Introduction
Gliomas are the most common type of primary brain
tumor with a global prevalence of 42.8% among primary central
nervous system tumors (1,2). According to the World Health
Organization (WHO), gliomas can be classified into four grades and
a higher grade reflects a higher degree of malignancy (3). Glioblastoma, a WHO grade IV glioma, is
the most aggressive type of glioma and accounts for ~49% of
malignant brain tumors (4,5). It is estimated that the annual
incidence of glioblastoma is ~3.23 per 100,000 individuals
(6). Temozolomide (TMZ) is the
first-line chemotherapy for glioblastoma and acts by inducing DNA
nucleotide mismatch, leading to glioblastoma cell damage, apoptosis
and cell cycle arrest in the G2/M phase (7–10).
However, TMZ resistance develops in nearly 50% of patients with
glioblastoma and is a contributor to poor prognosis (10–12).
Therefore, exploring potential mechanisms that lead to TMZ
resistance is worthwhile in improving the management of patients
with glioblastoma.
TMZ resistance can be induced by the overexpression
of O6-methylguanine-DNA methyltransferase and/or
deficiency in the DNA repair pathway (11). Recently, other mechanisms for TMZ
resistance have been proposed (13). The Akt/mTOR pathway plays a
fundamental role in glioblastoma pathology and progression, which
regulates multiple cellular processes, such as cell survival,
proliferation, angiogenesis, invasion and metastasis (14). Of note, this pathway also played a
fundamental role in TMZ resistance in glioblastoma according to
previous studies (15–17).
Adiponectin (ADN) is a protein consisting of 244
amino acids with a molecular weight of 28 kDa, which is secreted by
adipocytes and serves as a pivotal mediator of insulin sensitivity,
lipid metabolism and inflammation (18). Previous studies report that ADN was
closely involved in the pathology and progression of various types
of cancer, including glioblastoma (19–22).
For instance, one study indicated that ADN regulates pancreatic
cancer cell growth through the β-catenin pathway (23). Regarding glioblastoma, another study
illustrated that ADN regulates DNA synthesis, cell proliferation
and cell cycle arrest to modulate glioblastoma progression
(22). ADN also exerts fundamental
effects on drug resistance in cancer cells (24) and ADN regulates the sensitivity of
prostate cancer cells to doxorubicin (25). Another study proposed that ADN
modulated sunitinib sensitivity by abrogating the PI3K/Akt/NF-κB
pathway in renal cell carcinoma (26). Considering the involvement of ADN in
glioblastoma progression and its ability to modulate drug
resistance, it was hypothesized that ADN may also participate in
TMZ resistance in glioblastoma.
The current study intended to explore the engagement
of ADN in TMZ resistance in glioblastoma and the involvement of the
Akt/mTOR pathway during this process.
Materials and methods
Cell culture and ADN treatment
U251 (cat. no. SCSP-559; National Collection of
Authenticated Cell Cultures) and U87-MG (cat. no. TCHu138; National
Collection of Authenticated Cell Cultures) cells were maintained in
high-glucose Dulbecco's Modified Eagle's Medium (cat. no.
SH30022.01; Hyclone; Cytiva) containing 10% fetal bovine serum
(cat. no. SH30084.04; Hyclone; Cytiva) at 37°C in the presence of
5% CO2. They were subsequently treated with ADN (cat.
no. 1065-AP-050; R&D Systems, Inc.) at concentrations of 0.1,
0.5, 1.0 3.0 and 10.0 µg/ml. Subsequently, the cell growth rate was
determined by the Cell Counting Kit-8 (CCK-8) assay and the
phosphorylated (p)-Akt (Thr308), p-Akt (Ser473), Akt and p-mTOR
expression was analyzed by western blotting. The U87-MG cell line
used in the present study was the American Type Culture Collection
(ATCC) version, which is most probably a glioblastoma cell line of
unknown origin. The authenticity of the U87-MG cell line was
verified by Short Tandem Repeat (STR) Profiling. The STR Profiling
results of the U87-MG cell line were as follows: Amelogenin: X;
D5S818: 11, 12; D13S317: 8, 11; D7S820: 8, 9; D16S539: 12; vWA: 15,
17; TH01: 9.3; TPOX: 8; CSF1PO: 10, 11; D19S433: 15, 15.2; D21S11:
28, 32.2; D18S51: 13, 14, which were from the website of the
supplier (https://www.cellbank.org.cn/search-detail.php?id=211).
LY294002 treatment
LY294002 (a PI3K inhibitor; cat. no. ab120243;
Abcam) was dissolved in dimethyl sulfoxide (cat. no. PHR1309;
MilliporeSigma) for further treatment. Subsequently, U251 and
U87-MG cells were treated with 10 µM LY294002 in combination with
ADN for 24 h at 37°C. Furthermore, the apoptotic rate was assessed
by Annexin V/propidium iodide (AV/PI). The expression levels of
cleaved caspase 3 (c-caspase 3), caspase 3 and Bax were evaluated
by western blotting.
TMZ treatment
TMZ (cat. no. ab141055; Abcam) at concentrations of
0.1 and 1.0 mM were cultured with U251 and U87-MG cells,
respectively. ADN with concentrations of 1, 2 and 3 µg/ml were
cultured with cells in combination with TMZ at 0.1 and 1.0 mM at
37°C for 24 h. The mixture of 1.0 mM TMZ, 3 µg/ml ADN and 10 µM
LY294002 was also added to the cells. Finally, 1 mM TMZ or LY294002
was cultured with the cells in the presence of 3 µg/ml ADN at 37°C
for 24 h. Subsequently, the cell growth rate was assessed using
CCK-8, the apoptotic rate and cell cycle were evaluated using AV/PI
assay and cell cycle assay; the expression levels of c-caspase 3,
caspase 3, Bax, cyclin B1 and cyclin D1 were determined by western
blotting.
CCK-8 assay
The CCK-8 regent (cat. no. C0037; Beyotime Institute
of Biotechnology) with an amount of 50 µl was added and cultivated
with the cells for 1 h at 37°C. Subsequently, the optical density
value at 450 nm was measured with a microplate reader (Bio-Rad
Laboratories, Inc.). The cell growth rate was calculated.
AV/PI and cell cycle assays
An AV/PI kit (cat. no. C1062S; Beyotime Institute of
Biotechnology) was used to detect cell apoptosis. The cells were
digested with trypsin (cat. no. SH30042.01; Hyclone; Cytiva) for 1
min at room temperature and re-suspended. Following rinsing with
PBS, the cells were incubated with 5 µl AV and 5 µl PI at room
temperature in the dark for 15 min at room temperature. Finally,
the cells were measured with a CytoFLEX flow cytometer (Beckman
Coulter, Inc.). The data were analyzed with FlowJo X (FlowJo LLC).
The cells in quadrant 2 (Q2) and quadrant 4 (Q4) were considered as
apoptotic cells.
For the cell cycle assay, the cells were harvested
and re-suspended. Subsequently, they were fixed in 70% ethanol at
4°C overnight. The cells were centrifuged (800 × g at 4°C for 3
min) and collected and stained with PI solution (cat. no. ST1569;
Beyotime Institute of Biotechnology) for 30 min at room
temperature. A CytoFLEX flow cytometer (Beckman Coulter, Inc.) was
applied to detect the cells.
Western blotting
The cells were lysed with radio immunoprecipitation
assay buffer (cat. no. V900854; MilliporeSigma) and the protein
solution was quantified with the bicinchoninic acid kit (cat. no.
23225; Thermo Fisher Scientific, Inc.). The protein was denatured
at 100°C for 10 min following mixing with a loading buffer.
Subsequently, 10-µg protein samples were loaded into a 4–20% sodium
dodecyl sulfate polyacrylamide gel electrophoresis gel for
separation and transferred to the nitrocellulose membrane (cat. no.
