Introduction
Oral squamous cell carcinoma (OSCC) is the sixth most common type of cancer, with an incidence of ~377,713 cases worldwide, 62% of which occur in developing countries. Of OSCC cases, approximately one-third are recurrent. Metastasis, disease recurrence and drug resistance are key factors in the low (<60%) 5-year survival rate of patients with OSCC and a delay in metastasis contributes to prolonged survival of patients with OSCC (1–5).
The epithelial-mesenchymal transition (EMT) plays a key role in the pathogenesis of OSCC, specifically its progression from fibrotic transformation to metastasis. Polarized, quiescent epithelial cells become mesenchymal cells, giving cancer cells an invasive phenotype (1,5,6). The most widely studied factor in EMT induction is transforming growth factor (TGF)-β, which plays an important role in promoting tumor invasion and metastasis. The downregulation of E-cadherin is a distinctive marker of EMT progression and the increased expression of transcription factors such as Twist, Snail, Slug and ZEB1 is related to the establishment of invasive tumors (7–10).
The consumption of a diet rich in vegetables, legumes and cereals is strongly associated with the decreased manifestation of degenerative disorders such as cancer. Flavonoids are phenolic compounds synthesized in the secondary metabolism of plants. They are involved in the protection of plants from biotic stress factors (such as insect, microorganism and herbivore attacks) and abiotic stresses (such as drought, rain, frost and high temperatures). Flavonoids form a very diverse group and are classified as flavones, flavonols, isoflavonoids, flavanones, chalcones and anthocyanidins (11).
Polymethoxyflavones are compounds present in the peels of citrus fruits such as Citrus nobilis, from which the name nobiletin (Nob; 5,6,7,8,30′,4′-hexamethoxyflavone) is derived (Fig. 1), as well as tangerine (Citrus tangerina, which has the highest Nob content), bitter orange (Citrus aurantium) and mandarin orange (Citrus reticulata). Nob has been shown to have effects against several types of cancer, including breast (12–17), gastric (13), lung (14), liver (15) and bone (16) cancers and OSCC (18).
 |
Figure 1.
Chemical structures of nobiletin and 5-Demethylnobiletin.
|
Nob is degraded to 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone (5-DMN) via autolysis (18) or conversion in gastric juice (19). Nob uptake occurs in phases I and II of metabolism and in vivo, Nob demethylation is carried out by cytochrome P450 (20). Several investigations have shown that Nob metabolites are more potent than Nob in HT-29 cells derived from colon adenocarcinoma (18,21,22); the half maximal inhibitory concentration (IC50) of 5-DMN in these cells is 22 µM, whereas that of Nob is 46.5 µM. Researchers have concluded that the hydroxyl group at position 5 is involved in important molecular interactions in metabolic regulation (23).
Among the effects associated with Nob are cell cycle arrest via the mitogen-activated protein kinase and Akt pathways (24,25), the induction of apoptosis (26) and the suppression of cell proliferation (27). The antimetastatic activities of Nob include the regulation of matrix metalloproteinase 1–9 and tissue inhibitor of metalloproteinase 1 expression in human fibrosarcoma (28) and the inhibition of the TGF-β-induced migration and invasion of non-small cell lung cancer cells (29,30). Thus, Nob has a broad spectrum of mechanisms against cancer development. The present study demonstrated that Nob and 5-DMN inhibited cell proliferation and regulated the expression of N-cadherin, E-cadherin, Snail and Slug, suggesting their potential value in the treatment of OSCC.
Materials and methods
Reagents
Nob and 5-DMN were purchased from Merck. TGF-β was purchased from PeproTech, Inc. Actin, E-cadherin, N-cadherin, Slug and Snail were obtained from Santa Cruz Biotechnology, Inc.
Cell culture
FaDu cells derived from hypopharyngeal squamous cell carcinoma were obtained from the American Type Culture Collection and cultured by incubation in Dulbecco's modified Eagle's medium (MilliporeSigma) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 µU/ml penicillin and 100 µg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. Nob and 5-DMN (<0.5% of the total volume) were dissolved in dimethyl sulfoxide (DMSO).
Cell viability assay
Cell viability was determined via a 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. The cells [1×105/well, determined by a TC20 automated counter (Bio-Rad Laboratories, Inc.)] were seeded in 96-well plates at 37°C for 12 h. They were grown overnight and then treated with different doses of Nob and 5-DMN for 24, 48 and 72 h. Viability was measured after the addition of MTT (0.5 mg/ml) for 4 h. At the end of the assay, 150 µl of DMSO was added to dissolve the formazan crystals. The plates were read at 570 nm via a Synergy device (BioTek; Agilent Technologies, Inc.). The assay was repeated five times.
Colony-forming assay
FaDu cells (200/ml) were cultured in six-well plates at 37°C for 12 h and treated with TGF-β (3 pM) for 24 h. Nob (50 µM), 5-DMN (50 µM), or Nob (50 µM) plus 5-DMN (10 or 25 µM) was then added and the cells were cultured under normal conditions (5% CO2, 37°C) for 18 days. The medium was changed every 3 days. Colonies were fixed with 4% paraformaldehyde at 37°C, stained with Giemsa solution at 37°C for 30 min (MilliporeSigma) and then counted. The assay was performed three times.
