Introduction
In recent years, there has been a significant
increase in the incidence of oral malignancies associated with the
human papillomavirus (HPV). Globally, HPV has been implicated in
>38,000 cases of head and neck cancer, with ~30% classified as
oropharyngeal malignancies (1). HPV
types 16 and 18 are recognized as high-risk strains associated with
the development of oral squamous cell carcinoma (OSCC). The
infection status of HPV16 and HPV18 in OSCC shows that HPV16 is
present in 20–35% of cases, while HPV18 is present in 5–10% of
cases. Notably, India bears the highest burden of oral cancer in
Asia, constituting ~20% of all reported cases in Asia, primarily
due to delays in diagnosis that contributes to poor prognosis
(2,3). HPV infections are estimated to
contribute to ~20% of oral cancer cases and 60–80% of oropharyngeal
malignancies, which often exhibit distinct clinical responses and
survival rates. As such, HPV-positive tumors, especially
oropharyngeal cancers, respond better to radiation and
chemotherapy, likely due to increased radiosensitivity and enhanced
immune response. Patients with HPV-positive tumors typically have
higher survival rates, lower recurrence and reduced risk of
metastasis compared with HPV-negative patients. This favorable
prognosis has spurred research into less intensive treatment
options for these patients compared with that for HPV-negative
tumors (4). Besides HPV infection,
several key factors contribute to OSCC. These include tobacco use
(both smoking and smokeless forms), heavy alcohol consumption,
chewing betel quid, poor oral hygiene, dietary factors (such as low
intake of fruits and vegetables), sun exposure (particularly to the
lips), genetic predispositions and immunosuppression, all of which
increase the risk of developing OSCC (5). In addition to HPV16 and HPV18, other
high-risk HPV types that can cause OSCC include HPV31, HPV33,
HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58 and HPV59. Although
these types are less common compared with HPV16 and HPV18, they
still contribute to the development of OSCC (6). The presence of these high-risk HPV
types underscores the importance of regular screening and
preventive measures, such as HPV vaccination, to reduce the
incidence of HPV-related cancer (7). Understanding the broader spectrum of
HPV types involved in OSCC can aid in developing more comprehensive
strategies for prevention, early detection and treatment.
HPV is a non-enveloped virus known for its affinity
for infecting epithelial cells. Its genome consists of two helical
DNA strands within a spheroid structure, with the HPV family
comprising ~100 unique types (8–10).
Among the high-risk variants, HPV16 and HPV18 are most frequently
implicated, in 50 and 20% of cervical cancer cases, respectively
(11). Notably, these strains are
responsible for a notable proportion of oral cancer cases among
Indian betel quid chewers (12).
The viral genome encompasses three distinct segments: Early (E),
late (L) and upstream regulatory regions, with the E segment
containing six proteins: E1, E2, E4, E5, E6 and E7, which aid in
viral DNA replication and synthesis of new virus particles within
infected cells (13). Of particular
significance are the E6 and E7 oncoproteins, which serve a pivotal
role in HPV-associated carcinogenesis by promoting cell
proliferation, immortality and malignant transformation through
interactions with key cellular proteins such as p53 and
phosphorylated retinoblastoma protein (Rb) (14–16).
Protein-protein interaction (PPI) networks and analysis of gene
expression alterations associated with HPV infection provide
insights into the molecular mechanisms underlying HPV-mediated
oncogenesis (17–19). Structural analysis techniques can
further demonstrate the alterations in E6 and E7 proteins as they
interact with host proteins (20).
The integration of data from various omics sources and employing
bioinformatics tools can enable comprehensive exploration of
molecular alterations implicated in HPV-associated cancer.
The primary aim of the present study was to validate
the molecular profiles of HPV16 and 18 E6/E7 oncoproteins, and to
elucidate their association with OSCC and oral leukoplakia (OL).
This involved comparative analysis of differentially expressed
genes (DEGs), functional annotations, pathway interrogation, and
scrutiny of clinical and pathological indicators to detect
high-risk HPV types, mainly focusing on the E6 and E7
oncogenes.
Materials and methods
Structural validation
The FASTA file sequences for HPV16 and 18 E6/E7
proteins were obtained from UniProt (https://www.uniprot.org/). Online tools from Expasy
(Swiss Institute of Bioinformatics), such as Protparam (https://web.expasy.org/cgi-bin/protparam/protparam),
were used to analyze the molecular characteristics of these
proteins, including molecular weight, theoretical isoelectric
point, amino acid composition and average hydrophobicity. Protscale
(https://web.expasy.org/protscale/)
was used to predict the distribution of hydrophilic and hydrophobic
regions in the proteins. Furthermore, the 3D structures of the
HPV16 and 18 E6/E7 proteins were examined using the Phyre2
(http://www.sbg.bio.ic.ac.uk/~phyre2/) online software.
These structures were modelled using Modeller (version 2.0;
http://salilab.org/modeller/) and
visualized using Rasmol (version 2.7.5.2; http://www.openrasmol.org/software/rasmol/) and
Discovery Studio (2020; Dassault Systèmes SE; http://www.3ds.com/products/biovia/discovery-studio).
Data extraction
Raw gene expression data (datasets listed below)
from HPV16 and HPV18-infected OSCC tissues were pre-processed to
remove batch effects and normalize gene expression levels.
Predefined gene sets from the GEO database and Molecular Signatures
Database, were used to identify biological pathways and gene sets
of interest. Genes were ranked based on their differential
expression between HPV-positive and control (healthy) samples using
metrics such as log-fold change or a moderated t-statistic. Gene
set enrichment analysis (GSEA) was performed using software
provided by the Broad Institute, Inc. (https://www.gsea-msigdb.org/gsea/index.jsp). Default
settings were used unless otherwise specified. A focused search of
the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) for oral cancer
datasets using key words such as, ‘Oral cancer’, ‘HPV 16 and 18’
and ‘Homo sapiens’, and a filter for ‘Expression profile by
microarray’ resulted in 11 potential datasets (21). In total, three datasets were
selected after examining the requirement for complete clinical
information, and excluding datasets containing data from blood
samples, cancer cell lines or patients who had shown resistance to
earlier therapies. Thus, three gene expression profiles [accession
nos. GSE65858 (22), GSE42743
(23) and GSE6791 (24)] directly associated with OSCC and HPV
were chosen from GEO datasets for further study.
Microarray data pre-processing
Series matrix files for GSE65858, GSE42743 and
GSE6791 were obtained for the analysis. Initially, the RNA-seq data
from OSCC samples ensured that both HPV-positive and HPV-negative
samples were obtained. The raw RNA-seq count data were normalized
to obtain expression values using DESeq2 (Bioconductor) or edgeR
(Bioconductor) (https://bioconductor.org/packages/release/bioc/html/edgeR.html).
This file matched the order of samples in the gene expression
matrix. Common nomenclature was used to convert the probe
identifiers in each dataset into standardized gene symbols to
ensure consistent gene identification (25). A robust multi-array average approach
was used to normalize the datasets. Normalization was conducted
utilizing R software (version 4.3.2; http://cran.r-project.org/bin/windows/base/), which
standardized the gene expression data across all datasets by
harmonizing them in scale and distribution (26). This process was crucial for
mitigating systematic biases among samples or experimental
conditions, facilitating accurate comparisons and analyses across
datasets.
Identification of differentially
expressed genes (DEGs) in HPV-OSCC datasets
The GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) tool was used
to identify DEGs within the OSCC and HPV datasets. The GEO2R tool
produced a volcano plot that visually represents the variation in
gene expression on the x-axis and statistical significance
(P-value) on the y-axis, highlighting genes with significant
changes in expression levels. DEGs were selected using the
following criteria: P<0.01 and an absolute log-fold change
>1. The FunRich (version 3.1.3; http://www.funrich.org/) tool was used to create Venn
diagrams that showed the similarities and differences in DEGs
across the three microarray datasets.
PPI network and module analysis of
DEGs in HPV-OSCC datasets
DEGs from OSCC samples were used to create a network
that was explored using the STRING (https://string-db.org/) tool in the examination of
PPIs within HPV-OSCC. Interactions with a confidence value of
>0.4 were considered significant, which highlighted the
reliability of these linkages (27). The network was visually depicted
using Cytoscape (version 3.5.1; http://www.cytoscape.org), with the connections
between proteins shown as lines of different thicknesses to
illustrate interaction intensity. Hub genes were identified as
proteins related to ≥10 others, which indicated their importance
within the network. The present study utilized the MCODE
(https://apps.cytoscape.org/apps/mcode) and cytohubba
(https://apps.cytoscape.org/apps/cytohubba) plugins in
Cytoscape to identify closely connected gene clusters. By setting
specific parameters, such as a node score threshold of 0.2, a
k-core value of 2 and a maximum depth of 100, the plugin could find
and separate necessary modules or gene clusters that were closely
linked.
