Introduction
Rare follicular epithelium-derived hyalinizing
trabecular tumor (HTT) comprises 1% of thyroid tumors and is often
associated with favorable prognosis (1,2).
Previous studies indicate that HTT constitutes only 1% of all
thyroid tumors and can occur in individuals aged between 40–70
years (3,4). Due to its clinical rarity, there are
several uncertainties in the diagnosis and treatment of this
disease.
Whether HTT is benign or malignant remains
controversial. Although HTT is typically benign, it is considered a
borderline tumor with malignant potential due to reports of
invasion and metastasis in a number of cases (4,5). HTT
can transform malignantly into PTC (6).
HTT exhibits a prominent trabecular pattern,
abundant intratrabecular hyalinized stroma and characteristic
nuclear features of papillary carcinoma (7).
The cytological and histopathological diagnosis of
HTT is challenging (8) and it is
necessary to differentiate HTT from other diseases such as
papillary thyroid carcinoma, medullary thyroid carcinoma and
non-invasive follicular thyroid neoplasm with papillary-like
nuclear features as it guides the treatment process. Due to the
predominantly benign biological characteristics of HTT, surgical
resection followed by long-term follow-up is sufficient for its
management (9). Using
ultrasonography, histopathology and an analysis of the clinical
symptoms, the present study described a patient with HTT.
Case report
A 31-year-old female patient, during a routine
health checkup at the Affiliated Hospital of Shandong Second
Medical University (Weifang, China), in January 2022, was found to
have a thyroid mass on the right side. The patient did not undergo
any treatment for this condition until she presented to the
Affiliated Hospital of Shandong Second Medical University in June
2023. The patient had no symptoms upon the second admission for
treatment. The trachea of the patient was centrally located and not