HATF00010; MilliporeSigma). The membrane was blocked with skimmed
milk for 2 h at 37°C, followed by incubation with primary and
secondary antibodies overnight at 4°C and secondary antibodies at
37°C for 90 min. The primary antibodies used were for the following
proteins: Adiponectin receptor (ADIPOR) 1 (cat. no. APR06109G;
1:1,000; Epitomics, Inc.), ADIPOR2 (cat. no. APG01582G; 1:500;
Epitomics, Inc.), Akt (cat. no. 9272S; 1:1,000; Cell Signaling
Technology, Inc.), p-Akt (Thr308) (cat. no. 9275S; 1:500; Cell
Signaling Technology, Inc.), p-Akt (Ser473) (cat. no. 9271S; 1:500;
Cell Signaling Technology, Inc.), c-caspase 3 (cat. no. 9661S;
1:500; Cell Signaling Technology, Inc.), caspase 3 (cat. no. 9662S;
1:1,000; Cell Signaling Technology, Inc.), Bax (cat. no. 2772S;
1:1,000; Cell Signaling Technology, Inc.), p-mTOR (cat. no. 2983S;
1:500; Cell Signaling Technology, Inc.), cyclin B1 (cat. no.
ab32053; 1:1,000; Abcam), cyclin D1 (cat. no. ab16663; 1:1,000;
Abcam), β-actin (cat. no. 20536-1-AP; 1:4,000; Proteintech Group,
Inc.) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody
(cat. no. 10494-1-AP; 1:4,000; Proteintech Group, Inc.). Finally,
the horseradish peroxidase-conjugated secondary antibody (cat. no.
ab6721; 1:10,000; Abcam) was incubated with the membrane. The
protein bands were visualized with an enhanced chemiluminescence
kit (cat. no. 32209; Thermo Fisher Scientific, Inc.) and X-ray
film. The gray value was quantified by Gene Tools 3.7
(SynGene).
Statistical analysis
The SPSS software (v.26.0; IBM Corp.) was used for
statistical analysis. One-way ANOVA was applied for the comparison
among groups and Tukey's test was employed for post-hoc comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ADN increases growth and activates the
Akt/mTOR pathway in U251 and U87-MG cells
Considering that ADN binds to its receptors to exert
its functions, western blotting was applied to detect ADIPOR1 and
ADIPOR2 in U251 and U87-MG cells. It was found that ADIPOR1 and
ADIPOR2 were expressed in U251 and U87-MG cells, suggesting that
these two types of cells possessed the basis for ADN to exert its
functions (Fig. S1).
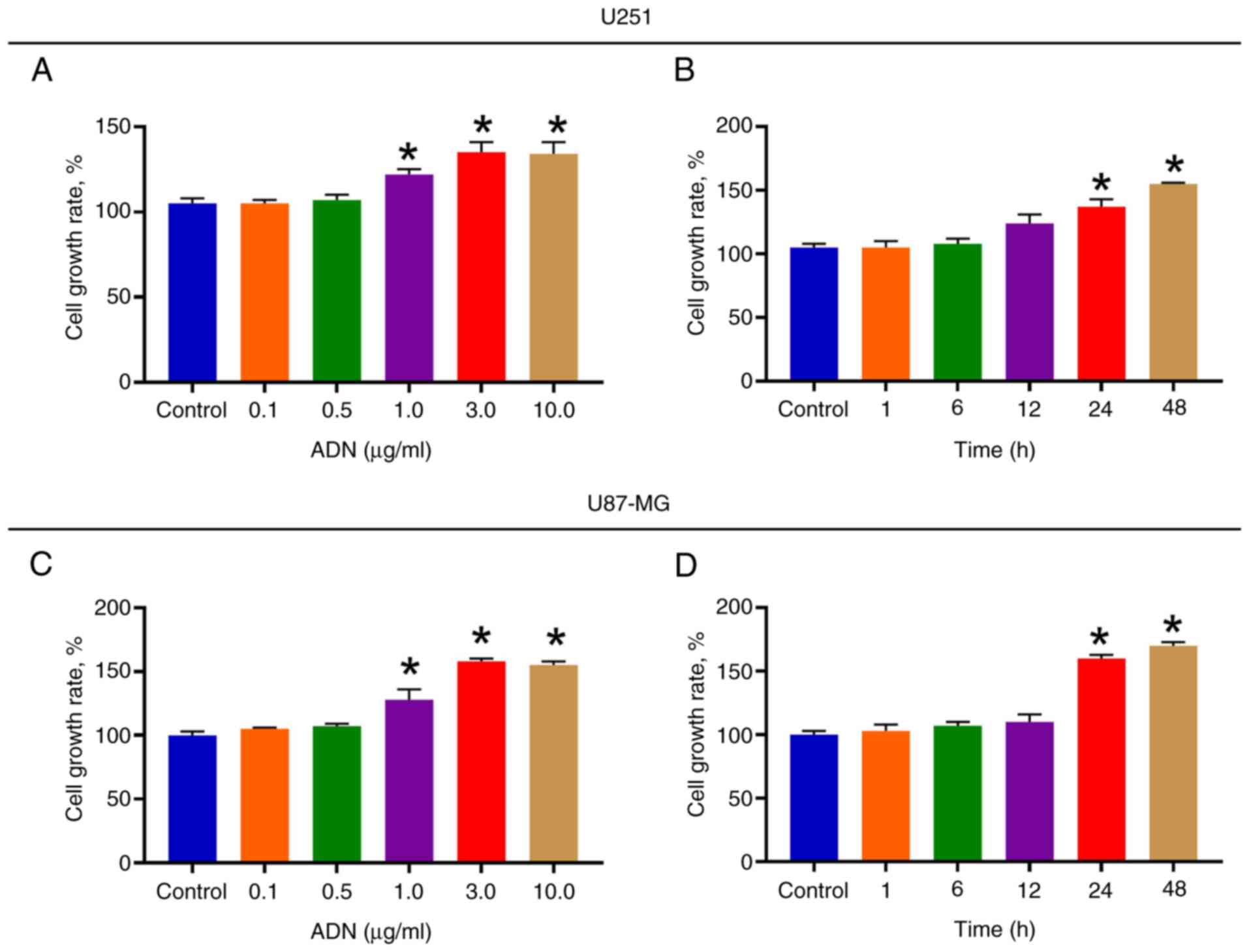
U251 cell growth rate was increased following
treatment with 1.0, 3.0 and 10.0 µg/ml ADN compared with that noted
in the control (all P<0.05; Fig.
1A). Subsequently, U251 cells were treated with 3.0 µg/ml ADN
at different time points. It was found that following ADN treatment
for 24 and 48 h, U251 cell growth rate was increased compared with
that of the control (both P<0.05; Fig. 1B). Similarly, the effect of ADN on
the U87-MG cell growth rate further indicated a
concentration-dependent (Fig. 1C)
and time-dependent (Fig. 1D) mode
of action. It was noted that when the treatment duration was 24 h,
ADN exhibited an optimal effect in promoting U251 and U87-MG cell
growth. In addition, compared with the treatment duration of 24 h,
the effect of ADN on U251 and U87-MG cell growth was not further
promoted after 48 h (both P>0.05). Therefore, the treatment
duration of ADN for 24 h was selected as the experimental condition
for subsequent experiments.
According to western blotting analysis (Fig. 2A), p-Akt (Thr308)/Akt was elevated
following 1.0 and 3.0 µg/ml treatment with ADN compared with that
of the control in U251 cells (both P<0.05; Fig. 2B). However, p-Akt (Ser473)/Akt was
not affected by ADN treatment at any concentration used in U251
cells (all P>0.05; Fig. 2C).
p-mTOR/GAPDH was increased following treatment with 1.0 and 3.0
µg/ml ADN compared with those of the control in U251 cells (both
P<0.05; Fig. 2D). The same
trends were also noted in U87-MG cells (Fig. 2E-H).
