Breast cancer is the most prevalent form of cancer
among women and its incidence and mortality rates are rising
annually (1). The initial symptoms
of breast cancer can be subtle, resulting in a majority of cases
being diagnosed in the advanced stages of the disease, which
exhibit aggressive characteristics and often a poor prognosis
(2). Surgical excision and
endocrine therapy are the mainstays in clinical practice (2,3).
However, these treatment methods can lead to an inadequate response
to chemotherapeutic drugs, side effects, susceptibility to drug
resistance (4), recurrence,
metastasis (5,6) and higher levels of patient suffering
and economic burden (7). Therefore,
the development of new anticancer medications is imperative to
hinder tumor growth and enhance the quality of life and survival
for patients with cancer.
Traditional Chinese Medicine has been utilized in
patients with a poor prognosis treated with radiotherapy and
chemotherapy, offering a potential novel approach to anticancer
treatment (8–10). The anticancer mechanism of active
ingredients found in Traditional Chinese Medicine have previously
been reported (11,12). According to a previous study, 18/247
anticancer drugs approved by the U.S. Food and Drug Administration
from 1981–2019 were derived from natural products (13). Increasing numbers of Traditional
Chinese Medicines, such as curcumin and Salvia miltiorrhiza,
have been used for anti-breast cancer treatment and as adjuvant
treatments that exhibit enhanced efficacy and reduced toxicity
(14,15).
Polysaccharides are complex sugar chains composed of
galactose, glucose, xylose, arabinose, rhamnose and other
monosaccharides. These monosaccharides link to form a higher-order
structure (41). Among these,
glucose and arabinose are particularly notable monosaccharides that
influence the activity of Radix Bupleuri. Polysaccharides
extracted from Radix Bupleuri have been reported to enhance
the functionality of macrophages and natural killer cells, boost
the body's immune response and exhibit antioxidant,
anti-inflammatory and immunomodulatory properties (42–45).
Flavonoids such as kaempferol, isorhamnetin and
quercetin have been identified as possessing the ability to
suppress the release and expression of certain pro-inflammatory
factors, such as TNF-α, inducible nitric oxide synthase, IL-1β,
IL-6 and IL-12p70 (46,47). Furthermore, flavonoids are capable
of inhibiting the generation of free radicals in the body,
scavenging free radicals, activating the body's anti-oxidant system
(48) and regulating inflammation
(49,50).
Traditional anticancer tools, such as surgery,
radiotherapy, chemotherapy, targeted therapy, hormonal therapy and
immunotherapy, aim to eliminate malignant cells, arrest disease
progression, alleviate symptoms and enhance patients' quality of
life. At present, small molecule therapeutic strategies are
gradually shifting towards the regulation of the tumor
microenvironment (TME) (51), as
well as targeted interventions of metabolic pathways (52) and protein lipidation modifications
(53), while delving into the
mechanisms of drug resistance formation (54) and potential for immunotherapy
(55). This section will focus on
the analysis of the regulatory mechanisms of tumor cells and their
microenvironment, with special attention to the effects of Radix
Bupleuri and its effective ingredients on breast cancer cell
stemness and metabolism. The present section is not only limited to
the cancer cells themselves but also reviewed the cells and
signaling molecules in the TME, as well as the inhibition of tumor
angiogenesis and drug resistance. In addition, the present section
explored novel ways in which Radix Bupleuri and its
effective ingredients may induce breast cancer cell death, such as
through ferroptosis and mitochondrial damage, to enhance the
overall effect of Radix Bupleuri against breast cancer. The
present section reviewed the anticancer effects of the Radix
Bupleuri and its effective ingredients in breast cancer, but
most of the studies on the main active components of Radix
Bupleuri against breast cancer are in vitro experimental
studies, while in vivo studies were limited (Table II).
The eukaryotic cell growth and proliferation process
encompasses replication and division, collectively referred to as
the cell cycle, consisting of the G1, S, G2 and M phases. The
advancement of the cell cycle is typically linked to an escalation
in proliferative proteins, such as Cyclin, Cyclin-dependent kinase
(CDK) and CDK inhibitor (56).
Disturbances in any of the regulatory factors can result in
abnormal cell cycle activity and uncontrolled cell proliferation,
leading to tumor formation (57).
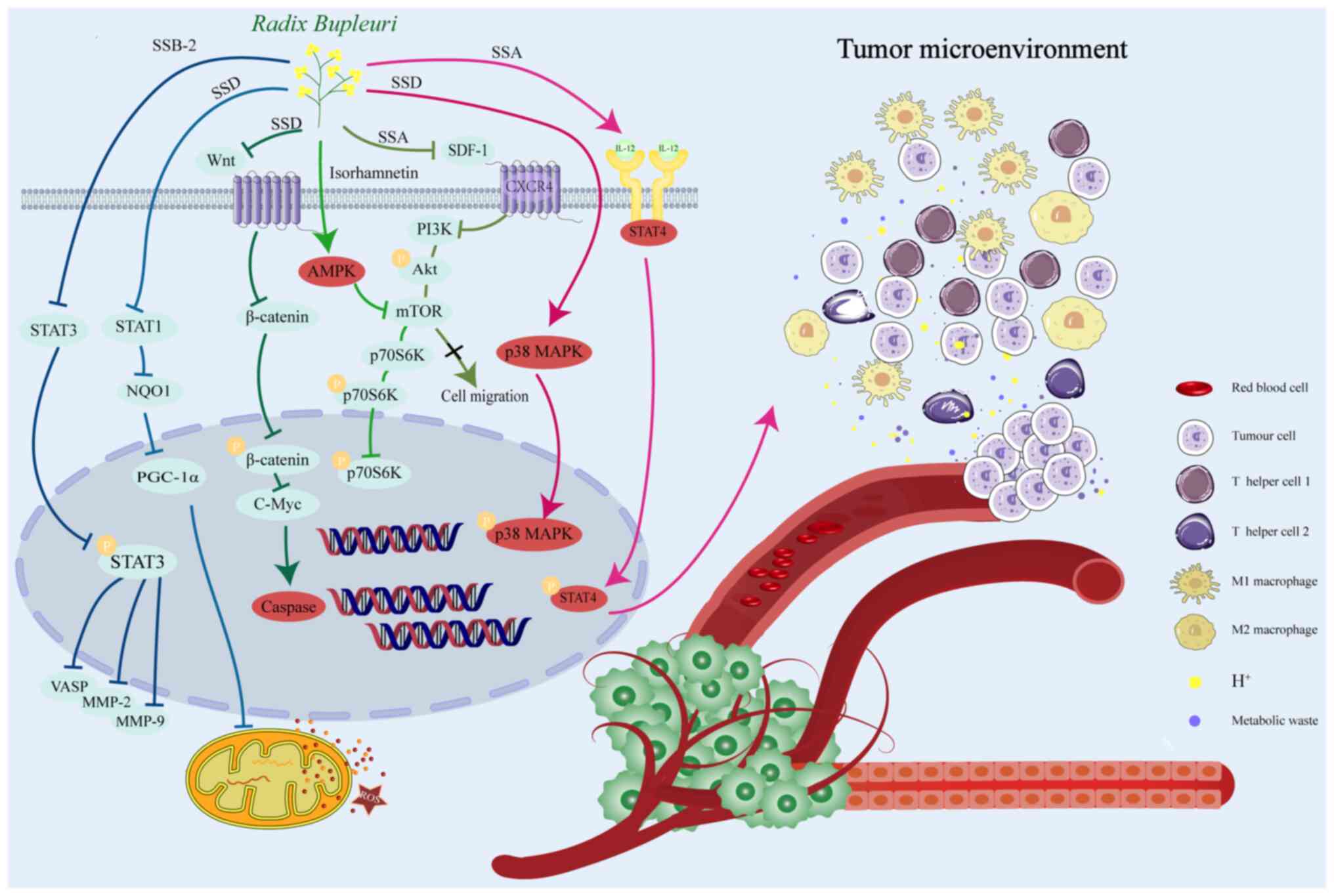
SSD was shown to induce a G0/G1 phase blockade in doxorubicin
(DOX)-resistant cells by decreasing the protein expression levels
of Cyclin D1 and CDK4, while promoting DOX-induced apoptosis in
vitro and in vivo by increasing cleaved Caspase 3
expression levels (58). p21 and
p27 act as negative regulators of the cell cycle in coordination
with cell cycle-associated kinases. Specifically, p21 halts cell
cycle progression through the G/S phase and corrects abnormal
centrosome replication, while p27 suppresses CDK expression,
causing cell cycle arrest in the G0/G1 phase, inhibiting centrosome
separation and impeding breast cancer cell proliferation (59). SSA induces apoptosis in the breast
cancer cell lines, MDA-MB-231 and MCF-7, through
p53/p21-independent and -dependent mechanisms (60). DNA synthesis during the S phase and
mitosis in the G2/M phase are crucial for sustaining malignant
tumor cell proliferation. Antitumor drugs primarily inhibit the
progression of these cell cycle phases (61,62).