Cell migration assays
The cell migration capacity was determined via wound healing and Transwell assays. FaDu cells were seeded in six-well plates and cultured to confluence. A 200-µl sterile pipette tip was then used to scratch a vertical line, followed by washing twice in sterile phosphate-buffered saline to remove the debris. The cells in Dulbecco's modified Eagle's medium without fetal bovine serum were then treated for 24 or 48 h with DMSO vehicle (control), 3 pM TGF-β1 + 50 µM Nob, 50 µM 5-DMN, or 50 µM Nob + 10 µM 5-DMN. A total of 15 images were obtained for each condition and the experiment was performed three times. For the wound healing assay, experiments were performed three time and images of each condition were captured with a Zeiss Primo-vert microscope (Zeiss AG). The data were analyzed with ImageJ 1.52a software (National Institutes of Health). The wound healing assay index was calculated using the following formula: Wound healing index=(X− Assay Group)/(X− Control Group)*100. For the Transwell assay, FaDu cells (n=10,000) were seeded in 24-well Transwell inserts (8-mm pore size) and treated with Nob or 5-DMN at different doses. After 24 h, the medium was removed and the cells were fixed with 4% paraformaldehyde at 37°C for 5 min. The membranes were then permeabilized with 100% methanol for 20 min and stained with thiazole orange (0.1 µM) for 15 min at 37°C. The membranes were visualized under a Polyvar fluorescence microscope, (Leica GmbH) as previously described (17).
Western blotting
FaDu cells (1×105) were seeded in six-well plates and treated with TGF-β (3 pM), + Nob (50 and 100 µM) or (B) 5-DMN (50 and 100 µM) and TGF-β (3 pM), + plus Nob (50 µM), 5-DMN (50 µM), Nob (50 µM) + 5-DMN (50 µM), or Nob (50 µM) + 5-DMN (100 µM) for 24 h. Lysates were prepared in 10 mM Tris-HCl, 0.15 mM NaCl, 1 M ethylenediaminetetraacetic acid, 20 mM NaF, 100 mM Na3VO4, 0.5% NP-40, 1% Triton, 1 mM phenylmethylsulfonyl fluoride (pH 6.8) and a protease inhibitor cocktail. The samples were centrifuged and the supernatants were recovered and stored at −80°C. Proteins were quantified via the Bradford assay and 40-µg aliquots were separated via 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (110 V; 2 h). The proteins were