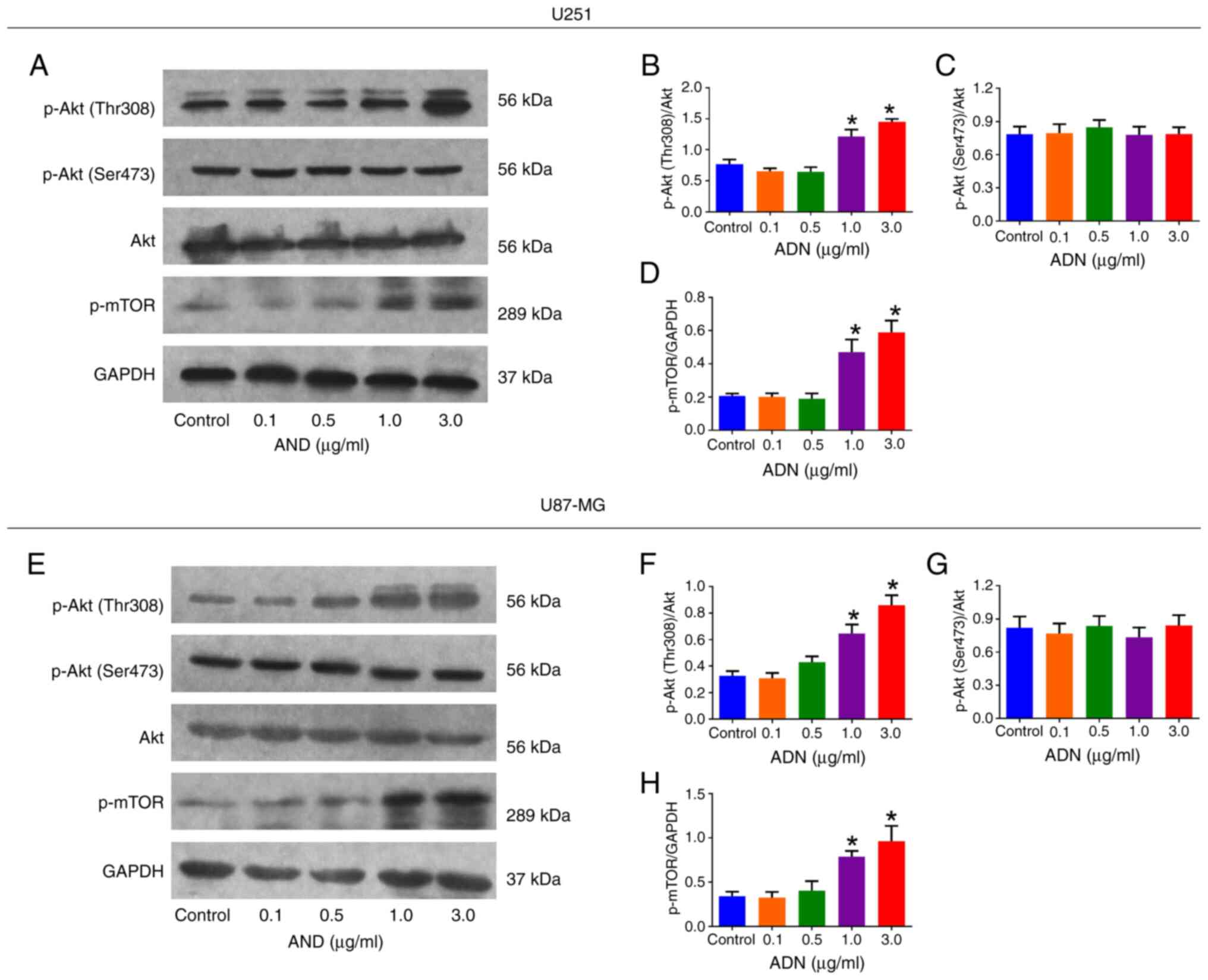 | Figure 2.ADN activates the Akt/mTOR pathway in
U251 and U87-MG cells. (A) Representative images of p-Akt (Thr308),
p-Akt (Ser473), Akt, p-mTOR and GAPDH by western blotting in U251
cells. Effect of different concentrations of ADN on (B) p-Akt
(Thr308)/Akt, (C) p-Akt (Ser473)/Akt and (D) p-mTOR/GAPDH in U251
cells. (E) Representative images of p-Akt (Thr308), p-Akt (Ser473),
Akt, p-mTOR and GAPDH by western blotting in U87-MG cells. Effect
of different concentrations of ADN on (F) p-Akt (Thr308)/Akt, (G)
p-Akt (Ser473)/Akt and (H) p-mTOR/GAPDH in U87-MG cells. *P<0.05
vs. control. ADN, adiponectin; p-, phosphorylated. |
Notably, when the concentration was 3.0 µg/ml, ADN
indicated outstanding ability to activate the Akt/mTOR pathway and
facilitate U251 and U87-MG cell growth. Therefore, 3.0 µg/ml ADN
was selected as the experimental condition for subsequent
experiments.
ADN activates the Akt/mTOR pathway to
inhibit apoptosis in U251 and U87-MG cells
According to the AV/PI assay (Fig. 3A), the apoptotic rate was decreased
by ADN compared with that of the vehicle (P<0.05); however, it
was increased by ADN + LY294002 compared with ADN in U251 cells
(P<0.05; Fig. 3B). western
blotting (Fig. 3C) indicated that
c-caspase 3/caspase3 (Fig. 3D) and
Bax/GAPDH (Fig. 3E) were decreased
by ADN compared with the vehicle (both P<0.05); however, they
were elevated by ADN + LY294002 compared with ADN in U251 cells
(both P<0.05). The same trends were noted in U87-MG cells
(Fig. 3F-J).
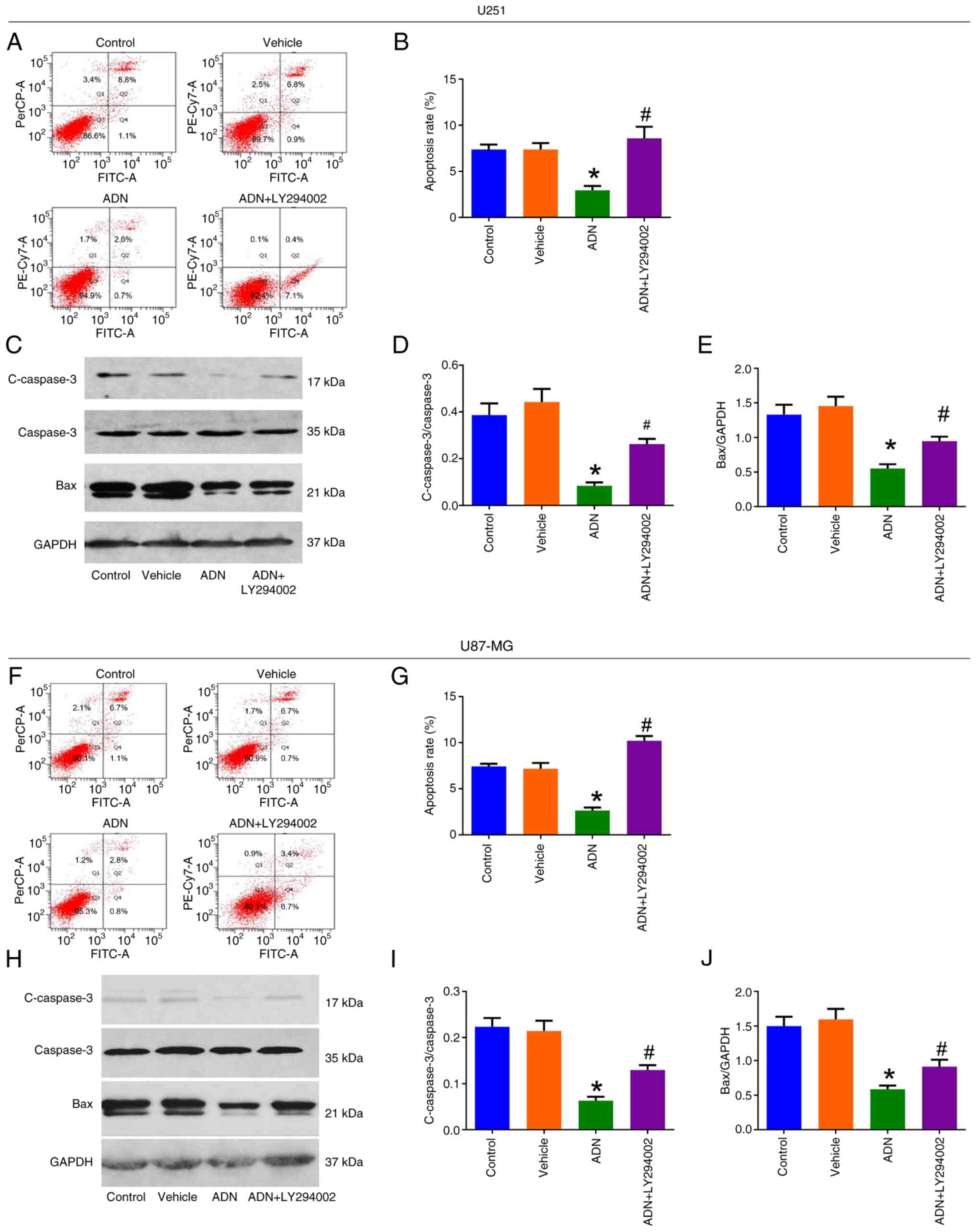 | Figure 3.LY294002 attenuates the inhibition of
ADN on U251 and U87-MG cell apoptosis. (A) Representative images of
U251 cell apoptosis by AV/PI assay and (B) comparison of apoptosis
rate among control, vehicle-treated, ADN-treated and ADN +
LY294002-treated U251 cells. (C) Representative images of c-caspase
3, caspase 3, Bax and GAPDH in U251 cells by western blotting and
comparison of (D) c-caspase 3/caspase 3 and (E) Bax/GAPDH among
control, vehicle-treated, ADN-treated and ADN + LY294002-treated
U251 cells. (F) Representative images of U87-MG cell apoptosis by
AV/PI assay and (G) comparison of apoptosis rate among control,
vehicle-treated, ADN-treated and ADN + LY294002-treated U87-MG
cells. (H) Representative images of c-caspase 3, caspase 3, Bax and
GAPDH in U87-MG cells by western blotting and comparison of (I)
c-caspase 3/caspase 3 and (J) Bax/GAPDH among control,