The flavonoid, isorhamnetin, exhibits high anticancer activity,
downregulating the protein expression levels of Cyclin B1 and CDK1
and inducing G2/M phase blockade, thus preventing the proliferation
of MCF-7 cells (63).
Mitogen-activated protein kinase (MAPK) serves a
crucial role in apoptosis induction. The downregulation of the p38
MAPK signaling pathway reduces the metastasis of breast cancer
cells in the bone-tropic mouse mammary tumor virus-driven polyoma
virus middle T oncoprotein transgenic (MMTV-PyMT) Bo1 mouse model
(64–66). Additionally, SSD induces the
apoptosis of MDA-MB-231 cells through the p38 MAPK signaling
pathway by enhancing the p38its phosphorylation level and
expression levels (67).
Excessive endoplasmic reticulum stress (ERS), or ER
dysfunction, results in the persistent accumulation of unfolded and
misfolded proteins in the ER, leading to an imbalance in cellular
homeostasis, which can induce apoptosis (68). Calnexin monitors intracellular
Ca2+ homeostasis by assisting in folding misfolded
proteins and serving an essential role in ERS (69). SSD acts as an adenosine triphosphate
(ATP)ase sarcoplasmic/ER Ca2+ transporting inhibitor in
apoptosis-deficient cancer cells, such as MCF-7, and induces ERS
and autophagic cell death by disrupting intracellular
Ca2+ homeostasis via the
Ca2+/calmodulin-dependent protein kinase 2/mammalian
target of rapamycin (mTOR) pathway (70). Wnt/β-catenin signaling
pathway-related genes are highly expressed in triple-negative
breast cancer (TNBC) (71). After
incubation with SSD, the protein and mRNA expression levels of
β-catenin in the cytoplasm and nucleus of the TNBC cells, SUM-159,
HCC1937, MDA-MB-468 and MDA-MB-231, were significantly decreased,
as well as the expression of downstream target genes, such as c-myc
and CyclinD1, indicating that SSD inhibits the proliferation of
TNBC cells by significantly inhibiting β-catenin and its downstream
target genes. SSD also induces Caspase-dependent apoptosis and
inhibits the proliferation of SUM-159, HCC1937, MDA-MB-468 and
MDA-MB-231 cells (72).
Therefore, saikosaponins and flavonoids inhibit the
development of breast cancer cells by negatively regulating cell
cycle-related proteins. Saikosaponins also regulate
apoptosis-related genes, downregulate the Wnt/β-catenin and p38
MAPK signaling pathways, reduce the expression levels of calnexin,
serve a vital role in inhibiting the proliferation and promoting
the apoptosis and autophagy of breast cancer cells and exhibit a
concentration and time dependent-effect compared with the clinical
breast cancer drug, paclitaxel, in which saikosaponins have a
higher potency (72).
Invasive metastatic recurrence is the leading cause
of death in patients with breast cancer (73). Epithelial-mesenchymal transition
(EMT) is an important mechanism for initiating the process of
malignant phenotypic transformation and the development of
metastasis in breast cancer (74).
Matrix metalloproteinases (MMPs) degrade the extracellular matrix
(ECM) and promote the metastatic process of tumors, particularly
MMP-2 and MMP-9 (75,76). SSA was shown to inhibit TNBC
invasive metastasis by downregulating the MMP-9 and MMP-2
expression levels in SUM-149 and MDA-MB-231 cells (26). Phosphorylated signal transducers and
activators of transcription 3 (p-STAT3) promotes the invasive
metastasis of TNBC by mediating the EMT (77). Vasodilator stimulated phosphoprotein
(VASP) can modulate actin polymerization and promote breast cancer
development (78,79). SSB-2 suppresses the proliferation
and migration of MCF-7 cells by inhibiting the STAT3 signaling
pathway and reducing the expression levels of VASP, MMP-2 and MMP-9
(80).
The phosphatidylinositol-3-kinase (PI3K)/protein
kinase B (AKT)/mTOR pathway can regulate tumor cell growth,
proliferation, survival, angiogenesis and other processes (81). The stromal cell-derived factor-1
(SDF-1)/chemokine receptor (CXCR4) axis is involved in the invasion
and migration of MDA-MB-231 cells (82), and the crosstalk between the
CXCR4/SDF-1 axis and the AKT/mTOR pathway occurs in circulating
tumor cells (83). CXCR4 expression
is associated with breast cancer growth, angiogenesis and distant
metastasis and is an important indicator of infiltrative metastasis
and poor prognosis (84,85). When compared with normal breast
cancer cells, CXCR4 is highly expressed in TNBC cell lines
(86). Results from an animal study
showed that SSA downregulates the expression of CXCR4 in mouse lung
high metastatic breast cancer luciferase cells (4T1-LUC cells) and
exhibits anti-growth and anti-metastatic effects on TNBC through
multiple signaling pathways, including PI3K/AKT/mTOR and MMPs
(26).
Accordingly, different saikosaponins can inhibit the
invasive and metastatic potential of breast cancer cells and
prevent the occurrence and development of breast cancer through
various signal transduction pathways such as the STAT3, VASP, MMPs,
CXCR4/SDF-1, PI3K/Akt and Akt/mTOR pathways.
Solid tumor cells have the potential to proliferate
indefinitely, and to support cell proliferation and survival, tumor
cells rewire their metabolism to a glycolytic phenotype to satisfy
their escalating bioenergetic demands, with TNBC being the breast
cancer subtype most reliant on glycolysis for energy gain (87). Through the Warburg effect, the
breakdown of glucose into lactic acid allows efficient access to
the ATP required for unlimited proliferation of tumor cells, the
raw material for the synthesis of tumor cell biomolecules and the
precursors essential for other metabolic pathways, ultimately
promoting tumor progression (88,89).
SSA reduces the upregulation of p-STAT3 and p-AKT in MDA-MB-231 and
MCF-7 cells and decreases lactate and ATP production and glucose
uptake in tumor cells through downregulation of the AKT/STAT3
signaling pathway, inhibiting the proliferation of tumor cells
(90).
Bioinformatics and network pharmacology analyses
have shown that flavonoids and saikosaponins can regulate lipid
metabolism-related genes and improve the depressive symptoms of
patients with breast cancer (91,92).
Saikosaponins can increase hepatic uptake of circulating fatty
acids, promote mitochondrial respiration in fatty acid oxidation,
repair imbalanced lipid metabolism and promote intracellular
cholesterol efflux, high-density lipoprotein (HDL) remodeling and
the clearance of low-density lipoprotein (LDL) particles and bile
acid synthesis, significantly modulating cholesterol clearance
(93,94). Certain commonly used drugs for the
treatment of breast cancer, such as exemestane and chloroquine, are
hepatotoxic and have major adverse effects (95,96).
Saikosaponins have been reported to be effective liver protectants
and Chaihu Liver Protection Tablets have been clinically used to
combat oxidative stress and lipid peroxidation reactions associated
with lipid overload (97).
The main component of the breast is adipose tissue
and mammary glands, in which cancer-associated adipocytes can
promote the proliferation and metastasis of breast cancer cells
(98–100). SSA and SSD inhibit adipocyte
3T3-L1 production via the AMP-activated protein kinase (AMPK) and
MAPK signaling pathways (101).