Gene Ontology and pathway enrichment
analysis
Functional and pathway enrichment analysis is
important for understanding the biological significance of
identified DEGs and gene clusters (28). FOR GO analysis, the default
enrichment statistic based on the Kolmogorov-Smirnov-like running
sum statistic was employed. Gene set permutations were performed to
assess statistical significance and calculate the false discovery
rate (FDR). To ensure robustness, 1,000 permutations were used.
Gene sets with FDR q-values <0.05 were considered significantly
enriched. The Enrichr tool (https://maayanlab.cloud/Enrichr/) was employed to
study biological functions and pathways associated with increased
and decreased hub genes.
Additionally, a Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway analysis was performed to identify pathways
significantly enriched with the identified DEGs. To evaluate the
significance of these pathways, criteria (normalization of DEGs)
including a Benjamini-Hochberg adjusted P<0.05 were applied. A
combined score based on the Jaccard coefficient (50%) and overlap
coefficient (50%) >0.5 was considered statistically
significant.
In vitro studies
A total of 100 formalin-fixed paraffin-embedded
(FFPE) tissue blocks were utilized, accessed from a tissue/sample
databank held at the archives of Meenakshi Academy of Higher
Education and Research University (Chennai, India). The tissue
samples, which were collected over 5 years between May 2015 and
June 2019, were obtained with ethics approval from the
Institutional Review Board of Meenakshi Ammal Dental College and
Hospital (approval no. MADC/IRB-XI/2017/235; February 7, 2023;
Chennai, India). Patients had provided consent for their tissues to
be used in future research. The sample set had 20 blocks from
normal mucosa (NM) in healthy individuals (n=20), 40 blocks from
patients with OL and 40 blocks from patients with OSCC. The age
range of the healthy individuals was 32–50 years, with a mean age
of 24 years and an age range of 18–29 years (male donors, n=10;
female donors, n=10). Patients with OL had a mean age of 45 years,
ranging from 32–50 years (male patients, n=28; female patients,
n=12). Meanwhile, patients with OSCC were older, with a mean age of
50 years and an age range of 35–60 years (male patients, n=25;
female patients, n=15). Gene expression levels of HPV16 and 18,
along with their oncogenes E6 and E7, were detected using reverse
transcription-quantitative PCR (RT-qPCR).
Tissue sectioning and
deparaffinization
Sample preparation involved obtaining 10-µm slices
from each FFPE tissue block. The sections were subsequently
collected in 2 ml microcentrifuge tubes, ensuring an even
distribution from the three groups. To remove the paraffin, 1 cc
xylene preheated at 60°C for 10 min was added to each tube
containing the tissue slices. The cells from the tissue pellet were
lysed using a homogenizer, and the samples were centrifuged at a
speed of 11,200 × g (deparaffinization at 60°C and inactivation at
95°C). During homogenization, Proteinase K (typically 20–50 µl, as
per protocol) was added to digest the tissue. Once digestion was
complete, samples were incubated at 95°C for 10 min to inactivate
Proteinase K, ensuring no interference in subsequent analytical
steps.
Extraction of DNA
The DNA extraction process utilized a commercially
available DNA isolation kit, QIAamp DNA FFPE Tissue Kit (Qiagen
India Pvt. Ltd.). After the wax was removed, the tissue pellet was
combined with 180 µl animal tissue lysis buffer and homogenized
using a homogenizer. Subsequently, 20 µl proteinase K was added to
the tubes containing the samples. The tubes were placed in an
orbital shaking incubator at 56°C for 1–3 h until the tissue
disintegrated. Next, 200 µl ethanol (96–100%) was added to the
mixture. Following that, the mixture underwent 15 sec
pulse-vortexing, then a brief centrifugation. The DNA extraction
process utilized a commercially available DNA isolation kit, QIAamp
DNA FFPE Tissue Kit (Qiagen India Pvt. Ltd.). After the paraffin
wax was removed, the tissue pellet was combined with 180 µl animal
tissue lysis buffer and homogenized using a homogenizer.
Subsequently, 20 µl proteinase K was added to the tubes containing
the samples. The tubes were placed in an orbital shaking incubator
at 56°C for 1–3 h until the tissue fully disintegrated. Following
this, 200 µl ethanol (96–100%) was added to the mixture, and the
tubes were pulse-vortexed for 15 sec, followed by a brief
centrifugation at 6,000 × g for 10 sec.
RT-quantitative PCR (qPCR)
For RT-qPCR, cDNA was synthesized using the
PrimeScript™ RT Master Mix (Takara Bio Inc.) following
the manufacturer's protocol, with incubation at 37°C for 15 min for
cDNA synthesis and inactivation at 85°C for 5 sec. qPCR analyses
were performed on the qPCR MX3000P system (Agilent Technologies
Inc.) using the KAPA SYBR® FAST qPCR Kit (KAPA
Biosystems; Roche Life Science) with SYBR Green dye, which
specifically binds to double-stranded DNA. Primers targeting HPV16,
HPV18, 16-E6, 16-E7, 18-E6 and 18-E7 were used, with GAPDH as the
reference gene, using forward (5′-TGCACCACCAACTGCTTAGC-3′) and
reverse (5′-GGCATGGACTGTGGTCATGAG-3′) primers due to the stable
expression of GAPDH across samples. Reactions were performed in
triplicate with a no-template negative control. Thermocycling
conditions included an initial denaturation at 95°C for 3 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec.
Melting curve analysis was conducted from 59 to 95°C to ensure
product specificity. Quantification followed the 2−ΔΔCq
method (29), using Cq values for
consistency. Samples were categorized as HPV-positive if Cq values
for HPV16 or HPV18 primers fell below a set threshold, indicating
detectable HPV DNA, and were confirmed by E6 and E7 region
amplification, while samples with Cq values above the threshold
were classified as HPV-negative.
Patients were categorized into HPV-positive and
HPV-negative groups based on the presence or absence of HPV DNA, as
determined by qPCR amplification of specific HPV genotypes (HPV16
and HPV18). If the Cq values for the HPV16 or HPV18 primers were
below a predefined threshold, the sample was classified as
HPV-positive. If no amplification was detected or if the Cq values
were above the threshold, indicating no detectable HPV DNA, the
sample was classified as HPV-negative. The HPV-positive cases were
further confirmed by the amplification of the E6 and E7 regions of
the HPV16 and HPV18 genomes, while the GAPDH gene served as a
control for normalization.
