Introduction
The liver is a common site for malignant tumor
metastasis and patients with liver metastases (LIM) have poor
survival rates (1,2). Currently, immune checkpoint inhibitors
(ICIs) are the standard treatment for various solid tumors
(3). However, clinical studies on
lung cancer, melanoma, breast cancer and gastric cancer have shown
that patients with LIM benefit less from ICI therapy compared to
those with metastases at other sites (4–8). For
instance, a sub-analysis of two Phase III clinical trials on
metastatic non-small cell lung cancer reported that the 3-year
survival rate and median survival time for the LIM subgroup were
considerably worse than those for the non-LIM subgroup (8 vs. 17%
and 6.8 vs. 11.1 months, respectively) (9). Similarly, a meta-analysis of various
cancer types demonstrated that the efficacy of ICI therapy is
greatly reduced when LIM is present (10).
This diminished efficacy of ICI therapy is likely
due to the unique anatomical structure and physiological functions
of the liver (11). The liver
features a dual venous system with an abundant blood supply, which
offers optimal conditions for tumor cell colonization. By absorbing
blood from the digestive tract, the liver is constantly exposed to
food and intestinal flora antigens, maintaining an immune-tolerant
state (12,13). Yu et al (14) reported that ICI therapy was less
beneficial for patients with LIM than for those with lung, bone or
brain metastases. In addition, the authors confirmed in mouse
models that LIM alters the systemic immune microenvironment by
attracting peripheral T cells to the liver, where they are
subsequently eliminated by macrophages (14).
Owing to its non-invasive and reproducible nature
and efficiency, high-intensity focused ultrasound (HIFU) is
increasingly being employed to treat LIM. Zhou et al
(15) presented a clinical study on
the efficacy of HIFU in patients with gastric cancer with LIM who
were contraindicated for either hepatectomy or radiofrequency
ablation. The results showed that HIFU improved long-term prognosis
without any considerable increase in the occurrence of adverse
events (AEs) (15). Similar
findings were reported in colorectal cancer with LIM (16). HIFU, delivered via a specialized
transducer, can concentrate ultrasound energy at a focal point,
generating transient high temperatures (65–100°C) that induce
coagulative necrosis in tumor tissues (17,18).
Concurrently, the cavitation effect induces the rupture of the cell
and nuclear membranes, rendering the cells incapable of
proliferation (19–21). Numerous preclinical studies have
confirmed that HIFU can enhance the efficacy of ICI therapy
(22–25). The potential mechanisms for the
enhanced antitumor response to ICI therapy after HIFU treatment
include the release of large amounts of tumor antigens or antigen
fragments, damage-associated molecular patterns, inflammatory
chemokines, cytokines and increased expression of
interferon-stimulating genes. These factors promote the activation
of innate immune cells, facilitate antigen presentation to adaptive
immune cells and attenuate the immunosuppressive tumor
microenvironment by reducing the activity of regulatory T cells and
myeloid-derived suppressor cells (23–25).
In recent years, emerging clinical data on prostate
and breast cancers have highlighted the potential of HIFU to
modulate the immune system (26,27).
However, limited clinical reports have focused on the effects of
this technology on other cancers in real-world settings.
Considering that HIFU is already being used to treat LIM in
numerous types of solid malignancies, the question arises of
whether the combination of HIFU with ICI therapy can lead to
improved efficacy in patients with LIM. In the present study, a
retrospective cohort study was conducted using data from newly
diagnosed patients with solid malignancies and LIM who received
first-line ICI therapy to explore the efficacy of HIFU in this
subgroup.
Materials and methods
Patient selection and data
collection
The medical records of 94 patients with advanced
metastatic solid malignant LIM who were treated at Mianyang Central
Hospital (Mianyang, China) between January 2018 and December 2023
were retrospectively reviewed. The study protocol was approved by
the Ethics Committee of Mianyang Central Hospital (Mianyang, China;
file no. S20240390-01) and the study was conducted in accordance
with the principles of the Declaration of Helsinki. The Ethics
Committee granted an exemption from informed consent due to the
retrospective nature of the study and no specimens were collected
from the patients.
The inclusion criteria were as follows: i) Newly
clinically and pathologically diagnosed advanced metastatic solid
malignancy; ii) concurrent LIM confirmed in at least two types of
imaging or pathology tests, iii) Eastern Cooperative Oncology Group
scores ≥1; iv) completion of at least two courses of a first-line
regimen of ICI-based therapy with subsequent therapeutic effect
evaluation using imaging; and v) availability of comprehensive
medical records, imaging data and follow-up information.
The exclusion criteria were as follows: i) Patients
with a history of cancer but with relapse confined to the liver
without pathological confirmation; ii) patients diagnosed with
advanced cancer who received small-molecule tyrosine kinase
inhibitors based on gene mutations during first-line ICI-based
therapy; iii) patients with unknown metastatic sites or survival
status; iv) patients with a history of ICI administration; or v)
lack of target lesions that could be used for evaluating the
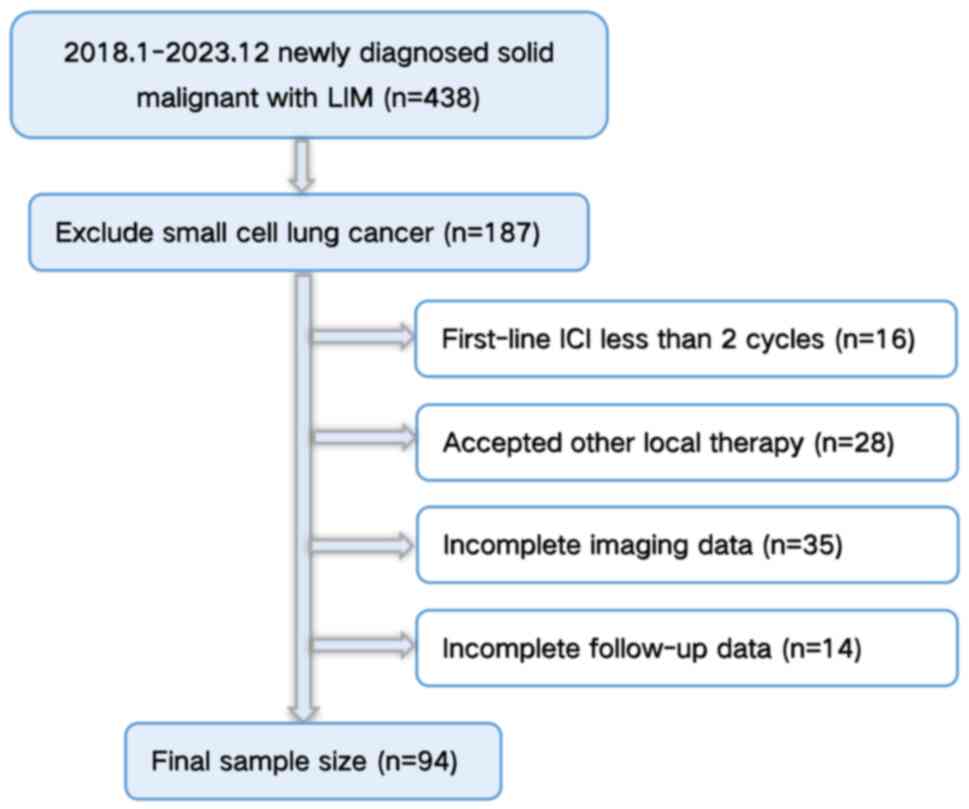
efficacy of systemic therapy. A flowchart of the participant
selection process is shown in Fig.