vehicle-treated, ADN-treated and ADN + LY294002-treated U87-MG
cells. *P<0.05 vs. vehicle; #P<0.05 vs. ADN. ADN,
adiponectin; c-caspase, cleaved caspase. |
ADN activates the Akt/mTOR pathway to
increase U251 and U87-MG cells in the S phase
According to cell cycle assay results (Fig. 4A), the proportion of U251 cells in
the S phase was increased by ADN treatment compared with the
vehicle (P<0.05); however, it was reduced by ADN + LY294002
compared with ADN (P<0.05; Fig.
4B). Western blotting (Fig. 4C)
indicated that cyclin B1/β-actin (Fig.
4D) and cyclin D1/β-actin (Fig.
4E) were increased following treatment with ADN compared with
the vehicle (P<0.05); however, they were decreased by ADN +
LY294002 compared with ADN in U251 cells (P<0.05). Similar
trends were noted in U87-MG cells (Fig.
4F-J).
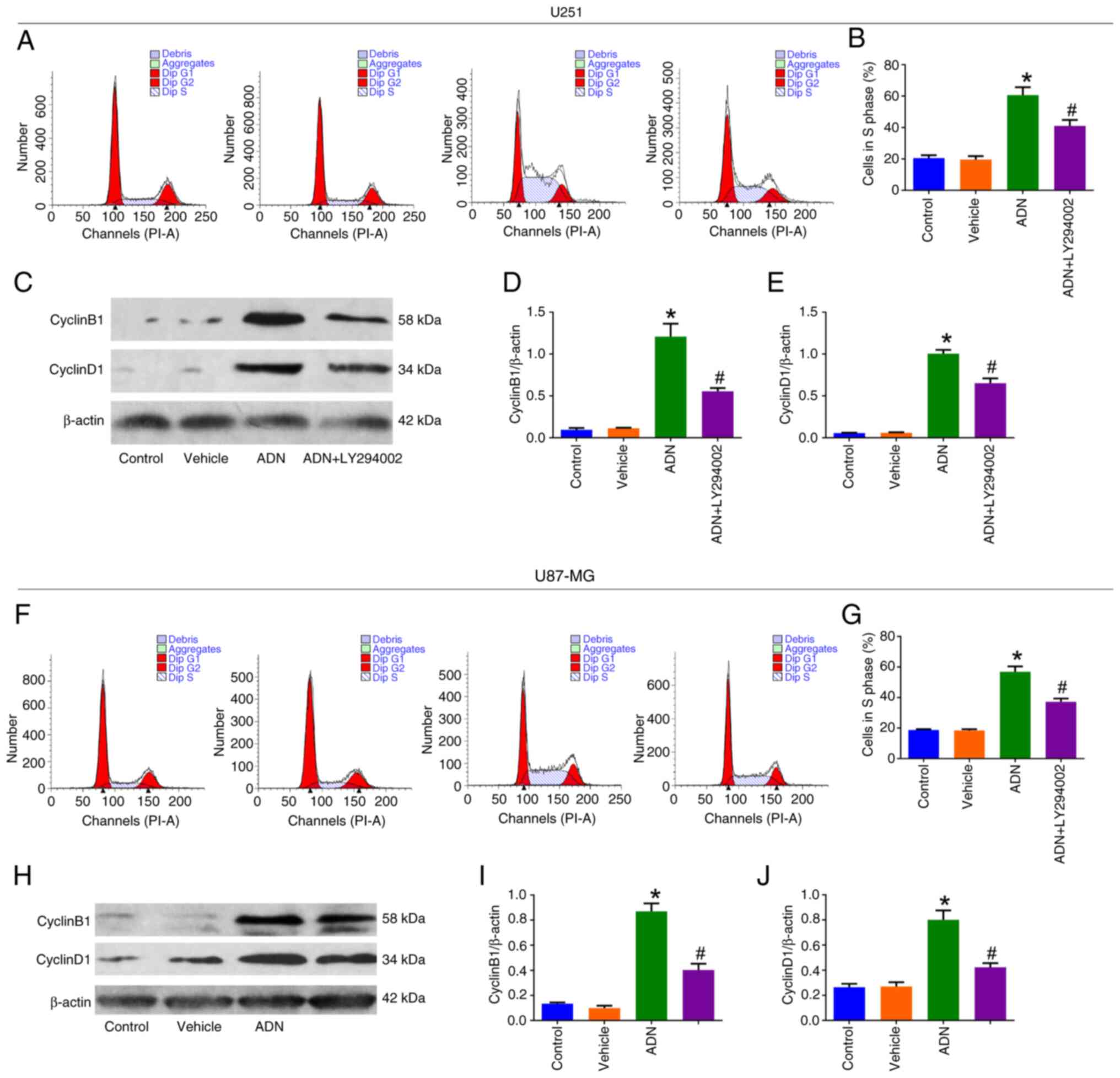 | Figure 4.LY294002 attenuates ADN-induced U251
and U87-MG cells in the S phase. (A) Representative images of U251
cell cycle analysis by cell cycle assay and (B) comparison of the
proportion of cells in the S phase among control, vehicle-treated,
ADN-treated and ADN + LY294002-treated U251 cells. (C)
Representative images of cyclinB1, cyclinD1 and β-actin in U251
cells by western blotting and comparison of (D) cyclinB1/β-actin
and (E) cyclinD1/β-actin among control, vehicle-treated,
ADN-treated and ADN + LY294002-treated U251 cells. (F)
Representative images of U87-MG cell cycle analysis by cell cycle
assay and (G) comparison of the proportion of cells in the S phase
among control, vehicle-treated, ADN-treated and ADN +
LY294002-treated U87-MG cells. (H) Representative images of
cyclinB1, cyclinD1 and β-actin in U87-MG cells by western blotting
and comparison of (I) cyclinB1/β-actin and (J) cyclinD1/β-actin
among control, vehicle-treated, ADN-treated and ADN +
LY294002-treated U87-MG cells. *P<0.05 vs. vehicle;
#P<0.05 vs. ADN. ADN, adiponectin. |
ADN facilitates TMZ resistance in U251
and U87-MG cells
Different concentrations of TMZ were applied to
treat the glioblastoma cell lines. It was noted that treatment with
1.0 mM TMZ indicated an optimal effect on inhibiting glioblastoma
cell line growth; therefore, this concentration was selected as the
optimal treatment condition for the subsequent step. In addition,
it was found that only 3.0 µg/ml ADN+1.0 mM TMZ increased
glioblastoma cell line growth compared with 1.0 mM TMZ (both
P<0.05; Fig. S2A and B).