Statistical methods
Data concerning HPV16 and 18, and their E6 and E7
proteins, were consolidated into an Excel spreadsheet (version 16;
Microsoft Corporation). Data were analyzed using SPSS (version 16;
SPSS, Inc.). Fisher's exact tests were performed to ascertain
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Prediction of HPV genotypes 16 and 18
E6/E7 oncoprotein structures
The physicochemical characteristics of HPV genotypes
16 and 18 E6/E7 oncoproteins were analyzed using the FASTA
sequences sourced from UniProt (Table
I). Hydrophilic and hydrophobic properties were assessed to
understand their interactions in aqueous environments, which
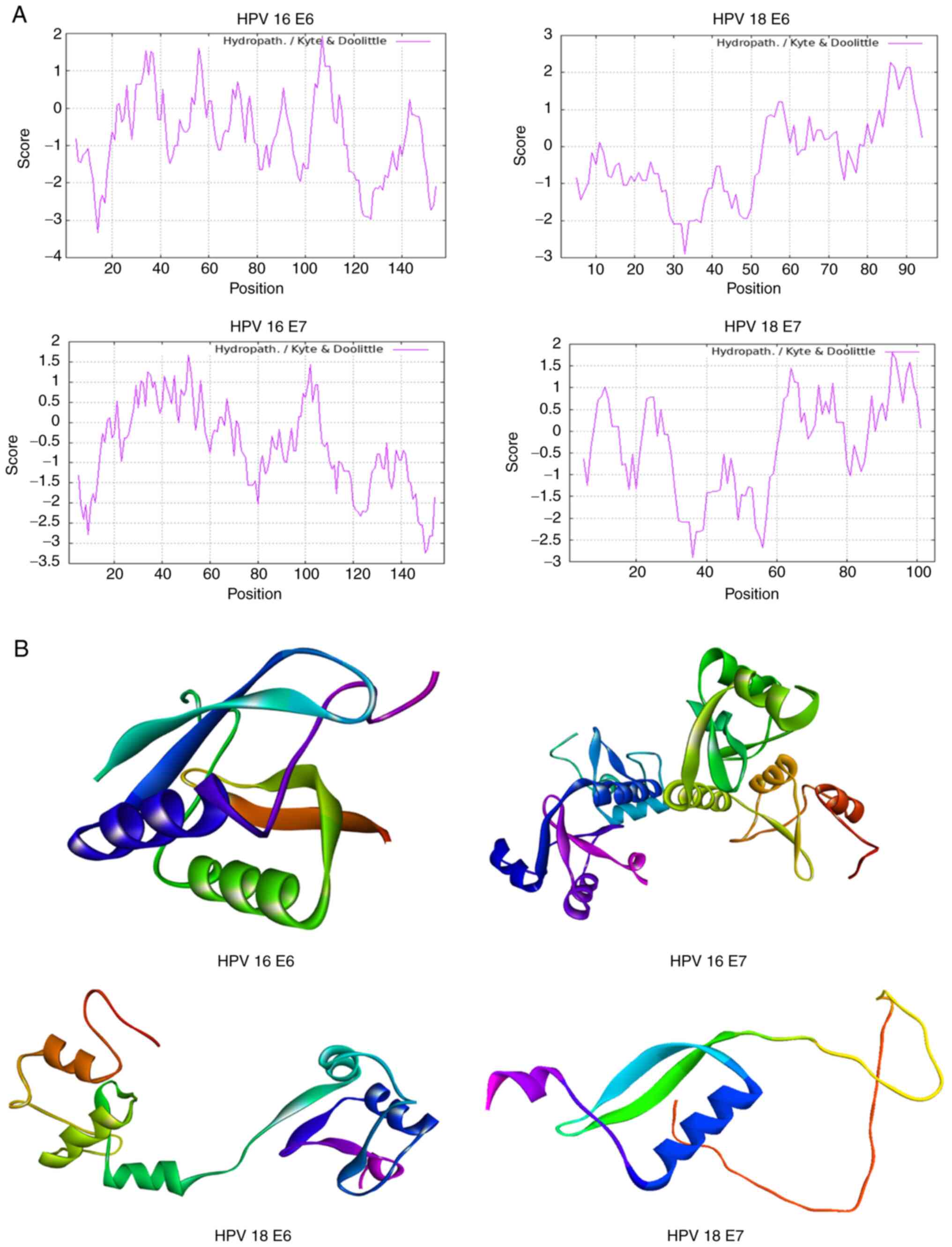
provided insights into their biological activities (Fig. 1A). Moreover, employing Modeller
software, the study conducted a structural modeling of the E6 and
E7 oncoproteins. The predicted 3D structures were achieved with a
high degree of similarity, and these models were visualized using
Rasmol, providing a clear depiction in Fig. 1B. This step offers a tangible
representation of the proteins' potential structural
conformations.
 | Table I.Structure prediction for HPV16 and 18
E6/E7 oncoproteins. |
Table I.
Structure prediction for HPV16 and 18
E6/E7 oncoproteins.
| HPV type | NCBI accession
no. | Sequence | Length, aa | Molecular weight,
kDa | Isoelectric point,
pH | Aliphatic
index |
|---|
| HPV16 | NP 041325.1
(E6) |
MHQKRTAMFQDPQERPRKLPQLCTELQ | 158 | 19.18 | 9.16 | 68.48 |
|
|
|
TTIHDIILECVYCKQQLLRREVYDFAFRDL |
|
|
|
|
|
|
|
CIVYRDGNPYAVCDKCLKFYSKISEYRH |
|
|
|
|
|
|
|
YCYSLYGTTLEQQYNKPLCDLLIRCINCQ |
|
|
|
|
|
|
|
KPLCPEEKQRHLDKKQRFHNIRGRWTGR |
|
|
|
|
|
|
|
CMSCCRSSRTRRETQL |
|
|
|
|
|
| NP 041326.1
(E7) |
MHGDTPTLHEYMLDLQPETTDLYCYEQL | 98 | 11.02 | 4.20 | 78.57 |
|
|
|
NDSEEEDEIDGPAGQAEPDRAHYNIVTFC |
|
|
|
|
|
|
|
CKCDSTLRLCVQSTHVDIRTLEDLLMGTL |
|
|
|
|
|
|
| GIVCPICSQKP |
|
|
|
|
| HPV18 | NP 040310.1
(E6) |
MARFEDPTRRPYKLPDLCTELNTSLQDIE | 158 | 18.87 | 8.95 | 78.99 |
|
|
|
ITCVYCKTVLELTEVFEFAFKDLFVVYRDS |
|
|
|
|
|
|
|
IPHAACHKCIDFYSRIRELRHYSDSVYGDT |
|
|
|
|
|
|
|
LEKLTNTGLYNLLIRCLRCQKPLNPAEKLR |
|
|
|
|
|
|
|
HLNEKRRFHNIAGHYRGQCHSCCNRARQ |
|
|
|
|
|
|
| ERLRRRETQV |
|
|
|
|
|
| NP 040311.1
(E7) |
MHGPKATLQDIVLHLEPQNEIPVDLLCHEQ | 105 | 11.99 | 4.70 | 71.59 |
|
|
|
LSDSEEENDEIDGVNHQHLPARRAEPQRH |
|
|
|
|
|
|
|
TMLCMCCKCEARIELVVESSADDLRAFQQ |
|
|
|
|
|
|
|
LFLNTLSFVCPWCASQQ |
|
|
|
|
GSEA of HPV-associated pathways
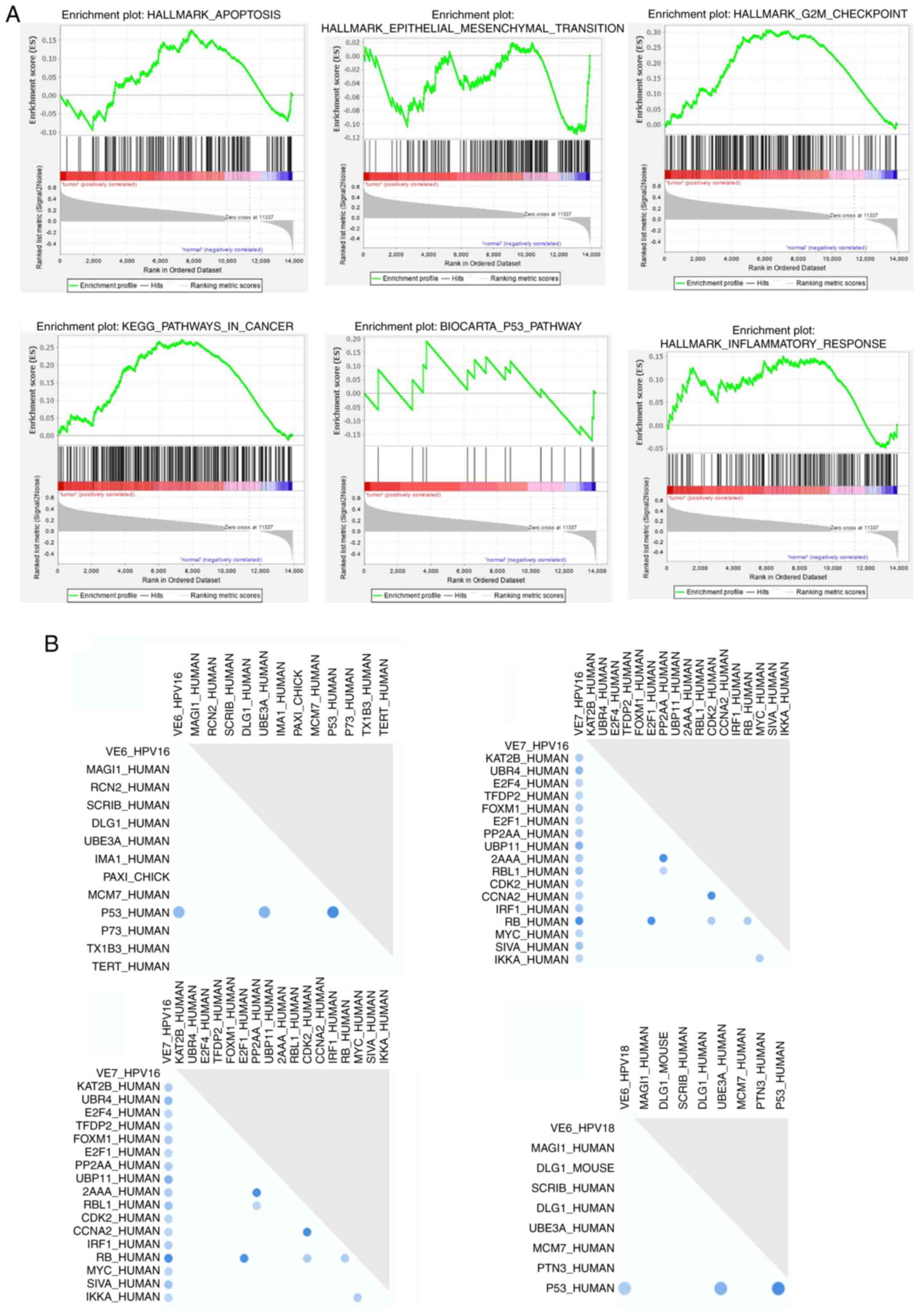
GSEA demonstrated upregulation of genes involved in
critical pathways, such as the epithelial-mesenchymal transition
(EMT), G2/M checkpoint, inflammation regulation,
apoptosis suppression, p53 and cancer-related pathways (Fig. 2A). Furthermore, Fig. 2B illustrates the binary relationship
between HPV 16 and 18 E6/E7 oncoproteins, and their regulation of
p53 and Rb proteins in human datasets. These findings strongly
suggest that the primary focus of this study, which is the role of
HPV 16 and 18 E6/E7 oncoproteins in the regulation of p53,
contributes significantly to the process of oral
carcinogenesis.