1.
The collected data included sex, age, primary tumor
pathology, first-line and subsequent treatment regimens, HIFU plan
details, time of diagnosis of the advanced solid malignancies with
LIM, time of disease progression and time of death. The
immunotherapy and chemotherapy regimens for the patients included
in the study followed the guidelines of the Chinese Society of
Clinical Oncology for the specific cancer type (28–31).
Oligometastasis was defined as the involvement of no
more than five metastatic nodules and a maximum of three organs
(32).
HIFU lesions selection and ablations
methods
Patients were divided into a HIFU group and non-HIFU
group based on whether they received HIFU treatment during
first-line treatment.
The selection of HIFU lesions requires comprehensive
evaluation of multiple factors, including tumor staging, clinical
symptoms, skin condition in the treatment area, lesion size, lesion
location and compression to adjacent tissue or organ. A few basic
conditions were required to be met before application: i) The
targeted lesions must be located within the focus of the ultrasound
transducer; ii) the ultrasound path should not be obstructed by
bony structures or gas-containing tissues; and iii) the presence of
substantially calcified arterial walls in the channel of ultrasound
therapy is contraindicated for HIFU treatment. Lesions that were
more likely to alleviate discomfort symptoms or reduce the
compression of tumors on adjacent blood vessels or bile ducts were
prioritized. Thus, the largest lesions are usually selected for
HIFU following the selection criteria. HIFU surgeries were commonly
completed within 5 h under general anesthesia, with a total HIFU
duration of usually <3,000 sec.
HIFU ablations were scheduled in advance. A sagittal
or axial orientation, which facilitates real-time monitoring of the
lesion and adjacent tissue structures, was chosen as the scanning
direction for guided imaging. The treatment protocol for each layer
was formulated with a spacing of 3 or 5 mm, determined by the left
and right or upper and lower diameters of the target tumor.
Typically, treatment commenced at the maximum level of the lesion
and the deeper side of the lesion was treated before the
superficial side. The doses were evaluated during the initial
treatment session or whenever a new high dose was proposed, with
adjustments made based on the ultrasound imaging and patient
status.
HIFU was performed using the model-JC200 Focused
Ultrasound Tumor Therapeutic System integrated therapy machine from
Chongqing Haifu Medical Technology Co., Ltd. Transducers with focal
lengths of 115 and 165 mm were often used based on the depth of
tumor treatment. All patients underwent HIFU under intravenous
anesthesia, with real-time evaluation of ablation efficacy based on
intraoperative monitoring of ultrasound grayscale changes.
Treatment efficacy evaluation
Treatment efficacy in each patient was assessed by
two experienced radiologists using computed tomography or magnetic
resonance images, following the Response Evaluation Criteria In
Solid Tumours (version 1.1) (33).
If their evaluations differed, the images were reviewed until a
consensus was reached. If other lesions could be evaluated for
treatment efficacy, those treated with HIFU were not considered
target lesions. However, in the absence of other assessable
lesions, those treated using HIFU were designated as target
lesions. AEs, including those related to ICI and chemotherapy
within 30 days after the last treatment with first-line ICI therapy
were graded according to the National Cancer Institute-Common
Terminology Criteria for Adverse Events (version 4.0) (34).
In the current study, progression-free survival
(PFS) refers to the time from the beginning of first-line treatment
to the observation of disease progression in patients; overall
survival (OS) refers to the time from the confirmation of diagnosis
as cancer with liver metastases to death caused by any reason.
Statistical analysis
Demographic information and clinicopathological
characteristics were summarized and presented using counts and
percentages. Differences between qualitative variables and
continuous variables were analyzed using χ2 statistics
and analysis of variance, respectively. Survival probabilities were
calculated using the Kaplan-Meier method with GraphPad 10.0 and
survival curves were compared using the log-rank test. The
follow-up duration was estimated with the reverse Kaplan-Meier
method. Statistical significance was set at a P-value of
<0.05.
Results
Patient characteristics
Demographic data and clinicopathological
characteristics were well-balanced between the treatment groups
(Table I). A total of 94 eligible
patients diagnosed with LIM who received first-line ICI between
February 2018 and December 2023 were included. The mean age of the
94 patients was 60.98 years (range, 36.0–81.0 years). Of these, 75
(79.79%) were between 50 and 75 years of age and 71.28% were men.
Gastric carcinoma was the most common primary tumor, followed by
non-small cell lung cancer and esophageal carcinoma. Among the
patients, 97.87% (n=92) had metastases in organs other than the
liver and 65.96% (n=62) had metastases in at least three organs
(including the liver). The lungs and bones were the most common
sites of metastases, at 52.13% (n=49) and 41.49% (n=39),
respectively. Adenocarcinoma was the most common histological
subtype, accounting for 67.02% (n=63) of the cases. There were no
differences between the HIFU group and non-HIFU group in terms of
sex, age, primary disease, metastasis organs, histology,
oligometastasis and systemic therapy regimens (P=0.309, 0.713,
0.053, 0.406, 0.404, 0.941 and 0.844, respectively). However, there
was a significant difference in the distribution of organ
metastases between the two groups (P=0.033). There was no case of
single organ metastasis in the HIFU group but 3.03% (n=2) in the
non-HIFU group. Furthermore, the incidence of metastasis to 2, 3,
and 4 organs was significantly higher in the HIFU group compared to
the non-HIFU group. A total of 88 patients received combination
therapy that included ICI, whereas six patients received ICI
monotherapy (two patients in the HIFU group). Metastatic sites and
liver metastasis details of 94 patients with LIM who accepted
first-line systemic therapy including ICI are presented in Table SI.
 | Table I.Clinicopathological variables of 94
patients with liver metastases who accepted first-line systemic
therapy including ICI. |
Table I.