deviated. A palpable mass ~2×2 cm in size was present on the right
side of the thyroid, with a firm texture, clear borders and no
tenderness. The mass moved up and down with swallowing. The left
side of the thyroid was normal and the lymph nodes in the neck were
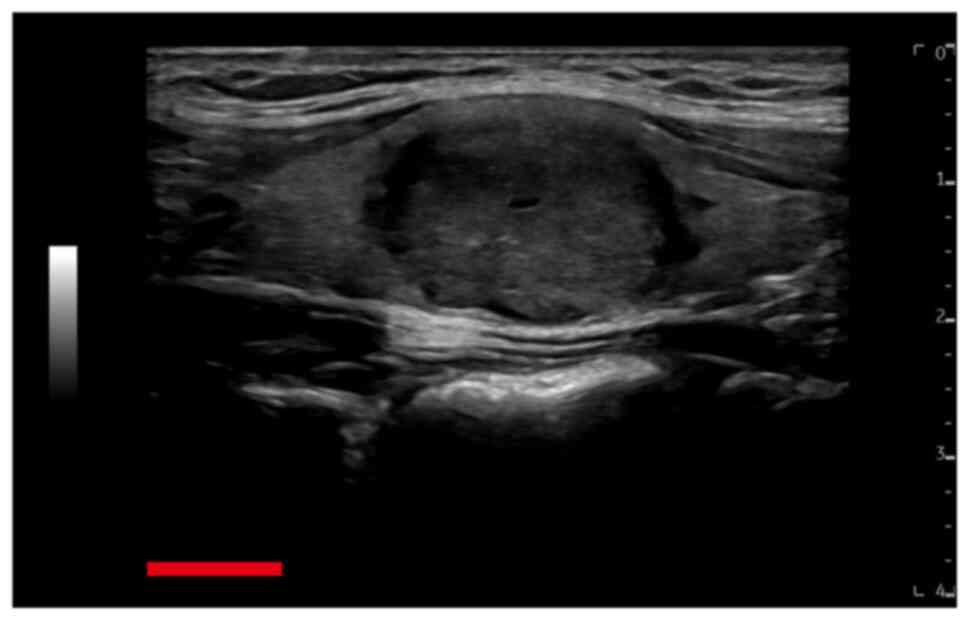
not enlarged. Ultrasound examination revealed a hypoechoic nodule
that was 2.2×2.0×1.5 cm in dimension on the right side of the
thyroid (Fig. 1), which, using the
thyroid imaging reporting and data system (TIRADS), was classed as
TIRADS 3 (10). Solitary cystic
thyroid nodules with a maximum diameter of 3 mm were observed on
the left side of the thyroid (TIRADS 2), no similar cystic nodules
in the right lobe of the thyroid were observed. The patient had no
previous history of thyroid disease, and a routine blood test
(including white and red blood cell count, hemoglobin, hematocrit,
mean corpuscular volume, mean corpuscular hemoglobin, mean
corpuscular hemoglobin concentration) showed no abnormalities. The
following day, the patient underwent the total removal of the right
lobe of the thyroid under general anesthesia. A lymph node
resection was not carried out because: i) HTT is not classified as
a malignant tumor and therefore lymph node dissection is not
routinely performed (11); ii) the
preoperative ultrasound did not reveal any abnormal enlarged lymph
nodes; iii) the patient is 31 years old and relatively young, and
there was a preference for maintaining quality of life; and iv)
upon reviewing the literature, the majority of patients were
treated with only complete resection of the tumor followed by
surveillance (2,12,13).
Therefore, lymph node biopsy was not performed during surgery.
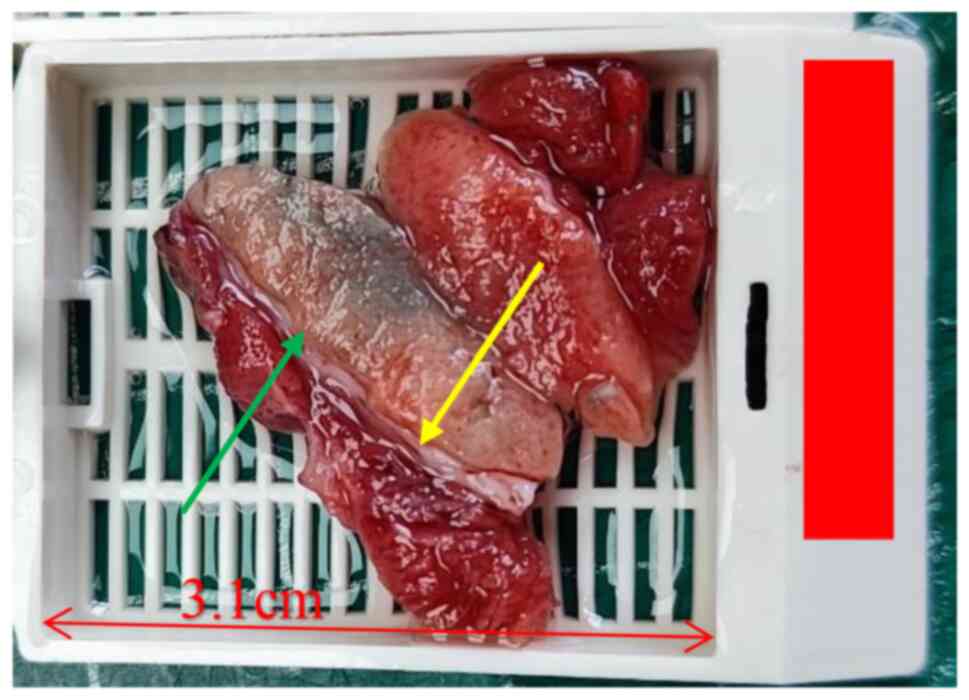
Macroscopic examination revealed a piece of
grayish-red tissue measuring 4.0×3.0×2.5 cm, with a visible
grayish-yellow to grayish-brown nodule measuring 2.1×2.0×1.8 cm on
the cut surface (Fig. 2).