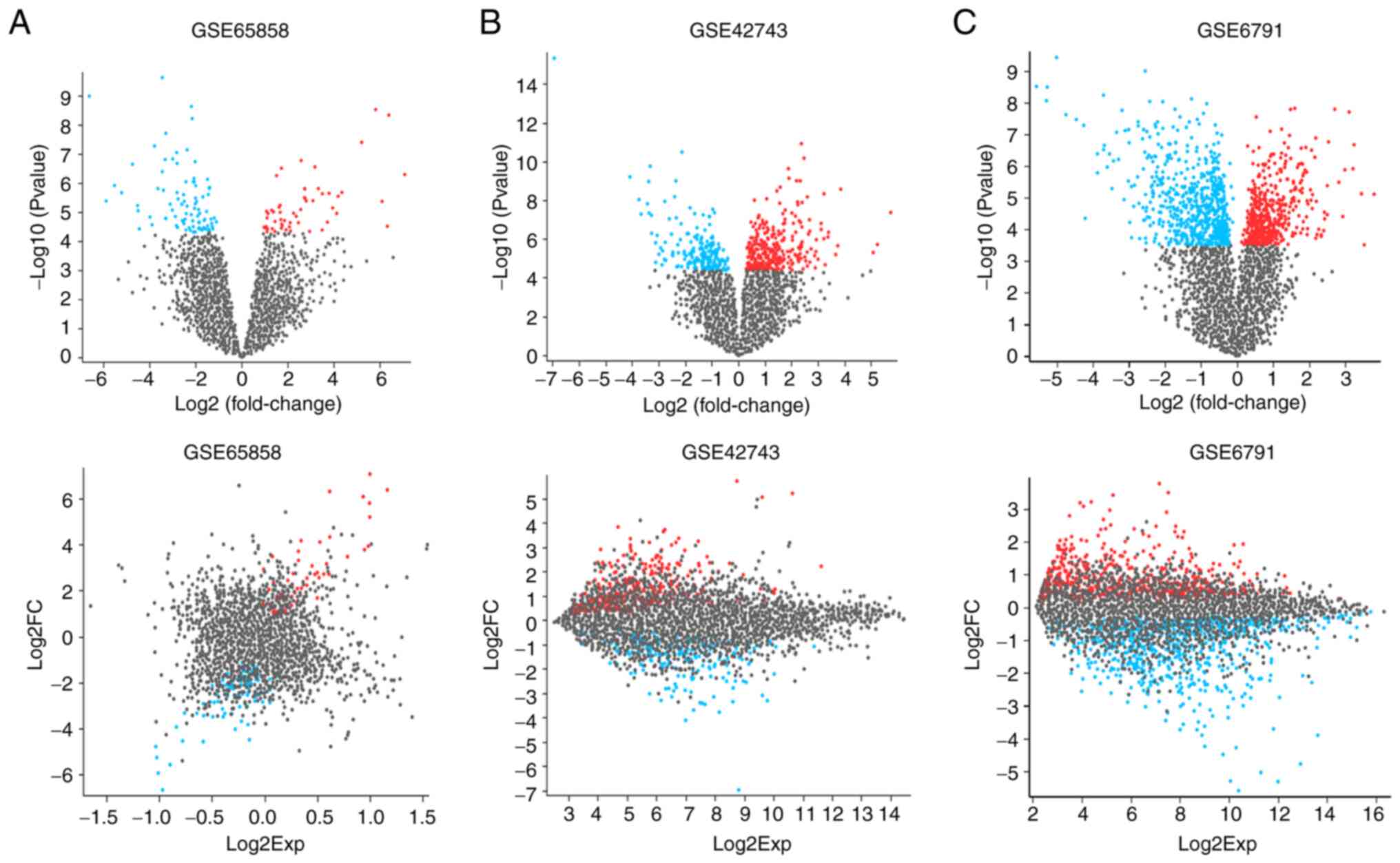
DEGs in OSCC
The DEGs between the OSCC and normal healthy
tissues, as determined from the GEO datasets, are illustrated in
Fig. 3A-C using volcano plots and
mean difference plots, which indicate the differences in gene
expression between the groups. The study suggests that
HPV-associated OSCC may contribute to oral tumorigenesis through
the regulation of specific pathways. Targeting these pathways could
potentially yield improved therapeutic outcomes in the context of
HPV-associated OSCC. This approach underscores the importance of
understanding the molecular mechanisms underlying this type of oral
cancer for the development of more effective treatment
strategies.
Identification of key pathways in
HPV-associated OSCC
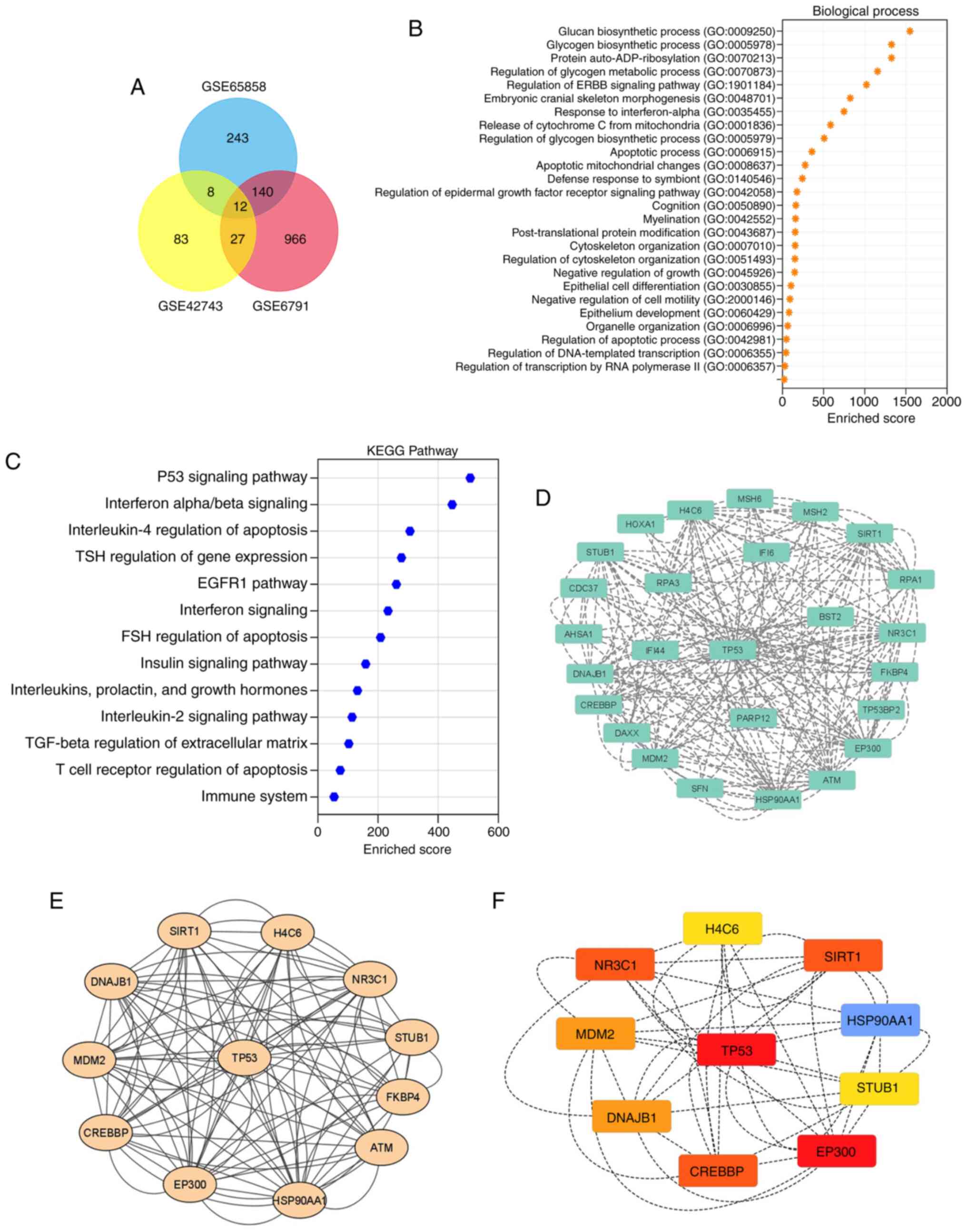
DEG analysis across the datasets identified 1,145,
130 and 403 DEGs in the GSE6791, GSE42743 and GSE65858 datasets,
respectively, all associated with HPV-related OSCC. This suggests
potential as therapeutic targets or biomarkers, as modulating these
genes may disrupt cancer-promoting pathways and enhance the
efficacy of immunotherapies or other targeted treatments (Fig. 4A and B). The results of KEGG pathway
analysis revealed significant enrichment in the ‘Interferon
alpha/beta signaling’, ‘Interleukin-4 regulation of apoptosis’,
‘TSH regulation of gene expression’ and ‘EGFR1 pathway’ related to
OSCC (Fig. 4C). Additionally,
protein interaction predictions for HPV-associated OSCC were
visualized using the STRING tool and a comprehensive network
analysis was conducted with Cytoscape, utilizing plugins such as
MCODE and cytoHubba (Fig. 4D).
