, Prati B, Tana C and Meschi T: Gut microbiota, muscle
mass and function in aging: A focus on physical frailty and
sarcopenia. Nutrients. 11:16332019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aliwa B, Horvath A, Traub J, Feldbacher N,
Habisch H, Fauler G, Madl T and Stadlbauer V: Altered gut
microbiome, bile acid composition and metabolome in sarcopenia in
liver cirrhosis. J Cachexia Sarcopenia Muscle. 14:2676–2691. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Zhang Y, Lane NE, Wu J, Yang T, Li
J, He H, Wei J, Zeng C and Lei G: Population-based metagenomics
analysis reveals altered gut microbiome in sarcopenia: Data from
the Xiangya Sarcopenia Study. J Cachexia Sarcopenia Muscle.
13:2340–2351. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee YA, Song SW, Jung SY, Bae J, Hwang N
and Kim HN: Sarcopenia in community-dwelling older adults is
associated with the diversity and composition of the gut
microbiota. Exp Gerontol. 167:1119272022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shan Z, Cheng N, Zhu J, Chen F and Ji J:
Meilibana: Analysis of intestinal flora in elderly Uygur patients
with sarcopenia. Immun Inflamm Dis. 12:e10972024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu X, Wu J, Tang J, Xu Z, Zhou B, Liu Y,
Hu F, Zhang G, Cheng R, Xia X, et al: Prevotella copri alleviates
sarcopenia via attenuating muscle mass loss and function decline. J
Cachexia Sarcopenia Muscle. 14:2275–2288. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tseng CW, Kyme P, Low J, Rocha MA, Alsabeh
R, Miller LG, Otto M, Arditi M, Diep BA, Nizet V, et al:
Staphylococcus aureus Panton-Valentine leukocidin contributes to
inflammation and muscle tissue injury. PLoS One. 4:e63872009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Avila-Novoa MG, Solis-Velazquez OA,
Guerrero-Medina PJ, Gonzalez-Gomez JP, Gonzalez-Torres B,
Velazquez-Suarez NY, Martínez-Chávez L, Martínez-Gonzáles NE, De la
Cruz-Color L, Ibarra-Velázquez LM, et al: Genetic and compositional
analysis of biofilm formed by Staphylococcus aureus isolated from
food contact surfaces. Front Microbiol. 13:10017002022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu S, Yi J, Zhang YG, Zhou J and Sun J:
Leaky intestine and impaired microbiome in an amyotrophic lateral
sclerosis mouse model. Physiol Rep. 3:e123562015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang Y, Ogbu D, Garrett S, Xia Y and Sun
J: Aberrant enteric neuromuscular system and dysbiosis in
amyotrophic lateral sclerosis. Gut Microbes. 13:19968482021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bin-Jumah MN, Gilani SJ, Hosawi S,
Al-Abbasi FA, Zeyadi M, Imam SS, Alshehri S, Ghoneim MM, Nadeem MS
and Kazmi I: Pathobiological relationship of excessive dietary
intake of Choline/L-Carnitine: A TMAO precursor-associated
aggravation in heart failure in sarcopenic patients. Nutrients.
13:34532021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hata S, Okamura T, Kobayashi A, Bamba R,
Miyoshi T, Nakajima H, Hashimoto Y, Majima S, Senmaru T, Okada H,
et al: Gut Microbiota Changes by an SGLT2 inhibitor,
luseogliflozin, alters metabolites compared with those in a low
carbohydrate diet in db/db Mice. Nutrients. 14:35312022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Potgens SA, Brossel H, Sboarina M, Catry
E, Cani PD, Neyrinck AM, Delzenne NM and Bindels LB: Klebsiella
oxytoca expands in cancer cachexia and acts as a gut pathobiont
contributing to intestinal dysfunction. Sci Rep. 8:123212018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ma WW, Huang ZQ, Liu K, Li DZ, Mo TL and
Liu Q: The role of intestinal microbiota and metabolites in
intestinal inflammation. Microbiol Res. 288:1278382024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bourqqia-Ramzi M, Mansilla-Guardiola J,
Munoz-Rodriguez D, Quarta E, Lombardo-Hernandez J,
Murciano-Cespedosa A, Conejero-Meca FJ, Mateos González Á, Geuna S,
Garcia-Esteban MT and Herrera-Rincon C: From the Microbiome to the
Electrome: Implications for the Microbiota-Gut-Brain Axis. Int J
Mol Sci. 25:62332024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang J, Yu Y and Wang J: Protein
nutritional support: The classical and potential new mechanisms in
the prevention and therapy of sarcopenia. J Agric Food Chem.