Hematoxylin and eosin (H&E) staining and
immunohistochemical staining were carried out and examined using an
Olympus BX53 light microscope. The tumor specimens were fixed in
10% neutral formalin at room temperature for ~48 h, then embedded
in paraffin and cut into 4-µm slices. H&E staining was
performed at room temperature, with hematoxylin staining for 5 min,
followed by eosin staining for 2 min. Immunohistochemical staining
was performed using the following pre-diluted primary antibodies
(ready-to-use) provided by Guangzhou LBP Medical Science and
Technology Co., Ltd.: anti-thyroglobulin (TG, cat. no. IM138),
anti-cytokeratin 19 (CK19, cat. no. IM378), anti-thyroid
transcription factor 1 (TTF-1, cat. no. IM301), anti-calcitonin
(CT, cat. no. IM382), anti-galectin-3 (cat. no. IR365),
anti-chromogranin A (CgA, cat. no. IM053), anti-CD34 (cat. no.
IM034), anti-podoplanin (D2-40, cat. no. IM070), and anti-Ki-67
(cat. no. IR098). For immunohistochemical staining, paraffin blocks
were sectioned at a thickness of 3 µm. These sections were then
deparaffinized in an alcohol gradient (xylene, 100% ethanol, 95%
ethanol, 75% ethanol, ethanol-free water) and subjected to
high-temperature (97°C) antigen retrieval for 21 min using EnVision
FLEX Target Retrieval Solution (pH=9.0; cat. no. DM828; Agilent
Technologies, Inc.). After cooling to room temperature, the
sections were rinsed with Tris-buffered saline solution (cat. no.
DM831, Agilent Technologies, Inc.). The primary antibodies were
incubated at room temperature for 25 min. After rinsing with
Tris-buffered saline solution, the sections were treated with a
peroxidase blocking reagent (ready-to-use, cat. no. SM801, Agilent
Technologies, Inc.) for 15 min at room temperature, followed by the
application of the secondary antibody (ready-to-use, Dako EnVision
FLEX/HRP detection reagent, cat. no. SM802, Agilent Technologies,
Inc.) and incubated in the dark at room temperature for 20 min.
After rinsing, the sections were developed using EnVision FLEX DAB
(cat. no. DM827, Agilent Technologies, Inc.), observed under a
microscope, and the development time was controlled. Subsequently,
the sections were counterstained with hematoxylin and