Disorders of glucose and lipid metabolism predispose individuals to
insulin resistance, abnormal glucose tolerance and altered lipid
metabolism, all of which are risk factors for the development of
breast cancer (102,103). It has been previously reported
that SSA can be used to reduce the levels of blood glucose,
triglyceride, free fatty acid, total cholesterol, LDL and HDL in
mice, significantly reduce liver weight and fat accumulation,
downregulate the expression of TNF-α and NF-κB, and upregulate the
expression of fibroblast growth factor-21 and recombinant autophagy
related protein 7, which stimulates the autophagy of cells and
improves insulin resistance (104).
Therefore, saikosaponins can affect the glucose
metabolism of breast cancer cells by regulating the production of
aerobic enzymolysis products and inhibiting the activity of
cytokines. Together with flavonoids, they can also serve a role in
lipid regulation. Targeting the regulation of the AMPK and the MAPK
signaling pathway and other aspects could enable treatments to
target multiple breast tumors. The pharmacological effects of the
active ingredients of Radix Bupleuri are shown in Table II.
The TME consists of cancer cells, stromal cells,
cytokines, chemokines and other factors that serve a crucial role
in breast cancer development, progression and drug resistance
(105). Tumor-associated
macrophages (TAMs) are the most abundant immune cell group in
breast cancer, participate in every stage of cancer progression and
are associated with tumor malignant progression (105,106). TAMs are typically divided into M1
and M2 types, with M1 TAMs contributing to the dormancy of
metastatic breast cancer cells and M2 TAMs promoting tumor growth
(105). Polysaccharides can
upregulate the activity of macrophages, effectively inhibit the
release of key factors of inflammatory response and immune
regulation, exert anti-inflammatory activity, prevent tissue damage
caused by excessive inflammation and may enhance the TME in breast
cancer (43,44). SSD can significantly enhance the
phagocytic ability of macrophages, increase their acid phosphatase
levels and promote the expression of immune-related antigens on the
cell surface (107).
Tumor infiltrating T cells are associated with
improved clinical prognosis and survival in patients with breast
cancer. As a crucial component of the TME, T cells serve a
significant role in the immune response to cancer (108). The balance between T helper (Th)1
and Th2 cells is particularly important, as a shift from Th1 to Th2
promotes breast cancer progression (109). SSA activates the IL-12/STAT4
pathway, leading to a significant increase in the infiltration of
CD8+ and CD4+ T cells in tumors. This
activation occurs through the upregulation of IL-12, IL-12R and
STAT4 gene expression, as well as the increased expression levels
of IL-12, IL-12R and p-STAT4 proteins (25). CD8+ T cells exert
tumor-killing activity by interacting with tumor antigens and
releasing perforin, granzyme and cytokines, which directly or
indirectly kill tumor cells. Additionally, this process promotes a
shift in the Th1/Th2 balance towards a Th1 response, thereby
inhibiting the development and progression of breast cancer
(25).
Therefore, polysaccharides and saikosaponins jointly
regulate the TME, which are important components of Radix
Bupleuri for immune regulation.
The process of tumor angiogenesis includes
endothelial cell activation, ECM degradation and endothelial cell
migration, angiogenesis and extension into the tumor and is
regulated by various cytokines in the TME (110). Vascular endothelial growth factor
(VEGF) is an important angiogenesis factor and blocking VEGF is a
potential strategy for the treatment of invasive breast cancer
(111). The VEGF receptor 2
(VEGFR2) signaling pathway and its downstream proteins are an
important signaling pathway that regulates endothelial cell
function during angiogenesis (112). Clinically, patients with breast
cancer who experience lymph node metastasis often harbor high
expression levels of hypoxia inducible factor 1 subunit α (HIF-1α)
and VEGF. The recurrence and metastasis of breast cancer may be
related to the upregulation of HIF-1α and VEGF, promoting the
angiogenesis of breast cancer and other related factors (113,114). SSA inhibits the phosphorylation of
VEGFR2 and the activity of downstream protein kinases,
phospholipase C-γ-1, focal adhesion kinase, Src and AKT, reducing
tumor angiogenesis and subsequently inhibiting the growth of mouse
breast cancer 4T1 cells (115).
Thus, by downregulating the expression level or activity of
angiogenesis-related proteins, saikosaponins inhibit tumor
angiogenesis and limit the growth and metastasis of breast
tumors.
At present, surgical resection is still the main
treatment option for patients with breast cancer and postoperative
radiotherapy or chemotherapy are often used to further improve
patient prognosis (116).
Radiotherapy and chemotherapy both non-selectively kill cancer
cells and can cause toxic side effects (117). Traditional Chinese Medicine has
been used for the prevention and treatment of breast cancer
(118). Traditional Chinese
Medicine has been reported to target tumors and can be combined
with Western medicine to enhance the efficacy of these medicines
and reduce potential toxicity (119).
During or after chemotherapy, the expression levels
of multidrug resistance protein (MDR)1 and breast cancer resistance
protein, ABCG2, can increase significantly (120). A mechanism of action of MDR is the
upregulation of p-glycoprotein (P-gp), and the direct inhibition of
P-gp upregulation by SSA and SSD in vitro reverses MDR in
MCF-7 cells (121,122). Isorhamnetin mediates the
inhibition of proliferation and the induction of apoptosis in
MCF7/adriamycin (ADR) and MDAMB-231/DOX cells by enhancing the
phosphorylation of AMPK, decreasing the phosphorylation levels of
mTOR and p70S6K, inhibiting the expression levels of B-cell
lymphoma-2 and increasing the cleavage of Caspase 3 (63). In addition, isorhamnetin also
downregulates the expression level of P-gp, leading to increased
intracellular DOX accumulation, increased toxicity, promotion of
apoptosis and the restriction of tumor growth in vivo
(63).
Moderate levels of reactive oxygen species (ROS) can
activate various signaling pathways to promote tumor development
(123,124). However, excessive ROS levels that
cannot be effectively balanced by the antioxidant system can lead
to oxidative stress, promoting apoptosis and the death of cancer
cells. Drug-resistant cells produce higher levels of ROS compared
with non-drug-resistant cells. To maintain redox homeostasis in an
environment with high ROS levels, drug-resistant cancer cells
enhance their antioxidant capacity by increasing the synthesis of
reduced glutathione (GSH) and upregulating antioxidant enzymes,
including NAD(P)H:quinone oxidoreductase 1 (NQO1) (125). NQO1 is significantly upregulated
in various types of solid tumors, suggesting its potential
involvement in cellular defense during oncogenesis (126). NQO1 is reported to be a
STAT1-regulated gene in MCF-7/DOX and MDA-MB-231/DOX-resistant
strain cells, as STAT1 expression levels are positively correlated
with NQO1 expression levels (58).
Molecular docking has shown that SSD may interact with the active
site of NQO1, forming two hydrogen bonds with the Leu103 and Tyr128
residues, suggesting that SSD has a strong binding affinity with
the NQO1 protein (58).
Subsequently, in vitro and in vivo experiments have
been performed to confirm that SSD downregulates the
STAT1/NQO1/peroxisome proliferator-activated receptor
γcoactivator-1 α (PGC-1α) signaling pathway (58). After SSD inhibits NQO1, the
consumption of nicotinamide adenine dinucleotide phosphate (NADPH)
and nicotinamide adenine dinucleotide (NADH) decreases (58). The redox imbalance in the cell is in
a high ROS state, and the antioxidants, GSH, NADPH and NADH, are
consumed in large quantities in response to oxidative stress, which
ultimately leads to a decrease in their levels (58), which leads to an increase in
intracellular oxidative stress levels and ultimately induces
MCF-7/DOX and MDA-MB-231/DOX apoptosis (58).
Patients with breast cancer treated with radiation
and chemotherapy may have adverse reactions, such as insomnia, bone
marrow suppression, leukopenia and depression (127). Radix Bupleuri, as the main
ingredient of Xiao Chaihu Tang, Jia Wei Chaihu Gui Jiang
Tang and other Traditional Chinese Medicine compound tonics,
has been reported to clinically improve the postoperative adverse
effects in patients with breast cancer, reduce or slow down the
secretion of tumor markers and improve the efficacy of certain
chemotherapeutic agents such as paclitaxel and cisplatin (27,128).
