Introduction
Pancreatic ductal adenocarcinoma (PDA) is the most
frequent malignant tumor of the exocrine pancreas (1,2) and
the incidence continues to increase yearly (3). Due to the difficulty of obtaining an
early diagnosis, the rapid progression of the disease and the poor
prognosis, the majority of patients are already in an advanced
stage of cancer at the time of diagnosis (4). When curative surgical resection is
possible, the 5-year survival rate is less than 5% (5,6).
Chemotherapy is a cornerstone treatment for patients with
unresectable or metastatic PDA. The development of combined
regimens, including Gemcitabine with albumin-bound paclitaxel and
FOLFIRINOX, has improved the survival of patients with metastatic
PDA (7). Other therapeutic
strategies are adopted for KRAS-mutated and wild-type PDA patients.
KRAS is mutated in >90% of PDA cases (8), therefore ongoing clinical trials have
been Only focused on inhibiting the more common allele variants
G12D, G12V. Only a small percentage of patients KRAS wild-type are
eligible for therapies targeting molecular alterations such as
BRCA1, BRCA2, NTRK, ROS1, ALK, RET, NRG1, BRAF, MSI-H status
(8).
The Claudin protein family is composed of no less
than 27 transmembrane proteins, which are assigned by category into
classical and non-classical types based on their sequence
characteristics (9–11). Claudins are important components of
tight junctions and form a paracellular barrier to control the flow
of molecules between cells (10).
Claudin 18 belongs to the non-classical type and is normally
expressed in gastric and lung cells (11). Claudin 18 has two isoforms: Claudin
18.1 (expressed in lung tissues) and Claudin 18.2 (CLDN18.2)
(expressed in gastric tissues) (12–14).
CLDN18.2 is expressed in normal gastric mucosa cells
and is retained in most gastric and gastroesophageal junction
adenocarcinomas (15,16). Moreover, it is aberrantly expressed
in 60–90% of PDA (17,18).
After malignant transformation, CLDN18.2 can undergo
phosphorylation and exposure to the membrane cell surface, becoming
enabled to bind monoclonal antibodies (19,20).
Based on these characteristics, CLDN18.2 was considered optimal for
therapeutic target (21), and
Zolbetuximab (ZBT) was developed as a first chimeric immunoglobulin
G1 monoclonal antibody highly specific for CLDN18.2 (22). ZBT binds to CLDN18.2 on the tumor
cell surface and stimulates cellular and soluble immune effectors
that activate both antibody-dependent cytotoxicity and
complement-dependent cytotoxicity (23). ZBT is currently being undergoing
clinical testing in gastric and pancreatic tumors.
Our study aimed to evaluate CLDN18.2 expression on
both pancreatic surgical specimens and FNABs, to investigate its
possible prognostic role, as well as therapeutic together with
upcoming targeted drugs.
Materials and methods
The current study enrolled 70 patients diagnosed
with PDA between February 2015 and November 2023 at the National
Institute of Gastroenterology of Castellana Grotte, Italy.
PDA specimens included either fine needle aspiration
biopsies (42 FNABs from metastatic cancers) or surgical samples (28
resections from non metastatic cancers). For each surgical
specimen, the chosen block included normal as well as neoplastic
tissue. Follow-up data and the dates of patients' deaths were
collected from the Institute records.
Serum CEA and CA19-9 levels (Elecsys Cobas 8000,
Roche, Basel Switzerland) were known for all patients.
Tissue specimens were sectioned into 4 µm thickness
slices, mounted on Apex Bond Slides (Leica Biosystems), and used
for immunohistochemical analysis. Immunohistochemical staining
procedures were carried out on a BOND III automated immunostainer
(Leica Biosystems, Wetzlar Germany), from deparaffinization to
counterstaining with hematoxylin, using the Bond Polymer Refine
Detection Kit (Leica Biosystems). For Claudin 18.2 detection, a
rabbit monoclonal antibody (clone EPR19202, Abcam, Cambridge, UK)
at 1:200 dilution was used. Antigen retrieval was performed using
BOND Epitope Retrieval Solution 2 (Leica Biosystems).
CLDN 18.2 immunostaining was evaluated using the
Histoscore (H-score) (24), defined
as a method combining both percentages of positive-expression cells
in the tissue and immunostaining intensities (1+, 2+, 3+). Only
membranous staining was retained for scoring. The H-score was
calculated according to the formula: (0 × percentage of no reactive
cells) + (1 × percentage of weakly stained cells) + (2 × percentage
of intermediately stained cells) + (3 × percentage of strongly
stained cells). Thus, the H-score ranged from 0 to a maximum of
300. A sample was considered positive with an H-score ≥5.
Statistical analysis
Patients' characteristics are reported as mean and
standard deviation (M±SD) for continuous variables, and as
frequency and percentage (%) for categorical variables. To test the
association between the independent groups (Claudine Positive vs.