coverslipped.
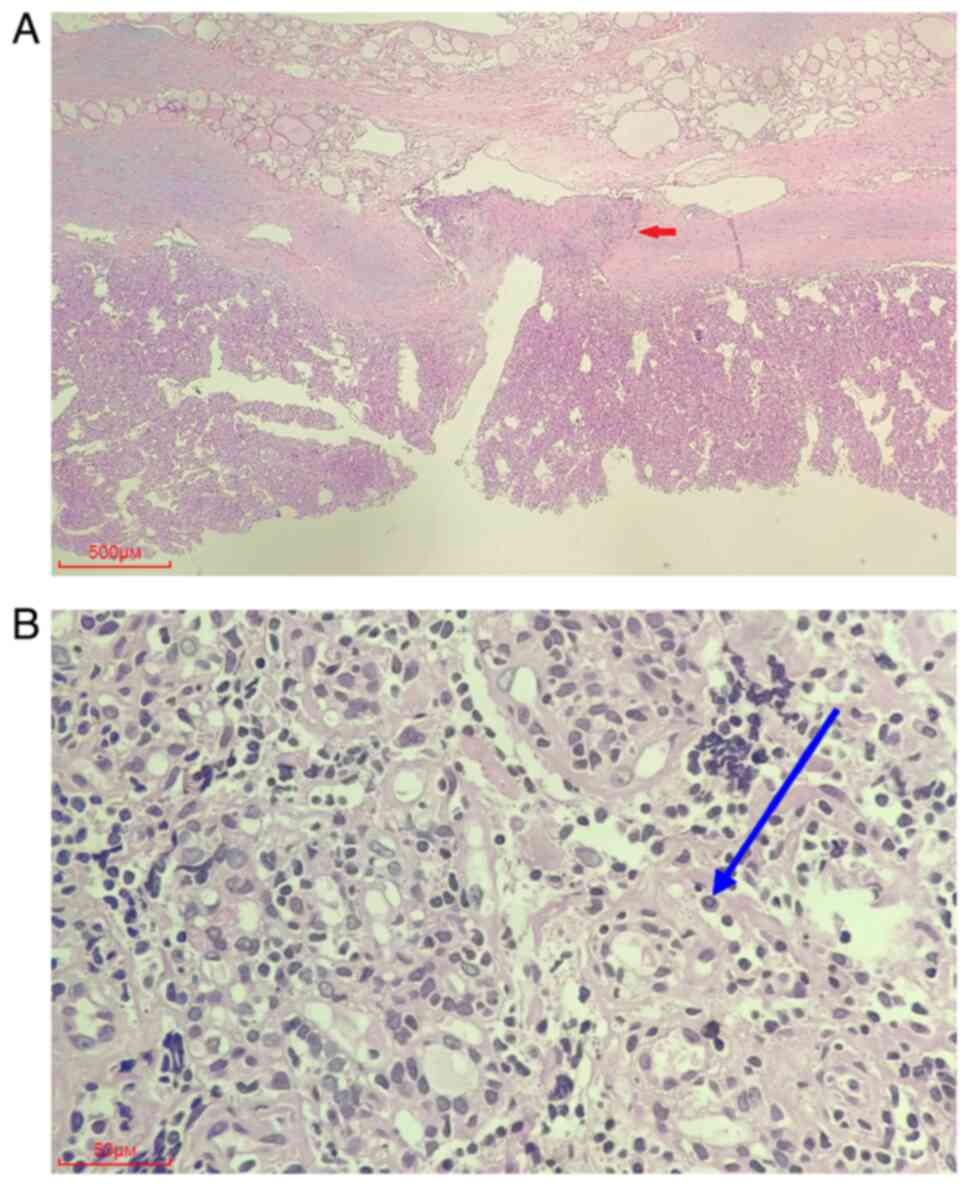
In the right thyroid lobe, the tumor was surrounded
by a fibrous capsule, but the capsule was invaded by tumor cells.
Tumor cells were arranged in trabeculae-like structures. Stromal
hyaline material was observed between the trabeculae, and nuclear
grooves and nuclear pseudoinclusions were observed in the nucleus
(Fig. 3). The immunohistochemical
staining results demonstrated that the tumor cells were positive
for TG, CK19 and TTF-1 and focally positive for galectin-3
(Fig. S1A-D). The hotspots (areas
that exhibit the highest density of Ki-67 staining) in the tumor
revealed a positive rate of 5% for Ki-67, but the staining was
negative for CT and CgA (Fig. S1E and
F). These results were consistent with HTT. The
immunohistochemistry results for CD34 and D2-40 did not reveal the
presence of tumor cells within the blood vessels or lymphatic
vessels (Fig. S1G and H).
Furthermore, based on the absence of red blood cells within the
‘spaces’, it was considered that the vessel-like dilated spaces
were not actual blood vessels but rather thyroid follicles. Close
follow-up of the patient was recommended.
The patient has recovered post operation. Thyroid
function tests at the 1-month postsurgical follow-up indicated no
abnormalities. At the 3-month follow-up, a thyroid ultrasound
examination also revealed no issues. A thyroid ultrasound and
thyroid function test 7-months after the surgery of the patient
indicated no abnormalities (Fig.
S2).
Discussion
In 1987, the study by Carney et al (1) provided a detailed report on the tumor
histopathology of 11 patients with HTT, which was then named
hyalinizing trabecular adenoma. The 2004 classification by the
World Health Organization (WHO) provides a distinct category for
this type of tumor, designating it as HTT and classifying it as
having a low malignant potential (2). The 2017 WHO Classification of Tumors
of Endocrine Organs also uses this categorization (7). However, studies suggest that HTT is a
benign tumor (3,14,15),
despite a small number of reports describing cases of HTT with
capsular invasion and distant metastasis (15–17).