The stability of subcellular organelles and metal
ions is essential for various physiological and biochemical
processes, such as homeostasis of the internal environment,
regulation of cellular metabolism, substance synthesis, signal
transmission and energy conversion (129). Staphylococcus aureus
infection can cause an increase in inflammatory markers such as
myeloperoxidase, TNF-α and IL-1 β in mice, as well as an
accumulation of Fe2+; it significantly increases the
expression levels of malondialdehyde (MDA) and reduces the
expression levels of GSH, indicating that S. aureus induces
ferroptosis in normal breast cells, leading to the development of
mastitis. Additionally, S. aureus reduces the expression
levels of sirtuin 1 (SIRT1), nuclear factor erythroid 2-related
factor 2 (Nrf2) and heme oxygenase 1 (HO-1); however, SSA treatment
increases the expression levels of SIRT1, Nrf2 and HO-1 in a
dose-dependent manner, indicating that SSA inhibits S.
aureus-induced mastitis by activating the SIRT1/Nrf2 signaling
pathway (34).
The study of signaling pathways has implications for
the treatment of certain diseases and future biotechnological
innovations. A number of relevant signaling pathways activated by
Radix Bupleuri active ingredients cause anti-breast cancer
effects (Fig. 2).
The incidence and mortality rates of breast cancer
continue to rise on a global scale (1). Current treatment approaches focus on
precise treatment methods, minimizing the extent of breast
resection (138) and utilizing a
combination of radiotherapy, chemotherapy and immunotherapy
post-surgery to enhance patient quality of life and extend survival
duration. However, there are several issues with these treatments,
such as adverse reactions and drug resistance. Traditional Chinese
Medicine may exhibit anticancer properties that target various
cellular signaling pathways (139,140). For instance, Radix
Bupleuri, a component of Traditional Chinese Medicine, contains
a diverse array of active ingredients with promising anticancer
applications. Saikosaponins, polysaccharides and flavonoids found
in Radix Bupleuri have been shown to regulate cell cycle
proteins, inhibit breast cancer cell proliferation and induce
apoptosis. Furthermore, Radix Bupleuri and its constituents
have been reported to prevent tumor cell invasion and metastasis,
influence tumor cell metabolism, modulate TAMs, enhance the TME,
reduce MDR protein expression levels in tumor cells and aid in the
efficacy of radiotherapy and chemotherapy by boosting immune system
activity.
Nevertheless, current research is focused on
identifying changes in protein expression levels and signaling
pathways, with specific targets of this treatment yet to be fully
understood. New technologies such as multi-omics studies, network
pharmacology and molecular docking, offer novel perspectives for
gaining a deeper insight into the mechanism of action of Radix
Bupleuri as an adjuvant chemotherapy agent. While combining
Radix Bupleuri with conventional chemotherapy drugs shows
potential in reversing drug resistance, rigorous clinical trials
are essential to assess the safety and efficacy of this treatment
approach. The adoption of modern ultrafine grinding technology and
nanotechnology can improve the efficient utilization and absorption
of herbal components, reduce toxicity, enhance chemotherapeutic
efficacy and improve the acceptance of herbal medicines by young
patients.
However, current studies in this area have a number
of limitations. First, according to bibliometric analysis, SSD is
the most studied Radix Bupleuri monomer as an anti-breast
cancer drug, but there are no detailed reports on the safety and
toxicology of SSD, which may limit its clinical development.
Second, current research is limited to in vitro cell and
in vivo animal experiments and there is little clinical
trial data. Third, the current research on the mechanism of action
of Radix Bupleuri is lacking in depth, especially the study
of the targets of compounds, and there are no consistent reports on
the concentrations and treatment durations of these compounds that
are required to induce anticancer effects.
Not applicable.
The present study was funded by the Sichuan Science and
Technology Program (grant nos. 2022YFS0623, 2024YFFK0346 and
2024NSFSC0561), the Sichuan Science and Technology Program Joint
Innovation Grant (grant no. 2022YFS0623-B3), the Southwest Medical
University Grant (grant nos. 2021ZKZD005 and 2022QN117), and the
Southwest Medical University College Student Innovation and
Entrepreneurship Training Program (grant no. 2024314).
Not applicable.
Conceptualization was conducted by SJ and WY;
formal analysis and data interpretation of the literature were
conducted by SJ, CL, TH and WY; literature analysis was conducted
by SJ, CL, DL, FZ and WW; writing of the original draft was
conducted by SJ, CL, TH and WY; reviewing and editing of the
manuscript was conducted by SJ, TH and WY; construction of the
figures was conducted by SJ and CL; supervision was conducted by
SJ, TH and WY; project administration was conducted by FZ, TH and
WY. Data authentication is not applicable. All authors have read
and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nolan E, Lindeman GJ and Visvader JE:
Deciphering breast cancer: From biology to the clinic. Cell.
186:1708–1728. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gradishar WJ, Moran MS, Abraham J,
Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ,
Chew H, et al: NCCN Guidelines® Insights: Breast cancer,
version 4.2023. J Natl Compr Canc Netw. 21:594–608. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Z, Shen H, Xu J, Fang Z, Wo G, Ma Y,
Yang K, Wang Y, Yu Q and Tang JH: GATA3 mediates doxorubicin
resistance by inhibiting CYB5R2-catalyzed iron reduction in breast
cancer cells. Drug Resist Updat. 69:1009742023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cancer Genome Atlas Network, .
Comprehensive molecular portraits of human breast tumours. Nature.
490:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang Y, Zhang H, Song X and Yang Q:
Metastatic heterogeneity of breast cancer: Molecular mechanism and
potential therapeutic targets. Semin Cancer Biol. 60:14–27. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bai X, Ni J, Beretov J, Graham P and Li Y:
Cancer stem cell in breast cancer therapeutic resistance. Cancer
Treat Rev. 69:152–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang R, Lee YG, Dhandapani S, Baek NI, Kim
KP, Cho YE, Xu X and Kim YJ: 8-paradol from ginger exacerbates
PINK1/Parkin mediated mitophagy to induce apoptosis in human
gastric adenocarcinoma. Pharmacol Res. 187:1066102023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YX, Gao QY, Zou TH, Wang BM, Liu SD,
Sheng JQ, Ren JL, Zou XP, Liu ZJ, Song YY, et al: Berberine versus
placebo for the prevention of recurrence of colorectal adenoma: A
multicentre, double-blinded, randomised controlled study. Lancet
Gastroenterol Hepatol. 5:267–275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Chen Z, Luo J, Zhang J, Sang AM,
Cheng ZS and Li XY: Corrigendum to ‘Salidroside postconditioning
attenuates ferroptosis-mediated lung ischemia-reperfusion injury by
activating the Nrf2/SLC7A11 signaling axis’ [Int. Immunopharmacol.
115 (2023) 109731]. Int Immunopharmacol. 115:1097312023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Rokavec M, Huang Z and Hermeking H:
Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress
colorectal cancer metastasis. Cell Death Differ. 30:1771–1785.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu K, Xia Y, Cheng P, Li Y, He L, Tao L,
Wei Z and Lu Y: Synergistic potentiation of the anti-metastatic
effect of a Ginseng-Salvia miltiorrhiza herbal pair and its
biological ingredients via the suppression of CD62E-dependent
neutrophil infiltration and NETformation. J Adv Res. S2090–1232.
2024.
|
|
13
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the nearly four decades from 01/1981
to 09/2019. J Nat Prod. 83:770–803. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Sun J, Li C, Qiao H and Hussain
Z: Functionalization of curcumin nanomedicines: A recent promising
adaptation to maximize pharmacokinetic profile, specific cell
internalization and anticancer efficacy against breast cancer. J
Nanobiotechnology. 21:1062023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang P, Liu W and Wang Y: The mechanisms
of tanshinone in the treatment of tumors. Front Pharmacol.
14:12822032023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ni LJ, Zhang LG, Hou J, Shi WZ and Guo ML:
A strategy for evaluating antipyretic efficacy of Chinese herbal
medicines based on UV spectra fingerprints. J Ethnopharmacol.
124:79–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng Y, Gao X, Meng M, Xue H and Qin X:
Multi-omics reveals the mechanisms of antidepressant-like effects
of the low polarity fraction of Bupleuri Radix. J Ethnopharmacol.