Claudine Negative), Chi-Squared test or Fisher's test where
necessary were used for categorical variables, while the Wilcoxon
ranksum test (Mann-Whitney) was used to compare continuous
variables.
Survival probability was explored using the non
parametric Kaplan-Meier method, and the equality of survival curves
was analyzed with the log-rank test.
To test the null hypothesis of non-association, the
two-tailed probability level was set at 0.05. The analyses were
conducted with Stata Statistical Software: Release 18, StataCorp,
2023, StataCorp LLC.: College Station, TX, USA.
Results
The clinicopathological features of the 70 patients
(32 women and 38 men) are listed in Table I. Median patient age was 69 years
(range 44–84 ys). Most patients (67.14%) had pancreatic head
cancer, that was well- or moderately-differentiated (Table I). Twelve (17.14%) cancers were
located at the isthmus, eleven (15.71%) at the body/tail and were
all poorly differentiated. The pathological tumor stage and node
stage were assessed only in surgical specimens. Seven (25%) of 28
surgical specimens were classified as pT3/4 vs. 21 (75%) as pT1/T2.
Twenty-one (75%) cases had lymph node invasion (pN1/2) vs. 7 (25%)
classified as N0. Serum carcinoma embryonic antigen (CEA) levels
were positive (cut-off >3 ng/ml) in 44 patients (69%) at the
time of diagnosis and carbohydrate antigens 19-9 (CA 19-9) (cut-off
>27 U/ml) in 53 patients (82%).
 | Table I.Clinicopathological patient
characteristics by CLDN18.2 expression. |