transferred to polyvinylidene fluoride membranes, blocked with 5% skimmed milk at 37°C for 1 h and incubated with the following primary antibodies: Anti-E-cadherin (sc-71008) (1:10,000), anti-N-cadherin (sc-59987) (1:10,000), anti-Slug (sc-166476) (1:15,000), anti-Snail (sc-271977) (1:10,000) and anti-actin (sc-47778) (1:5,000; all from Santa Cruz Biotechnology, Inc.). The membranes were incubated overnight at 4°C, washed three times with washing buffer and incubated for 2 h with secondary antibody [anti-rabbit (sc-2004), anti-goat (sc-2354) immunoglobulin G horseradish peroxidase conjugate (1:10,000); Santa Cruz Biotechnology, Inc.]. The membranes were exposed to luminol reagent for 15 min (Santa Cruz Biotechnology, Inc.). The bands were analyzed with the ImageQuant LAS 500 program, software version 1.1.0. (General Electric, Healthcare Bio-Sciences AB Bjorkgatan 30).
Data analysis
Experiments were performed three times in triplicate. Statistical analysis was performed with GraphPad Prism 5 (Dotmatics). Data were shown as mean ± standard deviation. Paired Student's t-test (two-way) was used for comparison between groups. Between-group comparisons were performed by one-way analysis of variance (ANOVA) followed by Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of Nob and 5-DMN on cell proliferation
Nob significantly inhibited the growth of FaDu cells in a dose- and time-dependent manner. The IC50 values of Nob in FaDu cells at 24, 48 and 72 h were 31.81, 22.65 and 19.92 µM, respectively (Figs. 2A and 3A). 5-DMN treatment had a greater inhibitory effect, yielding IC50 values of 42.03, 11.02 and 5.84 µM at 24, 48 and 72 h, respectively (Figs. 2B and 3B). In the combined treatment, the two agents had a synergistic cytotoxic effect, yielding IC50 values of 7.13, 6.59 and 4.9 µM at 24, 48 and 72 h, respectively (Figs. 2C and 3C). The IC50 values obtained at 24 h were used as the treatment doses in subsequent assays.
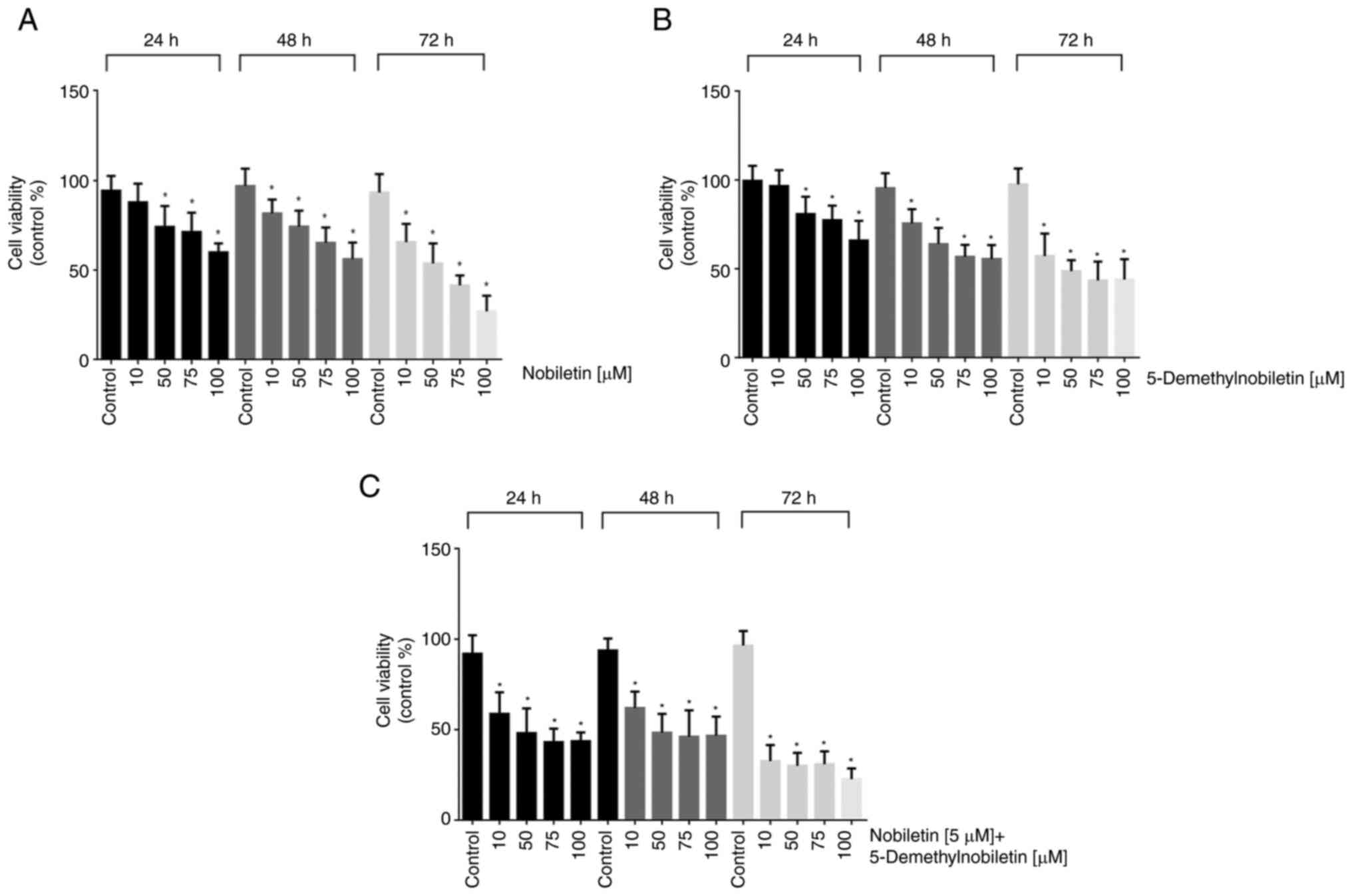 |
Figure 2.
Nob and 5-DMN reduced FaDu cell viability, as determined by the MTT assay. The cells were treated with different concentrations of (A) Nob, (B) 5-DMN and (C) Nob plus 5-DMN for 24, 48 and 72 h. *P˂0.05.
|
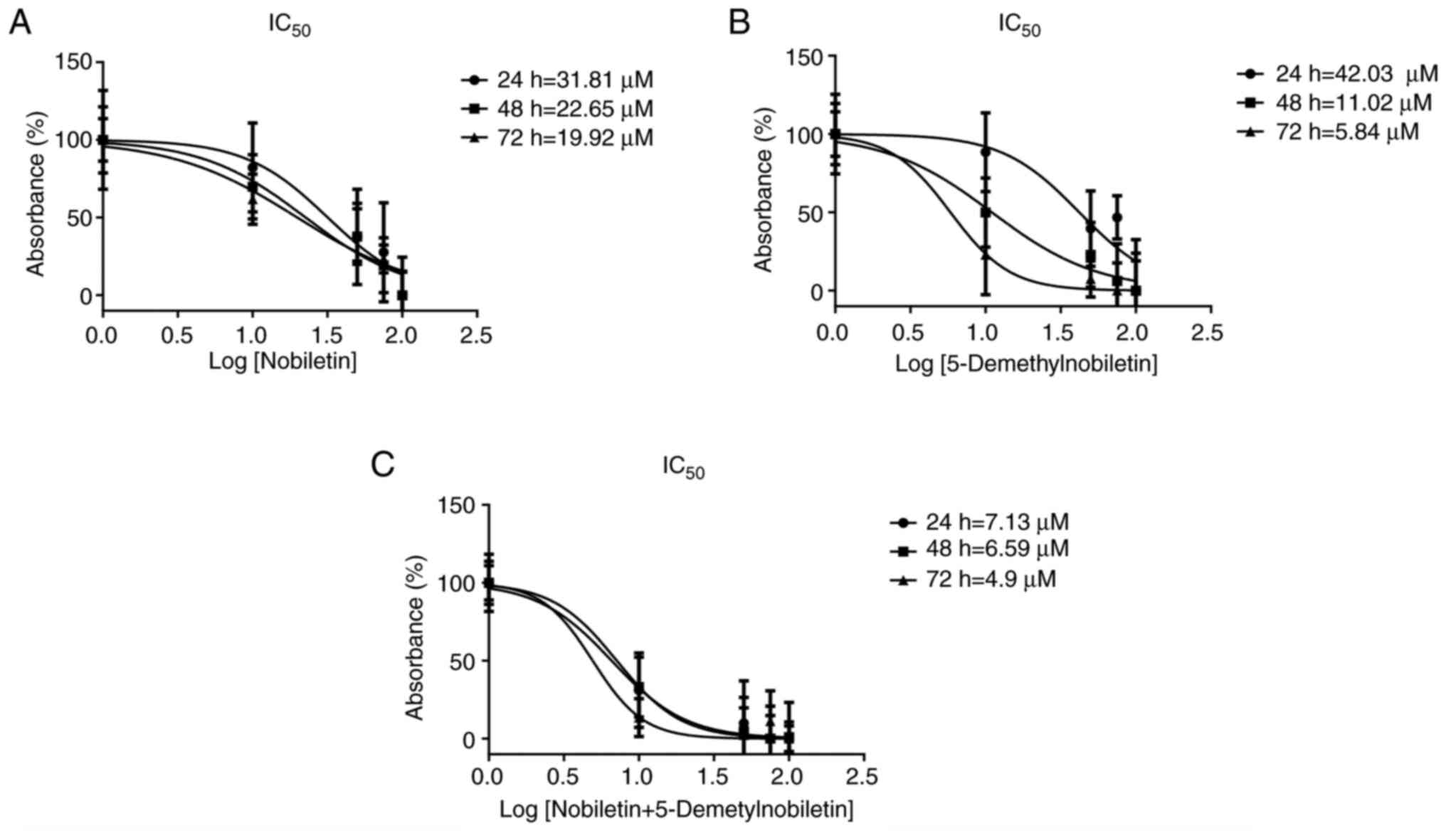 |
Figure 3.