Clinicopathological variables of 94
patients with liver metastases who accepted first-line systemic
therapy including ICI.
| Variable | Total (n=94) | With HIFU
(n=28) | Systemic therapy
only (n=66) | P-value |
|---|
| Sex |
|
|
| 0.309 |
|
Male | 67 (71.28) | 22 (78.57) | 45 (68.18) |
|
|
Female | 27 (28.72) | 6 (21.43) | 21 (31.81) |
|
| Age, years (mean,
60.98; range, 36.0–81.0) |
|
|
| 0.713 |
|
≤50 | 12 (12.77) | 4 (14.28) | 8 (12.12) |
|
|
51-65 | 45 (47.87) | 11 (39.29) | 34 (51.51) |
|
|
65-75 | 30 (31.91) | 11 (39.29) | 19 (28.79) |
|
|
>75 | 7 (7.45) | 2 (7.14) | 5 (7.58) |
|
| Primary
disease |
|
|
| 0.053 |
| Lung
carcinoma | 28 (29.79) | 10 (35.71) | 18 (27.27) |
|
| Gastric
carcinoma | 36 (38.29) | 9 (32.14) | 27 (40.91) |
|
|
Esophageal carcinoma | 11 (11.70) | 4 (14.29) | 7 (10.60) |
|
|
Gallbladder carcinoma | 2 (2.13) | 0 (0) | 2 (3.03) |
|
|
Cholangiocarcinoma | 7 (7.45) | 3 (10.72) | 4 (6.06) |
|
|
Pancreatic carcinoma | 4 (4.26) | 1 (3.57) | 3 (4.55) |
|
|
Nasopharyngeal carcinoma | 2 (2.13) | 0 (0) | 2 (3.03) |
|
| Other
carcinoma | 4 (4.26) | 1 (3.57) | 3 (4.55) |
|
| Metastasis to other
sites |
|
|
| 0.406 |
|
LUM | 49 (52.13) | 18 (64.29) | 31 (46.97) |
|
|
BOM | 39 (41.49) | 13 (46.43) | 26 (39.39) |
|
|
BRM | 9 (9.57) | 1 (3.57) | 8 (12.12) |
|
|
Others | 86 (91.49) | 24 (85.71) | 62 (93.94) |
|
| Number of organs
with metastases |
|
|
| 0.033 |
| 1 | 2 (2.13) | 0 (0) | 2 (3.03) |
|
| 2 | 30 (31.91) | 9 (32.14) | 21 (31.82) |
|
| 3 | 35 (37.23) | 11 (39.29) | 24 (36.36) |
|
| 4 | 23 (24.47) | 7 (25.00) | 16 (24.24) |
|
| 5 | 4 (4.26) | 1 (3.57) | 3 (4.55) |
|
| Histology |
|
|
| 0.404 |
| AC | 63 (67.02) | 20 (71.43) | 43 (65.15) |
|
|
SCC | 27 (28.72) | 8 (28.57) | 19 (28.79) |
|
|
Other | 4 (4.26) | 0 (0) | 4 (6.06) |
|
|
Oligometastasis |
|
|
| 0.941 |
|
Yes | 7 (7.45) | 2 (7.14) | 5 (7.58) |
|
| No | 87 (92.55) | 26 (92.86) | 61 (92.42) |
|
| Systemic therapy
regimens |
|
|
| 0.844 |
|
Chemotherapy + ICI | 88 (93.62) | 26 (92.86) | 62 (93.94) |
|
| ICI
only | 6 (6.38) | 2 (7.14) | 4 (6.06) |
|
Of the 94 patients, seven were classified as having
oligometastases (five men and two women). Of these patients, two
received HIFU as first-line treatment. Of the seven patients with
oligometastases, four had gastric carcinoma and all experienced
recurrence after radical surgery, in accordance with the guidelines
for gastric cancer LIM (35,36).
Of the seven patients, two had esophageal carcinoma and one had
non-small cell lung cancer. The clinicopathological characteristics
of the seven patients with oligometastases are presented in
Table SII.
A total of 28 patients received HIFU during
first-line ICI treatment, while 66 patients received systemic
therapy with ICI only. As of the data cutoff date of June 11, 2024,
the median follow-up duration was 13.8 months. Details on the
application of HIFU to the 28 patients, including treatment site
and time, total energy, energy and time at different output powers,
are presented in Table II. During
first-line treatment, the 28 patients underwent 36 HIFU treatments.
A total of 26 patients received HIFU after the start of ICI
therapy; two patients with newly diagnosed lung cancer underwent
liver HIFU within one week before immunotherapy while waiting for
genetic testing. Among the 28 patients, six underwent HIFU ablation
twice and one underwent three HIFU sessions.
 | Table II.Key parameters of 36 HIFU ablation
programs. |
Table II.
Key parameters of 36 HIFU ablation
programs.