Table I.
Clinicopathological patient
characteristics by CLDN18.2 expression.
|
Parametersa | Total cohort
(n=70) | Claudine-negative
(n=35) | Claudine-positive
(n=35) | P-value |
|---|
| Sex, male, n
(%) | 38 (54.29) | 19 (54.29) | 19 (54.29) | 0.99 |
| Age, years | 68.90±8.42 | 70.46±8.55 | 67.34±8.10 | 0.15 |
| Samples, n (%) |
|
|
| 0.46 |
|
FNAB | 42 (60.00) | 19 (54.29) | 23 (65.71) |
|
|
Surgical specimens | 28 (40.00) | 16 (45.71) | 12 (34.29) |
|
| Localization, n
(%) |
|
|
| 0.99 |
|
Head | 47 (67.14) | 24 (68.57) | 23 (65.71) |
|
|
Isthmus | 12 (17.14) | 6 (17.14) | 6 (17.14) |
|
| Body +
tail | 11 (15.71) | 5 (14.29) | 6 (17.14) |
|
| Histological
grading, n (%) |
|
|
| 0.04 |
| G1 +
G2 | 47 (67.14) | 19 (54.29) | 28 (80.00) |
|
| G3 | 23 (32.86) | 16 (45.71) | 7 (20.00) |
|
| Tumor stage, n
(%) |
|
|
| 0.66 |
| T1 +
T2 | 21 (75.00) | 11 (68.75) | 10 (83.33) |
|
| T3 +
T4 | 7 (25.00) | 5 (31.25) | 2 (16.67) |
|
| Node stage, n
(%) |
|
|
| 0.02 |
| N0 | 7 (25.00) | 1 (6.00) | 6 (50.00) |
|
| N1 +
N2 | 21 (75.00) | 15 (94.00) | 6 (50.00) |
|
| CEA | 17.82±32.61 | 26.57±44.74 | 10.20±13.10 | 0.06 |
| CA 19-9 |
2402.25±4435.15 |
2015.95±3562.89 |
2774.24±5180.95 | 0.55 |
| Status (died), n
(%) | 54 (78.26) | 30 (85.71) | 24 (70.59) | 0.13 |
| Median
survival | 5 (0.00–14.00) | 6.00
(0.00–17.00) | 5.00
(0.00–14.00) | 0.90 |
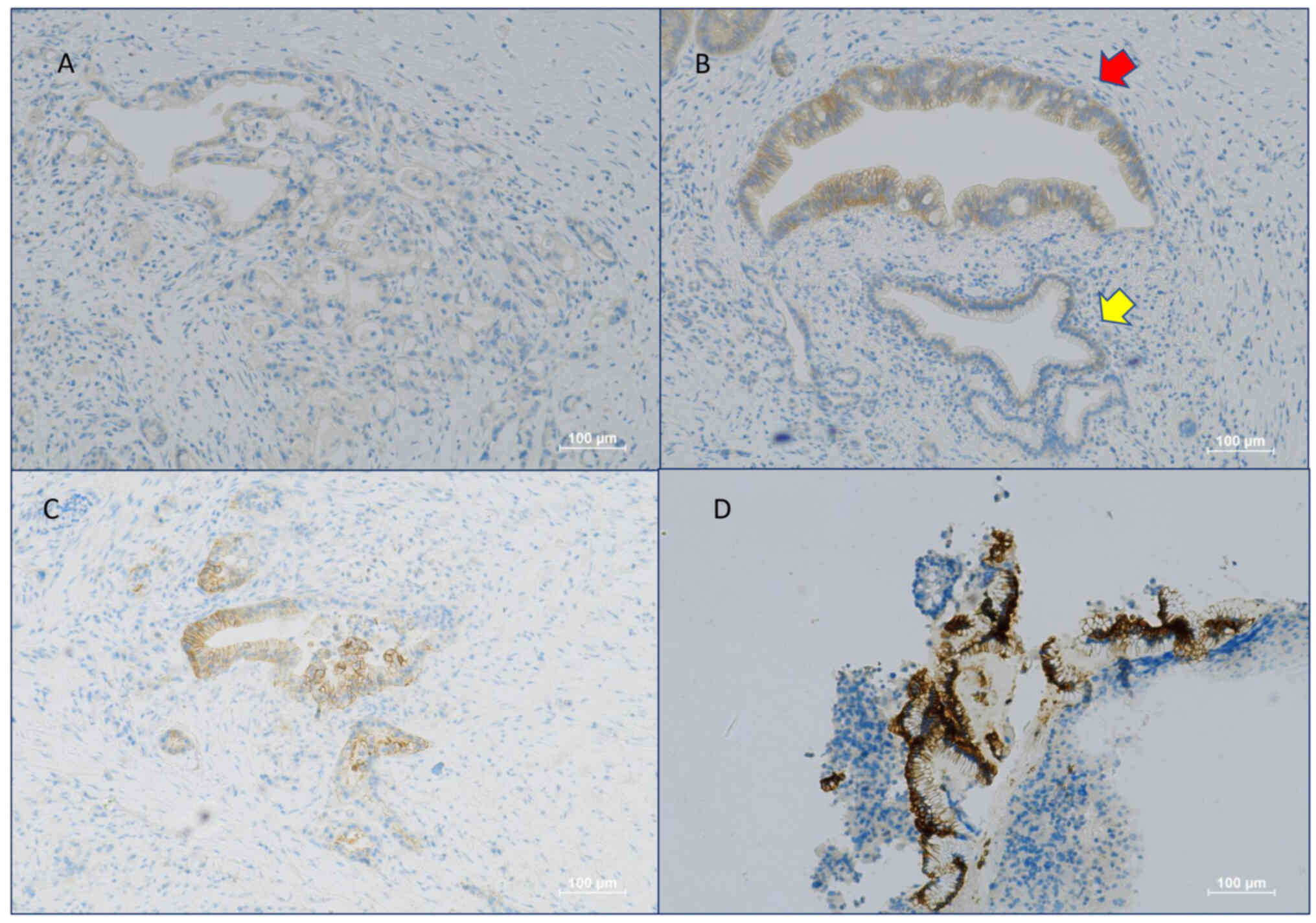
CLDN18.2 staining was not detectable in any of the
normal pancreatic tissue cells (Fig.