256:1128062020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Zhang S, Hu M, Chen Y, Wang W,
Zhang K, Kuang H and Wang Q: An integrative metabolomics and
network pharmacology method for exploring the effect and mechanism
of Radix Bupleuri and Radix Paeoniae Alba on
anti-depression. J Pharm Biomed Anal. 189:1134352020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Li S, Yu J, Wang W, Du Z, Gao S,
Ma Y, Tang R, Liu T, Ma S, et al: Saikosaponin B2 ameliorates
depression-induced microglia activation by inhibiting
ferroptosis-mediated neuroinflammation and ER stress. J
Ethnopharmacol. 316:1167292023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YX, Du Y, Liu XF, Yang FX, Wu X, Tan
L, Lu YH, Zhang JW, Zhou F and Wang GJ: A hepatoprotection study of
Radix Bupleuri on acetaminophen-induced liver injury based
on CYP450 inhibition. Chin J Nat Med. 17:517–524. 2019.PubMed/NCBI
|
|
21
|
Chen LL, Xia LY, Zhang JP, Wang Y, Chen
JY, Guo C and Xu WH: Saikosaponin D alleviates cancer cachexia by
directly inhibiting STAT3. Phytother Res. 37:809–819. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia R, Gu Z, He Q, Du J, Cao L, Jeney G,
Xu P and Yin G: Anti-oxidative, anti-inflammatory and
hepatoprotective effects of Radix Bupleuri extract against
oxidative damage in tilapia (Oreochromis niloticus) via Nrf2 and
TLRs signaling pathway. Fish Shellfish Immunol. 93:395–405. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SO, Park JY, Jeon SY, Yang CH and Kim
MR: Saikosaponin a, an active compound of Radix Bupleuri,
attenuates inflammation in hypertrophied 3T3-L1 adipocytes via
ERK/NF-κB signaling pathways. Int J Mol Med. 35:1126–1132. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren M, McGowan E, Li Y, Zhu X, Lu X, Zhu
Z, Lin Y and He S: Saikosaponin-d Suppresses COX2 Through
p-STAT3/C/EBPβ signaling pathway in liver cancer: A novel mechanism
of action. Front Pharmacol. 10:6232019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao X, Liu J, Ge S, Chen C, Li S, Wu X,
Feng X, Wang Y and Cai D: Saikosaponin A inhibits breast cancer by
regulating Th1/Th2 balance. Front Pharmacol. 10:6242019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Zhao L, Han X, Wang Y, Mi J, Wang
C, Sun D, Fu Y, Zhao X, Guo H and Wang Q: Saikosaponin A inhibits
triple-negative breast cancer growth and metastasis through
downregulation of CXCR4. Front Oncol. 9:14872019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Gan J, Han M, Wu Y, Liu L, Zhao Y
and Zhao R: Comparison of structure and the synergistic
anti-hepatocellular carcinoma effect of three polysaccharides from
vinegar-baked Radix Bupleuri. Int J Biol Macromol.
282:1367552024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng YL, Lee SC, Lin SZ, Chang WL, Chen
YL, Tsai NM, Liu YC, Tzao C, Yu DS and Harn HJ: Anti-proliferative
activity of Bupleurum scrozonerifolium in A549 human lung cancer
cells in vitro and in vivo. Cancer Lett. 222:183–193. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Witek Janusek L, Tell D and Mathews HL:
Mindfulness based stress reduction provides psychological benefit
and restores immune function of women newly diagnosed with breast
cancer: A randomized trial with active control. Brain Behav Immun.
80:358–373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cui B, Luo Y, Tian P, Peng F, Lu J, Yang
Y, Su Q, Liu B, Yu J, Luo X, et al: Stress-induced epinephrine
enhances lactate dehydrogenase A and promotes breast cancer
stem-like cells. J Clin Invest. 129:1030–1046. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Wang N, Zheng Y, Yang B, Wang S,
Wang X, Pan B and Wang Z: Naringenin in Si-Ni-San formula inhibits
chronic psychological stress-induced breast cancer growth and
metastasis by modulating estrogen metabolism through FXR/EST
pathway. J Adv Res. 47:189–207. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim S, Chen J, Cheng T, Gindulyte A, He J,
He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, et al: PubChem 2023
update. Nucleic Acids Res. 51:D1373–D1380. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan B, Yang R, Ma Y, Zhou S, Zhang X and
Liu Y: A systematic review of the active saikosaponins and extracts
isolated from Radix Bupleuri and their applications. Pharm
Biol. 55:620–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao L, Jin L and Yang B: Saikosaponin A
alleviates Staphylococcus aureus-induced mastitis in mice by
inhibiting ferroptosis via SIRT1/Nrf2 pathway. J Cell Mol Med.
27:3443–3450. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu Y, Hu X, Cao Y, Zhang Z and Zhang N:
Saikosaponin a inhibits lipopolysaccharide-oxidative stress and
inflammation in Human umbilical vein endothelial cells via
preventing TLR4 translocation into lipid rafts. Free Radic Biol
Med. 89:777–785. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong H, Han X, Hao M, Yang Q, Lyu Q, Tang
D, Shen Z, Wang K, Kuang H, Cao G, et al: Nanodrug rescues liver
fibrosis via synergistic therapy with H2O2 depletion and
Saikosaponin b1 sustained release. Commun Biol. 6:1842023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sinha SK, Shakya A, Prasad SK, Singh S,
Gurav NS, Prasad RS and Gurav SS: An in-silico evaluation of
different Saikosaponins for their potency against SARS-CoV-2 using
NSP15 and fusion spike glycoprotein as targets. J Biomol Struct
Dyn. 39:3244–3255. 2021.PubMed/NCBI
|
|
38
|
Chiang LC, Ng LT, Liu LT, Shieh DE and Lin
CC: Cytotoxicity and anti-hepatitis B virus activities of
saikosaponins from Bupleurum species. Planta Med. 69:705–709. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu Y and Lai Y: Pharmacological
properties and derivatives of saikosaponins-a review of recent
studies. J Pharm Pharmacol. 75:898–909. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wan H, Zhou L, Wu B, Han W, Sui C and Wei
J: Integrated metabolomics and transcriptomics analysis of roots of
Bupleurum chinense and B. scorzonerifolium, two sources of
medicinal Chaihu. Sci Rep. 12:223352022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang P, Ji X, Xia J, Xu M, Hao F, Tong H
and Jiao L: Structure and potential anti-fatigue mechanism of
polysaccharides from Bupleurum Chinense DC. Carbohydr Polym.
306:1206082023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang ZD, Li H, Wan F, Su XY, Lu Y, Chen
DF and Zhang YY: Polysaccharides extracted from the roots of
Bupleurum Chinense DC modulates macrophage functions. Chin J Nat
Med. 15:889–898. 2017.PubMed/NCBI
|
|
43
|
Tong H, Tian D, Li T, Wang B, Jiang G and
Sun X: Inhibition of inflammatory injure by polysaccharides from
Bupleurum Chinense through antagonizing P-selectin. Carbohydr
Polym. 105:20–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng XQ, Li H, Yue XL, Xie JY, Zhang YY,
Di HY and Chen DF: Macrophage immunomodulatory activity of the
polysaccharides from the roots of Bupleurum smithii var.
parvifolium. J Ethnopharmacol. 130:363–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie JY, Di HY, Li H, Cheng XQ, Zhang YY
and Chen DF: Bupleurum chinense DC polysaccharides attenuates
lipopolysaccharide-induced acute lung injury in mice.
Phytomedicine. 19:130–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi H, He J, Li X, Han J, Wu R, Wang D,
Yang F and Sun E: Isorhamnetin, the active constituent of a Chinese
herb Hippophae rhamnoides L, is a potent suppressor of
dendritic-cell maturation and trafficking. Int Immunopharmacol.