The study by Sambade et al (18) reports a case with HTT with minimal
capsular invasion, as well as another case with metastasis to a
regional lymph node. Additionally, the study by Molberg and
Albores-Saavedra (17) details 3
cases in which the tumors reveal capsular and/or vascular invasion,
classifying these as minimally invasive carcinomas. However, a
number of studies suggest that this may be due to the misdiagnosis
of papillary thyroid carcinoma (PTC) as HTT (3,19). In
the present case, the tumor cells invaded the capsule (albeit
without capsular penetration). Therefore, we hypothesize that it is
inappropriate to classify HTT as a benign tumor, as this may
mislead clinicians regarding the malignant potential of HTT. The
present report provided new evidence of the invasive capability of
HTT.
HTT is more common in women compared with men,
although this is debated (4). With
a mean onset age of 47 years, it does not typically present with
noticeable clinical symptoms (20).
The etiology of HTT is not established. However, the study by Casey
et al (6) reveals rearranged
during transfection gene/PTC mutations in a subset of HTTs,
suggesting that HTT is a form of PTC.
In the diagnostic process for nearly all thyroid
nodules, the initial course of action typically involves conducting
an ultrasound examination followed by a fine-needle aspiration
(FNA) biopsy (4). The main
ultrasound finding that indicates HTT is the presence of a single,
clearly defined, oval or round, solid hypoechoic nodule without
microcalcifications and with peri- or intranodular vascularity
(8,13); however, these diagnostic features
are not specific for HTT. Furthermore, upon FNA, both HTTs and PTCs
can demonstrate hypercellularity, psammoma bodies and cellular
atypia, including cytoplasmic invaginations, nuclear grooves and
nuclear pseudoinclusions, contributing to the diagnostic complexity
(9,16). Therefore, additional diagnostic
methods are needed for the diagnosis of HTT. Commonly used methods
include histopathological and molecular diagnoses (3).
On gross examination, HTT is usually well
circumscribed or encapsulated, and its colors usually vary from
yellow to tan. By contrast, PTC is typically white and lacks a
capsule (18).
Under the microscope, the histological features of
HTT originating from follicular cells include a trabecular
arrangement of the tumor cells, transparency between trabecular
cells and an acidophilic cytoplasm. The tumor cells typically have
a decreased nuclear-to-cytoplasmic ratio compared with normal
cells, often with nuclear grooves and pseudoinclusions (3,4,21).
Furthermore, HTT often coexists with lymphocytic thyroiditis and/or
multinodular goiter in the surrounding tumor tissue (3,21). An
important immunohistochemical antibody used to differentiate
between HTT and PTC is mindbomb homolog-1 (MIB-1; a monoclonal
antibody of Ki-67), which can be used to detect Ki-67 on the cell
membrane of HTT cells (8).
Additionally, the hyaline material of HTT stains positive with
periodic acid-Schiff staining (21).
Molecular testing of FNA biopsy samples can notably
increase accuracy of preoperative FNA diagnoses. This prevents the
misdiagnosis and over-treatment of patients, the additional and
unnecessary surgical risks, decreased quality of life, and
unwarranted healthcare expenses (22). One commonly used molecular test for
HTT, is a test for paired-box gene 8 (PAX8)-GLI-similar 3 (GLIS3)
rearrangement, which is present in 93% of HTT cases (22) and, to the best of our knowledge, is
not found in PTC. The PAX8-GLIS3 rearrangement can lead to the
overexpression of GLIS, which upregulates the production of various
collagens and transparent matrices, including type IV collagen.
This leads to the morphological features of HTT, which is
characterized by the deposition of hyalinized material (7,23).
Additionally, HTT lacks the BRAF V600E mutation (13) that is frequently observed in PTC
(24). Furthermore, there is not an
upregulation of microRNA in HTT, further distinguishing it from PTC
(25).