68:4098–4108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mendes J, Simoes CD, Martins JO and Sousa
AS: Inflammatory bowel disease and sarcopenia: A focus on muscle
strength - narrative review. Arq Gastroenterol. 60:373–382. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Agostini D, Gervasi M, Ferrini F,
Bartolacci A, Stranieri A, Piccoli G, Barbieri E, Sestili P, Patti
A, Stocchi V and Donati Zeppa S: An integrated approach to skeletal
muscle health in aging. Nutrients. 15:18022023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu S, Chen X, Cai R, Chen X, Zhang J, Xie
J and Shen M: Sulfated Chinese yam polysaccharides alleviate
LPS-induced acute inflammation in mice through modulating
intestinal microbiota. Foods. 12:17722023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li C, Wang Y, Zhao X, Li J, Wang H, Ren Y,
Sun H, Zhu X, Song Q and Wang J: Comparative analysis of intestinal
inflammation and microbiota dysbiosis of LPS-Challenged Piglets
between Different Breeds. Animals (Basel). 14:6652024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bian AL, Hu HY, Rong YD, Wang J, Wang JX
and Zhou XZ: A study on relationship between elderly sarcopenia and
inflammatory factors IL-6 and TNF-α. Eur J Med Res. 22:252017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xuekelati S, Maimaitiwusiman Z, Bai X,
Xiang H, Li Y and Wang H: Sarcopenia is associated with
hypomethylation of TWEAK and increased plasma levels of TWEAK and
its downstream inflammatory factor TNF-α in older adults: A
case-control study. Exp Gerontol. 188:1123902024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
den Besten G, van Eunen K, Groen AK,
Venema K, Reijngoud DJ and Bakker BM: The role of short-chain fatty
acids in the interplay between diet, gut microbiota, and host
energy metabolism. J Lipid Res. 54:2325–2340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kang M, Kang M, Yoo J, Lee J, Lee S, Yun
B, Song M, Kim JM, Kim HW, Yang J, et al: Dietary supplementation
with Lacticaseibacillus rhamnosus IDCC3201 alleviates sarcopenia by
modulating the gut microbiota and metabolites in
dexamethasone-induced models. Food Funct. 15:4936–4953. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu Y, Mao T, Wang Y, Qi X, Zhao W, Chen H,
Zhang C and Li X: Effect of gut microbiota-mediated tryptophan
metabolism on inflammaging in frailty and sarcopenia. J Gerontol A
Biol Sci Med Sci. 79:glae0442024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ciernikova S, Sevcikova A, Mladosievicova
B and Mego M: Microbiome in cancer development and treatment.
Microorganisms. 12:242023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gellhaus B, Boker KO, Schilling AF and
Saul D: Therapeutic consequences of targeting the
IGF-1/PI3K/AKT/FOXO3 axis in sarcopenia: A narrative review. Cells.
12:27872023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Abuduwaili H, Kamoshita K, Ishii KA,
Takahashi K, Abuduyimiti T, Qifang L, Isobe Y, Goto H, Nakano Y,
Takeshita Y, et al: Selenoprotein P deficiency protects against
immobilization-induced muscle atrophy by suppressing
atrophy-related E3 ubiquitin ligases. Am J Physiol Endocrinol
Metab. 324:E542–E552. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
He P, Du G, Qin X and Li Z: Reduced energy
metabolism contributing to aging of skeletal muscle by serum
metabolomics and gut microbiota analysis. Life Sci. 323:1216192023.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang X, Yang G, Jiang S, Ji B, Xie W, Li
H, Sun J and Li Y: Causal relationship between gut microbiota,
metabolites, and sarcopenia: A mendelian randomization study. J
Gerontol A Biol Sci Med Sci. 79:glae1732024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cailleaux PE, Dechelotte P and Coeffier M:
Novel dietary strategies to manage sarcopenia. Curr Opin Clin Nutr
Metab Care. 27:234–243. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lapauw L, Rutten A, Dupont J, Amini N,
Vercauteren L, Derrien M, Raes J and Gielen E: Associations between
gut microbiota and sarcopenia or its defining parameters in older
adults: A systematic review. J Cachexia Sarcopenia Muscle. Aug
27–2024.(Epub ahead of print). View Article : Google Scholar
|
|
76
|
Han DS, Wu WK, Liu PY, Yang YT, Hsu HC,
Kuo CH, Wu MS and Wang TG: Differences in the gut microbiome and
reduced fecal butyrate in elders with low skeletal muscle mass.