| No. | Ablation site | Total energy,
J | Average power,
W | Actual power | Ablation volume,
cm3 | Ablation time,
sec | Gray scale
change |
|---|
| 1 | LL | 114,500 | 183 | 100 W, 20 sec,
3.2%; | 3.12 | 625 | MGC |
|
|
|
|
| 150 W, 170 sec,
27.2%; |
|
|
|
|
|
|
|
| 200 W, 435 sec,
69.6% |
|
|
|
| 2 | RL | 213,600 | 368 | 300 W, 76 sec,
13.74%; | 5.85 | 553 | MGC |
|
|
|
|
| 400 W, 477 sec,
86.26% |
|
|
|
|
| LAG | 182,950 | 314 | 300 W, 422 sec,
72.38%; | 6.22 | 583 | MGC |
|
|
|
|
| 350 W, 161 sec,
27.62% |
|
|
|
| 3 | RL | 836,000 | 400 | 400 W, 2,090 sec,
100% | 266.40 | 2,090 | OGC |
|
| HGS | 291,100 | 386 | 300 W, 105 sec,
3.93%; | 26.88 | 754 | MGC |
|
|
|
|
| 400 W, 649 sec,
86.07% |
|
|
|
| 4 | RL | 195,250 | 250 | 250 W, 781 sec,
100% | 9.72 | 781 | MGC |
|
| RAG | 318,600 | 389 | 300 W, 90 sec,
10.99%; | 26.77 | 819 | OGC |
|
|
|
|
| 400 W, 729 sec,
89.01% |
|
|
|
| 5 | RL | 365,200 | 400 | 400 W, 913 sec,
100% | 57.58 | 913 | OGC |
| 6 | RL | 328,400 | 364 | 300 W, 151 sec,
16.74%; | 98.26 | 902 | MGC |
|
|
|
|
| 350 W, 346 sec,
38.36%; |
|
|
|
|
|
|
|
| 400 W, 405 sec,
44.90% |
|
|
|
| 7 | LL, RL | 337,350 | 366 | 250 W, 83 sec,
9.00%; | 112.08 | 922 | MGC |
|
|
|
|
| 300 W, 190 sec,
0.61%; |
|
|
|
|
|
|
|
| 400 W, 649 sec,
70.39% |
|
|
|
| 8 | LL | 232,960 | 299 | 80 W, 147 sec,
18.87%; | 24.63 | 779 | MGC |
|
|
|
|
| 350 W, 632 sec,
81.13% |
|
|
|
|
| LL | 126,300 | 300 | 300 W; 421 sec;
100% | 24.32 | 421 | OGC |
| 9 | RL | 523,750 | 365 | 365 W, 1436 sec,
100% | 82.50 | 1,436 | OGC |
| 10 | RL | 130,200 | 350 | 350 W, 174 sec,
75.65% | 21.60 | 479 | OGC |
| 11 | RL | 1,042,000 | 400 | 400 W, 2,605 sec,
100% | 27.90 | 2,605 | OGC |
|
| LL | 542,600 | 379 | 300 W, 171 sec,
11.96%; | 112.02 | 1,430 | MGC |
|
|
|
|
| 350 W, 246 sec,
17.2%; |
|
|
|
|
|
|
|
| 400 W, 1,013 sec,
70.84% |
|
|
|
| 12 | LL | 405,300 | 399 | 300 W, 15 sec,
1.47%; | 8.1 | 1,017 | MGC |
|
|
|
|
| 400 W, 1,002 sec,
98.53% |
|
|
|
|
| LL | 592,800 | 384 | 300 W, 240 sec,
15.56%; | 132.8 | 1,542 | MGC |
|
|
|
|
| 400 W, 1,302 sec,
84.44% |
|
|
|
| 13 | LL, RL | 141,800 | 392 | 350 W, 60 sec,
16.57%; | 153.9 | 362 | MGC |
|
|
|
|
| 400 W, 302 sec,
83.43% |
|
|
|
| 14 | LL | 324,400 | 400 | 400 W, 811 sec,
100% | 122.55 | 811 | OGC |
| 15 | HGS | 527,150 | 391 | 250 W, 53 sec,
3.93%; | 35.5 | 1,348 | MGC |
|
|
|
|
| 300 W, 41 sec,
3.04%; |
|
|
|
|
|
|
|
| 400 W, 1,254 sec,
93.03% |
|
|
|
| 16 | RL, | 165,000 | 267 | 200 W, 174 sec,
28.16%; | 8.16 | 618 | MGC |
|
| PALN |
|
| 250 W, 153 sec,
24.76%; |
|
|
|
|
|
|
|
| 300 W, 198 sec,
32.04%; |
|
|
|
|
|
|
|
| 350 W, 93 sec,
15.5% |
|
|
|
| 17 | LL, RL | 250,200 | 333 | 200 W, 56 sec,
7.46%; | 21.32 | 751 | MGC |
|
|
|
|
| 300 W, 85 sec,
11.32%; |
|
|
|
|
|
|
|
| 350 W, 610 sec,
81.22% |
|
|
|
| 18 | LL | 417,800 | 397 | 350 W, 60 sec,
5.7%; | 30.9 | 1,052 | OGC |
|
|
|
|
| 400 W, 992 sec,
94.3% |
|
|
|
| 19 | HOP | 113,250 | 238 | 150 W, 20 sec,
4.21%; | 63.2 | 475 | OGC |
|
|
|
|
| 200 W, 206 sec,
43.37%; |
|
|
|
|
|
|
|
| 250 W, 113 sec,
23.79%; |
|
|
|
|
|
|
|
| 300 W, 136 sec,
28.63% |
|
|
|
| 20 | CL | 745,550 | 349 | 300 W, 255 sec,
11.93%; | 204.7 | 2,138 | OGC |
|
|
|
|
| 350 W, 1,683 sec,
78.72%; |
|
|
|
|
|
|
|
| 400 W, 200 sec,
9.35% |
|
|
|
| 21 | HGS | 278,400 | 332 | 200 W, 66 sec,
7.88%; | 36.72 | 838 | OGC |
|
|
|
|
| 300 W, 100 sec,
11.93%; |
|
|
|
|
|
|
|
| 350 W, 672 sec,
80.19% |
|
|
|
| 22 | LL | 60,000 | 200 | 200 W, 300 sec,
100% | 1.2 | 300 | MGC |
| 23 | RL | 1,210,000 | 400 | 400 W, 3,025 sec,
100% | 318.5 | 3,025 | OGC |
|
| RL | 1,161,600 | 400 | 400 W, 2,904 sec,
100% | 1,080 | 2,904 | MGC |
|
| RL | 956,360 | 399 | 310 W, 36 sec,
1.5%; | 154.0 | 2,399 | MGC |
|
|
|
|
| 400 W, 2,363 sec,
98.50% |
|
|
|
| 24 | LL | 476,800 | 400 | 400 W, 1,192 sec,
100% | 30.9 | 1,192 | OGC |
| 25 | RL | 826,400 | 400 | 400 W, 2,066 sec,
100% | 429.7 | 2,066 | OGC |
| 26 | LL | 187,500 | 300 | 300 W, 625 sec,
100% | 12.1 | 625 | MGC |
| 27 | RL | 874,800 | 400 | 400 W, 2,187 sec,
100% | 283.5 | 2,187 | MGC |
| 28 | RL | 735,700 | 391 | 300 W, 105 sec,
5.58%; | 37.2 | 1,883 | OGC |
|
|
|
|
| 350 W, 140 sec,
7.43%; |
|
|
|
|
|
|
|
| 400 W, 1,638 sec,
86.99% |
|
|
|
Survival analyses
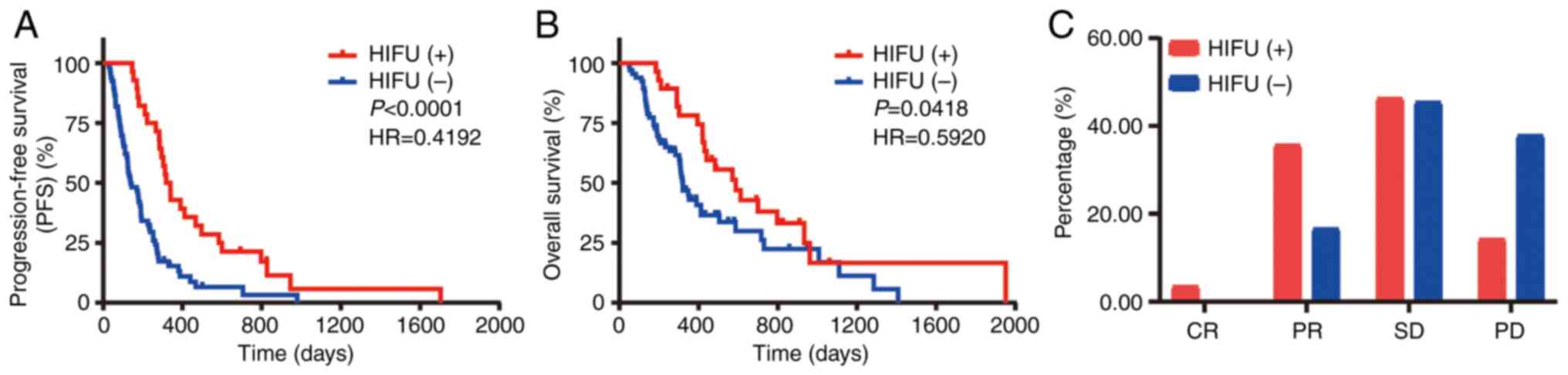
The Kaplan-Meier plots in Fig. 2 show the survival data for all
patients. Fig. 2A shows that the
patients who received HIFU had longer PFS [hazard ratio
(HR)=0.4192, 95% confidence interval (CI): 0.2747–0.6398,
P<0.001] than those who did not receive HIFU, with a 6-month PFS
rate of 85.71 vs. 42.42% and 1-year PFS rates of 46.43 and 12.12%,
respectively. The median PFS for the patients who received HIFU was
