Introduction
Advances in cancer treatments have improved the
prognosis of patients with gastrointestinal cancer. However,
gastrointestinal cancer accounts for >30% of cancer mortalities
(1). In advanced gastrointestinal
cancers, a high prevalence of cachexia can lead to a lower quality
of life (2). Cancer cachexia is a
complex disorder characterized by skeletal muscle loss. In cancer
cachexia, inflammatory cytokines released by the tumor can cause
systemic inflammation, deteriorated nutritional status and skeletal
muscle loss (3). Skeletal muscle
depletion and systemic inflammation can influence the outcomes of
patients with cancer. Indeed, our previous studies demonstrated
that the neutrophil-to-lymphocyte ratio (NLR) and the decrease of
the psoas muscle index (PMI) were notably associated with survival
in unresectable pancreatic cancer (4,5).
The cachexia index (CXI) is a novel index of
cachexia (6), evaluated using the
skeletal muscle index (SMI), serum albumin (ALB) levels and NLR
values. Thus, the CXI can reflect the skeletal muscle mass,
nutritional status and systemic inflammation in patients with
cancer. In clinical settings, the CXI has been associated with
survival in gastrointestinal cancers, including gastric, biliary
tract, pancreatic, colorectal and hepatocellular cancer (7–14). In
previous studies, the SMI was calculated using computed tomography
(CT) images for CXI assessment (7–14). In
recent years, bioelectrical impedance analysis (BIA) has been
widely used to evaluate body composition, as it is a simple and
inexpensive method without radiation exposure (15,16).
Overall, BIA is a widely used method to evaluate
sarcopenia in patients with cancer (15). However, few studies have assessed
the use of the CXI using BIA (16).
Therefore, the present study aimed to evaluate whether CXI values
calculated using BIA could predict survival in gastrointestinal
cancer.
Materials and methods
A total of 54 patients with gastrointestinal cancer
(colorectal, pancreatic, gastric, esophageal, biliary tract and
liver cancer) who underwent BIA at diagnosis between May 2021 and
April 2022 at Fukuchiyama City Hospital (Kyoto, Japan) were
retrospectively enrolled, regardless of their age, cancer staging,
presence of metastasis or prior medical history, including previous
cancer diagnoses. All medical records were retrospectively
reviewed.
Evaluation of the SMI was performed using BIA with
an InBody770 body composition analyzer (InBody Co., Ltd.) at
diagnosis. In addition to SMI, the body fat percentage and
extracellular water/total body water ratio (ECW/TBW) was evaluated.
Baseline characteristics, such as age, sex, primary tumor site,
resectability, and treatment were assessed. Biochemical test
results were also assessed, including white blood cell (WBC)
counts, neutrophil counts, lymphocyte counts, hemoglobin levels,
platelet counts, C-reactive protein (CRP) levels and ALB levels.
The SMI values were calculated using BIA. CXI values were
calculated using the following: SMI (kg/m2) × ALB
(g/dl)/NLR. Overall survival (OS) was assessed from the date of BIA
at diagnosis to the date of the last follow-up or mortality.
Firstly, the patients were divided into groups based
on their SMI values for each sex: Patients with low SMI (low-SMI
group) and patients with high SMI (high-SMI group). The SMI cut-off
values were determined as 7.0 kg/m2 for male patients
and 5.7 kg/m2 for female patients in accordance with the
Asian Working Group for Sarcopenia report (17,18).
The clinical features and prognoses of the two groups were then
assessed. Secondly, the patients were divided based on the median
CXI values for each sex: Low CXI (low-CXI group) and high CXI
(high-CXI group). The clinical features and prognoses of the two
groups were then assessed.
The present study was performed in accordance with
the Declaration of Helsinki and was approved by the ethical
committee of Fukuchiyama City Hospital (approval no. 5-57).
Statistical analysis
Statistical analyses were performed using R version
4.2.2 (R Foundation for Statistical Computing) and SPSS Statistics
27 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference. Continuous data are presented
as median (range) and categorical data are expressed as n (%).
Statistical analysis was performed using the Mann-Whitney U test,
Fisher's exact test or χ2 test. OS rates were evaluated
using the Kaplan-Meier method and the log-rank test.
Results
Baseline characteristics of enrolled
patients
Table I presents the
baseline characteristics of the patients in the present study. A
total of 34 (63.0%) male patients and 20 (37.0%) female patients
were enrolled. The median patient age was 72 years (range, 45–96).