Clin Nutr. 41:1491–1500. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
de Conti A, Tryndyak V, Koturbash I,
Heidor R, Kuroiwa-Trzmielina J, Ong TP, Beland FA, Moreno FS and
Pogribny IP: The chemopreventive activity of the butyric acid
prodrug tributyrin in experimental rat hepatocarcinogenesis is
associated with p53 acetylation and activation of the p53 apoptotic
signaling pathway. Carcinogenesis. 34:1900–1906. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ticinesi A, Nouvenne A, Cerundolo N,
Parise A, Mena P and Meschi T: The interaction between
Mediterranean diet and intestinal microbiome: Relevance for
preventive strategies against frailty in older individuals. Aging
Clin Exp Res. 36:582024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Dong J, Gu W, Yang X, Zeng L, Wang X, Mu
J, Wang Y, Li F, Yang M and Yu J: Crosstalk between polygonatum
kingianum, the miRNA, and gut microbiota in the regulation of lipid
metabolism. Front Pharmacol. 12:7405282021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Prukpitikul P, Sirivarasai J and Sutjarit
N: The molecular mechanisms underlying gut microbiota-miRNA
interaction in metabolic disorders. Benef Microbes. 15:83–96. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Huang LY, Lim AY, Hsu CC, Tsai YF, Fu TC,
Shyu YC, Peng SC and Wang JS: Sustainability of exercise-induced
benefits on circulating MicroRNAs and physical fitness in
community-dwelling older adults: A randomized controlled trial with
follow up. BMC Geriatr. 24:4732024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lee H, Kim YI, Nirmala FS, Kim JS, Seo HD,
Ha TY, Jang YJ, Jung CH and Ahn J: MiR-141-3p promotes
mitochondrial dysfunction in ovariectomy-induced sarcopenia via
targeting Fkbp5 and Fibin. Aging (Albany NY). 13:4881–4894. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Iannone F, Montesanto A, Cione E, Crocco
P, Caroleo MC, Dato S, Rose G and Passarino G: Expression patterns
of muscle-specific miR-133b and miR-206 correlate with nutritional
status and sarcopenia. Nutrients. 12:2972020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen FX, Shen Y, Liu Y, Wang HF, Liang CY
and Luo M: Inflammation-dependent downregulation of miR-532-3p
mediates apoptotic signaling in human sarcopenia through targeting
BAK1. Int J Biol Sci. 16:1481–1494. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jin J, Yang Z, Liu H, Guo M, Chen B, Zhu
H, Wang Y, Lin J, Wang S and Chen S: Effects of acupuncture on the
miR-146a-mediated IRAK1/TRAF6/NF-ĸB signaling pathway in rats with
sarcopenia induced by D-galactose. Ann Transl Med. 11:472023.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Okugawa Y, Yao L, Toiyama Y, Yamamoto A,
Shigemori T, Yin C, Omura Y, Ide S, Kitajima T, Shimura T, et al:
Prognostic impact of sarcopenia and its correlation with
circulating miR-21 in colorectal cancer patients. Oncol Rep.