1B). The expression of CLDN18.2 was evaluated solely in PDA
cells, excluding its expression in precancerous lesions like
pancreatic intraepithelial neoplasia. CLDN18.2 was positive in 35
(50%) PDA patients. Twenty-one (60%) of these had an H-score
>50. Twelve (34%) samples were scored up to 3+, fifteen (43%)
were scored up to 2+, eight (23%) were scored up to 1+
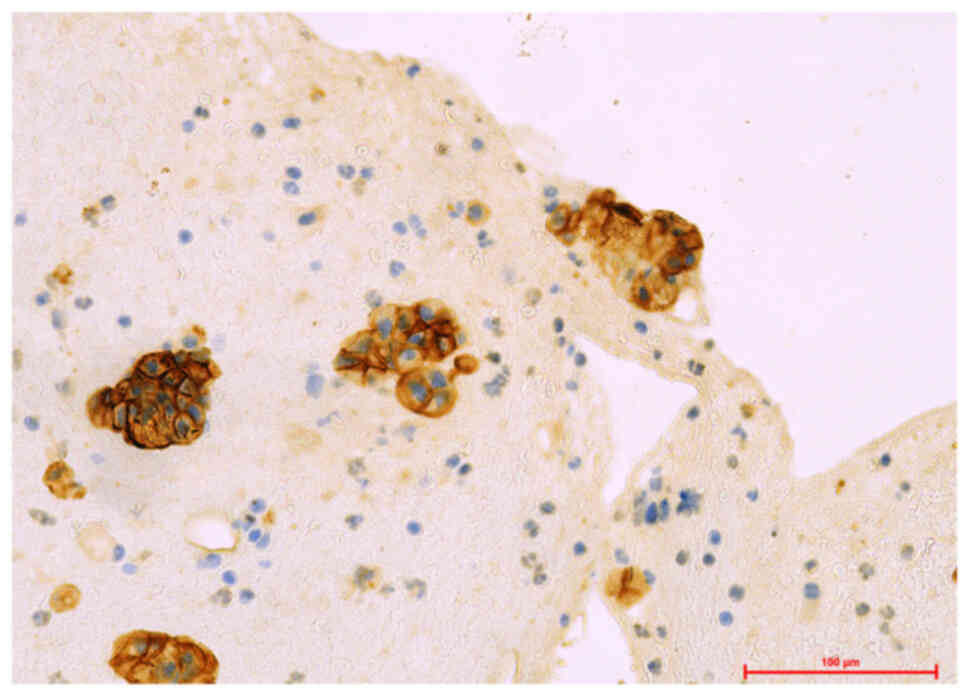
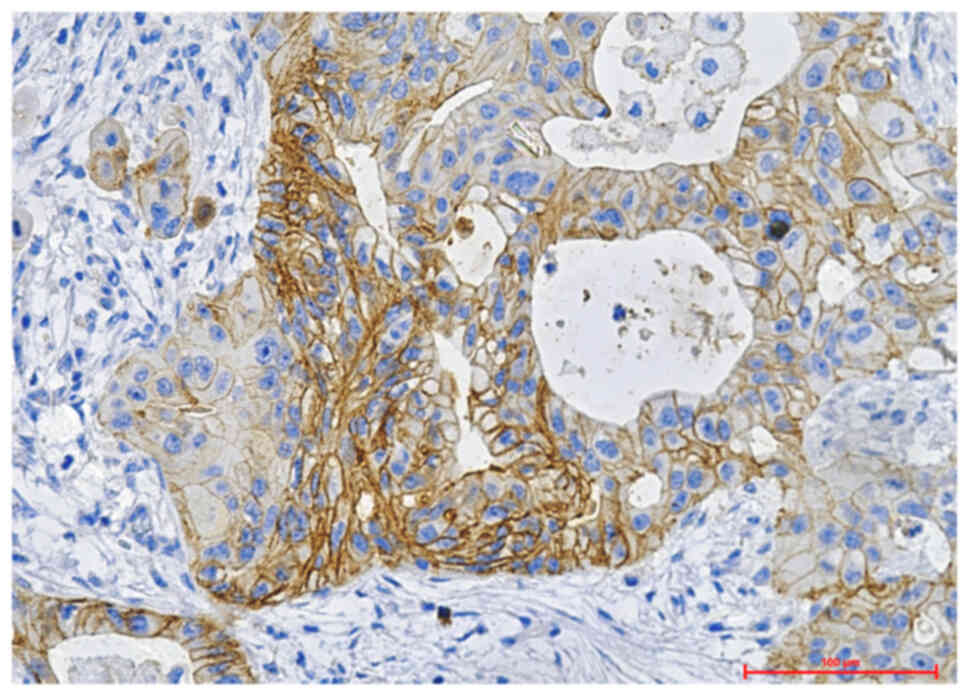
(representative images are shown in Fig. 1). A higher number of positive cases
was observed in the FNAB group (23 samples) compared to the
surgical specimens (12 samples) (67.71% vs. 34.29%) (Table I) (Fig.