The median follow-up period was 469 days (range, 31–684). There
were 20 patients (37.0%) with colorectal cancer, 12 (22.2%) with
pancreatic cancer, 11 (20.4%) with gastric cancer, 6 (11.1%) with
esophageal cancer, 4 (7.4%) with biliary tract cancer and 1 (1.9%)
with liver cancer. A total of 31 patients (57.4%) were diagnosed
with resectable cancer (resectable group) and 23 patients (42.6%)
were diagnosed with unresectable or recurrent cancer (unresectable
group). In the resectable group, 1 patient with gastric cancer
refused surgery, resulting in chemotherapy, and 1 patient with
gastric cancer received the best supportive care (BSC).
Furthermore, 1 patient with esophageal cancer underwent
chemoradiotherapy in addition to endoscopic submucosal dissection.
In the resectable group, 2 patients died, 1 patient with gastric
cancer receiving BSC died of pneumonia and 1 patient with gastric
cancer undergoing surgery died of recurrent peritoneal
dissemination. In the unresectable group, 18 patients (78.3%)
underwent chemotherapy, 3 patients (13.0%) underwent
chemoradiotherapy and 2 patients (8.7%) received BSC. The
hemoglobin concentration was significantly higher in the
unresectable group compared with the resectable group (13.1 vs.
11.9 g/dl; P=0.041). The CRP levels was also significantly higher
in the unresectable group compared with the resectable group (1.11
vs. 0.19 mg/dl; P=0.033). Furthermore, the NLR was significantly
higher in the unresectable group compared with the resectable group
(4.59 vs. 2.61; P=0.001). However, no significant differences were
demonstrated for SMI values between the unresectable and resectable
group in both female and male patients. Furthermore, in female
patients, the CXI was significantly lower in the unresectable group
compared with the resectable group (5.08 vs. 12.05; P=0.025).
However, in male patients, no significant difference in CXI was
observed between the unresectable and resectable groups (5.48 vs.
11.19; P=0.068).
 | Table I.Baseline characteristics of the
patients in the present study.a |
Table I.
Baseline characteristics of the
patients in the present study.a
| Characteristic | All cases, n=54 | Resectable group,
n=31 | Unresectable group,
n=23 | P-valueb |
|---|
| Age, years | 72 (45–96) | 72 (49–96) | 74 (45–91) | 0.581 |
| Sex |
|
|
| 0.254 |
| Male | 34 (63.0) | 22 (71.0) | 12 (52.2) |
|
|
Female | 20 (37.0) | 9 (29.0) | 11 (47.8) |
|
| ECOG-PS |
|
|
| >0.999 |
| 0 or
1 | 47 (87.0) | 27 (87.1) | 20 (87.0) |
