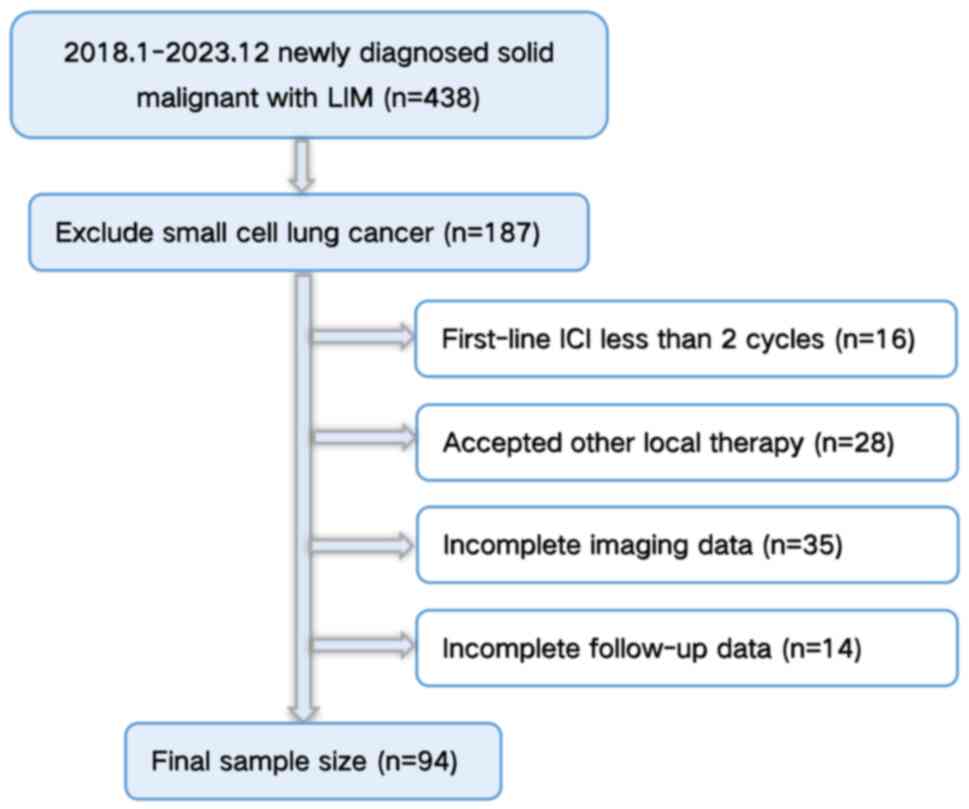
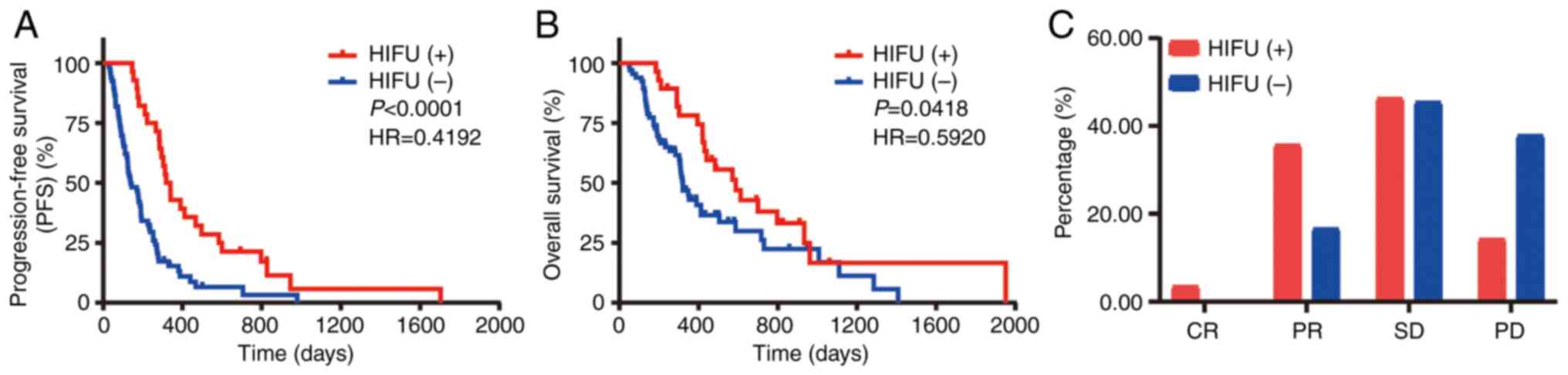
|
1
|
Disibio G and French SW: Metastatic
patterns of cancers: Results from a large autopsy study. Arch
Pathol Lab Med. 132:931–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsilimigras DI, Brodt P, Clavien PA,
Muschel RJ, D'Angelica MI, Endo I, Parks RW, Doyle M, de Santibañes
E and Pawlik TM: Liver Metastases. Nat Rev Dis Primers. 7:272021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Topalian SL, Taube JM and Pardoll DM:
Neoadjuvant checkpoint blockade for cancer immunotherapy. Science.