55:216–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
García-Mediavilla V, Crespo I, Collado PS,
Esteller A, Sánchez-Campos S, Tuñón MJ and González-Gallego J: The
anti-inflammatory flavones quercetin and kaempferol cause
inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and
reactive C-protein, and down-regulation of the nuclear factor
kappaB pathway in Chang Liver cells. Eur J Pharmacol. 557:221–229.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang X, Wang H, Shen C, Dong X, Li J and
Liu J: Effects of isorhamnetin on liver injury in heat
stroke-affected rats under dry-heat environments via oxidative
stress and inflammatory response. Sci Rep. 14:74762024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Haddouchi F, Chaouche TM, Ksouri R and
Larbat R: Leafy Stems of Phagnalon saxatile subsp. saxatile from
Algeriaas a Source of chlorogenic acids and flavonoids with
antioxidant activity: Characterization and quantification using
UPLC-DAD-ESI-MSn. Metabolites. 11:2802021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tao X, Hu X, Wu T, Zhou D, Yang D, Li X,
Fu Y, Zheng F, Yue H and Dai Y: Characterization and screening of
anti-melanogenesis and anti-photoaging activity of different
enzyme-assisted polysaccharide extracts from Portulaca
oleracea L. Phytomedicine. 116:1548792023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang YF, An ZY, Lin DH and Jin WL:
Targeting cancer cachexia: Molecular mechanisms and clinical study.
MedComm (2020). 3:e1642022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y, Wu MJ, Lu WC, Li YC, Chang CJ and
Yang JY: Metabolic switch regulates lineage plasticity and induces
synthetic lethality in triple-negative breast cancer. Cell Metab.
36:193–208.e8. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Duan N, Hua Y, Yan X, He Y, Zeng T, Gong
J, Fu Z, Li W and Yin Y: Unveiling alterations of epigenetic
modifications and chromatin architecture leading to lipid metabolic
reprogramming during the evolutionary trastuzumab adaptation of
HER2-positive breast cancer. Adv Sci (Weinh). 11:e23094242024.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Garcia-Martinez L, Zhang Y, Nakata Y, Chan
HL and Morey L: Epigenetic mechanisms in breast cancer therapy and
resistance. Nat Commun. 12:17862021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang F, Xiao Y, Ding JH, Jin X, Ma D, Li
DQ, Shi JX, Huang W, Wang YP, Jiang YZ and Shao ZM: Ferroptosis
heterogeneity in triple-negative breast cancer reveals an
innovative immunotherapy combination strategy. Cell Metab.
35:84–100.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Matthews HK, Bertoli C and de Bruin RAM:
Cell cycle control in cancer. Nat Rev Mol Cell Biol. 23:74–88.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Asghar U, Witkiewicz AK, Turner NC and
Knudsen ES: The history and future of targeting cyclin-dependent
kinases in cancer therapy. Nat Rev Drug Discov. 14:130–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Luo F, Yang J, Yang X, Mi J, Ye T, Li G
and Xie Y: Saikosaponin D potentiates the antineoplastic effects of
doxorubicin in drug-resistant breast cancer through perturbing
NQO1-mediated intracellular redox balance. Phytomedicine.
133:1559452024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hinz N and Jücker M: Distinct functions of
AKT isoforms in breast cancer: A comprehensive review. Cell Commun
Signal. 17:1542019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen JC, Chang NW, Chung JG and Chen KC:
Saikosaponin-A induces apoptotic mechanism in human breast
MDA-MB-231 and MCF-7 cancer cells. Am J Chin Med. 31:363–377. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Su M, Ren X, Du D, He H, Zhang D, Xie R,
Deng X, Zou C and Zou H: Curcumol β-cyclodextrin inclusion complex
enhances radiosensitivity of esophageal cancer under hypoxic and
normoxic condition. Jpn J Radiol. 41:1275–1289. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhu J, Ye L, Sun S, Yuan J, Huang J and
Zeng Z: Involvement of RFC3 in tamoxifen resistance in ER-positive
breast cancer through the cell cycle. Aging. 15:13738–13752. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang T, Xiao Y, Liu S, Luo F, Tang D, Yu Y
and Xie Y: Isorhamnetin induces cell cycle arrest and apoptosis by
triggering DNA damage and regulating the AMPK/mTOR/p70S6K signaling
pathway in doxorubicin-resistant breast cancer. Phytomedicine.
114:1547802023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Su X, Esser AK, Amend SR, Xiang J, Xu Y,
Ross MH, Fox GC, Kobayashi T, Steri V, Roomp K, et al: Antagonizing
integrin β3 increases immunosuppression in cancer. Cancer Res.
76:3484–3495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Murali B, Ren Q, Luo X, Faget DV, Wang C,
Johnson RM, Gruosso T, Flanagan KC, Fu Y, Leahy K, et al:
Inhibition of the stromal p38MAPK/MK2 pathway limits breast cancer
metastases and chemotherapy-induced bone loss. Cancer Res.
78:5618–5630. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Canovas B and Nebreda AR: Diversity and
versatility of p38 kinase signalling in health and disease. Nat Rev
Mol Cell Biol. 22:346–366. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fu R, Zhang L, Li Y, Li B, Ming Y, Li Z,
Xing H and Chen J: Saikosaponin D inhibits autophagosome-lysosome
fusion and induces autophagy-independent apoptosis in MDA-MB-231
breast cancer cells. Mol Med Rep. 22:1026–1034. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Han CC and Wan FS: New Insights into the
role of endoplasmic reticulum stress in breast cancer metastasis. J
Breast Cancer. 21:354–362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Paskevicius T, Farraj RA, Michalak M and
Agellon LB: Calnexin, more than just a molecular chaperone. Cells.
12:4032023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wong VK, Li T, Law BY, Ma ED, Yip NC,
Michelangeli F, Law CK, Zhang MM, Lam KY, Chan PL and Liu L:
Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell
death in apoptosis-defective cells. Cell Death Dis. 4:e7202013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pohl SG, Brook N, Agostino M, Arfuso F,
Kumar AP and Dharmarajan A: Wnt signaling in triple-negative breast
cancer. Oncogenesis. 6:e3102017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang J, Qi H, Zhang X, Si W, Xu F, Hou T,
Zhou H, Wang A, Li G, Liu Y, et al: Saikosaponin D from Radix
Bupleuri suppresses triple-negative breast cancer cell growth
by targeting β-catenin signaling. Biomed Pharmacother. 108:724–733.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Augimeri G, Gonzalez ME, Paolì A, Eido A,
Choi Y, Burman B, Djomehri S, Karthikeyan SK, Varambally S,
Buschhaus JM, et al: A hybrid breast cancer/mesenchymal stem cell
population enhances chemoresistance and metastasis. JCI Insight.
8:e1642162023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
He J, Chen S, Yu T, Chen W, Huang J, Peng
C and Ding Y: Harmine suppresses breast cancer cell migration and
invasion by regulating TAZ-mediated epithelial-mesenchymal
transition. Am J Cancer Res. 12:2612–2626. 2022.PubMed/NCBI
|
|
75
|
Kwon KR, Alam MB, Park JH, Kim TH and Lee
SH: Attenuation of UVB-Induced photo-aging by polyphenolic-rich
spatholobus suberectus stem extract via modulation of
MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients.
11:13412019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nakanishi M, Korechika A, Yamakawa H,
Kawabe N, Nakai K and Muragaki Y: Acidic microenvironment induction
of interleukin-8 expression and matrix metalloproteinase-2/-9
activation via acid-sensing ion channel 1 promotes breast cancer
cell progression. Oncol Rep. 45:1284–1294. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Qi Y, Wu H, Zhu T, Liu Z, Liu C, Yan C, Wu
Z, Xu Y, Bai Y, Yang L, et al: Acetyl-cinobufagin suppresses
triple-negative breast cancer progression by inhibiting the STAT3
pathway. Aging. 15:8258–8274. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li K, Zhang J, Tian Y, He Y, Xu X, Pan W,
Gao Y, Chen F and Wei L: The Wnt/β-catenin/VASP positive feedback
loop drives cell proliferation and migration in breast cancer.
Oncogene. 39:2258–2274. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tian Y, Xu L, He Y, Xu X, Li K, Ma Y, Gao
Y, Wei D and Wei L: Knockdown of RAC1 and VASP gene expression
inhibits breast cancer cell migration. Oncol Lett. 16:2151–2160.
2018.PubMed/NCBI
|
|
80
|
Ma Q, Gao FF, He X, Li K, Gao Y, Xu XL,
Jiang NH, Ding L, Song WJ, He YQ, et al: Antitumor effects of
saikosaponin b2 on breast cancer cell proliferation and migration.