Therefore, 3.0 µg/ml ADN and 1.0 mM TMZ were selected as the
conditions for subsequent experiments.
The AV/PI assay (Fig.
5A) suggested that the apoptotic rate was enhanced by TMZ
compared with vehicle (P<0.05); however, it was reduced by
ADN+TMZ compared with TMZ in U251 cells (P<0.05; Fig. 5B). Western blotting analysis
(Fig. 5C) revealed that the ratio
of c-caspase 3/caspase 3 (Fig. 5D)
and the ratio of Bax/GAPDH (Fig.
5E) were increased by TMZ compared with vehicle (all
P<0.05); however, they were reduced by ADN+TMZ compared with TMZ
in U251 cells (both P<0.05). The same trends were noted in
U87-MG cells (Fig. 5F-J).
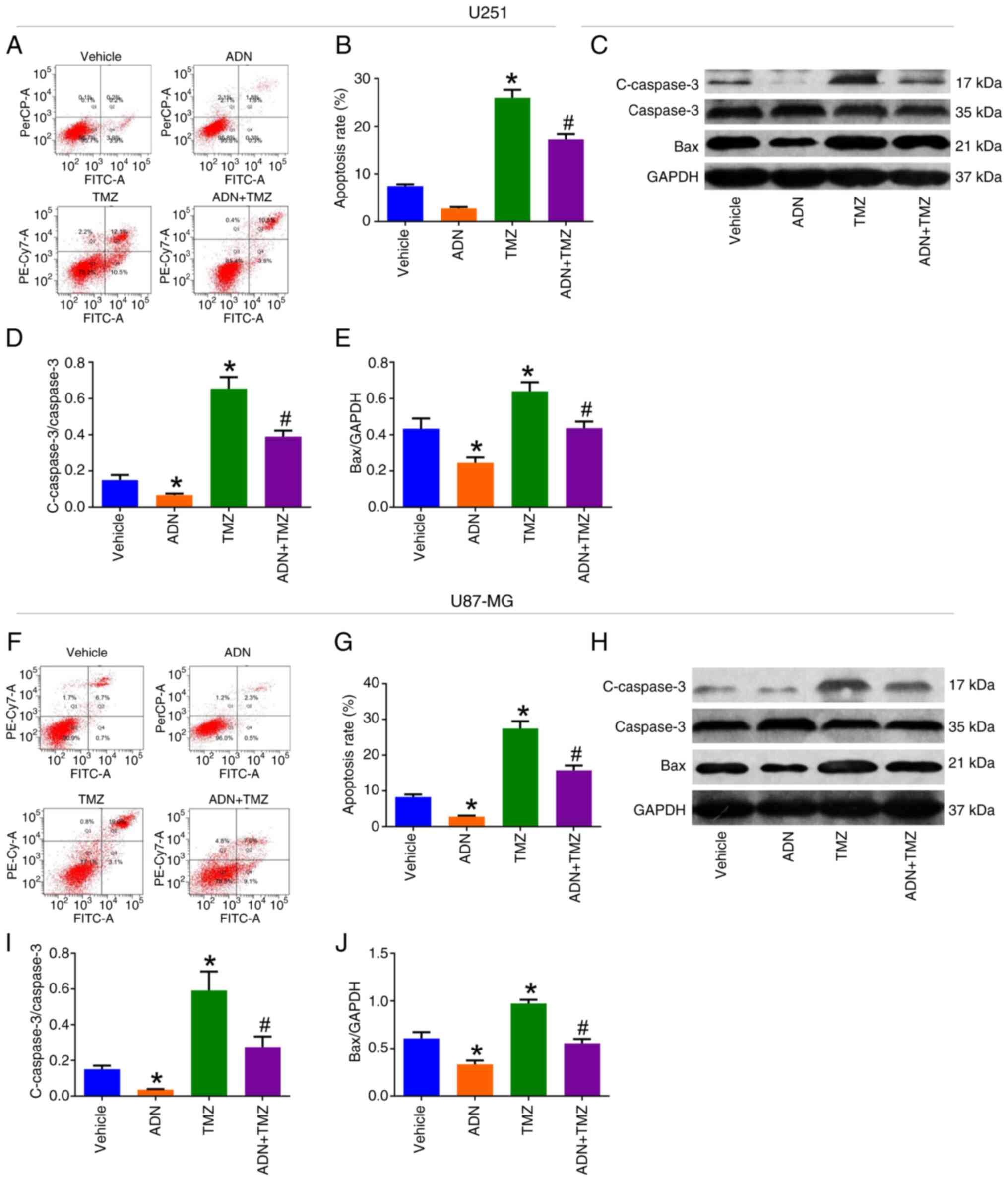 | Figure 5.ADN attenuates the promotion of TMZ
on U251 and U87-MG cell apoptosis. (A) Representative images of
U251 cell apoptosis by AV/PI assay and (B) comparison of apoptosis
rate among vehicle-treated, ADN-treated, TMZ-treated and
ADN+TMZ-treated U251 cells. (C) Representative images of c-caspase
3, caspase 3, Bax and GAPDH in U251 cells by western blotting and
comparison of (D) c-caspase 3/caspase 3 and (E) Bax/GAPDH among
vehicle-treated, ADN-treated, TMZ-treated and ADN + TMZ-treated
U251 cells. (F) Representative images of U87-MG cell apoptosis by
Annexin V/PI flow cytometry and (G) comparison of apoptosis rate
among vehicle-treated, ADN-treated, TMZ-treated and ADN +
TMZ-treated U87-MG cells. (H) Representative images of c-caspase 3,
caspase 3, Bax and GAPDH in U87-MG cells by western blotting and
comparison of (I) c-caspase 3/caspase 3 and (J) Bax/GAPDH among
vehicle-treated, ADN-treated, TMZ-treated and ADN+TMZ-treated
U87-MG cells. *P<0.05 vs. vehicle; #P<0.05 vs.