367:eaax01822020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bilen MA, Shabto JM, Martini DJ, Liu Y,
Lewis C, Collins H, Akce M, Kissick H, Carthon BC, Shaib WL, et al:
Sites of metastasis and association with clinical outcome in
advanced stage cancer patients treated with immunotherapy. BMC
Cancer. 19:8572019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tumeh PC, Hellmann MD, Hamid O, Tsai KK,
Loo KL, Gubens MA, Rosenblum M, Harview CL, Taube JM, Handley N, et
al: Liver metastasis and treatment outcome with anti-PD-1
monoclonal antibody in patients with melanoma and NSCLC. Cancer
Immunol Res. 5:417–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Welsh JW, Tang C, de Groot P, Naing A,
Hess KR, Heymach JV, Papadimitrakopoulou VA, Cushman TR, Subbiah V,
Chang JY, et al: Phase II trial of ipilimumab with stereotactic
radiation therapy for metastatic disease: Outcomes, toxicities, and
low-dose radiation-related abscopal responses. Cancer Immunol Res.
7:1903–1909. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Welsh J, Menon H, Chen D, Verma V, Tang C,
Altan M, Hess K, de Groot P, Nguyen QN, Varghese R, et al:
Pembrolizumab with or without radiation therapy for metastatic
non-small cell lung cancer: A Randomized Phase I/II Trial. J
Immunother Cancer. 8:e0010012020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Ji Q, Yan X, Lian B, Si L, Chi Z,
Sheng X, Kong Y, Mao L, Bai X, et al: The impact of liver
metastasis on anti-PD-1 monoclonal antibody monotherapy in advanced
melanoma: Analysis of five clinical studies. Front Oncol.
10:5466042020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vokes EE, Ready N, Felip E, Horn L, Burgio
MA, Antonia SJ, Arén Frontera O, Gettinger S, Holgado E, Spigel D,
et al: Nivolumab versus docetaxel in previously treated advanced
non-small-cell lung cancer (CheckMate 017 and CheckMate 057):
3-year update and outcomes in patients with liver metastases. Ann
Oncol. 29:959–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen XJ, Ren A, Zheng L, Zheng ED and
Jiang T: Pan-cancer analysis identifies liver metastases as
negative predictive factor for immune checkpoint inhibitors
treatment outcome. Front.Immunol. 12:6510862021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horst AK, Neumann K, Diehl L and Tiegs G:
Modulation of liver tolerance by conventional and nonconventional
antigen-presenting cells and regulatory immune cells. Cell Mol
Immunol. 13:277–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomson AW and Knolle PA:
Antigen-presenting cell function in the tolerogenic liver
environment. Nat Rev Immunol. 10:753–766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jenne CN and Kubes P: Immune surveillance
by the liver. Nat Immunol. 14:996–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu J, Green MD, Li S, Sun Y, Journey SN,
Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al: Liver
metastasis restrains immunotherapy efficacy via macrophage-mediated
T cell elimination. Nat Med. 27:152–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou B, He N, Hong J, Yang T, Ng DM, Gao
X, Yan K, Fan X, Zheng Z, Chen P, et al: HIFU for the treatment of
gastric cancer with liver metastases with unsuitable indications
for hepatectomy and radiofrequency ablation: A prospective and
propensity score-matched study. BMC Surg. 21:3082021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang T, Ng DM, Du N, He N, Dai X, Chen P,
Wu F, Chen B, Fan X, Yan K, et al: HIFU for the treatment of
difficult colorectal liver metastases with unsuitable indications
for resection and radiofrequency ablation: A phase I clinical
trial. Surg Endosc. 35:2306–2315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quesson B, Vimeux F, Salomir R, de Zwart
JA and Moonen CT: Automatic control of hyperthermic therapy based
on real-time Fourier analysis of MR temperature maps. Magn Reson
Med. 47:1065–1072. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tillander M, Hokland S, Koskela J, Dam H,
Andersen NP, Pedersen M, Tanderup K, Ylihautala M and Kohler M:
High intensity focused ultrasound induced in vivo large volume
hyperthermia under 3D MRI temperature control. Med Phys.