|
| ≥2 | 7 (13.0) | 4 (12.9) | 3 (13.0) |
|
| BMI,
kg/m2 | 21.5 (14.6–32.2) | 21.8 (14.6–32.2) | 21.1 (14.9–27.6) | 0.916 |
| Follow-up, days | 469 (31–684) | 561(148–684) | 267 (31–677) | <0.001 |
| Total
mortalities | 17 (31.5) | 2 (6.5) | 15 (65.2) | <0.001 |
| Primary tumor
site |
|
|
| 0.204 |
|
Colorectal cancer | 20 (37.0) | 14 (45.2) | 6 (26.1) |
|
|
Pancreatic cancer | 12 (22.2) | 6 (19.4) | 6 (26.1) |
|
| Gastric
cancer | 11 (20.4) | 8 (25.7) | 3 (13.0) |
|
|
Esophageal cancer | 6 (11.1) | 2 (6.5) | 4 (17.4) |
|
| Biliary
tract cancer | 4 (7.4) | 1 (3.2) | 3 (13.0) |
|
| Liver
cancer | 1 (1.9) | 0 (0.0) | 1 (4.3) |
|
| Treatment |
|
|
| <0.001 |
|
Surgery | 28 (51.9) | 28 (90.3) | 0 (0.0) |
|
|
Chemotherapy | 19 (35.2) | 1 (3.2) | 18 (78.3) |
|
|
Chemoradiotherapy | 4 (7.4) | 1 (3.2) | 3 (13.0) |
|
|
BSC | 3 (5.6) | 1 (3.2) | 2 (8.7) |
|
| WBC, /µl | 6,320
(3,210–21,840) | 6,100
(3,210–8,440) | 6,930
(3,930–21,840) | 0.120 |
| Hb, g/dl | 12.3
(8.3–15.8) | 11.9
(8.3–15.4) | 13.1
(9.6–15.8) | 0.041 |
| PLT,
×103/µl | 22.7
(11.9–51.9) | 24.0
(11.9–43.6) | 22.5
(12.0–51.9) | 0.649 |
| CRP, mg/dl | 0.295
(0.020–14.100) | 0.190
(0.020–4.930) | 1.110
(0.020–14.100) | 0.033 |
| ALB, g/dl | 4.0 (2.3–4.8) | 4.0 (2.7–4.8) | 3.8 (2.3–4.4) | 0.099 |
| NLR | 3.11
(0.72–9.53) | 2.61
(0.72–5.65) | 4.59
(1.36–9.53) | 0.001 |
| Body fat, % | 24.65
(3.70–45.60) | 24.90
(3.70–42.50) | 22.80
(9.90–45.60) | 0.643 |
| ECW/TBW | 0.394
(0.361–0.426) | 0.391
(0.361–0.426) | 0.395
(0.372–0.418) | 0.323 |
| SMI,
kg/m2 |
|
|
|
|
|
Male | 6.9 (5.1–8.9) | 6.85 (5.1–8.9) | 6.95 (6.2–8.2) | 0.986 |
|
Female | 5.45
(4.60–8.80) | 5.30
(4.60–6.60) | 5.70
(4.70–8.80) | 0.303 |
| CXI |
|
|
|
|
|
Male | 9.22
(1.94–39.25) | 11.19
(3.70–39.25) | 5.48
(1.94–21.29) | 0.068 |
|
Female | 6.24
(1.66–27.26) | 12.05
(2.77–27.26) | 5.08
(1.66–10.00) | 0.025 |
Clinical characteristics of the low-
and high-SMI groups
Table II presents
the clinical characteristics of the patients in the low- and
high-SMI groups. Body mass index (BMI) was significantly lower in
the low-SMI group compared with the high-SMI group (20.2 vs. 24.2;
P<0.001). However, there were no significant differences in age,
sex, primary tumor site, resectability, ALB or NLR between the two
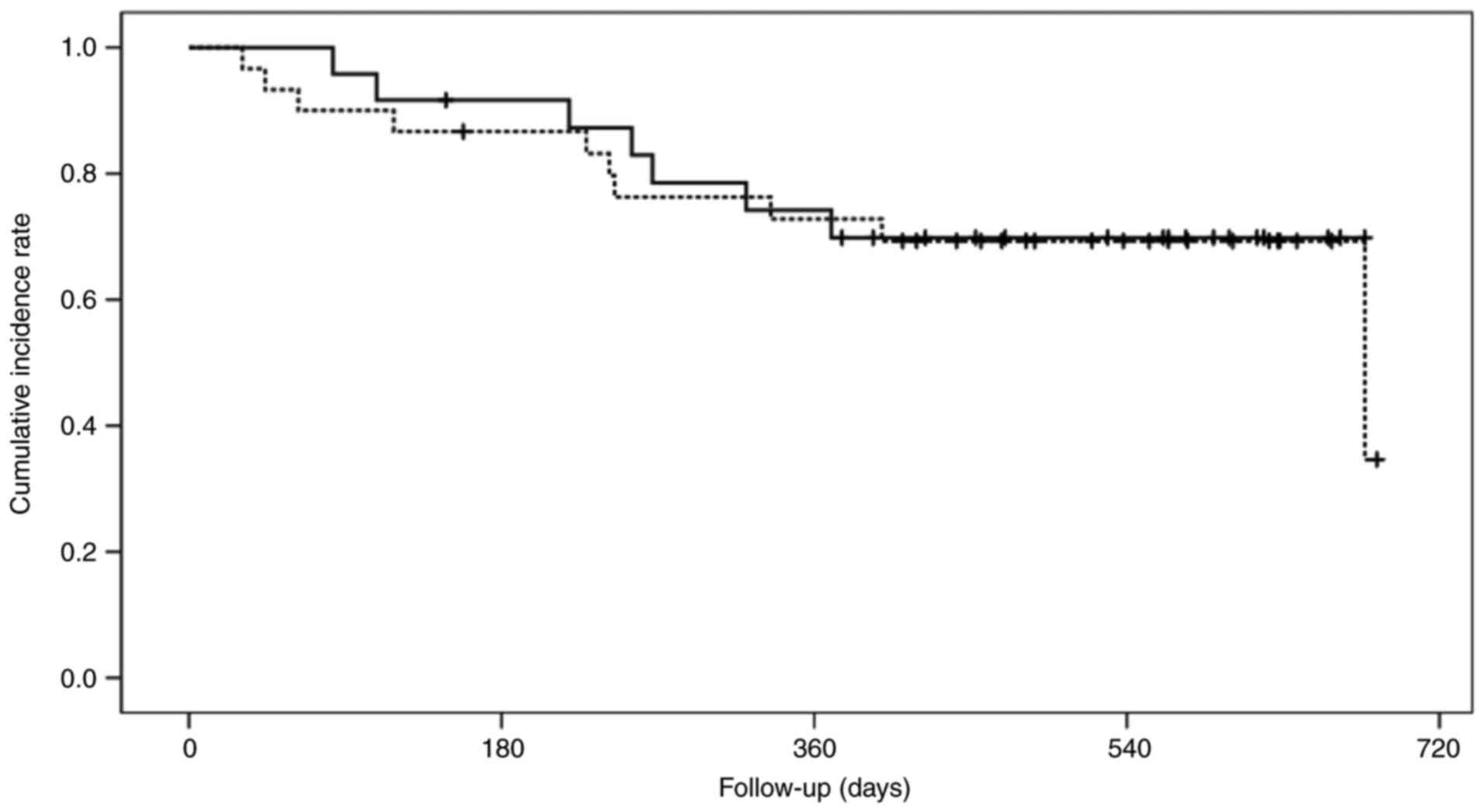
groups. Fig. 1 presents the OS of
the low- and high-SMI groups. The cumulative 1-year OS rates in the
low- and high-SMI groups were 72.8 and 74.2%, respectively
(P=0.782).