TMZ. ADN, adiponectin; TMZ, temozolomide; c-caspase, cleaved
caspase. |
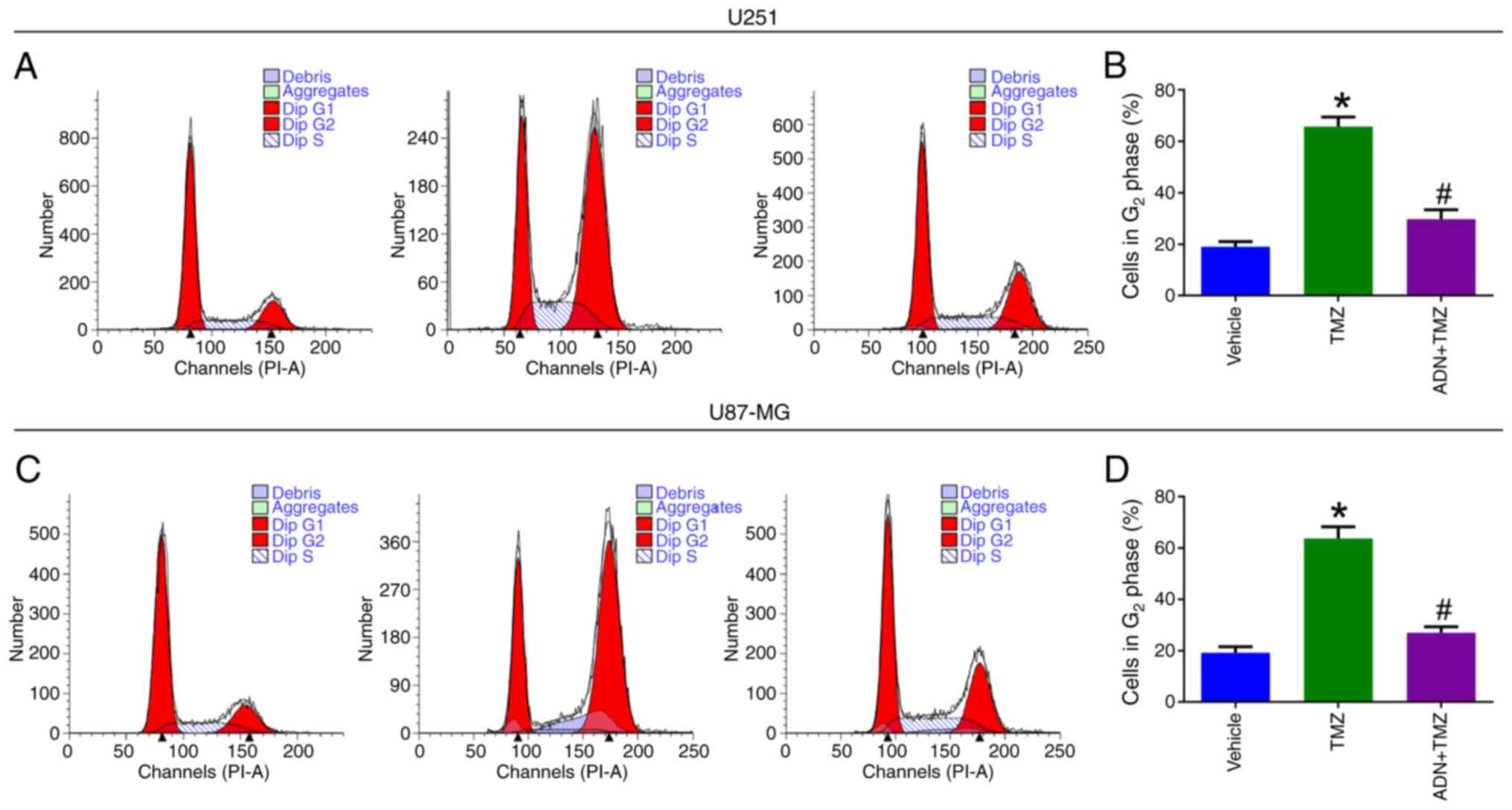
The cell cycle assay (Fig. 6A) suggested that the proportion of
U251 cells in the G2 phase was increased by TMZ compared
with that of the vehicle group (P<0.05), whereas it was
decreased in the ADN+TMZ group compared with that noted in the TMZ
group (P<0.05; Fig. 6B). The
same trends were noted in U87-MG cells (Fig. 6C and D).
ADN activates the Akt/mTOR pathway to
facilitate TMZ resistance in U251 and U87-MG cells
LY294002 was added to further explore the underlying
mechanism of ADN involved in TMZ resistance. It was found that the
U251 cell growth rate was reduced by TMZ + ADN + LY294002 compared
with TMZ+ADN (P<0.05; Fig.
S2A). The same trends were discovered in U87-MG cells (Fig. S2B).
Discussion
The actions of ADN are mediated via classical
receptors, which belong to the seven transmembrane domains receptor
family, such as ADIPOR1 and ADIPOR2 (27–29).
Notably, ADIPOR1 and ADIPOR2 are closely involved in the pathology
and progression of glioblastoma (30,31)
and several studies have reported that they are expressed in
various tumor tissues, including glioma (22,32–34).
According to a previous study, ADIPOR1 and ADIPOR2 are expressed in
83% (25/30) of human glioma tissues; in addition, 70% of glioma
tissues indicated co-expression of both receptors, which were also
expressed in glioblastoma cell lines (22). In line with this previous study
(22), the present study also
discovered that ADIPOR1 and ADIPOR2 were expressed in U251 and
U87-MG cells, suggesting that these two glioblastoma cell lines
possessed the basis for ADN to exert its functions. Subsequently,
it was noted that ADN could facilitate glioblastoma cell line
growth and cells in the S phase, while inhibiting apoptosis. A
possible reason might be that ADN can bind to ADIPOR1 and ADIPOR2
to regulate several downstream pathways, such as the adenosine
monophosphate-activated protein kinase (AMPK)/sirtuin 1, AMPK/mTOR
and extracellular signal-regulated kinase (ERK)1/2 pathways to
accelerate glioblastoma cell line proliferation, while inhibiting
apoptosis (22,30,31).
The Akt/mTOR pathway, a key regulator of multiple
cellular processes, is involved in the pathology and progression of
various types of cancer (14,35–37).
Regarding glioblastoma, previous studies have indicated that the
Akt/mTOR pathway regulates cell proliferation, apoptosis,
metastasis, autophagy and cell cycle arrest (38–40).
Notably, a previous study discovered that Akt phosphorylation can
be regulated by ADN, which is responsible for glioblastoma
progression (22). The current
study showed that ADN activated the Akt/mTOR pathway in
glioblastoma cell lines, which was consistent with a previous study
(22). It should be clarified that
Ser473 and Thr308 are two phosphorylation sites of Akt (41). However, it was found that ADN could
only lead to Akt phosphorylation at Thr308, while it could not
induce its phosphorylation at Ser473 in glioblastoma cell lines. It
is to notable that the addition of LY294002 reversed the effect of
ADN on glioblastoma cell line apoptosis and cells in the S phase.
The findings suggested that ADN might activate the Akt/mTOR pathway
to facilitate glioblastoma progression.
TMZ is the first-line chemotherapy for patients with
glioblastoma, whereas drug resistance to TMZ remains a challenging
problem and is a cause of poor prognosis (42,43).
U251 and U87-MG cell lines are widely used cell lines to establish
TMZ-resistant glioblastoma cell models according to previous
studies (44–48). Guided by these previous studies
(44–48), U251 and U87-MG cell lines we also
applied to explore the effect of ADN on TMZ resistance in
glioblastoma. It was found that ADN reversed the effect of TMZ on
apoptosis, cell cycle arrest and growth in glioblastoma cell lines,
which indicated that it contributed to TMZ resistance in
glioblastoma. However, the underlying mechanisms responsible for
ADN-induced TMZ resistance have not been reported by previous
studies. The Akt/mTOR pathway was found to be aberrantly activated
in primary glioblastoma samples and its activation could impair the
efficacy of TMZ treatment (16,49–51).
Therefore, it was further explored whether this pathway is involved
in the regulation of ADN on TMZ resistance in glioblastoma.
Notably, it was discovered that the addition of LY294002 reversed
the effect of ADN on TMZ resistance in glioblastoma cell lines.
Therefore, ADN might induce TMZ resistance by activating the
Akt/mTOR pathway in glioblastoma.
The current study observed that ADN facilitated
glioblastoma progression and induced TMZ resistance by activating
the Akt/mTOR pathway. However, the underlying mechanisms of ADN's
regulation of the Akt/mTOR pathway were unclear. According to a
previous study, it was hypothesized that ADN might bind to its
receptors (ADIPOR1 and ADIPOR2) to initiate the activation of PI3K,
which further activated the Akt/mTOR pathway, thereby promoting
glioblastoma progression and TMZ resistance (22). However, this hypothesis should be
validated by further experiments.
It should be clarified that this study applied 0.1,
0.5, 1.0, 3.0 and 10.0 µg/ml as the concentration gradient of ADN
to treat U251 and U87-MG cell lines. The setting of these
concentrations of ADN was exploratory in nature. Initially, the
present study tried to follow the experience of a relevant previous
study, which used 0.0025, 0.025 and 0.25 µg/ml as the concentration
gradient of ADN to treat U251 and U87-MG cell lines (22). However, after applying similar
concentrations of ADN (0.1 and 0.5 µg/ml), it was found that U251
and U87-MG cell line growth was unaffected. Consequently, it was
decided to use higher concentrations of ADN (1.0, 3.0 and 10.0
µg/ml) to treat these cell lines. It is hoped that the
concentration gradient of ADN set in the present study can provide
a reference for future researchers embarking on similar
investigations.