In practical pathological work, considering the
rarity of HTT compared with PTC and medullary carcinomas, which are
more common, pathologists may not be inclined to diagnose HTT
without further examination (such as using immunohistochemistry and
molecular testing); therefore, HTT requires a differential
diagnosis. Previous studies (9,21,26)
also highlight the importance of differentiating HTT from papillary
and medullary carcinomas. Although histopathology of PTC is similar
to HTT, MIB-1 is positively expressed on the cell membrane in HTT
but not in PTC (21). Therefore,
MIB-1 helps to distinguish between HTT and PTC. Medullary thyroid
carcinoma originates from the parafollicular cells of the thyroid,
generally with inconspicuous nucleoli and a lack of mitotic
figures. Immunohistochemical staining of medullary thyroid
carcinoma indicates a positive expression of CgA and CT and a
negative expression of TG, whereas HTT reveals opposite results
(27). The purpose of differential
diagnosis is to ensure that the possibility of HTT is not
overlooked during the diagnostic process.
In conclusion, although diagnosing HTT is
challenging, combining immunohistochemistry and molecular diagnoses
may improve the diagnostic accuracy. Although there is controversy
regarding the benign or malignant nature of HTT, the present case
provided evidence of its aggressive behavior. The close follow-up
of a patient may be necessary to accurately assess their condition
and guide the subsequent treatments.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Hanchao
Yang (Department of Pathology, Affiliated Hospital of Shandong
Second Medical University, Weifang, China) for their assistance in
capturing images using the microscope.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LZ and LG designed and conceived the study and
revised the manuscript. LZ, QM and ZS performed the research and
analyzed the data. LZ wrote the manuscript. LZ and ZS confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the present case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carney JA, Ryan J and Goellner JR:
Hyalinizing trabecular adenoma of the thyroid gland. Am J Surg
Pathol. 11:583–591. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayashi S, Bandoh N, Baba S, Hayashi M,
Goto T, Takahara M, Kato Y, Aimono E and Nishihara H: A case of
hyalinizing trabecular tumor of the thyroid: Diagnostic
significance of PAX8-GLIS3 fusion. Thyroid Res. 17:92024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nielsen L, Gallardo AMC, Alonso PP, Medina
LO, García EL, Del Arco CD, Jiménez RB, García LA, Blanco MC,
González JV, et al: Diagnostic clues for hyalinizing trabecular
tumor on fine needle aspiration cytology. Cytojournal. 20:192023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alsogair O, Alalawi AA, Alzahim AF, Saleem
MA, Aljohani FM and Alahmadi LS: Hyalinizing trabecular tumor of
the thyroid gland: A case report and literature review. Cureus.
15:e378452023.PubMed/NCBI
|
|
5
|
Umekita Y, Umeki K, Kawano F, Tanaka H and
Kataoka H: Unusual papillary thyroid carcinoma with hyalinizing
trabecular tumor-like feature in a young female patient: A case
report. J Med Case Rep. 17:1122023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Casey MB, Sebo TJ and Carney JA:
Hyalinizing trabecular adenoma of the thyroid gland identification
through MIB-1 staining of fine-needle aspiration biopsy smears. Am
J Clin Pathol. 122:506–510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nikiforova MN, Nikiforov YE and Ohori NP:
GLIS rearrangements in thyroid nodules: A key to preoperative
diagnosis of hyalinizing trabecular tumor. Cancer Cytopathol.
127:560–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito Y, Hirokawa M, Kousaka K, Ito M,
Kihara M, Miya A and Miyauchi A: Diagnosis and management of
hyalinizing trabecular tumor of the thyroid: A single-institution
experience. Endocr J. 68:1403–1409. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
An FX, Zhao Y, Liu HG, Wen WJ and Yin YH:
Fine Needle aspiration cytology of hyalinizing trabecular tumor of
the thyroid. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 44:1040–1044.