CytoHubba was used to identify hub genes from the PPI network,
whereas MCODE was used to identify the genetic sequence for the
specific proteins. This analysis unveiled the proteins associated
with the p53 pathway from the OSCC datasets, highlighting their
high nodal strength, closeness centrality, betweenness centrality
and radiality, as detailed in Table
II. These findings provide a comprehensive view of the
molecular interactions and key players (EP300, HSP90AA1, TP53,
CREBBP, NR3C1, SIRT1, MDM2, DNAJB1, H4C6 and STUB1) in the context
of HPV-associated OSCC, which could be pivotal for further research
and therapeutic development.
 | Table II.Targets in human
papillomavirus-associated oral squamous cell carcinoma
datasets. |
Table II.
Targets in human
papillomavirus-associated oral squamous cell carcinoma
datasets.
| Top DEGs | Betweenness | Closeness | Clustering
co-efficient | Degree | Radiality | MCODE | MCC method |
|---|
| EP300 | 5.20 | 11.0 | 0.45 | 22 | 2.27 | 7.00 | 8,640 |
| HSP90AA1 | 5.20 | 11.0 | 0.43 | 22 | 2.27 | 7.00 | 8,640 |
| TP53 | 5.20 | 11.0 | 0.43 | 22 | 2.27 | 7.00 | 8,640 |
| CREBBP | 3.41 | 10.5 | 0.43 | 20 | 2.18 | 6.37 | 7,200 |
| NR3C1 | 1.80 | 10.0 | 0.41 | 18 | 2.09 | 6.37 | 7,200 |
| SIRT1 | 1.78 | 10.0 | 0.41 | 18 | 2.09 | 6.37 | 7,200 |
| MDM2 | 1.23 | 10.0 | 0.40 | 18 | 2.09 | 6.37 | 6,480 |
| DNAJB1 | 1.23 | 10.0 | 0.40 | 18 | 2.09 | 6.00 | 6,480 |
| H4C6 | 0.33 | 09.0 | 0.37 | 14 | 1.90 | 5.78 | 1,440 |
| STUB1 | 0.28 | 09.0 | 0.36 | 14 | 1.90 | 5.78 | 1,440 |
E6/E7 expression profile associated
with cellular signaling
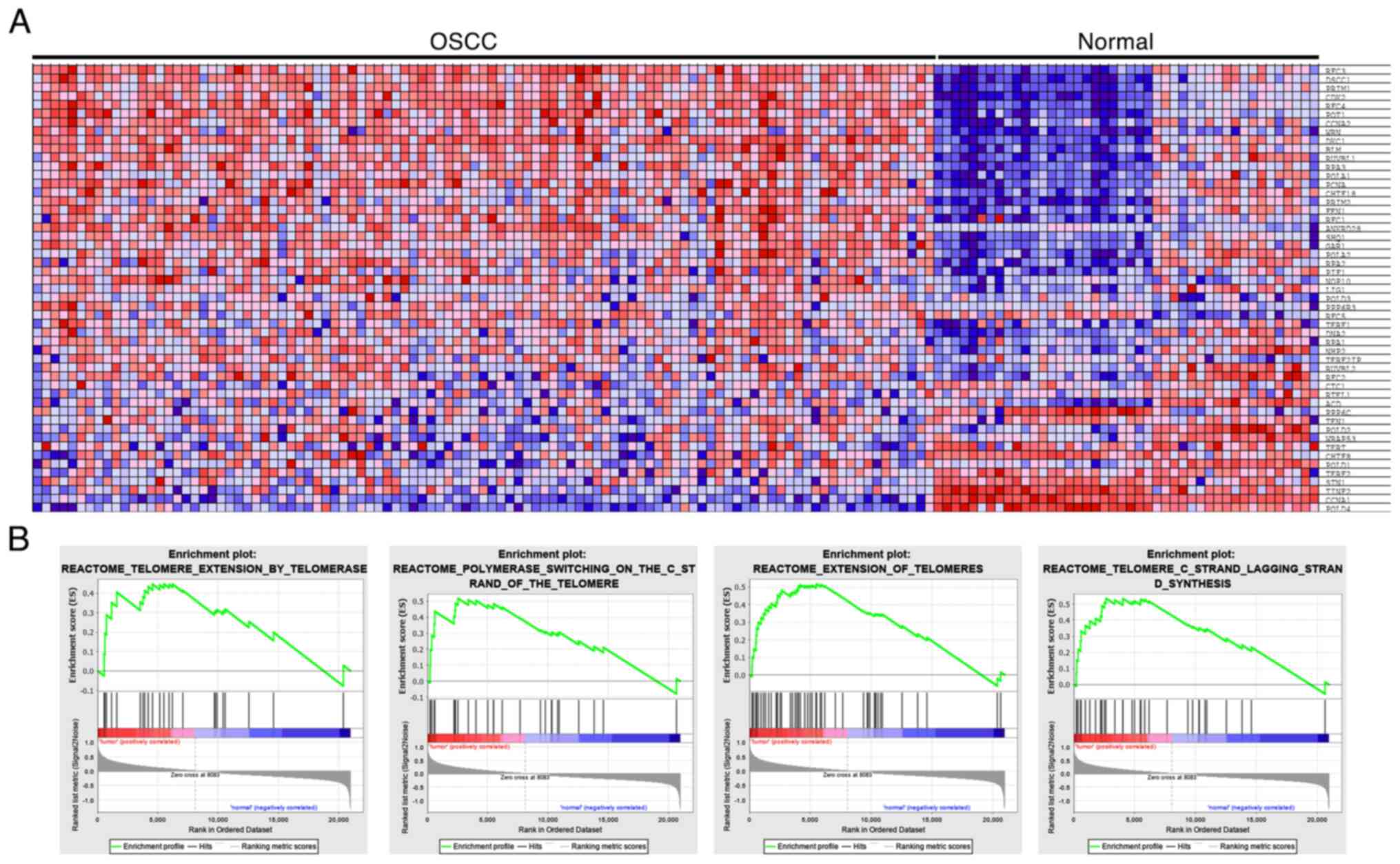
Heat map cluster analysis was conducted on DEGs from
the GEO database and demonstrated that genes associated with
HPV-related oral cancer were implicated in telomere regulation
(Fig. 5A). GSEA plots (Fig. 5B) showed that HPV E6 and E7
genotypes may significantly impact the regulation, elongation and
synthesis of telomeres, particularly on the lagging strand.
Notably, these genes are associated with E6/E7 and play a role in
influencing oral tumorigenesis, oncogenic pathways and telomeric
regulation in oral cancer. These results provide compelling
evidence of the connection between HPV-associated oral cancer and
the regulation of telomeres, shedding light on the mechanisms
underlying oral tumorigenesis.