IC50 in FaDu cells. IC50 curves of (A) nobiletin, (B) 5-Demethylnobiletin and (C) both agents in FaDu cells after 24, 48 and 72 h. IC50, half maximal inhibitory concentration.
|
Nob and 5-DMN suppress TGF-β-induced cellular morphological changes
After 24 h of TGF-β1 treatment, the morphology of the cells changed from cuboidal (Fig. 4A) to an elongated spindle shape (Fig. 4B). The cells recovered their cuboidal morphology when exposed to NOB (10 and 50 µM; Fig. 4C and D). Similar results were obtained with 5-DMN (10 and 50 µM) treatment, but this treatment induced cell detachment and clustering (Fig. 4E and F).
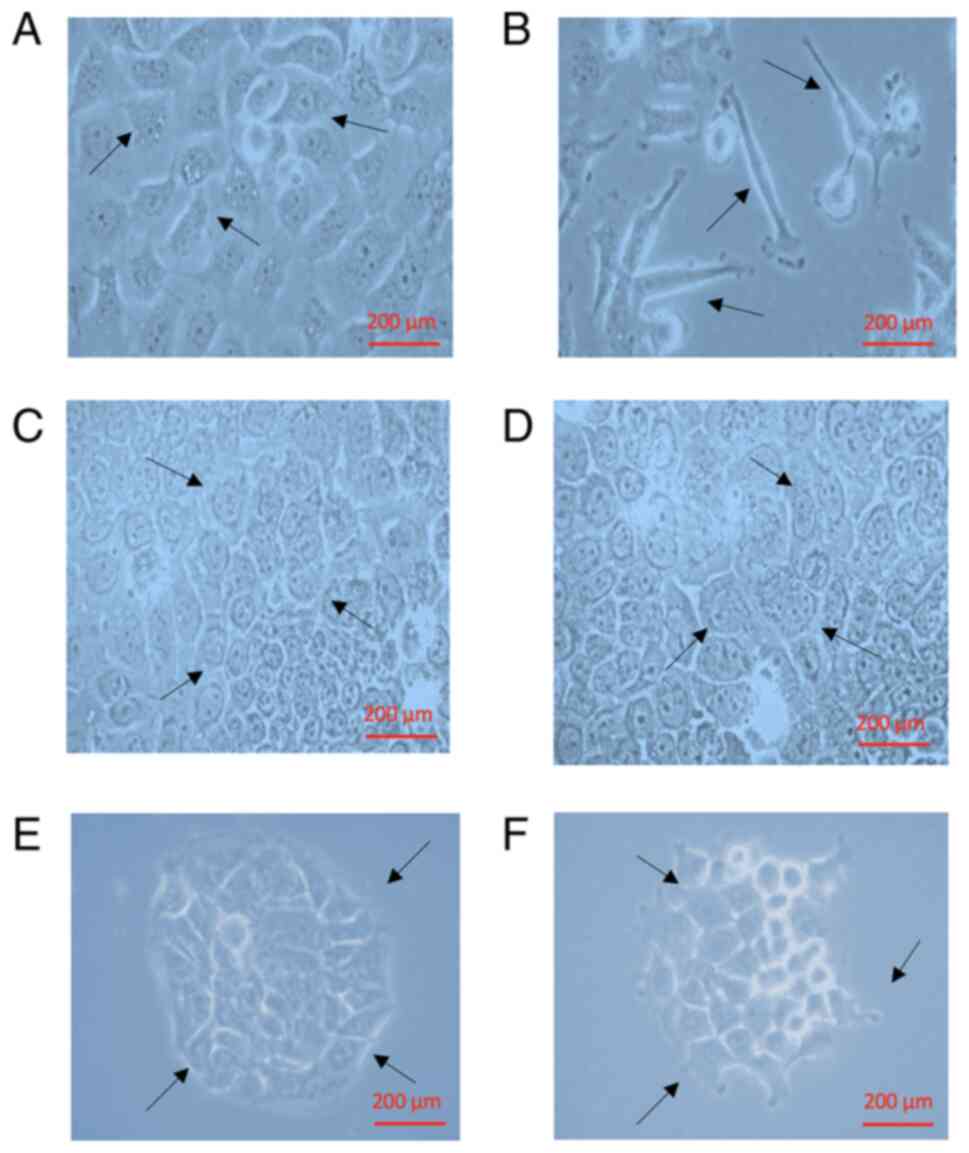 |
Figure 4.
Effects of TGF on the morphological characteristics of FaDu cells. (A) Control, Dulbecco's modified Eagle's medium + 10% fetal bovine serum; (B) + TGF-β1 (3 pM); (C) TGF-β1 + Nob (10 µM); (D) TGF-β1 + Nob (50 µM); (E) TGF-β1 + 5-DMN (10 µM); or (F) TGF-β1 + 5-DMN (50 µM). Scale bar, 200 µm. TGF, transforming growth factor; Nob, nobiletin; 5-DMN, 5-Demethylnobiletin. The arrows represent the morphological changes of FaDu cells.
|
Effects of Nob and 5-DMN on cell migration
The results of the wound-healing assay differed significantly between the control and TGF-β1-treated cells, with no significant difference observed between Nob- and 5-DMN treated cells or between either of these groups and TGF-β1-treated cells. Combined treatment with Nob and 5-DMN yielded significant differences between the groups at 24 and 48 h (Fig. 5). In the Transwell assay, compared with TGF-β1, Nob significantly suppressed migration, but 5-DMN and the combination treatment did not (Fig. 6). These results suggested that Nob played an important role in cell migration.