43:1539–1549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coussios CC, Farny CH, Haar GT and Roy RA:
Role of acoustic cavitation in the delivery and monitoring of
cancer treatment by high-intensity focused ultrasound (HIFU). Int J
Hyperthermia. 23:105–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ebbini ES and ter Haar G:
Ultrasound-guided therapeutic focused ultrasound: current status
and future directions. Int J Hyperthermia. 31:77–89. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ter Haar G: HIFU Tissue Ablation: Concept
and devices. Adv Exp Med Biol. 880:3–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sheybani ND, Witter AR, Thim EA, Yagita H,
Bullock TNJ and Price RJ: Combination of thermally ablative focused
ultrasound with gemcitabine controls breast cancer via adaptive
immunity. J Immunother Cancer. 8:e0010082020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Silvestrini MT, Ingham ES, Mahakian LM,
Kheirolomoom A, Liu Y, Fite BZ, Tam SM, Tucci ST, Watson KD, Wong
AW, et al: Priming is key to effective incorporation of
image-guided thermal ablation into immunotherapy protocols. JCI
Insight. 2:e905212017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han X, Wang R, Xu J, Chen Q, Liang C, Chen
J, Zhao J, Chu J, Fan Q, Archibong E, et al: In situ thermal
ablation of tumors in combination with nano-adjuvant and immune
checkpoint blockade to inhibit cancer metastasis and recurrence.
Biomaterials. 224:1194902019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chavez M, Silvestrini MT, Ingham ES, Fite
BZ, Mahakian LM, Tam SM, Ilovitsh A, Monjazeb AM, Murphy WJ,
Hubbard NE, et al: Distinct immune signatures in directly treated
and distant tumors result from TLR adjuvants and focal ablation.
Theranostics. 8:3611–3628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karwacki J, Kiełbik A, Szlasa W, Sauer N,
Kowalczyk K, Krajewski W, Saczko J, Kulbacka J, Szydełko T and
Małkiewicz B: Boosting the immune response-combining local and
immune therapy for prostate cancer treatment. Cells. 11:27932022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sinha M, Zhang L, Subudhi S, Chen B,
Marquez J, Liu EV, Allaire K, Cheung A, Ng S, Nguyen C, et al:
Pre-existing immune status associated with response to combination
of sipuleucel-T and ipilimumab in patients with metastatic
castration-resistant prostate cancer. J Immunother Cancer.
9:e0022542021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu YL, Planchard D, Lu S, Sun H, Yamamoto
N, Kim DW, Tan DSW, Yang JC, Azrif M, Mitsudomi T, et al: Pan-Asian
adapted clinical practice guidelines for the management of patients
with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative
endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 30:171–210.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang FH, Zhang XT, Li YF, Tang L, Qu XJ,
Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, et al: The Chinese Society
of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis
and treatment of gastric cancer, 2021. Cancer Commun (Lond).
41:747–795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang LL, Chen YP, Chen CB, Chen MY, Chen
NY, Chen XZ, Du XJ, Fang WF, Feng M, Gao J, et al: The Chinese
Society of Clinical Oncology (CSCO) clinical guidelines for the
diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun
(Lond). 41:1195–1227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muro K, Lordick F, Tsushima T,
Pentheroudakis G, Baba E, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, et
al: Pan-Asian adapted ESMO clinical practice guidelines for the
management of patients with metastatic oesophageal cancer: A
JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann
Oncol. 30:34–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dingemans AC, Hendriks LEL, Berghmans T,
Levy A, Hasan B, Faivre-Finn C, Giaj-Levra M, Giaj-Levra N, Girard
N, Greillier L, et al: Definition of synchronous oligometastatic
non-small cell lung Cancer-A consensus report. J Thorac Oncol.