Recently, several studies have revealed the
potential mechanisms regarding glioblastoma progression and
relapse, which are conducive to improving the management of
glioblastoma patients (52–54). Nevertheless, the prognosis of
glioblastoma patients is unsatisfactory and one of the contributors
is TMZ resistance, which is developed in ~50% of glioblastoma
patients (10). Therefore,
investigating potential mechanisms contributing to TMZ resistance
is crucial to improving the prognosis of patients with
glioblastoma. The current study found that ADN was responsible for
glioblastoma progression and TMZ resistance. The findings provided
perspectives that ADN and its downstream Akt/mTOR pathway might be
potential therapeutic targets to reverse TMZ resistance in patients
with glioblastoma. Meanwhile, considering the involvement of ADN in
glioblastoma progression and TMZ resistance, ADN may serve as a
potential marker for patients with glioblastoma receiving TMZ.
Therefore, the detection of ADN by enzyme-linked immunosorbent
assay and reverse transcription PCR might assist in predicting the
prognosis of patients with glioblastoma receiving TMZ. Overall, the
findings might be conducive to enhancing the management of patients
with glioblastoma. To achieve this goal, further in vivo
experiments are required to validate the effect of ADN on
glioblastoma progression and TMZ resistance. Further clinical
studies are warranted to verify the potential of ADN to serve as a
biomarker for patients with glioblastoma receiving TMZ.
Several limitations of this study should be noted.
i) The underlying mechanism of the regulation of ADN on the
Akt/mTOR pathway should be investigated by further experiments. ii)
The glioblastoma cell lines used in this study included U251 and
U87-MG cell lines, which could not fully represent the
glioblastoma. Further studies could consider applying primary
glioblastoma cells or other glioblastoma cell lines, such as A172,
LN229 and LN18 cell lines, to validate the findings of this study.
iii) Further in vivo experiments were required to confirm
the engagement of ADN in glioblastoma progression and TMZ
resistance.
In conclusion, the present study demonstrated that
ADN activated the Akt/mTOR pathway to facilitate cell cycle,
inhibited cell apoptosis and induced TMZ resistance in
glioblastoma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Role of collagen receptor
DDR2 in cerebral vasospasm: A Youth project of Shaanxi Natural
Science Basic Research Program (grant no. 2016SF-033).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JY and JH contributed to the conception and design
of the study. PS and FL were responsible for the acquisition of
data. KH, JW and YC were responsible for analysis and
interpretation of the data. SS contributed to the methodology of
the overal study design and wrote the first draft of the
manuscript. WM contributed to the interpretation of the data and
manuscript revision.. JY and JH confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van den Bent MJ, Geurts M, French PJ,
Smits M, Capper D, Bromberg JEC and Chang SM: Primary brain tumours
in adults. Lancet. 402:1564–1579. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salari N, Ghasemi H, Fatahian R, Mansouri
K, Dokaneheifard S, Shiri MH, Hemmati M and Mohammadi M: The global
prevalence of primary central nervous system tumors: A systematic
review and meta-analysis. Eur J Med Res. 28:392023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weller M, Wen PY, Chang SM, Dirven L, Lim
M, Monje M and Reifenberger G: Glioma. Nat Rev Dis Primers.
10:332024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grochans S, Cybulska AM, Simińska D,
Korbecki J, Kojder K, Chlubek D and Baranowska-Bosiacka I:
Epidemiology of glioblastoma multiforme-literature review. Cancers
(Basel). 14:24122022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schaff LR and Mellinghoff IK: Glioblastoma
and other primary brain malignancies in adults: A review. JAMA.
329:574–587. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller KD, Ostrom QT, Kruchko C, Patil N,
Tihan T, Cioffi G, Fuchs HE, Waite KA, Jemal A, Siegel RL and
Barnholtz-Sloan JS: Brain and other central nervous system tumor
statistics, 2021. CA Cancer J Clin. 71:381–406. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodríguez-Camacho A, Flores-Vázquez JG,
Moscardini Martelli J, Torres-Ríos JA, Olmos-Guzmán A, Ortiz-Arce
CS, Cid-Sánchez DR, Pérez SR, Macías-González MDS,
Hernández-Sánchez LC, et al: Glioblastoma Treatment:
State-of-the-Art and Future Perspectives. Int J Mol Sci.
23:72022022. View Article : Google Scholar
|
|
8
|
Czarnywojtek A, Borowska M, Dyrka K, Van
Gool S, Sawicka-Gutaj N, Moskal J, Kościński J, Graczyk P, Hałas T,
Lewandowska AM, et al: Glioblastoma Multiforme: The latest
diagnostics and treatment techniques. Pharmacology. 108:423–431.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aldoghachi AF, Aldoghachi AF, Breyne K,
Ling KH and Cheah PS: Recent Advances in the therapeutic strategies
of glioblastoma multiforme. Neuroscience. 491:240–270. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karachi A, Dastmalchi F, Mitchell DA and
Rahman M: Temozolomide for immunomodulation in the treatment of
glioblastoma. Neuro Oncology. 20:1566–1572. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomar MS, Kumar A, Srivastava C and
Shrivastava A: Elucidating the mechanisms of Temozolomide
resistance in gliomas and the strategies to overcome the
resistance. Biochim Biophys Acta Rev Cancer. 1876:1886162021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teraiya M, Perreault H and Chen VC: An
overview of glioblastoma multiforme and temozolomide resistance:
Can LC-MS-based proteomics reveal the fundamental mechanism of
temozolomide resistance? Front Oncol. 13:11662072023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Daisy Precilla S, Biswas I, Kuduvalli SS
and Anitha TS: Crosstalk between PI3K/AKT/mTOR and WNT/β-Catenin
signaling in GBM-Could combination therapy checkmate the collusion?
Cell Signal. 95:1103502022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khabibov M, Garifullin A, Boumber Y,
Khaddour K, Fernandez M, Khamitov F, Khalikova L, Kuznetsova N, Kit
O and Kharin L: Signaling pathways and therapeutic approaches in
glioblastoma multiforme (Review). Int J Oncol. 60:692022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo L and Wu Z: FOXM1-mediated NUF2
expression confers temozolomide resistance to human glioma cells by
regulating autophagy via the PI3K/AKT/mTOR signaling pathway.
Neuropathology. 42:430–446. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zając A, Sumorek-Wiadro J, Langner E,
Wertel I, Maciejczyk A, Pawlikowska-Pawlęga B, Pawelec J, Wasiak M,
Hułas-Stasiak M, Bądziul D, et al: Involvement of PI3K pathway in
glioma cell resistance to temozolomide treatment. Int J Mol Sci.
22:51552021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong J, Guo G, Guo L, Wang Z, Chen Z, Nan
Y, Cao Y, Li R, Yang X, Dong J, et al: Amlexanox enhances
temozolomide-induced antitumor effects in human glioblastoma cells
by inhibiting IKBKE and the Akt-mTOR signaling pathway. ACS Omega.