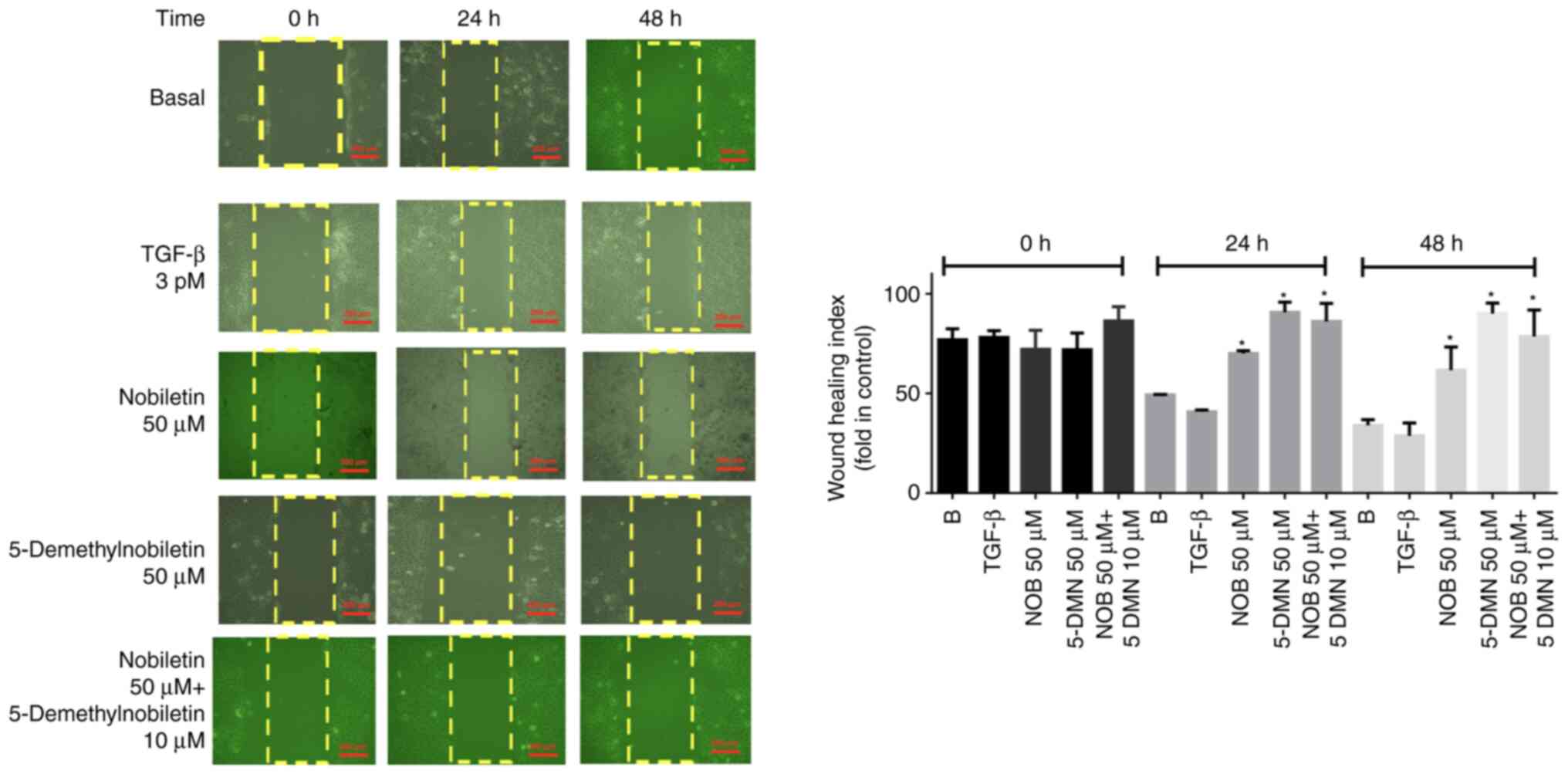 |
Figure 5.
Effects of Nob, 5-DMN and both agents on the TGF-β1-induced migration of FaDu cells. *P˂0.05. Scale bar, 200 µm. Nob, nobiletin; 5-DMN, 5-Demethylnobiletin; TGF, transforming growth factor.