2.38 times that of those who did not (95% CI: 1.502–3.772), at
10.95 and 4.60 months, respectively. Fig. 2B shows that OS was better for the
patients who received HIFU (HR=0.5920, 95% CI: 0.3633–0.9646,
P=0.0418) than in those who did not, with a 6-month OS rate of 100
vs. 74.24%, respectively. The 1-year OS rates were 78.57 and
34.85%, respectively. The median OS in the HIFU group was 1.84
times that in the non-HIFU group (95% CI: 1.087–3.106), at 19.6 and
10.67 months, respectively. The disease control rate (defined as
the ratio of the sum of complete response, partial response and
stable disease to the total number of subgroups) in the HIFU group
was 82.14% (23/28), higher than that in the systemic therapy group
with ICI only, which was 66.67% (44/66) (Fig. 2C).
AEs
The all-cause and immune-mediated AEs in the two
groups are presented in Table
III. All-cause AEs occurred in 96.42% (n=27) of the patients in
the HIFU group and in 95.45% (n=63) in the non-HIFU group.
Myelosuppression was the most frequent AE and the most common AE of
grade ≥3 in both groups, followed by elevated levels of alanine
aminotransferase (ALT)/aspartate aminotransferase (AST), fatigue
and decreased appetite. Both groups had one case of treatment
suspension due to ALT/AST elevation, with treatment resumed after
recovery. Diarrhea was more common in the HIFU than in the non-HIFU
group (10.71 and 0%, respectively). However, this difference is
unlikely to be meaningful, as intake of laxatives was necessary for
bowel preparation before abdominal HIFU. The most frequent
immune-mediated AEs were troponin elevation, skin reactions and
thyroid dysfunction. There was no significant difference in
immune-mediated AEs between the two groups and no immune-mediated
AEs of grade ≥3 in either group.
 | Table III.Summary of AEs. |
Table III.
Summary of AEs.
| A, Common AEs of
chemotherapy |
|---|
|
|---|
|
| With HIFU therapy
(N=28) | Systemic therapy
with ICI only (N=66) |
|---|
|
|
|
|
|---|
| Event | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
|---|
|
Myelosuppression | 27 (96.42) | 11 (39.29) | 60 (90.90) | 19 (28.79) |
| Vomiting | 4 (14.29) | 1 (3.57) | 7 (10.61) | 2 (3.03) |
| Fatigue | 12 (42.86) | 2 (7.14) | 27 (40.91) | 2 (4.55) |
| Nausea | 7 (25.00) | 0 | 18 (27.27) | 2 (3.03) |
| Diarrhea | 3 (10.71) | 0 | 2 (3.03) | 0 |
| Decreased
appetite | 10 (35.71) | 0 | 23 (34.85) | 0 |
| Hepatotoxicity |
|
|
|
|
| ALT/AST
elevation | 17 (60.71) | 1 (3.57) | 40 (60.61) | 1 (1.52) |
| Bilirubin
elevation | 13 (19.70) | 0 | 32 (48.48) | 0 |
|
| B,
Immune-mediated AEs |
|
|
| With HIFU
therapy (N=28) | Systemic therapy
with ICI only (N=66) |
|
|
|
|
| Event | Any
grade | Grade
≥3 | Any
grade | Grade
≥3 |
|
| Thyroid
dysfunction | 4 (14.29) | 0 | 14 (21.21) | 0 |
| Pneumonitis | 2 (7.14) | 0 | 2 (3.03) | 0 |
| Skin reactions | 4 (14.29) | 0 | 12 (18.18) | 0 |
| Renal
dysfunction | 3 (10.71) | 0 | 5 (7.58) | 0 |
| Troponin
elevation | 6 (21.43) | 0 | 13 (19.70) | 0 |
| Arthralgia | 3 (10.71) | 0 | 5 (7.58) | 0 |
| CCEP | 2 (7.14) | 0 | 8 (12.12) | 0 |
Discussion
Although the widely used ICIs have markedly improved
efficacy in the clinical treatment of various histologically
advanced cancers, their benefits for patients with LIM are limited
(4–10). Local treatment strategies for LIM,
such as radiotherapy and radiofrequency ablation, have been shown
to enhance the efficacy of ICI (37–40),
but the disadvantages of these local treatment methods cannot be
overlooked. For instance, radiotherapy is constrained by long
treatment duration and normal-tissue dose limitations, while
radiofrequency ablation is invasive and less reliable for tumors
close to the gallbladder, main biliary tract and major blood
vessels. HIFU, a recent technology for local treatment, has gained
traction in cancer therapy, particularly when LIM has occurred, due
to its non-invasive nature, efficiency, limited side effects and
reproducibility, regardless of dose limits (41). In the present retrospective cohort
study, the clinicopathological characteristics, survival and AEs of
94 patients diagnosed with LIM who received first-line ICI
treatment were analyzed. The findings indicate that HIFU prolonged
PFS and OS in newly diagnosed patients with LIM, with similar AEs
across the study groups.
Both preclinical and clinical studies have
demonstrated that HIFU enhances anti-tumor immunity. Preclinical
studies have shown that HIFU ablation can generate in situ
antigens, increase the activity of antigen-presenting cells such as
dendritic cells and promote innate immune cell activity (23–26).