 | Table II.Clinical characteristics of the
patients in the low- and high-skeletal muscle index
groups.a |
Table II.
Clinical characteristics of the
patients in the low- and high-skeletal muscle index
groups.a
| Characteristic | Low-SMI group
(n=30) | High-SMI group
(n=24) | P-value |
|---|
| Age, years | 74 (45–96) | 7 (49–83) | 0.559 |
| Sex |
|
| 0.778 |
|
Male | 18 (60.0) | 16 (66.7) |
|
|
Female | 12 (40.0) | 8 (33.3) |
|
| ECOG-PS |
|
| 0.443 |
| 0 or
1 | 25 (83.3) | 22 (91.7) |
|
| ≥2 | 5 (16.7) | 2 (8.3) |
|
| BMI
kg/m2 | 20.2
(14.6–26.1) | 24.2
(17.9–32.2) | <0.001 |
| Follow-up,
days | 475 (31–684) | 461 (83–677) | 0.993 |
| Total
mortalities | 10 (33.3) | 7 (29.2) | 0.777 |
| Primary tumor
site |
|
| 0.112 |
|
Colorectal cancer | 10 (33.3) | 10 (41.7) |
|
|
Pancreatic cancer | 8 (26.7) | 4 (16.7) |
|
| Gastric
cancer | 8 (26.7) | 3 (12.5) |
|
|
Esophageal cancer | 4 (13.3) | 2 (8.4) |
|
| Biliary
tract cancer | 0 (0.0) | 4 (16.7) |
|
| Liver
cancer | 0 (0.0) | 1 (4.2) |
|
| Resectability |
|
| 0.410 |
|
Resectable | 19 (63.3) | 12 (50.0) |
|
|
Unresectable | 11 (36.7) | 12 (50.0) |
|
| WBC, /µl | 6,435
(3,930–17,920) | 5,855
(3,210–21,840) | 0.870 |
| Hb, g/dl | 12.4
(8.7–15.4) | 11.9
(8.3–15.8) | 0.814 |
| PLT,
×103/µl | 23.8
(11.9–41.3) | 22.5
(12.3–51.9) | 0.781 |
| CRP, mg/dl | 0.35
(0.02–12.86) | 0.21
(0.02–14.10) | >0.999 |
| ALB, g/dl | 4.0 (2.6–4.8) | 4.0 (2.3–4.8) | 0.422 |
| NLR | 3.63
(0.86–9.26) | 2.84
(0.72–9.53) | 0.802 |
| Body fat, % | 24.65
(3.70–37.70) | 24.25
(9.90–45.60) | 0.560 |
| ECW/TBW | 0.394
(0.361–0.426) | 0.391
(0.379–0.416) | 0.958 |
Clinical characteristics of the low-
and high-CXI groups
Table III presents
the clinical characteristics of the patients in the low- and
high-CXI groups. Using the medians, the low-CXI group was
classified as patients with a CXI value <9.22 (male patients) or
<6.24 (female patients), while the high-CXI group was classified
as those with a CXI value ≥9.22 (male patients) or ≥6.24 (female
patients). There were no significant differences in age, sex,
primary tumor site or resectability between the two groups.
 | Table III.Clinical characteristics of the
patients in the low-and high-cachexia index groups.a |
Table III.