14:2109–2119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
National Cancer Institute, . Terminology
Criteria for Adverse Events (CTCAE). Version 4.0. 2009. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40
|
|
35
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2021 (6th edition).
Gastric Cancer. 26:1–25. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lorusso D, Xiang Y, Hasegawa K, Scambia G,
Leiva M, Ramos-Elias P, Acevedo A, Sukhin V, Cloven N, Pereira de
Santana Gomes AJ, et al: Pembrolizumab or placebo with
chemoradiotherapy followed by pembrolizumab or placebo for newly
diagnosed, high-risk, locally advanced cervical cancer
(ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind,
phase 3 clinical trial. Lancet. 403:1341–1350. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lim M, Weller M, Idbaih A, Steinbach J,
Finocchiaro G, Raval RR, Ansstas G, Baehring J, Taylor JW, Honnorat
J, et al: Phase III trial of chemoradiotherapy with temozolomide
plus nivolumab or placebo for newly diagnosed glioblastoma with
methylated MGMT promoter. Neuro Oncol. 24:1935–1949. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang ZY, Jiang AN, Yang W, Yan K, Wu W,
Wang S, Jiang BB, Sun LQ, Zhao K and Chen MH: Percutaneous
radiofrequency ablation is an effective method for local control of
liver metastases from lung cancer. Front Oncol. 12:8772732022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bachu VS, Kedda J, Suk I, Green JJ and
Tyler B: High-Intensity focused ultrasound: A review of mechanisms
and clinical applications. Ann Biomed Eng. 49:1975–1991. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou Q, Zhu XQ, Zhang J, Xu ZL, Lu P and
Wu F: Changes in circulating immunosuppressive cytokine levels of
cancer patients after high intensity focused ultrasound treatment.
Ultrasound Med Biol. 34:81–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu XQ, Lu P, Xu ZL, Zhou Q, Zhang J, Wang
ZB and Wu F: Alterations in immune response profile of
tumor-draining lymph nodes after high-intensity focused ultrasound
ablation of breast cancer patients. Cells. 10:33462021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eranki A, Srinivasan P, Ries M, Kim A,
Lazarski CA, Rossi CT, Khokhlova TD, Wilson E, Knoblach SM, Sharma
KV, et al: High-Intensity Focused Ultrasound (HIFU) triggers immune
sensitization of refractory murine neuroblastoma to checkpoint
inhibitor therapy. Clin Cancer Res. 26:1152–1161. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang X, Liao Y, Fan L, Lin B, Li J, Wu D,
Liao D, Yuan L, Liu J, Gao F, et al: High-intensity focused
ultrasound ablation combined with immunotherapy for treating liver
metastases: A prospective non-randomized trial. PLoS One.
19:e03065952024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dupré A, Pérol D, Blanc E, Peyrat P, Basso
V, Chen Y, Vincenot J, Kocot A, Melodelima D and Rivoire M:
Efficacy of high-intensity focused ultrasound-assisted hepatic
resection (HIFU-AR) on blood loss reduction in patients with liver
metastases requiring hepatectomy: Study protocol for a randomized
controlled trial. Trials. 18:572017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dupré A, Rivoire M, Metzger S, Cropet C,
Vincenot J, Peyrat P, Chen Y, Pérol D and Melodelima D:
Intra-operative high-intensity focused ultrasound in patients with
colorectal liver metastases: A prospective ablate-and-resect study.
Ultrasound Med Biol. 49:1845–1851. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fu J, Li WZ, McGrath NA, Lai CW, Brar G,
Xiang YQ and Xie C: Immune checkpoint inhibitor associated
hepatotoxicity in primary liver cancer versus other cancers: A
systematic review and meta-analysis. Front Oncol. 11:6502922021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li L, Li G, Rao B, Dong AH, Liang W, Zhu
JX, Qin MP, Huang WW, Lu JM, Li ZF and Wu YZ: Landscape of immune
checkpoint inhibitor-related adverse events in Chinese population.
Sci Rep. 10:155672020. View Article : Google Scholar : PubMed/NCBI
|