|
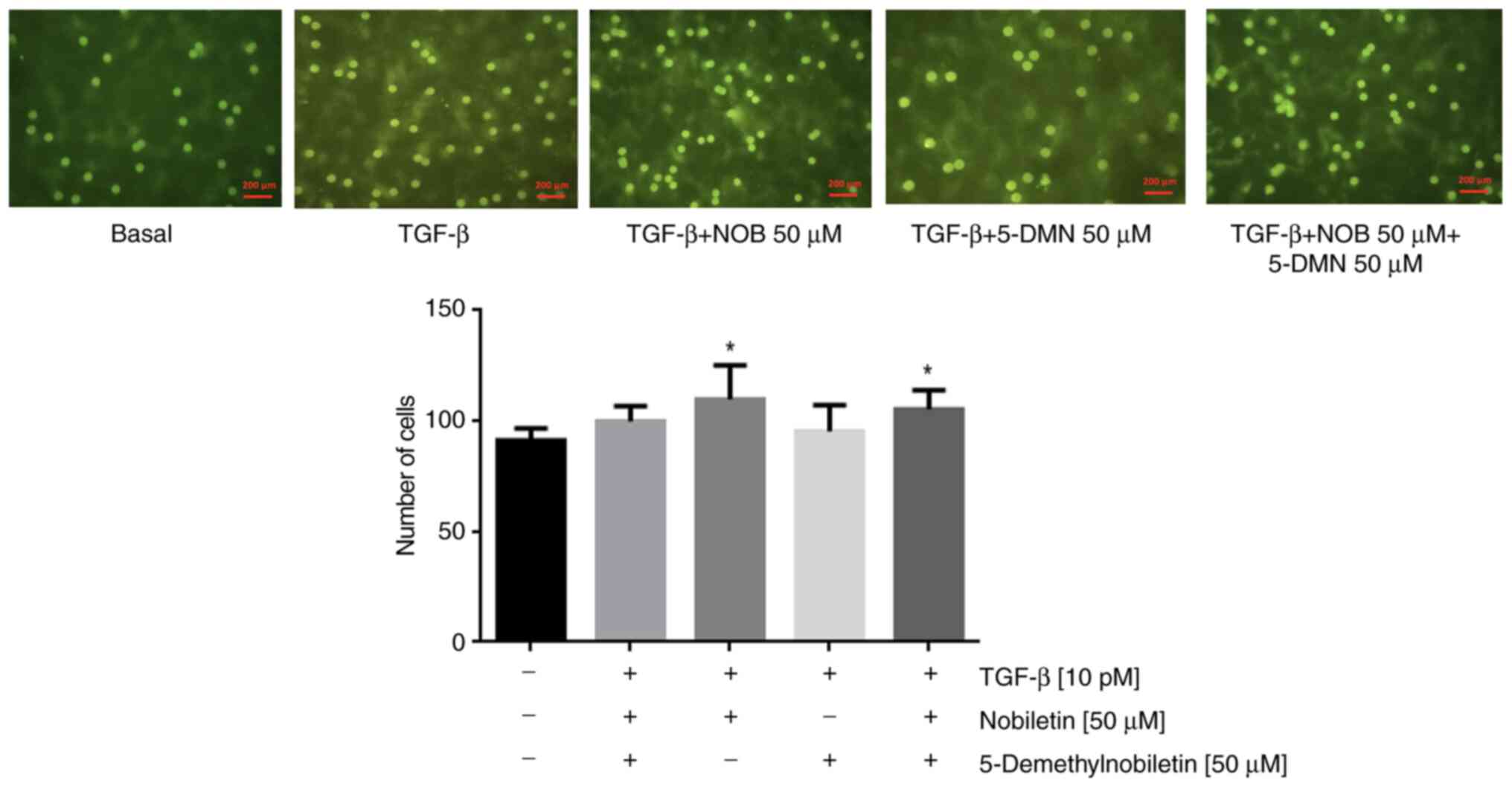 |
Figure 6.
Effects of Nob, 5-DMN and Nob + 5-DMN on the TGF-β-induced migration of FaDu cells incubated in DMEM + 10% SBF with TGF-β, TGF-β + Nob (50 µM), TGF-β + 5-DMN (50 µM), or TGF-β + Nob (50 µM) + 5-DMN (50 µM). *P˂0.05. Scale bar, 200 µm. Nob, nobiletin; 5-DMN, 5-Demethylnobiletin; TGF, transforming growth factor.
|
Effects of Nob and 5-DMN on colony formation
In the colony formation assay, compared with the control, TGF-β1 treatment significantly increased colony formation. Nob and/or 5-DMN treatment significantly reversed the effect of TGF-β1, reducing the number of colonies formed and inhibiting cell survival (Fig. 7).
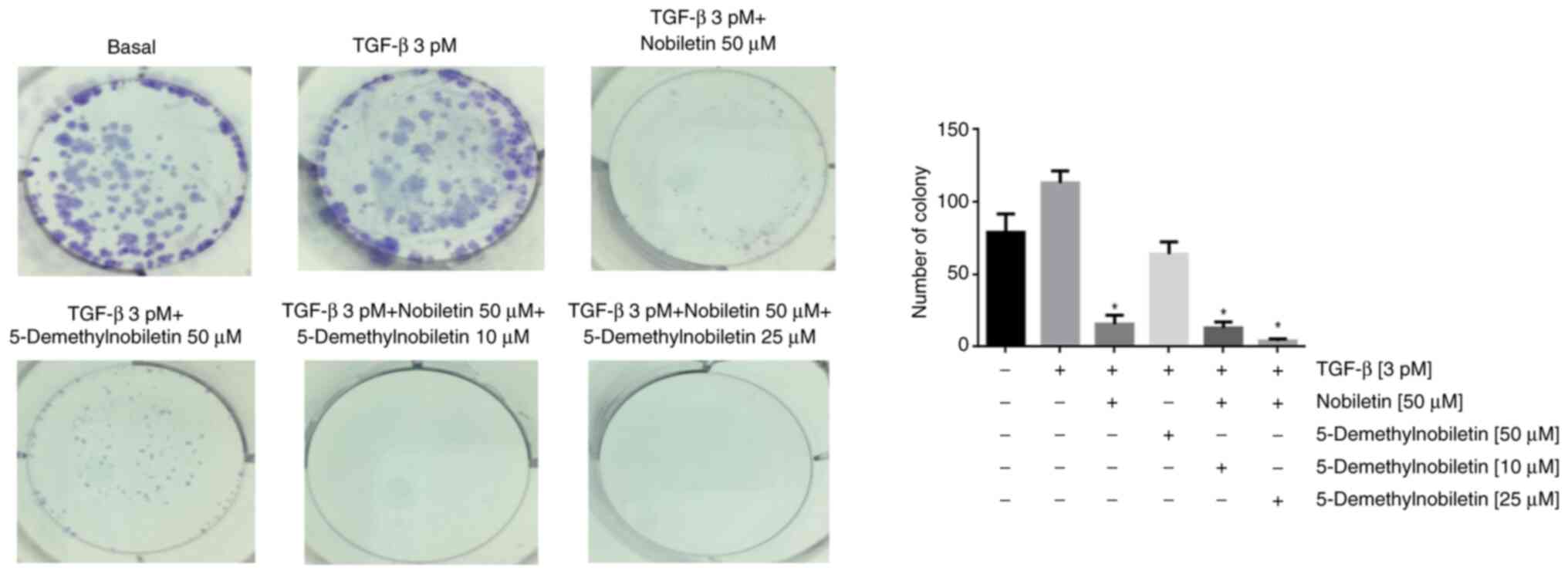 |
Figure 7.