Zhou et al (41) observed
decreased serum levels of immunosuppressive cytokines, including
VEGF, TGF-β1 and TGF-β2, in 15 solid tumors after HIFU treatment.
This suggests that HIFU not only directly destroys tumors but also
reduces the production of immunosuppressive cytokines secreted by
cancer cells. In addition, a prospective randomized clinical trial
by Zhu et al (42) revealed
that HIFU ablation induces marked infiltration of CD3, CD4, CD8, B
lymphocytes and natural killer (NK) cells in treated breast
lesions, with notable increases in the numbers of FasL(+),
granzyme(+) and perforin(+) tumor-infiltrating lymphocytes
post-HIFU treatment. These findings suggest that HIFU can restore
anti-tumor immunity, and the increased infiltration of T
lymphocytes provides mechanistic support for the efficacy of ICI.
Eranki et al (43) found
that HIFU can effectively induce immune sensitization in a
previously unresponsive murine neuroblastoma model and is a
promising novel and efficacious immuno-adjuvant modality to
overcome therapeutic resistance. HIFU alone causes upregulation of
the expression of splenic and lymph node NK cells and circulating
IL-2, IFN-γ and damage-associated molecular patterns, whereas the
levels of immune regulators such as CD4+forkhead box p3+, IL-10 and
VEGF-A are reduced considerably. Combining HIFU with α-cytotoxic T
lymphocyte antigen-4 and α-programmed death ligand 1 markedly
enhanced the anti-tumor response, improving survival from 0 to
62.5%, and, importantly, led to abscopal effects (43). However, these studies did not
explore the combination of HIFU and ICI in real-world patients with
advanced cancer. To our knowledge, only one article, published by
our team in 2024, has focused on this novel combination therapy in
patients with LIM, confirming the feasibility and safety of the
method in clinical settings, although survival data were not
presented (44). In the present
study, it was reported that HIFU with ICI prolonged PFS in patients
with advanced cancers and LIM. Based on these results, it may be
speculated that HIFU may reverse the unfavorable immune
microenvironment caused by LIM. In our opinion, this longer PFS may
be caused by HIFU's inferences to the micro-immune environment.
However, the effect was temporary, as resistance to ICI developed
and the condition progressed after approximately six months. Thus,
the method could not completely alter the deterioration of the
immune microenvironment caused by multiple LIM and multiple organ
metastases.
HIFU combined with ICI represents a new therapeutic
model for patients with LIM; however, numerous issues, such as
safety, treatment sequence and timing, require further exploration.
Dupré et al (45,46) confirmed the safety of HIFU ablation
before surgical resection of liver tumors and its effectiveness in
reducing intraoperative bleeding in patients with LIM.
Although these results support the safety of HIFU
therapy, surgical candidates often have a limited tumor burden in
the liver and the entire body, whereas patients in the present
study had multiple LIM. Furthermore, the proportion of patients
with elevated ALT, AST and bilirubin levels was much higher among
the 94 patients in the present cohort. Previous meta-analyses and
studies reported liver toxicity incidences of 16–37.8% with
immunotherapy (47,48), whereas in the present study, the
incidence in both the HIFU and non-HIFU groups exceeded 60%. This
result may be due to the damage caused by the occurrence of
multiple LIM, chemotherapy drugs and ICIs rather than HIFU itself.
Zhou et al (15) reported
that HIFU treatment improved the long-term prognosis of patients
with gastric cancer without any notable increase in AEs in patients
with LIM without extrahepatic metastasis who could not undergo
surgery or radiofrequency ablation. This study supports the safety
and survival benefits of HIFU, but it included patients without
extrahepatic metastases. LIM are frequently accompanied by
metastases to other organs, which also explains the higher
incidence of grade 1–2 AEs, such as troponin elevation,
hepatotoxicity and renal dysfunction, in both groups compared to
previous results. These observations suggest the necessity of
closer monitoring of relevant indicators in patients with advanced
cancer with LIM undergoing ICI therapy. Importantly, no
immune-mediated AEs of grade ≥3 were observed in either group.
The present study has certain limitations. First, it
was a retrospective clinical study from a single institution that
included only 94 patients across eight cancer types. Therefore, the
possibility of incomplete or inaccurate records inherent in
retrospective research or the influence of specific environments
and populations in a single institution center cannot be excluded.
Second, it was not possible to provide the size and quantity of the
LIM lesions. There were too many patients with multiple liver
metastases and numerous small lesions, even diffuse small lesions,
making it difficult to count the number. Furthermore, most of these
patients also had multiple pulmonary and bone metastases, which
further reduced the significance of the size of liver metastases.
However, the largest tumor was found in the liver of every patient.
Third, due to the gradual onset of immunotherapy, physicians often
recommended this treatment for patients with an anticipated
survival exceeding two months, thereby introducing a potential
selection bias. Fourth, patients who received one or two cycles of
ICI but without further imaging review were excluded, as the
efficacy of ICI was uncertain in these cases. Finally, as HIFU is a
novel method in cancer treatment, limited clinical evidence about
its efficiency on the immune system has been reported previously.
In the present study, it was not observed that HIFU may improve the
immune environment by ablating liver metastases, and this was not
included in the lesions' selection items. However, this approach
may have inflated the objective response rate. Of note, seven
patients with oligometastases were potentially curable according to
the guidelines for oligometastasis of lung carcinoma (32), and 2 of 7 accepted HIFU after
failing in the pre-treatment evaluation for other local curative
treatments. Nonetheless, this study suggests that HIFU may enhance
ICI efficacy in patients with LIM, with mild and manageable
AEs.
In conclusion, the present retrospective study
indicated that HIFU prolonged the PFS and OS of first-line
ICI-based treatment in newly diagnosed patients with advanced
cancer with LIM, with manageable safety and tolerability.
Therefore, the efficacy of HIFU in patients with LIM undergoing ICI
treatment warrants further prospective clinical investigation.
Given the good safety of this combination strategy and that up to
50% of patients with various cancer types will either present with
or develop LIM during the course of their disease, it is indicated
that it can be further promoted in clinical practice for liver and
extrahepatic lesions. This treatment strategy may also be
considered for advanced cancers without LIM. To further clarify the
efficacy of HIFU in immunotherapy for LIM, animal studies are in
planning in our group to further investigate the efficacy of HIFU
in immunotherapy for LIM.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was financially supported by the State Key
Laboratory of Ultrasound in Medicine and Engineering (grant nos.