39:1555–1564. 2018.PubMed/NCBI
|
|
87
|
Wang W, Liu W, Xu J and Jin H: MiR-33a
targets FOSL1 and EN2 as a clinical prognostic marker for
sarcopenia by glioma. Front Genet. 13:9535802022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
He N, Zhang Y, Zhang Y, Feng B, Zheng Z
and Ye H: Circulating miR-29b decrease in response to sarcopenia in
patients with cardiovascular risk factors in older Chinese. Front
Cardiovasc Med. 9:10943882022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Qaisar R, Karim A, Muhammad T, Shah I and
Khan J: Circulating MicroRNAs as biomarkers of accelerated
sarcopenia in chronic heart failure. Glob Heart. 16:562021.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Faraldi M, Sansoni V, Vitale J, Perego S,
Gomarasca M, Verdelli C, Messina C, Sconfienza LM, Banfi G,
Corbetta S and Lombardi G: Plasma microRNA signature associated
with skeletal muscle wasting in post-menopausal osteoporotic women.
J Cachexia Sarcopenia Muscle. 15:690–701. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
He N, Zhang YL, Zhang Y, Feng B, Zheng Z,
Wang D, Zhang S, Guo Q and Ye H: Circulating MicroRNAs in plasma
decrease in response to sarcopenia in the elderly. Front Genet.
11:1672020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Salamanna F, Contartese D, Ruffilli A,
Barile F, Bellavia D, Marchese L, Manzetti M, Viroli G, Faldini C
and Giavaresi G: Sharing circulating Micro-RNAs between
osteoporosis and sarcopenia: A systematic review. Life (Basel).
13:6022023.PubMed/NCBI
|
|
93
|
Li Z, Liu C, Li S, Li T, Li Y, Wang N, Bao
X, Xue P and Liu S: BMSC-derived exosomes inhibit
dexamethasone-induced muscle atrophy via the miR-486-5p/FoxO1 Axis.
Front Endocrinol (Lausanne). 12:6812672021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Che J, Xu C, Wu Y, Jia P, Han Q, Ma Y,
Wang X and Zheng Y: MiR-1290 promotes myoblast differentiation and
protects against myotube atrophy via Akt/p70/FoxO3 pathway
regulation. Skelet Muscle. 11:62021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ahmad N, Kushwaha P, Karvande A, Tripathi
AK, Kothari P, Adhikary S, Khedgikar V, Mishra VK and Trivedi R:
MicroRNA-672-5p identified during weaning reverses osteopenia and
sarcopenia in ovariectomized mice. Mol Ther Nucleic Acids.
14:536–549. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Stewart-Hunt L, Pratt-Phillips S,
McCutcheon LJ and Geor RJ: Dietary energy source and physical
conditioning affect insulin sensitivity and skeletal muscle glucose
metabolism in horses. Equine Vet J. Suppl (38):355–360. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Barres R and Zierath JR: The role of diet
and exercise in the transgenerational epigenetic landscape of T2DM.
Nat Rev Endocrinol. 12:441–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Critchlow AJ, Williams RM and Alexander
SE: The PoWeR of exercise: Exploring the anti-ageing effects of
exercise through epigenetic modifications to skeletal muscle. J
Physiol. 601:1175–1177. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Sohi G and Dilworth FJ: Noncoding RNAs as
epigenetic mediators of skeletal muscle regeneration. FEBS J.
282:1630–1646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Pinheiro A and Naya FJ: The Key Lnc (RNA)s
in cardiac and skeletal muscle development, regeneration, and
disease. J Cardiovasc Dev Dis. 8:842021.PubMed/NCBI
|
|
101
|
Human Microbiome Project Consortium, .
Structure, function and diversity of the healthy human microbiome.
Nature. 486:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Quigley EM: Gut bacteria in health and
disease. Gastroenterol Hepatol (N Y). 9:560–569. 2013.PubMed/NCBI
|
|
103
|
Knight R, Vrbanac A, Taylor BC, Aksenov A,
Callewaert C, Debelius J, Gonzalez A, Kosciolek T, McCall LI,
McDonald D, et al: Best practices for analysing microbiomes. Nat
Rev Microbiol. 16:410–422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Collins SL, Stine JG, Bisanz JE, Okafor CD
and Patterson AD: Bile acids and the gut microbiota: Metabolic
interactions and impacts on disease. Nat Rev Microbiol. 21:236–247.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lapiere A and Richard ML: Bacterial-fungal
metabolic interactions within the microbiota and their potential
relevance in human health and disease: A short review. Gut
Microbes. 14:21056102022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wagner J, Kancherla J, Braccia D,
Matsumara J, Felix V, Crabtree J, Mahurkar A and Corrada Bravo H:
Interactive exploratory data analysis of integrative human
microbiome project data using metaviz. F1000Res. 9:6012020.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Rahman S, Ikram AR, Azeem F, Tahir Ul
Qamar M, Shaheen T and Mehboob-Ur-Rahman: Precision genome editing
with CRISPR-Cas9. Methods Mol Biol. 2788:355–372. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li Y, Li C, Yan J, Liao Y, Qin C, Wang L,
Huang Y, Yang C, Wang J, Ding X, et al: Polymeric micellar
nanoparticles for effective CRISPR/Cas9 genome editing in cancer.