Mol Med Rep. 20:1943–1951. 2019.PubMed/NCBI
|
|
81
|
Miricescu D, Totan A, Stanescu S II,
Badoiu SC, Stefani C and Greabu M: PI3K/AKT/mTOR signaling pathway
in breast cancer: From molecular landscape to clinical aspects. Int
J Mol Sci. 22:1732020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang J, Chen J, Wo D, Yan H, Liu P, Ma E,
Li L, Zheng L, Chen D, Yu Z, et al: LRP6 ectodomain prevents
SDF-1/CXCR4-induced breast cancer metastasis to lung. Clin Cancer
Res. 25:4832–4845. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xin Y, Hu B, Li K, Hu G, Zhang C, Chen X,
Tang K, Du P and Tan Y: Circulating tumor cells with
metastasis-initiating competence survive fluid shear stress during
hematogenous dissemination through CXCR4-PI3K/AKT signaling. Cancer
Lett. 590:2168702024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jankowski K, Kucia M, Wysoczynski M, Reca
R, Zhao D, Trzyna E, Trent J, Peiper S, Zembala M, Ratajczak J, et
al: Both hepatocyte growth factor (HGF) and stromal-derived
factor-1 regulate the metastatic behavior of human rhabdomyosarcoma
cells, but only HGF enhances their resistance to radiochemotherapy.
Cancer Res. 63:7926–7935. 2003.PubMed/NCBI
|
|
86
|
Gupta N, Mohan CD, Shanmugam MK, Jung YY,
Chinnathambi A, Alharbi SA, Ashrafizadeh M, Mahale M, Bender A,
Kumar AP, et al: CXCR4 expression is elevated in TNBC patient
derived samples and Z-guggulsterone abrogates tumor progression by
targeting CXCL12/CXCR4 signaling axis in preclinical breast cancer
model. Environ Res. 232:1163352023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Schreier A, Zappasodi R, Serganova I,
Brown KA, Demaria S and Andreopoulou E: Facts and perspectives:
Implications of tumor glycolysis on immunotherapy response in
triple negative breast cancer. Front Oncol. 12:10617892022.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Pavlova NN, Zhu J and Thompson CB: The
hallmarks of cancer metabolism: Still emerging. Cell Meta.
34:355–377. 2022. View Article : Google Scholar
|
|
89
|
Arundhathi JRD, Mathur SR, Gogia A, Deo
SVS, Mohapatra P and Prasad CP: Metabolic changes in triple
negative breast cancer-focus on aerobic glycolysis. Mol Biol Rep.
48:4733–4745. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang Y, Dai K, Xu D, Fan H, Ji N, Wang D,
Zhao Y and Liu R: Saikosaponin A alleviates glycolysis of breast
cancer cells through repression of Akt/STAT3 pathway. Chem Biol
Drug Des. 102:115–125. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhu Q, Han Y, He Y, Meng P, Fu Y, Yang H,
He G, Long M and Shi Y: Quercetin inhibits neuronal Ferroptosis and
promotes immune response by targeting lipid metabolism-related gene
PTGS2 to alleviate breast cancer-related depression. Phytomedicine.
130:1555602024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Luo K, Dai RJ, Zeng YB, Chang WJ, Deng YL
and Lv F: Triterpenoid saponins from Bupleurum marginatum and their
anti-liver fibrotic activities. J Asian Nat Prod Res. 26:858–864.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fan J, Li X, Li P, Li N, Wang T, Shen H,
Siow Y, Choy P and Gong Y: Saikosaponin-d attenuates the
development of liver fibrosis by preventing hepatocyte injury.
Biochem Cell Biol. 85:189–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chang GR, Lin WL, Lin TC, Liao HJ and Lu
YW: The ameliorative effects of saikosaponin in
Thioacetamide-induced liver injury and non-alcoholic fatty liver
disease in mice. Int J Mol Sci. 22:113832021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Decensi A, Dunn BK, Puntoni M, Gennari A
and Ford LG: Exemestane for breast cancer prevention: a critical
shift? Cancer Discov. 2:25–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Costedoat-Chalumeau N, Dunogué B, Leroux
G, Morel N, Jallouli M, Le Guern V, Piette JC, Brézin AP, Melles RB
and Marmor MF: A critical review of the effects of
hydroxychloroquine and chloroquine on the eye. Clin Rev Allergy
Immunol. 49:317–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zheng Q, Li X, Huang N, Li F, Ge J, Wang
D, Sun R and Liu R: Saikosaponins ameliorate hyperlipidemia in rats
by enhancing hepatic lipid and cholesterol metabolism. J
Ethnopharmacol. 305:1161102023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang F and Liu S: Mechanistic insights of
adipocyte metabolism in regulating breast cancer progression.
Pharmacol Res. 155:1047412020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hoy AJ, Balaban S and Saunders DN:
Adipocyte-tumor cell metabolic crosstalk in breast cancer. Trends
Mol Med. 23:381–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu L, Wu Y, Zhang C, Li Y, Zeng Y, Zhang
C, Li R, Luo D, Wang L, Zhang L, et al: Cancer-associated
adipocyte-derived G-CSF promotes breast cancer malignancy via Stat3
signaling. J Mol Cell Biol. 12:723–737. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lim SH, Lee HS, Han HK and Choi CI:
Saikosaponin A and D inhibit Adipogenesis via the AMPK and MAPK
signaling pathways in 3T3-L1 adipocytes. Int J Mol Sci.
22:114092021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Boyle P, Boniol M, Koechlin A, Robertson
C, Valentini F, Coppens K, Fairley LL, Boniol M, Zheng T, Zhang Y,
et al: Diabetes and breast cancer risk: A meta-analysis. Br J
Cancer. 107:1608–1617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhao P, Xia N, Zhang H and Deng T: The
metabolic syndrome is a risk factor for breast cancer: A systematic
review and Meta-analysis. Obes Facts. 13:384–396. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lee MS, Noh JW and Lee BC: Effects of
Saikosaponin-A on insulin resistance in obesity: Computational and
animal experimental study. Chem Pharm Bull (Tokyo). 72:365–373.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mittal S, Brown NJ and Holen I: The breast
tumor microenvironment: Role in cancer development, progression and
response to therapy. Expert Rev Mol Diagn. 18:227–243. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Munir MT, Kay MK, Kang MH, Rahman MM,
Al-Harrasi A, Choudhury M, Moustaid-Moussa N, Hussain F and Rahman
SM: Tumor-associated macrophages as multifaceted regulators of
breast tumor growth. Int J Mol Sci. 22:56262021. View Article : Google Scholar
|
|
107
|
Yao RY, Zou YF and Chen XF: Traditional
use, pharmacology, toxicology, and quality control of species in
genus Bupleurum L. Chin Herb Med. 5:245–255. 2013.PubMed/NCBI
|
|
108
|
Virassamy B, Caramia F, Savas P, Sant S,
Wang J, Christo SN, Byrne A, Clarke K, Brown E, Teo ZL, et al:
Intratumoral CD8+ T cells with a tissue-resident memory
phenotype mediate local immunity and immune checkpoint responses in
breast cancer. Cancer Cell. 41:585–601.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Xiao Y, Huang Y, Jiang J, Chen Y and Wei
C: Identification of the prognostic value of Th1/Th2 ratio and a
novel prognostic signature in basal-like breast cancer. Hereditas.
160:22023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Talmadge JE and Fidler IJ: AACR centennial
series: The biology of cancer metastasis: historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xu Z, Goel HL, Burkart C, Burman L, Chong
YE, Barber AG, Geng Y, Zhai L, Wang M, Kumar A, et al: Inhibition
of VEGF binding to neuropilin-2 enhances chemosensitivity and
inhibits metastasis in triple-negative breast cancer. Sci Transl
Med. 15:eadf11282023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Claesson-Welsh L and Welsh M: VEGFA and
tumour angiogenesis. J Intern Med. 273:114–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Srivastava N, Usmani SS, Subbarayan R,
Saini R and Pandey PK: Hypoxia: Syndicating triple negative breast
cancer against various therapeutic regimens. Front Oncol.