2020KFKT015, 2020KFKT018, 2023KFKT020 and 2022KFKT011). The funder
had no role in the study design, data collection and analysis,
decision to publish or preparation of the manuscript.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conceptualization, XD, GF and WC; methodology, XD
and FG; software, JL and YL; validation, DW and XD; formal
analysis, JL, YL, YZ and ML; investigation, YL, YZ, XY, BL and LN;
resources, ML; data curation, ML, YL and XD; writing-original draft
preparation, YL and BL; writing-review and editing, JL, YZ, XD and
WC; visualization, DW; supervision, BL and YZ; project
administration, BL, GF and JL. All authors have read and agreed to
the published version of the manuscript. YL and XD checked and
confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
The clinical trial protocol was approved by the
Ethics Committee of Mianyang Central Hospital (file no.
S20240390-01) and conducted in accordance with the principles of
the Declaration of Helsinki. The Ethics Committee granted an
exemption from informed consent due to the retrospective nature of
the study, and no specimens were collected from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HIFU
|
high-intensity focused ultrasound
thermal ablation
|
|
LIM
|
liver metastasis
|
|
ICI
|
immune checkpoint inhibitor
|
|
AEs
|
adverse events
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Disibio G and French SW: Metastatic
patterns of cancers: Results from a large autopsy study. Arch
Pathol Lab Med. 132:931–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsilimigras DI, Brodt P, Clavien PA,
Muschel RJ, D'Angelica MI, Endo I, Parks RW, Doyle M, de Santibañes
E and Pawlik TM: Liver Metastases. Nat Rev Dis Primers. 7:272021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Topalian SL, Taube JM and Pardoll DM:
Neoadjuvant checkpoint blockade for cancer immunotherapy. Science.
367:eaax01822020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bilen MA, Shabto JM, Martini DJ, Liu Y,
Lewis C, Collins H, Akce M, Kissick H, Carthon BC, Shaib WL, et al:
Sites of metastasis and association with clinical outcome in
advanced stage cancer patients treated with immunotherapy. BMC
Cancer. 19:8572019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tumeh PC, Hellmann MD, Hamid O, Tsai KK,
Loo KL, Gubens MA, Rosenblum M, Harview CL, Taube JM, Handley N, et
al: Liver metastasis and treatment outcome with anti-PD-1
monoclonal antibody in patients with melanoma and NSCLC. Cancer
Immunol Res. 5:417–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Welsh JW, Tang C, de Groot P, Naing A,
Hess KR, Heymach JV, Papadimitrakopoulou VA, Cushman TR, Subbiah V,
Chang JY, et al: Phase II trial of ipilimumab with stereotactic
radiation therapy for metastatic disease: Outcomes, toxicities, and
low-dose radiation-related abscopal responses. Cancer Immunol Res.
7:1903–1909. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Welsh J, Menon H, Chen D, Verma V, Tang C,
Altan M, Hess K, de Groot P, Nguyen QN, Varghese R, et al:
Pembrolizumab with or without radiation therapy for metastatic
non-small cell lung cancer: A Randomized Phase I/II Trial. J
Immunother Cancer. 8:e0010012020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Ji Q, Yan X, Lian B, Si L, Chi Z,
Sheng X, Kong Y, Mao L, Bai X, et al: The impact of liver
metastasis on anti-PD-1 monoclonal antibody monotherapy in advanced
melanoma: Analysis of five clinical studies. Front Oncol.
10:5466042020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vokes EE, Ready N, Felip E, Horn L, Burgio
MA, Antonia SJ, Arén Frontera O, Gettinger S, Holgado E, Spigel D,
et al: Nivolumab versus docetaxel in previously treated advanced
non-small-cell lung cancer (CheckMate 017 and CheckMate 057):
3-year update and outcomes in patients with liver metastases. Ann
Oncol. 29:959–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen XJ, Ren A, Zheng L, Zheng ED and
Jiang T: Pan-cancer analysis identifies liver metastases as
negative predictive factor for immune checkpoint inhibitors
treatment outcome. Front.Immunol. 12:6510862021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horst AK, Neumann K, Diehl L and Tiegs G:
Modulation of liver tolerance by conventional and nonconventional
antigen-presenting cells and regulatory immune cells. Cell Mol
Immunol. 13:277–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomson AW and Knolle PA:
Antigen-presenting cell function in the tolerogenic liver
environment. Nat Rev Immunol. 10:753–766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jenne CN and Kubes P: Immune surveillance
by the liver. Nat Immunol. 14:996–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu J, Green MD, Li S, Sun Y, Journey SN,
Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al: Liver
metastasis restrains immunotherapy efficacy via macrophage-mediated
T cell elimination. Nat Med. 27:152–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou B, He N, Hong J, Yang T, Ng DM, Gao
X, Yan K, Fan X, Zheng Z, Chen P, et al: HIFU for the treatment of
gastric cancer with liver metastases with unsuitable indications
for hepatectomy and radiofrequency ablation: A prospective and
propensity score-matched study. BMC Surg. 21:3082021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang T, Ng DM, Du N, He N, Dai X, Chen P,
Wu F, Chen B, Fan X, Yan K, et al: HIFU for the treatment of
difficult colorectal liver metastases with unsuitable indications
for resection and radiofrequency ablation: A phase I clinical
trial. Surg Endosc. 35:2306–2315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quesson B, Vimeux F, Salomir R, de Zwart
JA and Moonen CT: Automatic control of hyperthermic therapy based
on real-time Fourier analysis of MR temperature maps. Magn Reson
Med. 47:1065–1072. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tillander M, Hokland S, Koskela J, Dam H,
Andersen NP, Pedersen M, Tanderup K, Ylihautala M and Kohler M:
High intensity focused ultrasound induced in vivo large volume
hyperthermia under 3D MRI temperature control. Med Phys.
43:1539–1549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coussios CC, Farny CH, Haar GT and Roy RA:
Role of acoustic cavitation in the delivery and monitoring of
cancer treatment by high-intensity focused ultrasound (HIFU). Int J
Hyperthermia. 23:105–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ebbini ES and ter Haar G:
Ultrasound-guided therapeutic focused ultrasound: current status
and future directions. Int J Hyperthermia. 31:77–89. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ter Haar G: HIFU Tissue Ablation: Concept
and devices. Adv Exp Med Biol. 880:3–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sheybani ND, Witter AR, Thim EA, Yagita H,
Bullock TNJ and Price RJ: Combination of thermally ablative focused
ultrasound with gemcitabine controls breast cancer via adaptive
immunity. J Immunother Cancer. 8:e0010082020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Silvestrini MT, Ingham ES, Mahakian LM,
Kheirolomoom A, Liu Y, Fite BZ, Tam SM, Tucci ST, Watson KD, Wong
AW, et al: Priming is key to effective incorporation of
image-guided thermal ablation into immunotherapy protocols. JCI
Insight. 2:e905212017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han X, Wang R, Xu J, Chen Q, Liang C, Chen
J, Zhao J, Chu J, Fan Q, Archibong E, et al: In situ thermal
ablation of tumors in combination with nano-adjuvant and immune
checkpoint blockade to inhibit cancer metastasis and recurrence.