2–Fig. 3). The analytic results
showed that histologic grading and node stage were significantly
associated with CLDN18.2 expression (P=0.04; P=0.02) (Table I). Mean serum CEA values were lower
in patients who were CLDN18.2 positive (Table I). The other clinicopathological
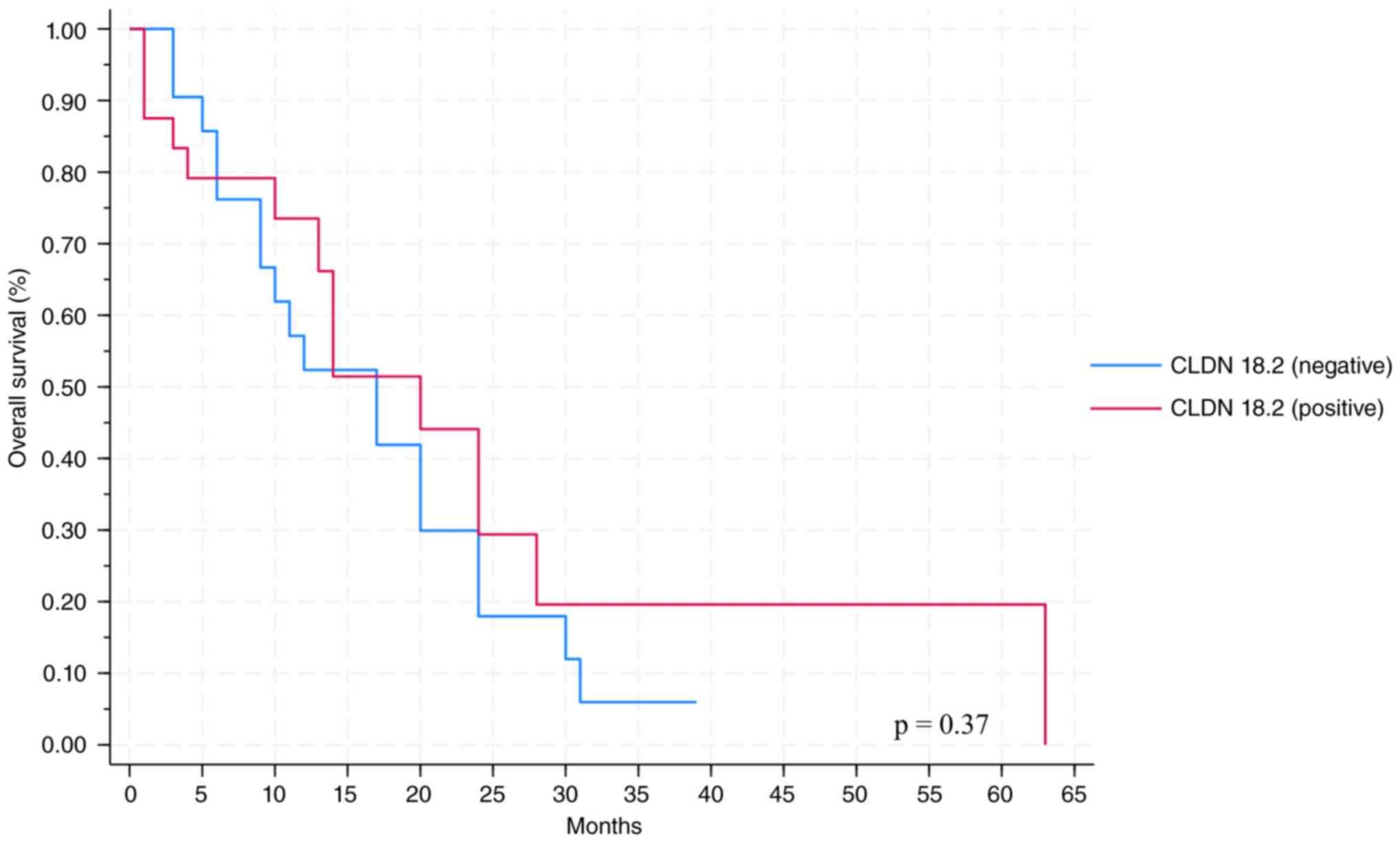
characteristics showed no significant association (Table I). Positive CLDN18.2 immunostaining
was not associated with survival outcomes (Fig. 4). Median OS was 5 months in the
positive group vs. six months in the negative samples (Table I).
Discussion
To the best of our knowledge, ours is the first
study that evaluated the expression of CLDN18.2 also on pancreatic
FNAB. It is known that curative resection is not possible for the
majority of these patients, so very often the diagnosis is made on
FNAB, being the only material available for further evaluation.
Unlike in other studies (17,22,24–27),
we also evaluated the presence of the protein on biopsy slices, in
order to verify its expression on samples with poor cellularity. We
demonstrated a higher percentage of CLDN18.2 positive specimens in
the FNAB group, likely due to hypofixation problems that are more
frequent in surgical samples. Therefore, the increased expression
of this marker on FNAB could be useful both to resolve a doubtful
histological diagnosis and to decide the possible eligibility of
patients for the target drug. Chemosensitivity of PDA is moderate
and so targeted therapies are of high interest. This study was
conducted to evaluate whether CLDN18.2 immunoreactivity can be
considered an adequate indication for ZBT target therapy. Claudins
are appropriate targets for anticancer treatment due to their
dysregulated location following carcinogenesis (6). In fact, while in normal cells they are
present at the level of tight junctions and therefore not reachable
by the targeting antibodies, carcinogenesis alters their
localization and makes them good targets (6).