Colony formation of FaDu cells incubated with TGF-β1 and exposed to Nob, 5-DMN or Nob + 5-DMN. *P˂0.05. TGF, transforming growth factor; Nob, nobiletin; 5-DMN, 5-Demethylnobiletin.
|
Nob and 5-DMN inhibit the effects of TGF-β1 on EMT
Nob and 5-DMN inhibited the expression of the EMT markers N-cadherin, Snail and Slug in FaDu cells incubated with TGF-β1 in a dose-dependent manner, subsequently increasing E-cadherin expression (Fig. 8A). The combination treatment increased the expression of E-cadherin and decreased the expression of N-cadherin, Slug and Snail (Fig. 8B). These data suggested that Nob and 5-DMN regulate FaDu cell proliferation, migration and invasion and that these agents combined have additional effects on EMT marker expression. In addition, Nob, 5-DMN and combination treatment induced the expression of Bax and suppressed the expression of Bcl2, highlighting the roles of these flavonoids in the regulation of apoptosis (Fig. 8B).
 |
Figure 8.
Expression levels of E-cadherin, N-cadherin, Slug and Snail. FaDu cells were treated with TGF-β (3 pM), + plus (A) Nob or (B) 5-DMN for 24 h. Actin was used as the loading control. *P˂0.05. TGF, transforming growth factor; Nob, nobiletin; 5-DMN, 5-Demethylnobiletin.
|
Discussion
Cancer imposes a high public health burden and has a high mortality rate. As cancer progresses, tumor cells undergo a number of changes in the expression of adhesion molecules, leading to their detachment from the basal layer and the acquisition of invasive phenotypic characteristics (that is, EMT). Epithelial cells acquire a mesenchymal architecture that allows them to invade new positions.
Hypopharyngeal squamous cell carcinoma is a common head and neck cancer with a relatively high incidence in men and is associated with alcohol consumption, tobacco use and Papillomavirus infection. When this type of cancer is diagnosed, it has typically already metastasized to the lymph nodes and lungs, resulting in a very low survival rate and major side effects that reduce the quality of life of patients (29).
The present study evaluated the effect of Nob and 5-DMN on EMT in FaDu cells derived from a hypopharyngeal squamous cell carcinoma. Nob has attracted increasing research interest because of its multiple benefits in the treatment of diseases such as diabetes (31), osteoporosis (32) and neurodegenerative diseases (33). Like other flavonoids, it has anti-inflammatory (34) and antioxidant (35) activities and hepatoprotective and cardioprotective (36) functions. These flavones are obtained naturally from citrus peel and despite their broad spectrum of functions, some research indicates that 5-DMN has greater biological activity than the parent flavones (22,37,38).
As 5-DMN has shown greater potency in the inhibition of colon cancer growth (18), the present study compared the effects of Nob and 5-DMN on TGF-β1-induced EMT in the FaDu cell line, which is derived from a squamous cell carcinoma of the hypopharynx. The present study is important because 5-DMN is extracted from citrus peel and is a metabolic product derived from Nob (39). In the present study, 5-DMN had greater potency than Nob in the inhibition of cell proliferation, but no significant difference in migration or the regulation of EMT marker expression was observed between the treatment groups.
5-DMN has been reported to inhibit colon cancer cell proliferation and induce apoptosis (23), with an IC50 of 42.03 µM, similar to the value obtained in the present study at 24 h. In a lung cancer cell line, treatment with the synthetic form yielded an IC50 of 21.8 µM and resulted in cell cycle regulation and the promotion of apoptosis (40). In the A459 cell line, Nob treatment yielded an IC50 of 100 µM (41), which was greater than that reported for FaDu cells in the present study. Among the effects associated with 5-DMN are induction of apoptosis via a reduction of the mitochondrial membrane potential (in neuroblastoma) and cell cycle arrest (42). However, no study has examined the effect of 5-DMN on EMT to the best of the authors' knowledge. In lung adenocarcinoma cells, Nob decreases cell viability and inhibits TGF-β1-induced EMT at a dose of 100 µM and reduces Snail, Slug and Twist expression levels at a dose of 20 µM. The present study obtained similar results for these doses. Similarly, TGF-β1-stimulated colony formation was suppressed (29), in the FaDu cell line. Similar results have been reported for glioma cells treated with 15 µM Nob (43).
The present study compared the effect of Nob and 5-DMN on EMT in an in vitro model. It was found that TGF-β-induced EMT was effectively blocked by both agents and the results are similar to those obtained with other flavonoids, such as biochanin A (44), silibinin (45) and scutellarin, in gastric cancer (46).
The results of the present study revealed that Nob and 5-DMN inhibited proliferation and cell migration. However, combined treatment with both agents did not result in a synergistic inhibitory effect. These results were similar to those reported with silibinin (45). The present study found that Nob and 5-DMN reversed the effects of TGF-β on the expression of EMT markers in a manner similar to that reported in renal cell carcinoma subjected to hypoxia (47).
The present study revealed that either agent alone or the combination of both agents inhibited EMT and altered the Bax/Bcl2 ratio. However, the study has limitations that need to be addressed in further investigations. First, it did not determine the mechanism of action for EMT suppression and apoptosis induction and, second, the role of migration and apoptosis needs to be assessed in an in vivo setting.