Biomaterials. 224:1194902019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chavez M, Silvestrini MT, Ingham ES, Fite
BZ, Mahakian LM, Tam SM, Ilovitsh A, Monjazeb AM, Murphy WJ,
Hubbard NE, et al: Distinct immune signatures in directly treated
and distant tumors result from TLR adjuvants and focal ablation.
Theranostics. 8:3611–3628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karwacki J, Kiełbik A, Szlasa W, Sauer N,
Kowalczyk K, Krajewski W, Saczko J, Kulbacka J, Szydełko T and
Małkiewicz B: Boosting the immune response-combining local and
immune therapy for prostate cancer treatment. Cells. 11:27932022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sinha M, Zhang L, Subudhi S, Chen B,
Marquez J, Liu EV, Allaire K, Cheung A, Ng S, Nguyen C, et al:
Pre-existing immune status associated with response to combination
of sipuleucel-T and ipilimumab in patients with metastatic
castration-resistant prostate cancer. J Immunother Cancer.
9:e0022542021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu YL, Planchard D, Lu S, Sun H, Yamamoto
N, Kim DW, Tan DSW, Yang JC, Azrif M, Mitsudomi T, et al: Pan-Asian
adapted clinical practice guidelines for the management of patients
with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative
endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 30:171–210.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang FH, Zhang XT, Li YF, Tang L, Qu XJ,
Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, et al: The Chinese Society
of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis
and treatment of gastric cancer, 2021. Cancer Commun (Lond).
41:747–795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang LL, Chen YP, Chen CB, Chen MY, Chen
NY, Chen XZ, Du XJ, Fang WF, Feng M, Gao J, et al: The Chinese
Society of Clinical Oncology (CSCO) clinical guidelines for the
diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun
(Lond). 41:1195–1227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muro K, Lordick F, Tsushima T,
Pentheroudakis G, Baba E, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, et
al: Pan-Asian adapted ESMO clinical practice guidelines for the
management of patients with metastatic oesophageal cancer: A
JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann
Oncol. 30:34–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dingemans AC, Hendriks LEL, Berghmans T,
Levy A, Hasan B, Faivre-Finn C, Giaj-Levra M, Giaj-Levra N, Girard
N, Greillier L, et al: Definition of synchronous oligometastatic
non-small cell lung Cancer-A consensus report. J Thorac Oncol.
14:2109–2119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
National Cancer Institute, . Terminology
Criteria for Adverse Events (CTCAE). Version 4.0. 2009. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40
|
|
35
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2021 (6th edition).
Gastric Cancer. 26:1–25. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lorusso D, Xiang Y, Hasegawa K, Scambia G,
Leiva M, Ramos-Elias P, Acevedo A, Sukhin V, Cloven N, Pereira de
Santana Gomes AJ, et al: Pembrolizumab or placebo with
chemoradiotherapy followed by pembrolizumab or placebo for newly
diagnosed, high-risk, locally advanced cervical cancer
(ENGOT-cx11/GOG-3047/KEYNOTE-A18): A randomised, double-blind,
phase 3 clinical trial. Lancet. 403:1341–1350. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lim M, Weller M, Idbaih A, Steinbach J,
Finocchiaro G, Raval RR, Ansstas G, Baehring J, Taylor JW, Honnorat
J, et al: Phase III trial of chemoradiotherapy with temozolomide
plus nivolumab or placebo for newly diagnosed glioblastoma with
methylated MGMT promoter. Neuro Oncol. 24:1935–1949. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang ZY, Jiang AN, Yang W, Yan K, Wu W,
Wang S, Jiang BB, Sun LQ, Zhao K and Chen MH: Percutaneous
radiofrequency ablation is an effective method for local control of
liver metastases from lung cancer. Front Oncol. 12:8772732022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bachu VS, Kedda J, Suk I, Green JJ and
Tyler B: High-Intensity focused ultrasound: A review of mechanisms
and clinical applications. Ann Biomed Eng. 49:1975–1991. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou Q, Zhu XQ, Zhang J, Xu ZL, Lu P and
Wu F: Changes in circulating immunosuppressive cytokine levels of
cancer patients after high intensity focused ultrasound treatment.
Ultrasound Med Biol. 34:81–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu XQ, Lu P, Xu ZL, Zhou Q, Zhang J, Wang
ZB and Wu F: Alterations in immune response profile of
tumor-draining lymph nodes after high-intensity focused ultrasound
ablation of breast cancer patients. Cells. 10:33462021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eranki A, Srinivasan P, Ries M, Kim A,
Lazarski CA, Rossi CT, Khokhlova TD, Wilson E, Knoblach SM, Sharma
KV, et al: High-Intensity Focused Ultrasound (HIFU) triggers immune
sensitization of refractory murine neuroblastoma to checkpoint
inhibitor therapy. Clin Cancer Res. 26:1152–1161. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang X, Liao Y, Fan L, Lin B, Li J, Wu D,
Liao D, Yuan L, Liu J, Gao F, et al: High-intensity focused
ultrasound ablation combined with immunotherapy for treating liver
metastases: A prospective non-randomized trial. PLoS One.
19:e03065952024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dupré A, Pérol D, Blanc E, Peyrat P, Basso
V, Chen Y, Vincenot J, Kocot A, Melodelima D and Rivoire M:
Efficacy of high-intensity focused ultrasound-assisted hepatic
resection (HIFU-AR) on blood loss reduction in patients with liver
metastases requiring hepatectomy: Study protocol for a randomized
controlled trial. Trials. 18:572017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dupré A, Rivoire M, Metzger S, Cropet C,
Vincenot J, Peyrat P, Chen Y, Pérol D and Melodelima D:
Intra-operative high-intensity focused ultrasound in patients with
colorectal liver metastases: A prospective ablate-and-resect study.
Ultrasound Med Biol. 49:1845–1851. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fu J, Li WZ, McGrath NA, Lai CW, Brar G,
Xiang YQ and Xie C: Immune checkpoint inhibitor associated
hepatotoxicity in primary liver cancer versus other cancers: A
systematic review and meta-analysis. Front Oncol. 11:6502922021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li L, Li G, Rao B, Dong AH, Liang W, Zhu
JX, Qin MP, Huang WW, Lu JM, Li ZF and Wu YZ: Landscape of immune
checkpoint inhibitor-related adverse events in Chinese population.
Sci Rep. 10:155672020. View Article : Google Scholar : PubMed/NCBI
|