Clinical characteristics of the
patients in the low-and high-cachexia index groups.a
| Characteristic | Low-CXI group
(n=27) | High-CXI group
(n=27) | P-value |
|---|
| Age, years | 74 (50–96) | 71 (45–91) | 0.709 |
| Sex |
|
| 1.000 |
|
Male | 17 (63.0) | 17 (63.0) |
|
|
Female | 10 (37.0) | 10 (37.0) |
|
| ECOG-PS |
|
| 0.100 |
| 0 or
1 | 21 (77.8) | 26 (96.3) |
|
| ≥2 | 6 (22.2) | 1 (3.7) |
|
| BMI
kg/m2 | 21.0
(14.6–27.6) | 21.8
(17.1–32.2) | 0.416 |
| Follow-up,
days | 399 (31–638) | 564 (148–684) | 0.002 |
| Total
mortalities | 12 (44.4) | 5 (18.5) | 0.077 |
| Primary tumor
site |
|
| 0.894 |
|
Colorectal cancer | 9 (33.3) | 11 (40.7) |
|
|
Pancreatic cancer | 5 (18.6) | 7 (25.9) |
|
| Gastric
cancer | 6 (22.2) | 5 (18.5) |
|
|
Esophageal cancer | 4 (14.8) | 2 (7.4) |
|
| Biliary
tract cancer | 2 (7.4) | 2 (7.4) |
|
| Liver
cancer | 1 (3.7) | 0 (0.0) |
|
| Resectability |
|
| 0.098 |
|
Resectable | 12 (44.4) | 19 (70.4) |
|
|
Unresectable | 15 (55.6) | 8 (29.6) |
|
| WBC, /µl | 6,930
(3,960–21,840) | 5,590
(3,210–8,440) | 0.034 |
| Hb, g/dl | 12.1
(8.7–15.8) | 12.9
(8.3–15.1) | 0.345 |
| PLT,
×103/µl | 22.8
(11.9–51.9) | 22.6
(12.0–43.6) | 0.972 |
| CRP, mg/dl | 1.11
(0.02–14.10) | 0.17
(0.02–2.35) | 0.005 |
| ALB, g/dl | 3.8 (2.3–4.7) | 4.0 (3.3–4.8) | 0.025 |
| NLR | 5.03
(2.69–9.53) | 2.09
(0.72–3.25) | <0.001 |
| SMI,
kg/m2 |
|
|
|
|
Male | 6.6 (5.1–8.2) | 7.4 (6.3–8.9) | 0.009 |
|
Female | 5.15
(4.60–8.80) | 5.60
(5.20–6.60) | 0.425 |
| Body fat, % | 24.9
(6.8–45.6) | 23.6
(3.7–42.5) | 0.802 |
| ECW/TBW | 0.395
(0.361–0.426) | 0.391
(0.372–0.411) | 0.197 |
The ALB levels were significantly lower in the
low-CXI group compared with the high-CXI group (3.8 vs. 4.0 g/dl;
P=0.025), whilst the NLR was significantly higher in the low-CXI
group compared with that in the high-CXI group (5.03 vs. 2.09;
P<0.001). In male patients, the SMI was significantly lower in
the low-CXI group compared with the high-CXI group (6.6 vs. 7.4;
P=0.009). However, in female patients, no significant differences
in SMI were observed between the low- and high-CXI groups (5.15 vs.
5.6; P=0.425). Additionally, WBC counts were significantly higher
in the low-CXI group compared with the high-CXI group (6,930 vs.
5,590 WBC/µl; P=0.034) and CRP was also significantly higher in the
low-CXI group compared with the high-CXI group (1.11 vs. 0.17
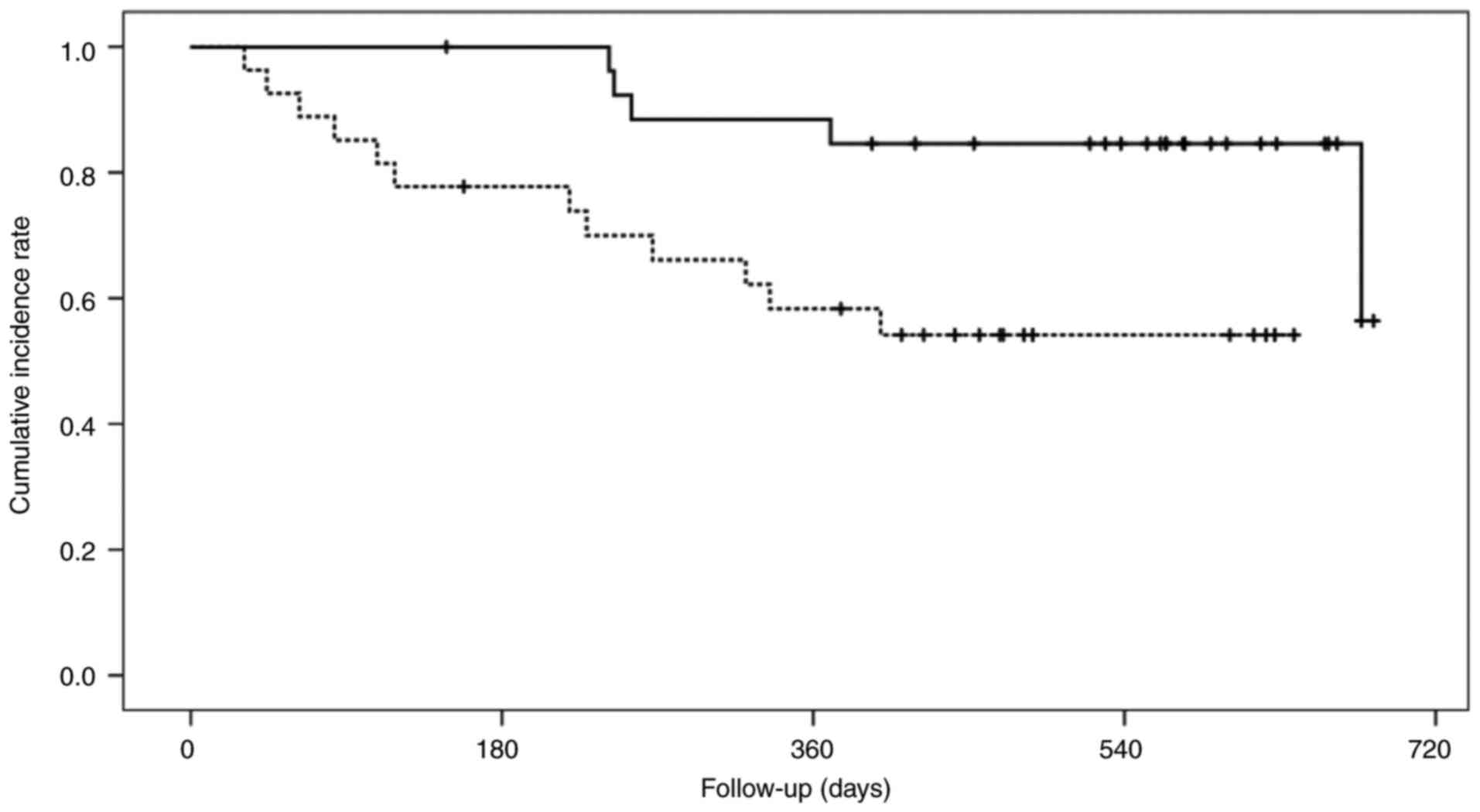