The phase II clinical trial (28,29)
demonstrated that ZBT in combination with first-line chemotherapy
significantly improved the overall survival, progression-free
survival and the objective response rate, with acceptable safety
and tolerability in patients with CLDN 18.2-positive advanced
recurrent gastric cancers and gastroesophageal junction cancers
compared with those who received chemotherapy alone. Recently, ZBT
combined with chemotherapy demonstrated a survival benefit in
patients with CLDN18.2-positive and HER-2-negative gastric or
gastroesophageal junction cancers in the global phase III SPOTLIGHT
and GLOW trials (30,31).
Türeci et al (32) demonstrated that, using human
peripheral blood mononuclear cells and serum as effectors, ZBT
induced ADCC (Antibody-Dependent Cell-Mediated Cytotoxicity) and
CDC (Complement Dependent Cytotoxicity) against human pancreatic
cancer cells in ex vivo models. They also revealed that ZBT
suppressed tumor development and lung metastasis formation in human
pancreatic cancer cell lines transduced with lentiviral
claudin-18.2 in mouse xenograft models (32). Furthermore, they demonstrated that
CLDN18.2 expression on the cell surface was increased by
gemcitabine or 5-fluorouracil in vitro administration
(32). If this finding were
supported by other studies it would mean that even if pancreatic
cancer cells are not killed by chemotherapy, the patients could
become newly eligible for ZBT therapy, owing to the increased
expression of CLDN18.2.
More recently, a randomized open-label phase 2 study
(NCT03816163) assessed the safety and efficacy of Gemcitabine and
nab-paclitaxel alone or with ZBT in patients with PDA and high
CLDN18.2 expression (>75% of positive tumor cells) (25).
Considering the importance that CLDN18.2 might have
as a potential therapeutic marker for PDA, we correlated its
expression with clinicopathological features and clinical outcomes.
Pancreatic tumor samples showed heterogeneous CLDN18.2 expression
as regards the level of surface expression measured as staining
intensity of positive cells and also the fraction of stained cells
within a single tumor sample. We defined tumors as CLDN18.2
positive if a proportion of ≥5% of all evaluable tumor cells showed
membrane-specific staining. Noteworthily, PDA often has a prominent
desmoplastic and stromal-dominant component with few tumor cells.
Therefore, evaluating the sample for the expression of a target
molecule and determining the positive fraction of the target
molecule is surely a relevant analysis. Each sample was evaluated
by two independent pathologists.
Our results showed that a considerable number of
patients were immunoreactive to CLDN18.2 (Table I) and the majority of them (21/35,
60%) had a Histoscore >50. This indicates that even if clinical
benefit will require a high expression of CLDN18.2, a considerable
number of PDA patients will still be eligible.
Previous articles (22,24,25,33)
reported controversial results for the expression of CLDN18.2 in
normal pancreatic tissue. Some investigators have reported a weak
expression of CLDN18.2 in normal pancreatic tissue (25), whereas others have found no
expression (22,24,33).
In agreement with the latter, our data confirm that it was not
expressed in any type of normal pancreatic cell (Fig. 1B yellow arrow). Therefore, it
resulted an ideal therapeutic target because it showed a high and
specific expression in the tumor and no expression in normal
pancreatic tissues.
In agreement with Zhang and Lyu (17,27),
we found a significant correlation between CLDN18.2 expression and
tumor histologic grading, with well- or moderately-differentiated
tumors yielding a higher prevalence of positive samples (Table I). In our study, the node stage was
assessed only in 28 samples. Correlation analysis highlighted that
the proportion of CLDN18.2 positive tumors was significantly higher
in lymph-node negative tumors, in contrast to previously reported
findings (22,26) but in agreement with Park S et
al (25). As already
demonstrated in other studies (24,25),
the expression of CLDN18.2 is associated with the prognostic
factors mentioned above but was not correlated with patient
survival. This result is possible because grading and nodal status
do not always correlate with survival.
This study suffers from some limitations that need
to be highlighted. First, this study has a small number of
patients. Second, the detection method and the cut-off used are
arbitrary. Therefore, more large-scale studies using detection
methods based on the results of ongoing clinical trials of ZBT are
needed in order to better identify patients eligible for targeted
therapy.