In vitro analysis
The prevalence of HPV16 and 18, and their E6 and E7
oncoproteins, were compared across OSCC, OL and NM using Fisher's
exact test (Table III). HPV16 E6
and E7 oncoproteins were significantly associated with OSCC, which
indicated their higher prevalence in cancerous tissues. The HPV18
E7 oncoprotein was also significantly associated with OSCC. By
contrast, no significant differences were found for the overall
prevalence of HPV16 and HPV18. Additionally, the prevalence of
HPV16 and 18, and their E6 and E7 oncoproteins, was compared among
patients according to tumor location (Table IV), patient habits (Table V) and sex distribution (Table VI) among the OSCC, OL and NM
groups. The analyses highlighted distinct patterns in the
prevalence of HPV16 and 18, specifically in their oncoproteins E6
and E7, across OSCC, OL and NM groups. HPV16 oncoproteins E6 and E7
were significantly more prevalent in OSCC cases, with the E7
oncoprotein showing the highest association with OSCC, suggesting a
strong link between HPV16 E7 and malignancy. Similarly, HPV18 E7
was significantly associated with OSCC, while no significant
difference was found in the general prevalence of HPV16 or HPV18
across the three groups. In examining tumor location, a higher
number of HPV-positive cases with E6 and E7 expression were found
in OSCC at non-tongue sites compared with OL and NM. Regarding
patient habits, HPV positivity (including E6 and E7 expression) was
more prevalent among OSCC patients with tobacco use, notably in
HPV16 E7-positive cases, further supporting an association between
these oncoproteins and OSCC in patients with habits. Sex
distribution data showed that males had a higher prevalence of
HPV-positive cases across OSCC and OL groups, with no HPV
positivity in NM, suggesting a sex-linked pattern in HPV infection
prevalence within OSCC and OL.
 | Table III.Prevalence of HPV16 and 18, and E6
and E7 oncoproteins, in OSCC (n=40), OL (n=40) and NM (n=20)
groups. |
Table III.
Prevalence of HPV16 and 18, and E6
and E7 oncoproteins, in OSCC (n=40), OL (n=40) and NM (n=20)
groups.
| HPV type and
oncoprotein | OSCC, n (%) | OL, n (%) | NM, n (%) |
P-valuea |
|---|
| HPV-16 | 3 (7.5) | 5 (12.5) | 0 (0) | >0.999 |
| E6 | 7 (17.5) | 2 (5.0) | 0 (0) | 0.0152 |
| E7 | 8 (20) | 0 (0) | 0 (0) | 0.0000229 |
| HPV-18 | 6 (15) | 6 (15) | 0 (0) | 0.1577 |
| E6 | 0 (0) | 0 (0) | 0 (0) | - |
| E7 | 6 (15) | 0 (0) | 0 (0) | 0.0070 |
 | Table IV.Positivity of HPV16 and 18, and E6
and E7 oncoproteins, according to location on the tongue and other
sites among OSCC (n=40), OL (n=40) and NM (n=20) groups. |
Table IV.
Positivity of HPV16 and 18, and E6
and E7 oncoproteins, according to location on the tongue and other
sites among OSCC (n=40), OL (n=40) and NM (n=20) groups.
| A, Tongue |
|---|
|
|---|
|
|
| HPV16 | HPV18 |
|---|
|
|
|
|
|
|---|
| Group | No. of cases | HPV16 positive | E6 | E7 | HPV18 positive | E6 | E7 |
|---|
| OSCC | 13 | 2 | 2 | 3 | 0 | 0 | 1 |
| OL | 8 | 1 | 0 | 0 | 3 | 0 | 0 |
| NM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| B, Other
sites |
|
|
|
| HPV16 | HPV18 |
|
|
|
|
|
| Group | No. of
cases | HPV16
positive | E6 | E7 | HPV18
positive | E6 | E7 |
|
| OSCC | 27 | 1 | 5 | 5 | 6 | 0 | 5 |
| OL | 32 | 4 | 2 | 0 | 3 | 0 | 0 |
| NM | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
 | Table V.Positivity for HPV16 and 18, and E6
and E7 oncoproteins, in patients with or without tobacco habits
among OSCC (n=40), OL (n=40) and NM (n=20) groups. |
Table V.
Positivity for HPV16 and 18, and E6
and E7 oncoproteins, in patients with or without tobacco habits
among OSCC (n=40), OL (n=40) and NM (n=20) groups.
| A, With habits |
|---|
|
|---|
|
|
| HPV16 | HPV18 |
|---|
|
|
|
|
|
|---|
| Group | No. of cases | HPV16 positive | E6 | E7 | HPV18 positive | E6 | E7 |
|---|
| OSCC | 25 | 1 | 3 | 7 | 2 | 0 | 3 |
| OL | 34 | 4 | 2 | 0 | 4 | 0 | 0 |
| NM | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| B, Without
habits |
|
|
|
| HPV16 | HPV18 |
|
|
|
|
|
| Group | No. of
cases | HPV16
positive | E6 | E7 | HPV18
positive | E6 | E7 |
|
| OSCC | 15 | 2 | 4 | 1 | 4 | 0 | 3 |
| OL | 6 | 1 | 0 | 0 | 2 | 0 | 0 |
| NM | 20 | 0 | 0 | 0 | 0 | 0 | 0 |
 | Table VI.Distribution of HPV-positive and
negative cases in OSCC (n=40), OL (n=40) and NM (n=20) groups
according to sex. |
Table VI.
Distribution of HPV-positive and
negative cases in OSCC (n=40), OL (n=40) and NM (n=20) groups
according to sex.
| A, HPV
positive |
|---|
|
|---|
| Group | No. of cases | Male | Female |
|---|
| OSCC | 17 | 12 | 5 |
| OL | 10 | 9 | 1 |
| NM | 0 | 0 | 0 |
|
| B, HPV
negative |
|
| Group | No. of
cases | Male | Female |
|
| OSCC | 23 | 16 | 7 |
| OL | 30 | 29 | 1 |
| NM | 20 | 10 | 10 |
Discussion
HPV, particularly HPV16, has emerged as a key
etiological factor in a subset of OSCC cases. Unlike traditional
risk factors, such as tobacco and alcohol use, HPV-associated OSCC
predominantly affects younger individuals, often with no history of
smoking or heavy drinking (30).
Understanding the molecular mechanisms underlying HPV-driven OSCC
is important for early detection, prognosis and targeted therapies.
The primary aim of the present study was to investigate the
structural features of HPV16 and 18 E6/E7 oncoproteins through a
physicochemical investigation. Modeler software was used to
successfully create 3D structural models demonstrating how the E6
and E7 oncoproteins were spatially arranged. The identification of
DEGs in HPV-associated OSCC, including EP300, HSP90AA1, TP53,
CREBBP, NR3C1, SIRT1, MDM2, DNAJB1, H4C6 and STUB1, demonstrated
critical insights into the molecular mechanisms of the disease.
These genes may serve pivotal roles across various pathways
essential for cancer development, such as the EMT, G2/M
cell cycle checkpoint, cancer-associated pathways, regulation of
inflammatory responses, suppression of apoptosis and the p53
pathway. EMT is important in cancer metastasis, as it enables
epithelial cells to gain migratory and invasive properties
(31). Through epigenetic
modifications, genes such as EP300 and SIRT1 regulate transcription
factors pivotal for EMT (32),
which illustrates the complexity of cancer metastasis at the
epigenetic level. The G2/M checkpoint of the cell cycle
ensures DNA integrity before mitosis, with TP53 serving a critical
role in its activation in response to DNA damage (33). The balance between TP53 and its
negative regulator, MDM2, is key for cell cycle control (34), pointing to potential areas of
intervention in cancer treatment. HSP90AA1 helps in protein folding
by synthesizing cellular protein folds, thereby helping the tumor
cells evade cellular stress and increasing tumor survival (35). CREBBP and EP300 modify transcription
factors needed for growth and proliferation (36). The improper translation of these two
genes can cause cells to grow and survive without control, which
are fundamental characteristics of cancer cells. NR3C1 is involved
in anti-inflammatory reactions (37), whereby controlling inflammation
could be a therapeutic target. Apoptosis suppression, facilitated
by the interplay between TP53 and MDM2, and the role of SIRT1,
underscores the evasion of programmed cell death as a cancer
hallmark. The p53 pathway, central to DNA damage response and
cellular stress, is disrupted in HPV-OSCC, particularly by the HPV
E6 protein promoting p53 degradation (38). This disruption highlights the
significance of the p53 pathway in maintaining cellular homeostasis
and preventing cancer development. This suggests that the
degradation of p53 by HPV E6 in HPV-OSCC disrupts the cellular
stress response and impairs the ability of cells to repair DNA or
undergo apoptosis, promoting oncogenesis and therapeutic
resistance.