mg/dl; P=0.005). Fig. 2 shows the
OS of the low- and high-CXI groups. The cumulative 1-year OS rate
was significantly lower in the low-CXI group compared with the
high-CXI groups (58.3 vs. 88.5%; P=0.012).
Discussion
In the present study, SMI and CXI values using BIA
in patients with gastrointestinal cancer were assessed. The results
demonstrated that the SMI had a lower impact on OS, whereas the CXI
at diagnosis was closely associated OS in gastrointestinal cancer.
Therefore, calculating CXI values using BIA may be valuable for
predicting OS in patients with gastrointestinal cancer.
Cancer cachexia is closely related to advanced
gastrointestinal cancer. The prevalence of cachexia is 88.9% in
patients with advanced pancreatic cancer, 76.5% in those with
advanced gastric cancer and 52.9% in those with advanced esophageal
cancer (2). Cancer cachexia may
reduce the effects of chemotherapy and increase
chemotherapy-related toxicities, particularly in older patients
with cancer (19). However,
previous studies have reported that skeletal muscle loss at
diagnosis may not influence the survival in advanced pancreatic
cancer (5,20). Recently, the CXI has emerged as an
improved prognostic index due to its ability to reflect systemic
inflammation and nutritional status in addition to skeletal muscle
mass, which are closely associated with cancer cachexia (3,21).
The CXI is frequently associated with the prognosis
of patients with certain malignancies (6–14,16,22–24).
Jafri et al (6) first
established the CXI using the SMI calculated from CT images and
reported that a lower CXI was associated with worse clinical
outcomes of patients with metastatic non-small cell lung cancer.
Thereafter, previous studies have reported that preoperative CXI
may be a prognostic factor for OS (7–10).
Furthermore, in patients with unresectable hepatocellular carcinoma
and gastric cancer undergoing chemotherapy, the CXI may be a
beneficial indicator to predict treatment response and prognosis
(11,12). Although the progression or prognosis
may differ among several gastrointestinal cancers, the CXI could be
a pivotal factor influencing prognosis across gastrointestinal
cancers. The present study included patients with resectable and
unresectable different gastrointestinal cancers as a preliminary
analysis due to the limited number of enrolled patients.