6:4289–4299. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khoramipour K, Chamari K, Hekmatikar AA,
Ziyaiyan A, Taherkhani S, Elguindy NM and Bragazzi NL: Adiponectin:
Structure, physiological functions, role in diseases, and effects
of nutrition. Nutrients. 13:11802021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naimo GD, Forestiero M, Paolì A, Malivindi
R, Gelsomino L, Győrffy B, Leonetti AE, Giordano F, Panza S,
Conforti FL, et al: ERα/LKB1 complex upregulates E-cadherin
expression and stimulates breast cancer growth and progression upon
adiponectin exposure. Int J Cancer. 153:1257–1272. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Illiano M, Nigro E, Sapio L, Caiafa I,
Spina A, Scudiero O, Bianco A, Esposito S, Mazzeo F, Pedone PV, et
al: Adiponectin down-regulates CREB and inhibits proliferation of
A549 lung cancer cells. Pulm Pharmacol Ther. 45:114–120. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nigro E, Orlandella FM, Polito R,
Mariniello RM, Monaco ML, Mallardo M, De Stefano AE, Iervolino PLC,
Salvatore G and Daniele A: Adiponectin and leptin exert
antagonizing effects on proliferation and motility of papillary
thyroid cancer cell lines. J Physiol Biochem. 77:237–248. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Porcile C, Di Zazzo E, Monaco ML, D'Angelo
G, Passarella D, Russo C, Di Costanzo A, Pattarozzi A, Gatti M,
Bajetto A, et al: Adiponectin as novel regulator of cell
proliferation in human glioblastoma. J Cell Physiol. 229:1444–1454.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang J, Fan Y, Zhang W, Shen Y, Liu T,
Yao M, Gu J, Tu H and Gan Y: Adiponectin suppresses human
pancreatic cancer growth through attenuating the β-Catenin
signaling pathway. Int J Biol Sci. 15:253–264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Yu C and Deng W: Roles and
mechanisms of adipokines in drug resistance of tumor cells. Eur J
Pharmacol. 899:1740192021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bub JD, Miyazaki T and Iwamoto Y:
Adiponectin as a growth inhibitor in prostate cancer cells. Biochem
Biophys Res Commun. 340:1158–1166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun G, Zhang X, Liu Z, Zhu S, Shen P,
Zhang H, Zhang M, Chen N, Zhao J, Chen J, et al: The
Adiponectin-AdipoR1 axis mediates tumor progression and tyrosine
kinase inhibitor resistance in metastatic renal cell carcinoma.
Neoplasia. 21:921–931. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bocian-Jastrzębska A, Malczewska-Herman A
and Kos-Kudła B: Role of leptin and adiponectin in carcinogenesis.
Cancers (Basel). 15:42502023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang H and Judd RL: Adiponectin regulation
and function. Compr Physiol. 8:1031–1063. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nigro E, Daniele A, Salzillo A, Ragone A,
Naviglio S and Sapio L: AdipoRon and other adiponectin receptor
agonists as potential candidates in cancer treatments. Int J Mol
Sci. 22:55692021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Chen J and Zhang J:
AdipoR1-mediated miR-3908 inhibits glioblastoma tumorigenicity
through downregulation of STAT2 associated with the AMPK/SIRT1
pathway. Oncol Rep. 37:3387–3396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jie C, Xuan W, Feng HD, Hua DM, Bo W, Fei
S and Hao Z: AdipoR2 inhibits human glioblastoma cell growth
through the AMPK/mTOR pathway. Eur J Med Res. 26:282021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Byeon JS, Jeong JY, Kim MJ, Lee SM, Nam
WH, Myung SJ, Kim JG, Yang SK, Kim JH and Suh DJ: Adiponectin and
adiponectin receptor in relation to colorectal cancer progression.
Int J Cancer. 127:2758–2767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jardé T, Caldefie-Chézet F,
Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F and
Vasson MP: Involvement of adiponectin and leptin in breast cancer:
Clinical and in vitro studies. Endocr Relat Cancer. 16:1197–1210.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williams CJ, Mitsiades N, Sozopoulos E,
His A, Wolk A, Nifli AP, Tseleni-Balafouta S and Mantzoros CS:
Adiponectin receptor expression is elevated in colorectal
carcinomas but not in gastrointestinal stromal tumors. Endocr Relat
Cancer. 15:289–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barzegar Behrooz A, Talaie Z, Jusheghani
F, Łos MJ, Klonisch T and Ghavami S: Wnt and PI3K/Akt/mTOR survival
pathways as therapeutic targets in glioblastoma. Int J Mol Sci.
23:13532022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fattahi S, Amjadi-Moheb F, Tabaripour R,
Ashrafi GH and Akhavan-Niaki H: PI3K/AKT/mTOR signaling in gastric
cancer: Epigenetics and beyond. Life Sci. 262:1185132020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miricescu D, Totan A, Stanescu S II,
Badoiu SC, Stefani C and Greabu M: PI3K/AKT/mTOR signaling pathway
in breast cancer: From molecular landscape to clinical aspects. Int
J Mol Sci. 22:1732020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng X, Li W, Xu H, Liu J, Ren L, Yang Y,
Li S, Wang J, Ji T and Du G: Sinomenine ester derivative inhibits
glioblastoma by inducing mitochondria-dependent apoptosis and
autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm Sin B.
11:3465–3480. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang R, Wang M, Zhang G, Bao Y, Wu Y, Li
X, Yang W and Cui H: E2F7-EZH2 axis regulates PTEN/AKT/mTOR
signalling and glioblastoma progression. Br J Cancer.
123:1445–1455. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang Y, Liu J, Hong W, Fei X and Liu R:
Arctigenin inhibits glioblastoma proliferation through the AKT/mTOR
pathway and induces autophagy. Biomed Res Int. 2020:35426132020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Manning BD and Toker A: AKT/PKB Signaling:
Navigating the Network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee SY: Temozolomide resistance in
glioblastoma multiforme. Genes Dis. 3:198–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chien CH, Hsueh WT, Chuang JY and Chang
KY: Dissecting the mechanism of temozolomide resistance and its
association with the regulatory roles of intracellular reactive
oxygen species in glioblastoma. J Biomed Sci. 28:182021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thanasupawat T, Glogowska A, Burg M, Krcek
J, Beiko J, Pitz M, Zhang GJ, Hombach-Klonisch S and Klonisch T:
C1q/TNF-related peptide 8 (CTRP8) promotes temozolomide resistance
in human glioblastoma. Mol Oncol. 12:1464–1479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun Q, Xu J, Yuan F, Liu Y, Chen Q, Guo L,
Dong H and Liu B: RND1 inhibits epithelial-mesenchymal transition
and temozolomide resistance of glioblastoma via AKT/GSK3-β pathway.
Cancer Biol Ther. 25:23217702024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang X, Jia L, Jin X, Liu Q, Cao W, Gao X,
Yang M and Sun B: NF-ĸB inhibitor reverses temozolomide resistance
in human glioma TR/U251 cells. Oncol Lett. 9:2586–2590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li H, Chen L, Li JJ, Zhou Q, Huang A, Liu
WW, Wang K, Gao L, Qi ST and Lu YT: miR-519a enhances
chemosensitivity and promotes autophagy in glioblastoma by
targeting STAT3/Bcl2 signaling pathway. J Hematol Oncol. 11:702018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang YN, Zhang XH, Wang YM, Zhang X and Gu
Z: miR-204 reverses temozolomide resistance and inhibits cancer
initiating cells phenotypes by degrading FAP-α in glioblastoma.
Oncol Lett. 15:7563–7570. 2018.PubMed/NCBI
|
|
49
|
Rao V, Kumar G, Vibhavari RJA, Nandakumar
K, Thorat ND, Chamallamudi MR and Kumar N: Temozolomide Resistance:
A Multifarious Review on Mechanisms Beyond O-6-Methylguanine-DNA
Methyltransferase. CNS Neurol Disord Drug Targets. 22:817–831.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Singh N, Miner A, Hennis L and Mittal S:
Mechanisms of temozolomide resistance in glioblastoma-a
comprehensive review. Cancer Drug Resist. 4:17–43. 2021.PubMed/NCBI
|
|
51
|
Chen L, Han L, Shi Z, Zhang K, Liu Y,
Zheng Y, Jiang T, Pu P, Jiang C and Kang C: LY294002 enhances
cytotoxicity of temozolomide in glioma by down-regulation of the
PI3K/Akt pathway. Mol Med Rep. 5:575–579. 2012.PubMed/NCBI
|
|
52
|
Dai X, Ye L, Li H, Dong X, Tian H, Gao P,
Dong J and Cheng H: Crosstalk between microglia and neural stem
cells influences the relapse of glioblastoma in GBM immunological
microenvironment. Clin Immunol. 251:1093332023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gao P, Wang H, Li H, Shu L, Han Z, Li S,
Cheng H and Dai X: miR-21-5p Inhibits the Proliferation, Migration,
and Invasion of Glioma by Targeting S100A10. J Cancer.
14:1781–1793. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou L, Li H, Yao H, Dai X, Gao P and
Cheng H: TMED family genes and their roles in human diseases. Int J
Med Sci. 20:1732–1743. 2023. View Article : Google Scholar : PubMed/NCBI
|