The results from the KEGG pathway analysis indicated
significant enrichment in the ‘Interferon alpha/beta signaling’,
‘Interleukin-4 regulation of apoptosis’, ‘TSH regulation of gene
expression’ and ‘EGFR1 pathway’ in the context of HPV-associated
OSCC, thus highlighting the intricate network of cellular signaling
cascades implicated in the pathogenesis of this disease. The
IFN-α/β signaling pathway has an important role in the innate
immune response to viral infections, including HPV (39). IFNs are cytokines that induce
antiviral states in cells, upregulating genes that inhibit viral
replication and spread. TSH-regulating gene expression serves an
important role in metabolic regulation and cellular proliferation
(40), which indicates that its
involvement in OSCC reflects the complex hormonal and metabolic
reprogramming that occurs during cancer progression. The EGFR1
signaling pathway is instrumental in cell growth, differentiation
and survival (41). The significant
enrichment of the EGFR1 signaling pathway in HPV-OSCC highlights
the importance of growth factor signaling in the development and
progression of this type of cancer. HPV oncoproteins are known to
interact with and dysregulate growth factor pathways to promote
cellular proliferation and evade apoptosis (42). PPI network analysis further
elucidated the extensive interactions of proteins within HPV-linked
OSCC datasets. This comprehensive examination of the molecular
interaction's sheds light on the key nodes and factors influencing
the development of oral cancer. Telomere regulation analysis
(through in vitro pathway study with molecular databases
analysis) demonstrated the potential role of E6 and E7 oncoproteins
in stabilizing telomeres, which suggests an association with
unregulated cell division in oral cancer. The results of in
vitro assays that assessed HPV presence across diverse tissue
types reinforced the epidemiological association between HPV
infection and the onset of oral malignancies. This examination
corroborated the molecular findings from other study analyses,
providing a more holistic depiction of HPV involvement in OSCC
(41,43). Fundamentally, the present findings
substantiated the association between HPV infection and OSCC,
emphasizing the significance of recognizing HPV as a pivotal
determinant in the prevention, diagnosis and management of oral
malignancies. Recognition of the inherent constraints, such as
potential biases in datasets, underscores the imperative for
ongoing validation and expansion of these findings.
The present study identified unique gene expression
profiles and molecular pathways in HPV16 and HPV18-infected OSCC
tissues, which differed significantly from non-HPV-related OSCC.
HPV-positive samples exhibited upregulation of E6 and E7 oncogenes,
which may disrupt the expression of tumor suppressor genes, such as
p53 and Rb, due to viral protein interactions. By contrast, in
non-HPV-related OSCC cases, these genes are often mutated or
altered through other carcinogenic pathways (43). The molecular pathways activated in
HPV-positive OSCC are distinct, with the PI3K/AKT pathway and cell
cycle regulation prominently affected by viral oncoproteins
(44–47). By contrast, non-HPV-related OSCC
shows alterations in pathways associated with tobacco and alcohol
exposure, such as the EGFR pathway and oxidative stress
responses.
Clinically, patients with HPV-positive OSCC
generally have an improved prognosis and respond differently to
treatment modalities, such as radiation and chemotherapy, compared
with those with non-HPV-related OSCC (48,49).
These differences underscore the importance of distinguishing
between HPV-related and non-HPV-related OSCC in clinical management
and treatment planning. Furthermore, the present research
identified specific biomarkers and potential therapeutic targets
unique to HPV16 and HPV18 infections. Therapeutic strategies
targeting the HPV oncoproteins E6 and E7 could provide more
effective treatments for HPV-positive OSCC, while different
approaches might be necessary for non-HPV-related cases.
Through structural validation, gene expression
analysis and in vitro experiments, the present study
demonstrated how HPV, particularly types 16 and 18 and their E6 and
E7 oncoproteins, contribute to oral cancer development. These
findings highlight the dysregulation of key pathways, such as EMT,
cell-cycle control and inflammation, shedding light on the
underlying mechanisms of HPV-associated OSCC. These results provide
important implications for targeted therapies and diagnostic
strategies in HPV-related oral cancer, underscoring the need for
further research to advance the current understanding and clinical
management of these diseases. Future studies should incorporate
protein interaction network analyses utilizing platforms such as
STRING and Cytoscape to elucidate the complex molecular
interactions and pathways implicated in these processes. Such
analyses will provide both visual and quantitative insights into
the underlying molecular dynamics. Moreover, it is crucial to
investigate the specific roles and functions of these key proteins
within the identified networks. Conducting thorough experimental
validation will strengthen the current findings and may also reveal
new therapeutic targets. Such future studies are essential for
translating this research into clinical applications for oral
cancer, aiming to enhance treatment strategies, improve patient
outcomes, and achieve more effective and lasting tumor suppression
and tissue regeneration. In conclusion, the increasing incidence of
mouth cancer linked to HPV, especially in the oropharyngeal area,
is a substantial global health concern. The main aims of the
present study were to investigate the structural properties of
HPV16 and 18 E6/E7 oncoproteins, and to identify associations
between HPV genotypes 16 and 18 and both OSCC and OL. The analysis
verified the presence of high-risk HPV types 16 and 18, together
with their corresponding E6/E7 oncogenes, in both OSCC and OL
through RT-qPCR analysis on DNA isolated from FFPE tissue samples.
The findings demonstrated increased occurrence of HPV18 in both the
OSCC and OL groups in comparison to HPV16. Moreover, further
investigation is required to identify small inhibitors that may be
potential targets for treating oral cancer linked with HPV.
Acknowledgements
Not applicable.
Funding
The authors would like to thank the Deanship of Scientific
Research at King Khalid University for supporting this work through
Large Group Project (no. RGP-2/504/45).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SFJH, SSA, KB, LT, IM, IHM, NSA and MIK contributed
to the conception and design of the present study. Data collection
was performed by SFJH, KB, LT and IM. Data analysis was performed
by SSA, LT. and IHM. The first draft of the manuscript was written
by SFJH, KB, LT, IM and MIK. Reviewing and editing of the
manuscript was performed by SSA, IHM and MIK. SFJH and IHM confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
The Declaration of Helsinki and was approved by the Institutional
Review Board of Meenakshi Ammal Dental College and Hospital
(Chennai, India; approval no. MADC/IRB-XI/2017/235; February 7,
2023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HPV
|
human papillomavirus
|
|
OSCC
|
oral squamous cell carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
GSEA
|
gene set enrichment analysis
|
|
DEGs
|
differentially expressed genes
|
|
GEO
|
Gene Expression Omnibus
|
|
PPI
|
protein-protein interaction
|
|
KEGG
|
Kyoto Encyclopedia of Genes And
Genomes
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Pinkiewicz M, Dorobisz K and Zatoński T:
Human papillomavirus-associated head and neck cancers. Where are we
now? A systematic review. Cancer Manag Res. 14:3313–3324. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
HPV Information Centre, . Human
Papillomavirus and Related Diseases Report: WORLD. https://hpvcentre.net/statistics/reports/XWX.pdfOctober
10–2024
|
|
3
|
Mallath MK, Taylor DG, Badwe RA, Rath GK,
Shanta V, Pramesh CS, Digumarti R, Sebastian P, Borthakur BB,
Kalwar A, et al: The growing burden of cancer in India:
Epidemiology and social context. Lancet Oncol. 15:e205–e212. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Powell SF, Vu L, Spanos WC and Pyeon D:
The key differences between human papillomavirus-positive and
-negative head and neck cancers: Biological and clinical
implications. Cancers (Basel). 13:52062021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang X, Wu J, Wang J and Huang R: Tobacco
and oral squamous cell carcinoma: A review of carcinogenic
pathways. Tob Induc Dis. 17:292019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Findik S, Findik S, Abuoğlu S, Cihan FG,
Ilter H and Iyisoy MS: Human papillomavirus (HPV) subtypes and
their relationships with cervical smear results in cervical cancer
screening: A community-based study from the central Anatolia region
of Turkey. Int J Clin Exp Pathol. 12:1391–1398. 2019.PubMed/NCBI
|
|
7
|
Pathak P, Pajai S and Kesharwani H: A
review on the use of the HPV vaccine in the prevention of cervical
cancer. Cureus. 14:e287102022.PubMed/NCBI
|
|
8
|
Comparetto C and Borruto F: Human
papillomavirus infection: Overview. Handbook on Human
Papillomavirus: Prevalence, Detection and Management. Nova Science
Publishers; Hauppauge, NY, USA: pp. 1–137. 2013
|
|
9
|
Kumaraswamy KL and Vidhya M: Human
papilloma virus and oral infections: An update. J Cancer Res Ther.