Subsequently, the results demonstrated that the OS rate of patients
in the low-CXI group was significantly lower compared with the
high-CXI group. By contrast, no differences were reported for age,
primary tumor site or resectability between the high- and low-CXI
groups. Collectively, the data demonstrate that the CXI could be
useful for predicting the prognosis of gastrointestinal cancer,
regardless of the treatment strategy.
In previous reports, the SMI in the evaluation of
CXI values was calculated by analyzing the skeletal muscle area at
the L3 level on CT images (7–14);
however, Okubo et al (16)
reported that CXI calculations using BIA may be a prognostic
indicator in elderly patients with non-Hodgkin's lymphoma. Notably,
BIA is a cost-effective, quick and non-invasive method that does
not involve radiation exposure. By contrast, a specialized software
is required when calculating the SMI using CT images. In addition,
BIA can provide other body composition data such as body fat
percentage and ECW/TBW. Collectively, BIA is an attractive and
accurate modality for measuring CXI or sarcopenia (15,25).
The findings of the present study also suggest that CXI
calculations using BIA could be an acceptable prognostic indicator
of gastrointestinal cancer. However, in patients with significant
ascites or edema, the SMI can be overestimated using the BIA method
(26). Moreover, BIA cannot be
performed in patients with a lower performance status, as it
requires maintaining a standing position for a few minutes
(16).
The present study has certain limitations. Firstly,
the present study was a retrospective, single-center analysis with
a limited number of cases, which introduced the possibility of
selection bias. Secondly, the observation period was short and the
number of patients in the resectable group who experienced relapse
or mortality was too small to determine the clinical significance.
Therefore, a larger prospective study with a longer follow-up
period is necessary in the future. Thirdly, the results of the
present study cannot be easily generalized to all types of
gastrointestinal cancer, as the primary tumor sites, patterns of
tumor progression and treatment modalities can vary widely.
Overall, the present study demonstrated that CXI
values determined using BIA may predict survival in patients with
gastrointestinal cancer.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Project Mirai Cancer
Research Grants.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NI, KoO and JS contributed to the study's conception
and design. Collection and assembly of data were performed by TOh,
NI, KoO, KeO, HS, TTs, TOk and JS. Data analysis and interpretation
were performed by TOh, NI, KoO, JS, TD, KI, OD and MM. TOh and NI
drafted the manuscript. KK, NY, KY, KU, TI, TTa, HK and YI revised
the manuscript. TTs, TOk and JS confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki and was approved by the ethical
committee of Fukuchiyama City Hospital (Kyoto, Japan; approval no.
5-57). The opt-out method was used to obtain informed consent due
to the retrospective design of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun L, Quan XQ and Yu S: An
epidemiological survey of cachexia in advanced cancer patients and
analysis on its diagnostic and treatment status. Nutr Cancer.
67:1056–1062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwai N, Okuda T, Sakagami J, Harada T,
Ohara T, Taniguchi M, Sakai H, Oka K, Hara T, Tsuji T, et al:
Neutrophil to lymphocyte ratio predicts prognosis in unresectable
pancreatic cancer. Sci Rep. 10:187582020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwai N, Okuda T, Oka K, Sakagami J, Harada
T, Ohara T, Hattori C, Taniguchi M, Sakai H, Hara T, et al:
Depletion of psoas muscle mass after systemic chemotherapy is
associated with poor prognosis in patients with unresectable
pancreatic cancer. Cancers (Basel). 13:38602021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jafri SH, Previgliano C, Khandelwal K and
Shi R: Cachexia index in advanced non-small-cell lung cancer
patients. Clin Med Insights Oncol. 9:87–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakashima K, Haruki K, Kamada T, Takahashi
J, Nakaseko Y, Ohdaira H, Furukawa K, Suzuki Y and Ikegami T:
Usefulness of the cachexia index as a prognostic indicator for
patients with gastric cancer. Ann Gastroenterol Surg. 7:733–740.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamura R, Haruki K, Shirai Y, Tanji Y,
Taniai T, Okui N, Furukawa K, Shiozaki H, Onda S and Ikegami T:
Preoperative cachexia index can predict the prognosis of
extrahepatic biliary tract cancer after resection. Surg Oncol.
44:1018252022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimagaki T, Sugimachi K, Mano Y, Onishi
E, Iguchi T, Nakashima Y, Sugiyama M, Yamamoto M, Morita M and Toh
Y: Cachexia index as a prognostic predictor after resection of
pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg.
7:977–986. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamada T, Haruki K, Nakashima K, Takahashi
J, Nakaseko Y, Suzuki N, Ohdaira H, Eto K, Ikegami T and Suzuki Y:
Prognostic significance of the cachexia index in patients with
stage I–III colorectal cancer who underwent laparoscopic surgery.