In conclusion, the results of this study seem to
suggest an attractive role for CLDN18.2 in PDA, for both diagnostic
and prognostic-therapeutic purposes. In fact, its absence in normal
tissue and high expression in neoplastic cells suggest that it may
be a very useful marker for diagnostic and prognostic-therapeutic
purposes. Its expression is correlated with grading and node stage
and the high percentage of positive samples could indicate that a
large number of patients may be eligible for ZBT.
Acknowledgments
The authors would like to thank Dr Victoria Mary
Pragnell (University of Bari Aldo Moro, Bari, Italy) for their
contribution to revising the English language.
Funding
This study was supported by a grant from the Italian Ministry of
Health (Ricerca Corrente 2023; grant no. 11).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
AMV conceptualized the study; IG and NL performed
the IHC method; AMV analyzed and interpreted the results; RD
performed the statistical analysis; GA and MTS analyzed and
interpreted the data and investigated for data in the literature;
PAI provided laboratory data; SV and CO enrolled patients and
contributed to conception and design of study; AMV performed
writing-review and editing; RA and CL contributed to the
acquisition of data and performed the supervision of the
manuscript. RA and CL confirm the authenticity of all raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the local scientific
committee and by the institutional ethics committee of the
Oncologic Institute Research Hospital of Bari, Italy, and was
performed in accordance with the Declaration of Helsinki. The study
individuals gave written consent for the laboratory investigations
and recording of their clinical data.
Patient consent for publication
Patients provided written informed consent for
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stoffel EM, Randall E, Brand RE and
Goggins M: Pancreatic cancer: Changing epidemiology and new
approaches to risk assessment, early detection, and prevention.
Gastroenterology. 164:752–765. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park W, Chawla A and O'Reilly EM:
Pancreatic cancer: A review. JAMA. 326:851–862. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Wu N, Pei B, Ma X and Yang W:
Claudin and pancreatic cancer. Front Oncol. 13:11362272023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren B, Cui M, Yang G, Wang H, Feng M, You
L and Zhao Y: Tumor microenvironment participates in metastasis of
pancreatic cancer. Mol Cancer. 17:1082018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sahin U, Koslowski M, Dhaene K, Usener D,
Brandenburg G, Seitz G, Huber C and Türeci O: Claudin-18 splice
variant 2 is a pan-cancer target suitable for therapeutic antibody
development. Clin Cancer Res. 14:7624–7634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugimoto M, Takagi T, Suzuki R, Konno N,
Asama H, Sato Y, Irie H, Okubo Y, Nakamura J, Takasumi M, et al:
Drug treatment for chemotherapy-induced peripheral neuropathy in
patients with pancreatic cancer. Fukushima J Med Sci. 68:1–10.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hosein AN, Dougan SK, Aguirre AJ and
Maitra A: Translational advances in pancreatic ductal
adenocarcinoma therapy. Nat Cancer. 3:272–286. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furuse M, Fujita K, Hiiragi T, Fujimoto K
and Tsukita S: Claudin-1 and −2: Novel integral membrane proteins
localizing at tight junctions with no sequence similarity to
occludin. J Cell Biol. 141:1539–1550. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nichols LS, Ashfaq R and Iacobuzio-Donahue
CA: Claudin 4 protein expression in primary and metastatic
pancreatic cancer: Support for use as a therapeutic target. Am J
Clin Pathol. 121:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hashimoto I and Oshima T: Claudins and
gastric cancer: An overview. Cancers (Basel). 14:2902022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krause G, Winkler L, Mueller SL, Haseloff
RF, Piontek J and Blasig IE: Structure and function of claudins.
Biochim Biophys Acta. 1778:631–645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lal-Nag M and Morin PJ: The claudins.
Genome Biol. 10:2352009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong JY, An JY, Lee J, Park S.H, Park JO,
Park YS, Lim HY, Kim KM, Kang WK and Kim ST: Claudin 18.2
expression in various tumor types and its role as a potential
target in advanced gastric cancer. Transl Cancer Res. 9:3367–3374.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pellino A, Brignola S, Riello E, Niero M,
Murgioni S, Guido M, Nappo F, Businello G, Sbaraglia M, Bergamo F,
et al: Association of CLDN18 protein expression with
clinicopathological features and prognosis in advanced gastric and
gastroesophageal junction adenocarcinomas. J Pers Med. 11:10952021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dottermusch M, Krüger S, Behrens H.M,
Halske C and Röcken C: Expression of the potential therapeutic
target claudin-18.2 is frequently decreased in gastric cancer:
Results from a large Caucasian cohort study. Virchows Arch.