2002.(In Chinese). PubMed/NCBI
|
|
10
|
Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo
B, Li J, Qian L, Cui L, Chen W, et al: 2020 Chinese guidelines for
ultrasound malignancy risk stratification of thyroid nodules: The
C-TIRADS. Endocrine. 70:256–279. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma JM, Wu LF, Wang G and Sun B: Progress
in diagnosis and treatment of thyroid hyaline beam tumor. Chin J
Pract Surg. 38:575–577. 2018.(In Chinese).
|
|
12
|
Cheng CH: Hyalinizing trabecular tumor, a
rare histologically unique tumor of the thyroid, coexisting with
papillary thyroid carcinoma. Tzu Chi Med J. 33:198–199. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rossi ED, Papotti M, Faquin W, Larocca LM
and Pantanowitz L: The diagnosis of hyalinizing trabecular tumor: A
difficult and controversial thyroid entity. Head Neck Pathol.
14:778–784. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong B, Xu Y, Xiao Y and Yu X: Comparison
of MIB-1-specific membrane staining in hyalinising trabecular tumor
using mainstream automated immunohistochemical staining platforms.
J Clin Lab Anal. 38:e251132024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carney JA, Hirokawa M, Lloyd RV, Papotti M
and Sebo TJ: Hyalinizing trabecular tumors of the thyroid gland are
almost all benign. Am J Surg Pathol. 32:1877–1889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gowrishankar S, Pai SA and Carney JA:
Hyalinizing trabecular carcinoma of the thyroid gland.
Histopathology. 52:529–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Molberg K and Albores-Saavedra J:
Hyalinizing trabecular carcinoma of the thyroid gland. Hum Pathol.
25:192–197. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sambade C, Franssila K, Cameselle-Teijeiro
J, Nesland J and Sobrinho-Simões M: Hyalinizing trabecular adenoma:
A misnomer for a peculiar tumor of the thyroid gland. Endocr
Pathol. 2:83–91. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Howard BE, Gnagi SH, Ocal IT and Hinni ML:
Hyalinizing trabecular tumor masquerading as papillary thyroid
carcinoma on fine-needle aspiration. ORL J Otorhinolaryngol Relat
Spec. 75:309–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu S: Hyalinizing trabecular tumor of the
thyroid: A case report. Asian J Surg. 46:5559–5560. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Huang X, Hu Y, Wang F, Du T, He W,
Chen L, Lang B, Pu Q and Chen H: Hyalinizing trabecular tumor of
the thyroid: A clinicopathological analysis of four cases and
review of the literature. Int J Clin Exp Pathol. 10:7616–7626.
2017.PubMed/NCBI
|
|
22
|
Mahjabin F, Gonsalves C, Drew PA, Mukhtar
F and Leon ME: Understanding and overcoming the pitfalls in
cytopathological diagnosis of hyalinizing trabecular tumor of
thyroid. Int J Surg Pathol. 32:91–96. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basili T, Dopeso H, Kim SH, Ferrando L,
Pareja F, Da Cruz Paula A, da Silva EM, Stylianou A, Maroldi A,
Marchiò C, et al: Oncogenic properties and signaling basis of the
PAX8-GLIS3 fusion gene. Int J Cancer. 147:2253–2264. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stojanović S, Šelemetjev S, Đorić I,
Janković Miljuš J, Tatić S, Živaljević V and Išić Denčić T:
BRAFV600E, BANCR, miR-203a-3p and miR-204-3p in risk stratification
of PTC patients. Biomedicines. 11:33382023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheu SY, Vogel E, Worm K, Grabellus F,
Schwertheim S and Schmid KW: Hyalinizing trabecular tumour of the
thyroid-differential expression of distinct miRNAs compared with
papillary thyroid carcinoma. Histopathology. 56:632–640. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jones DJ, Kieliszak CR, Patel SS and
Selinsky CR: Hyalinizing trabecular tumor of the thyroid gland and
its significant diagnostic issue. Thyroid Res. 10:72017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Podany P and Gilani SM: Hyalinizing
trabecular tumor: Cytologic, histologic and molecular features and
diagnostic considerations. Ann Diagn Pathol. 54:1518032021.
View Article : Google Scholar : PubMed/NCBI
|