13:11991052023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Al Kawas H, Saaid I, Jank P, Westhoff CC,
Denkert C, Pross T, Weiler KBS and Karsten MM: How VEGF-A and its
splice variants affect breast cancer development-clinical
implications. Cell Oncol (Dordr). 45:227–239. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang P, Lai X, Zhu MH, Long M, Liu XL,
Wang ZX, Zhang Y, Guo RJ, Dong J, Lu Q, et al: Saikosaponin A, a
triterpene saponin, suppresses angiogenesis and tumor growth by
blocking VEGFR2-mediated signaling pathway. Front Pharmacol.
12:7132002021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Nanda A, Hu J, Hodgkinson S, Ali S,
Rainsbury R and Roy PG: Oncoplastic breast-conserving surgery for
women with primary breast cancer. Cochrane Database Syst Rev.
10:Cd0136582021.PubMed/NCBI
|
|
117
|
Williams LJ, Kunkler IH, Taylor KJ, Dunlop
J, Piper T, Caldwell J, Jack W, Loane JF, Elder K, Bartlett JMS, et
al: Postoperative radiotherapy in women with early operable breast
cancer (Scottish Breast Conservation Trial): 30-year update of a
randomised, controlled, phase 3 trial. Lancet Oncol. 25:1213–1221.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li J, Wang S, Wang N, Zheng Y, Yang B,
Wang X, Zhang J, Pan B and Wang Z: Aiduqing formula inhibits breast
cancer metastasis by suppressing TAM/CXCL1-induced Treg
differentiation and infiltration. Cell Commun Signal. 19:892021.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wu TN, Chen HM and Shyur LF: Current
advancements of Plant-derived agents for Triple-negative breast
cancer therapy through deregulating cancer cell functions and
reprogramming tumor microenvironment. Int J Mol Sci. 22:135712021.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li W, Zhang H, Assaraf YG, Zhao K, Xu X,
Xie J, Yang DH and Chen ZS: Overcoming ABC transporter-mediated
multidrug resistance: Molecular mechanisms and novel therapeutic
drug strategies. Drug Resist Updat. 27:14–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Li C, Guan X, Xue H, Wang P, Wang M and
Gai X: Reversal of P-glycoprotein-mediated multidrug resistance is
induced by saikosaponin D in breast cancer MCF-7/adriamycin cells.
Pathol Res Pract. 213:848–853. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ye RP and Chen ZD: Saikosaponin A, an
active glycoside from Radix Bupleuri, reverses
P-glycoprotein-mediated multidrug resistance in MCF-7/ADR cells and
HepG2/ADM cells. Xenobiotica. 47:176–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yuan Z, Yu F, Zhang D and Wang H:
Profiling of the assembly of RecA nucleofilaments implies a
potential target for environmental factors to disturb DNA repair. J
Environ Sci (China). 102:283–290. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Xu J, Bi G, Luo Q, Liu Y, Liu T, Li L,
Zeng Q, Wang Q, Wang Y, Yu J and Yi P: PHLDA1 modulates the
endoplasmic reticulum stress response and is required for
resistance to oxidative Stress-induced cell death in human ovarian
cancer cells. J Cancer. 12:5486–5493. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang Y, Wang F, Shi L, Lu M, Lee KJ,
Ditty MM, Xing Y, He HZ, Ren X and Zheng SY: Nanoscale coordination
polymers enabling antioxidants inhibition for enhanced chemodynamic
therapy. J Control Release. 354:196–206. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Roy NJ, Save SN and Sharma VK, Lim SL,
Baskar N and Sharma VK: NAD(P)H:Quinone acceptor oxidoreductase 1
(NQO1) activatable Salicylamide H+/Cl transporters. Chemistry.
29:e2023014122023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Liu C, Cheng B, Zhao G and Yuan H: Process
analysis of anthracycline adverse reactions in breast cancer
patients with postoperative chemotherapy. J Investig Med.
70:1352–1357. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhao Y, Xu D, Wang J, Zhou D, Liu A, Sun
Y, Yuan Y, Li J and Guo W: The pharmacological mechanism of
chaihu-jia-longgu-muli-tang for treating depression: Integrated
meta-analysis and network pharmacology analysis. Front Pharmacol.
14:12576172023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Hu H, Xu Q, Mo Z, Hu X, He Q, Zhang Z and
Xu Z: New anti-cancer explorations based on metal ions. J
Nanobiotechnology. 20:4572022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhao B, Wei D, Long Q, Chen Q, Wang F,
Chen L, Li Z, Li T, Ma T, Liu W, et al: Altered synaptic currents,
mitophagy, mitochondrial dynamics in Alzheimer's disease models and
therapeutic potential of Dengzhan Shengmai capsules intervention. J
Pharm Anal. 14:348–370. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Begum HM and Shen K: Intracellular and
microenvironmental regulation of mitochondrial membrane potential
in cancer cells. WIREs Mech Dis. 15:e15952023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Song IS, Jeong YJ, Jeong SH, Kim JE, Han
J, Kim TH and Jang SW: Modulation of mitochondrial ERβ expression
inhibits Triple-negative breast cancer tumor progression by
activating mitochondrial function. Cell Physiol Biochem.
52:468–485. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wang P, Ren J, Tang J, Zhang D, Li B and
Li Y: Estrogen-like activities of saikosaponin-d in vitro: A pilot
study. Eur J Pharmacol. 626:159–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhong Y, Li J, Zhu X, Huang N, Liu R and
Sun R: A comprehensive review of bupleuri radix and its bioactive
components: With a major focus on treating chronic liver diseasess.
J Ethnopharmacol. 330:1182442024. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lan T, Wang W, Zeng XX, Tong YH, Mao ZJ
and Wang SW: Saikosaponin A triggers cell ferroptosis in
hepatocellular carcinoma by inducing endoplasmic reticulum
stress-stimulated ATF3 expression. Biochem Biophys Res Commun.
674:10–18. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Shi C, Sun L, Fang R, Zheng S, Yu M and Li
Q: Saikosaponin-A exhibits antipancreatic cancer activity by
targeting the EGFR/PI3K/Akt pathway. Curr Pharm Biotechnol.
24:579–588. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen M, Hu C, Yang L, Guo Q, Liang Y and
Wang W: Saikosaponin-D induces the pyroptosis of lung cancer by
increasing ROS and activating the NF-κB/NLRP3/caspase-1/GSDMD
pathway. J Biochem Mol Toxicol. 37:e234442023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
de Boniface J, Szulkin R and Johansson
ALV: Survival after breast conservation vs mastectomy adjusted for
comorbidity and socioeconomic status: A Swedish National 6-Year
Follow-up of 48 986 women. JAMA Surg. 156:628–637. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Song D, Chen M, Chen X, Xu J, Wu S, Lyu Y
and Zhao Q: Apoptosis induction and inhibition of invasion and
migration in gastric cancer cells by Isoorientin studied using
network pharmacology. BMC Complement Med Ther. 24:3092024.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wei Y, Li S, Li Z, Wan Z and Lin J:
Interpretable-ADMET: A web service for ADMET prediction and
optimization based on deep neural representation. Bioinformatics.
38:2863–2871. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Cheng W, Wu S, Yuan Z, Hu W, Yu X, Kang N,
Wang Q, Zhu M, Xia K, Yang W, et al: Pharmacokinetics, tissue
distribution, and excretion characteristics of a radix polygoni
multiflori extract in rats. Front Pharmacol. 13:8276682022.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Yu X, Xia K, Wu S, Wang Q, Cheng W, Ji C,
Yang W, Kang C, Yuan Z and Li Y: Simultaneous determination and
pharmacokinetic study of six components in beagle dog plasma by
UPLC-MS/MS after oral administration of Astragalus Membranaceus
aqueous extract. Biomed Chromatogr. 36:e54882022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Li X, Li X, Huang N, Liu R and Sun R: A
comprehensive review and perspectives on pharmacology and
toxicology of saikosaponins. Phytomedicine. 50:73–87. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Manoharan S, Deivendran B and Perumal E:
Chemotherapeutic potential of Saikosaponin D: Experimental
evidence. J Xenobiot. 12:378–405. 2022. View Article : Google Scholar : PubMed/NCBI
|