475:563–571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Z, Liu X, Zhou L, Zhang M and Liang
Z: Investigation of clinical application of claudin 18 isoform 2 in
pancreatic ductal adenocarcinoma: A retrospective analysis of 302
Chinese patients. Histol Histopathol. 37:1031–1040. 2022.PubMed/NCBI
|
|
18
|
Swisshelm K, Macek R and Kubbies M: Role
of claudins in tumorigenesis. Adv Drug Deliv Rev. 57:919–928. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang D, Huang G, Liu J and Wei W:
Claudin18.2-targeted cancer theranostics. Am J Nucl Med Mol
Imaging. 13:64–69. 2023.PubMed/NCBI
|
|
20
|
Kubota Y and Shitara K: Zolbetuximab for
Claudin 18.2-positive gastric or gastroesophageal junction cancer.
Ther Adv Med Oncol. 16:175883592312179672024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao W, Xing H, Li Y, Tian W, Song Y, Jiang
Z and Yu J: Claudin18.2 is a novel molecular biomarker for
tumor-targeted immunotherapy. Biomarker Res. 10:382022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wöll S, Schilitter AM, Dhaene K, Roller M,
Esposito I, Sahin U and Türeci Ö: Claudin 18.2 is a target for
IMAB362 antibody in pancreatic neoplasms. Int J Cancer.
134:731–739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh P, Toom S and Huang Y: Anti-claudin
18.2 antibody as new targeted therapy for advanced gastric cancer.
J Hematol Oncol. 10:1052017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Zhang CS, Dong XY, Hu Y, Duan BJ,
Bai J, Wu YY, Fan L, Liao XH, Kang Y, et al: Claudin 18.2 is a
potential therapeutic target for zolbetuximab in pancreatic ductal
adenocarcinoma. World J Gastrointest Oncol. 14:1252–1264. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park S, Shin K, Kim I.H, Hong T, Kim Y,
Suh J and Lee M: Clinicopathological features and prognosis of
resected pancreatic ductal adenocarcinoma patients with claudin-18
overexpression. J Clin Med. 12:53942023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kayikcioglu E and Yüceer RO: The role of
claudin 18.2 and HER-2 in pancreatic cancer outcomes. Medicine
(Baltimore). 102:e328822023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lyu SI, Fretter C, Simon AG, Spielmann SM,
Damanakis AI, Zhao Y, Bruns CJ, Schmidt T, Popp FC, Waldschmidt D,
et al: Extent and clinical significance of the therapy-relevant
tight junction protein claudin 18.2 in pancreatic ductal
adenocarcinoma-real-world evidence. Transl Oncol. 47:1020442024.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shain U, Türeci Ö, Manikhas G, Lordick F,
Rusyn A, Vynnychenko I, Dudon V, Bazin I, Bondarenko I, Melichar B,
et al: FAST: A randomised phase II study of zolbetuximab (IMAB362)
plus EOX versus EOX alone for first-line treatment of advanced
CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma.
Ann Oncol. 32:609–619. 2021. View Article : Google Scholar
|
|
29
|
Lordick F, Al-Batran SE, Ganguli A,
Morlock R, Sahin U and Türeci Ö: Patient-reported outcomes from the
phase II FAST trial of zolbetuximab plus EOX compared to EOX alone
as first-line treatment of patients with metastatic CLDN18.2+
gastroesophageal adenocarcinoma. Gastric Cancer. 24:721–730. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shah MA, Shitara K, Ajani JA, Bang YJ,
Enzinger P, Ilson D, Lordick F, Van Cutsem E, Gallego Plazas J,
Huang J, et al: Zolbetuximab plus CAPOX in CLDN18.2-positive
gastric or gastroesophageal junction adenocarcinoma: The
randomized, phase 3 GLOW trial. Nat Med. 29:2133–2141. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shitara K, Lordick F, Bang YJ, Enzinger P,
Ilson D, Shah MA, Van Cutsem E, Xu RH, Aprile G, Xu J, et al:
Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive,
HER2-negative, untreated, locally advanced unresectable or
metastatic gastric or gastro-oesophageal junction adenocarcinoma
(SPOTLIGHT): A multicentre, randomised, double-blind, phase 3
trial. Lancet. 401:1655–1668. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Türeci Ö, Mitnacht-Kraus R, Wöll S, Yamada
T and Sahin U: Characterization of zolbetuximab in pancreatic
cancer models. Oncoimmunology. 8:e15230962018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanaka M, Shibahara J, Fukushima N,
Shinozaki A, Umeda M, Ishikawa S, Kokudo N and Fukayama M:
Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J
Histochem Cytochem. 59:942–952. 2011. View Article : Google Scholar : PubMed/NCBI
|