Surg Today. 53:1064–1072. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goh MJ, Kang W, Jeong WK, Sinn DH, Gwak
GY, Paik YH, Choi MS, Lee JH, Koh KC and Paik SW: Prognostic
significance of cachexia index in patients with advanced
hepatocellular carcinoma treated with systemic chemotherapy. Sci
Rep. 12:76472022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsunaga T, Satio H, Sakano Y, Makinoya
M, Shimizu S, Shishido Y, Miyatani K, Hanaki T, Kihara K, Yamamoto
M, et al: Prognostic significance of the cachexia index in patients
with unresectable advanced gastric cancer receiving palliative
chemotherapy: A retrospective single-center study. Surg Today.
54:231–239. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong C, Wan Q, Zhao R, Zuo X, Chen Y and
Li T: Cachexia index as a prognostic indicator in patients with
gastric cancer: A retrospective study. Cancers (Basel).
14:44002022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanji Y, Furukawa K, Haruki K, Taniai T,
Onda S, Tsunematsu M, Shirai Y, Yanagaki M, Igarashi Y and Ikegami
T: Significant impact of cachexia index on the outcomes after
hepatic resection for colorectal liver metastases. Ann
Gastroenterol Surg. 6:804–812. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aleixo GFP, Shachar SS, Nyrop KA, Muss HB,
Battaglini CL and Williams GR: Bioelectrical impedance analysis for
the assessment of sarcopenia in patients with cancer: A systematic
review. Oncologist. 25:170–182. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okubo S, Shinmura K, Kadota S, Nakayasu M,
Kurosawa S, Nakayama H, Sakurai A, Ito C, Aisa Y and Nakazato T:
Evaluation of the cachexia index using a bioelectrical impedance
analysis in elderly patients with non-Hodgkin's lymphoma: A
single-center prospective study. Ann Hematol. 103:823–831. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen LK, Liu LK, Woo J, Assantachai P,
Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al:
Sarcopenia in Asia: Consensus report of the Asian working group for
sarcopenia. J Am Med Dir Assoc. 15:95–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen LK, Woo J, Assantachai P, Auyeung TW,
Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al: Asian
working group for sarcopenia: 2019 Consensus update on sarcopenia
diagnosis and treatment. J Am Med Dir Assoc. 21:300–307.e2. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caillet P, Liuu E, Raynaud Simon A,
Bonnefoy M, Guerin O, Berrut G, Lesourd B, Jeandel C, Ferry M,
Rolland Y and Paillaud E: Association between cachexia,
chemotherapy and outcomes in older cancer patients: A systematic
review. Clin Nutr. 36:1473–1482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basile D, Parnofiello A, Vitale MG,
Cortiula F, Gerratana L, Fanotto V, Lisanti C, Pelizzari G, Ongaro
E, Bartoletti M, et al: The IMPACT study: Early loss of skeletal
muscle mass in advanced pancreatic cancer patients. J Cachexia
Sarcopenia Muscle. 10:368–377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Tang X, Zhang J, Man C, Jiang D,
Xu Y, Zhang W, Gong D and Fan Y: Cachexia index as a predictor of
reduced survival in patients with gastrointestinal cancer: A
systematic review and meta-analysis. Nutr Cancer. 76:815–823. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Go SI, Park MJ and Lee GW: Clinical
significance of the cachexia index in patients with small cell lung
cancer. BMC Cancer. 21:5632021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Go SI, Park MJ, Park S, Kang MH, Kim HG,
Kang JH, Kim JH and Lee GW: Cachexia index as a potential biomarker
for cancer cachexia and a prognostic indicator in diffuse large
B-cell lymphoma. J Cachexia Sarcopenia Muscle. 12:2211–2219. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karmali R, Alrifai T, Fughhi IAM, Ng R,
Chukkapalli V, Shah P, Basu S, Nathan S, Szymanski-Grant K, Gordon
LI, et al: Impact of cachexia on outcomes in aggressive lymphomas.
Ann Hematol. 96:951–956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iwai N, Sakai H, Oka K, Sakagami J, Okuda
T, Hattori C, Taniguchi M, Hara T, Tsuji T, Komaki T, et al:
Predictors of response to anamorelin in gastrointestinal cancer
patients with cachexia: A retrospective study. Support Care Cancer.
31:1152023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kikuchi N, Uojima H, Hidaka H, Iwasaki S,
Wada N, Kubota K, Nakazawa T, Shibuya A, Kako M, Take A, et al:
Evaluation of skeletal muscle mass in patients with chronic liver
disease shows different results based on bioelectric impedance
analysis and computed tomography. Ann Nutr Metab. 78:336–344. 2022.
View Article : Google Scholar : PubMed/NCBI
|