Biomaterials. 309:1225732024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Adlard B, Donaldson SG, Odland JO, Weihe
P, Berner J, Carlsen A, Bonefeld-Jorgensen EC, Dudarev AA, Gibson
JC, Krümmel EM, et al: Future directions for monitoring and human
health research for the arctic monitoring and assessment programme.
Glob Health Action. 11:14800842018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Jackson R, Yao T, Bulut N, Cantu-Jungles
TM and Hamaker BR: Protein combined with certain dietary fibers
increases butyrate production in gut microbiota fermentation. Food
Funct. 15:3186–3198. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Modoux M, Rolhion N, Lefevre JH, Oeuvray
C, Nadvornik P, Illes P, Emond P, Parc Y, Mani S, Dvorak Z and
Sokol H: Butyrate acts through HDAC inhibition to enhance aryl
hydrocarbon receptor activation by gut microbiota-derived ligands.
Gut Microbes. 14:21056372022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wan F, Deng FL, Chen L, Zhong RQ, Wang MY,
Yi B, Liu L, Zhao HB and Zhang HF: Long-term chemically protected
sodium butyrate supplementation in broilers as an antibiotic
alternative to dynamically modulate gut microbiota. Poult Sci.
101:1022212022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Drut A, Mkaouar H, Kriaa A, Mariaule V,
Akermi N, Meric T, Sénécat O, Maguin E, Hernandez J and Rhimi M:
Gut microbiota in cats with inflammatory bowel disease and
low-grade intestinal T-cell lymphoma. Front Microbiol.
15:13466392024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yang Y, Huang S, Liao Y, Wu X, Zhang C,
Wang X and Yang Z: Hippuric acid alleviates dextran sulfate
sodium-induced colitis via suppressing inflammatory activity and
modulating gut microbiota. Biochem Biophys Res Commun.
710:1498792024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu M, Ma J, Xu J, Huangfu W, Zhang Y, Ali
Q, Liu B, Li D, Cui Y, Wang Z, et al: Fecal microbiota
transplantation alleviates intestinal inflammatory diarrhea caused
by oxidative stress and pyroptosis via reducing gut
microbiota-derived lipopolysaccharides. Int J Biol Macromol.
261((Pt 1)): 1296962024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wu R, Xiong R, Li Y, Chen J and Yan R: Gut
microbiome, metabolome, host immunity associated with inflammatory
bowel disease and intervention of fecal microbiota transplantation.
J Autoimmun. 141:1030622023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Monda V, Villano I, Messina A, Valenzano
A, Esposito T, Moscatelli F, Viggiano A, Cibelli G, Chieffi S,
Monda M and Messina G: Exercise modifies the gut microbiota with
positive health effects. Oxid Med Cell Longev. 2017:38319722017.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Mimee M, Tucker AC, Voigt CA and Lu TK:
Programming a human commensal bacterium, bacteroides
thetaiotaomicron, to sense and respond to stimuli in the murine gut
microbiota. Cell Syst. 2:2142016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Das S, Preethi B, Kushwaha S and
Shrivastava R: Therapeutic strategies to modulate gut microbial
health: Approaches for sarcopenia management. Histol Histopathol.
39:1395–1425. 2024.PubMed/NCBI
|
|
120
|
Li T, Yin D and Shi R: Gut-muscle axis
mechanism of exercise prevention of sarcopenia. Front Nutr.
11:14187782024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhang T, Cheng JK and Hu YM: Gut
microbiota as a promising therapeutic target for age-related
sarcopenia. Ageing Res Rev. 81:1017392022. View Article : Google Scholar : PubMed/NCBI
|