7:120–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balaram P, Nalinakumari KR, Abraham E,
Balan A, Hareendran NK, Bernard HU and Chan SY: Human
papillomaviruses in 91 oral cancers from Indian betel quid
chewers-high prevalence and multiplicity of infections. Int J
Cancer. 61:450–454. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rader JS, Tsaih SW, Fullin D, Murray MW,
Iden M, Zimmermann MT and Flister MJ: Genetic variations in human
papillomavirus and cervical cancer outcomes. Int J Cancer.
144:2206–2214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kornhaber MS, Florence T, Davis T and
Kingsley K: Assessment of oral human papillomavirus prevalence in
pediatric and adult patients within a multi-ethnic clinic
population. Dent J (Basel). 10:542022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamid NA, Brown C and Gaston K: The
regulation of cell proliferation by the papillomavirus early
proteins. Cell Mol Life Sci. 66:1700–1717. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rorke EA: Antisense human papillomavirus
(HPV) E6/E7 expression, reduced stability of epidermal growth
factor, and diminished growth of HPV-positive tumor cells. J Natl
Cancer Inst. 89:1243–1246. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wise-Draper TM and Wells SI:
Papillomavirus E6 and E7 proteins and their cellular targets. Front
Biosci. 13:1003–1017. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seville LL, Shah N, Westwell AD and Chan
WC: Modulation of pRB/E2F functions in the regulation of cell cycle
and in cancer. Curr Cancer Drug Targets. 5:159–170. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mittal S and Banks L: Molecular mechanisms
underlying human papillomavirus E6 and E7 oncoprotein-induced cell
transformation. Mutat Res Rev Mutat Res. 772:23–35. 2027.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Estêvão D, Costa NR, da Costa RMG and
Medeiros R: Hallmarks of HPV carcinogenesis: The role of E6, E7 and
E5 oncoproteins in cellular malignancy. Biochim Biophys Acta Gene
Regul Mech. 1862:153–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeo-Teh NSL, Ito Y and Jha S: High-risk
human papillomaviral oncogenes E6 and E7 target key cellular
pathways to achieve oncogenesis. Int J Mol Sci. 19:17062018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Macalino SJY, Basith S, Clavio NAB, Chang
H, Kang S and Choi S: Evolution of in silico strategies for
protein-protein interaction drug discovery. Molecules. 23:19632018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCormack ME, Lopez JA, Crocker TH and
Mukhtar MS: Making the right connections: Network biology and plant
immune system dynamics. Curr Plant Biol. 5:2–12. 2016. View Article : Google Scholar
|
|
22
|
Yang R, Klimentová J, Göckel-Krzikalla E,
Ly R, Gmelin N, Hotz-Wagenblatt A, Řehulková H, Stulík J, Rösl F
and Niebler M: Combined transcriptome and proteome analysis of
immortalized human keratinocytes expressing human papillomavirus 16
(HPV16) oncogenes reveals novel key factors and networks in
HPV-induced carcinogenesis. mSphere. 4:e00129–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chakraborty S, Hosen MI, Ahmed M and
Shekhar HU: Onco-multi-OMICS approach: A new frontier in cancer
research. Biomed Res Int. 2018:98362562018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Humphreys IR, Pei J, Baek M, Krishnakumar
A, Anishchenko I, Ovchinnikov S, Zhang J, Ness TJ, Banjade S, Bagde
SR, et al: Computed structures of core eukaryotic protein
complexes. Science. 374:eabm48052021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jimenez-Lopez JC, Gachomo EW, Sharma S and
Kotchoni SO: Genome sequencing and next-generation sequence data
analysis: A comprehensive compilation of bioinformatics tools and
databases. Am J Mol Biol. 3:115–130. 2013. View Article : Google Scholar
|
|
26
|
Wichmann G, Rosolowski M, Krohn K, Kreuz
M, Boehm A, Reiche A, Scharrer U, Halama D, Bertolini J, Bauer U,
et al: The role of HPV RNA transcription, immune response-related
gene expression and disruptive TP53 mutations in diagnostic and
prognostic profiling of head and neck cancer. Int J Cancer.
137:2846–2857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lohavanichbutr P, Méndez E, Holsinger FC,
Rue TC, Zhang Y, Houck J, Upton MP, Futran N, Schwartz SM, Wang P
and Chen C: A 13-gene signature prognostic of HPV-negative OSCC:
Discovery and external validation. Clin Cancer Res. 19:1197–1203.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pyeon D, Newton MA, Lambert PF, den Boon
JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH,
Smith EM, et al: Fundamental differences in cell cycle deregulation
in human papillomavirus-positive and human papillomavirus-negative
head/neck and cervical cancers. Cancer Res. 67:4605–4619. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sultan G, Zubair S, Tayubi IA, Dahms HU
and Madar IH: Towards the early detection of ductal carcinoma (a
common type of breast cancer) using biomarkers linked to the
PPAR(γ) signaling pathway. Bioinformation. 15:799–805. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tomaić V: Functional roles of E6 and E7
oncoproteins in HPV-induced malignancies at diverse anatomical
sites. Cancers (Basel). 8:952016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Helt AM and Galloway DA: Destabilization
of the retinoblastoma tumor suppressor by human papillomavirus type
16 E7 is not sufficient to overcome cell cycle arrest in human
keratinocytes. J Virol. 75:6737–6747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Porter VL and Marra MA: The drivers,
mechanisms, and consequences of genome instability in HPV-driven
cancers. Cancers (Basel). 14:46232022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Q, Yuan O, Cui H, Hu T, Xiao GG, Wei
J, Zhang H and Wu C: Bioinformatic analysis identifies HPV-related
tumor microenvironment remodeling prognostic biomarkers in head and
neck squamous cell carcinoma. Front Cell Infect Microbiol.
12:10079502022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feller L, Wood NH, Khammissa RAG and
Lemmer J: Human papillomavirus-mediated carcinogenesis and
HPV-associated oral and oropharyngeal squamous cell carcinoma. Part
1: Human papillomavirus-mediated carcinogenesis. Head Face Med.
6:142010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:172016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rubio K, Molina-Herrera A, Pérez-González
A, Hernández-Galdámez HV, Piña-Vázquez C, Araujo-Ramos T and Singh
I: EP300 as a molecular integrator of fibrotic transcriptional
programs. Int J Mol Sci. 24:123022023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meek DW: Tumour suppression by p53: A role
for the DNA damage response? Nat Rev Cancer. 9:714–723. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chinnam M, Xu C, Lama R, Zhang X, Cedeno
CD, Wang Y, Stablewski AB, Goodrich DW and Wang X: MDM2 E3 ligase
activity is essential for p53 regulation and cell cycle integrity.
PLoS Genet. 18:e10101712022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Calderwood SK, Sherman MY and Ciocca DR:
Heat shock proteins in cancer. Springer Science & Business
Media; 2007, View Article : Google Scholar
|
|
41
|
Attar N and Kurdistani SK: Exploitation of
EP300 and CREBBP lysine acetyltransferases by cancer. Cold Spring
Harb Perspect Med. 7:a0265342017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li L, Xing W, Jiang L, Chen D and Zhang G:
NR3C1 overexpression regulates the expression and alternative
splicing of inflammation-associated genes involved in PTSD. Gene.
859:1471992023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Smith PL, Lombardi G and Foster GR: Type I
interferons and the innate immune response-more than just antiviral
cytokines. Mol Immunol. 42:869–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Juul A and Jorgensen JOL: Growth hormone
in adults: Physiological and clinical aspects. 2nd edition.
Cambridge University Press; 2000, View Article : Google Scholar
|
|
45
|
Chen J: Signaling pathways in
HPV-associated cancers and therapeutic implications. Rev Med Virol.
25 (Suppl 1):S24–S53. 2015. View Article : Google Scholar
|
|
46
|
Shahoumi LA and Yeudall WA: Targeted
therapies for non-HPV-related head and neck cancer: Challenges and
opportunities in the context of predictive, preventive, and
personalized medicine. EPMA J. 10:291–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bolt R: Novel biomarkers in the management
of HPV-positive &-negative oropharyngeal carcinoma. PhD thesis;
University of Sheffield: 2016
|
|
48
|
Liouta G, Adamaki M, Tsintarakis A,
Zoumpourlis P, Liouta A, Agelaki S and Zoumpourlis V: DNA
methylation as a diagnostic, prognostic, and predictive biomarker
in head and neck cancer. Int J Mol Sci. 24:29962013. View Article : Google Scholar
|
|
49
|
Deutsch F, Regina Bullen I, Nguyen K, Tran
NH, Elliott M and Tran N: Current state of play for HPV-positive
oropharyngeal cancers. Cancer Treat Rev. 110:1024392022. View Article : Google Scholar : PubMed/NCBI
|