In conclusion, discrepancies in the effective concentrations of Nob and 5-DMN may be a function of differences in tissues and/or their sensitivity to flavonoids. Thus, further research on apoptosis mechanism and its role on xenograft mouse models studies should be conducted to determine their therapeutic effectiveness.
Acknowledgements
The authors would like to thank Dr Marina Macías‑Silva and Dr Marcela Sosa‑Garrocho (Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, Mexico City, Mexico) for their teachings on the management of TGF beta.
Funding
The present study was funded by the General Directorate of Academic Personnel Affairs of the National Autonomous University of Mexico (grant no. PAPIIT-202422) and Fundacion UNAM (grant no. UNA 290722 7Y5).
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
GGV wrote the manuscript and performed the western blotting, colony formation and migration assays. MRM performed the viability assay, western blotting and IC50 calculation. GGV and MRM confirm the authenticity of all the raw data. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Ali J, Sabiha B, Jan HU, Haider SA, Khan AA and Ali SS: Genetic etiology of oral cancer. Oral Oncol. 70:23–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA CancerJ Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jansen L, Buttmann-Schweiger N, Listl S, Ressing M, Holleczek B, Katalinic A, Luttmann S, Kraywinkel K and Brenner H; GEKID Cancer Survival Working Group, : Differences in incidence and survival of oral cavity and pharyngeal cancers between Germany and the United States depend on the HPV-association of the cancer site. Oral Oncol. 76:8–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE and Grandis JR: Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aires FT, Lin CS, Matos LL, Kulcsar MAV and Cernea CR: Risk factors for distant metastasis in patients with oral cavity squamous cell carcinoma undergoing surgical treatment. ORL J Otorhinolaryngol Relat Spec. 79:347–355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chi AC, Day TA and Neville BW: Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J Clin. 65:401–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y and Weinberg RA: Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front Med. 12:361–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gloushankova NA, Zhitnyak IY and Rubtsova SN: Role of epithelial-mesenchymal transition in tumor progression. Biochemistry (Mosc). 83:1469–1476. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D, Moers V, Lemaire S, et al: Identification of the tumour transition states occurring during EMT. Nature. 556:463–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nieto MA, Huang RY, Jackson RA and Thiery JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng XL, Ho SC, Mo XF, Lin FY, Zhang NQ, Luo H, Zhang X and Zhang CX: Association between flavonoids, flavonoid subclasses intake and breast cancer risk: A case-control study in China. Eur J Cancer Prev. 29:493–500. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Surichan S, Arroo RR, Ruparelia K, Tsatsakis AM and Androutsopoulos VP: Nobiletin bioactivation in MDA-MB-468 breast cancer cells by cytochrome P450 CYP1 enzymes. Food Chem Toxicol. 113:228–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen M, Li H, Zheng S, Shen J, Chen Y, Li Y, Yuan M, Wu J and Sun Q: Nobiletin targets SREBP1/ACLY to induce autophagy-dependent cell death of gastric cancer cells through PI3K/Akt/mTOR signaling pathway. Phytomedicine. 128:1553602024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uesato S, Yamashita H, Maeda R, Hirata Y, Yamamoto M, Matsue S, Nagaoka Y, Shibano M, Taniguchi M, Baba K and Ju-ichi M: Synergistic antitumor effect of a combination of paclitaxel and carboplatin with nobiletin from Citrus depressa on non-small-cell lung cancer cell lines. Planta Med. 80:452–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma X, Jin S, Zhang Y, Wan L, Zhao Y and Zhou L: Inhibitory effects of nobiletin on hepatocellular carcinoma in vitro and in vivo. Phytother Res. 28:560–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng HL, Hsieh MJ, Yang JS, Lin CW, Lue KH, Lu KH and Yang SF: Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget. 7:35208–35223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosas-Martínez M, González Rosales JA and Gutiérrez-Venegas G: Nobiletin regulates invasion, migration and metastasis in hypopharyngeal squamous cell carcinoma. Cell Mol Biol (Noisy-le-grand). 69:1–6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiou YS, Zheng YN, Tsai ML, Lai CS, Ho CT and Pan MH: 5-Demethylnobiletin more potently inhibits colon cancer cell growth than nobiletin in vitro and in vivo. J Food Bioact. 2:91–97. 2018. View Article : Google Scholar
|
|
19
|
Zheng J, Bi J, Johnson D, Sun Y, Song M, Qiu P, Dong P, Decker E and Xiao H: Analysis of 10 metabolites of polymethoxyflavones with high sensitivity by electrochemical detection in high-performance liquid chromatography. J Agric Food Chem. 63:509–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang F, Li T, Zhang XQ, Gong Y, Su H, Fan J, Wang L, Hu QD and Tan RZ: Screening of active components in Astragalus mongholicus Bunge and Panax notoginseng formula for anti-fibrosis in CKD: Nobiletin inhibits Lgals1/PI3K/AKT signaling to improve renal fibrosis. Ren Fail. 46:23750332024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Song M, Qiu P, Li F, Wang M, Zheng J, Wang Q, Xu F and Xiao H: A metabolite of nobiletin, 4′-demethylnobiletin and atorvastatin synergistically inhibits human colon cancer cell growth by inducing G0/G1 cell cycle arrest and apoptosis. Food Funct. 9:87–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiu P, Dong P, Guan H, Li S, Ho CT, Pan MH, McClements DJ and Xiao H: Inhibitory effects of 5-hydroxy polymethoxyflavones on colon cancer cells. Mol Nutr Food Res. 54 (Suppl 2):S244–S252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goh JXH, Tan LT, Goh JK, Chan KG, Pusparajah P, Lee LH and Goh BH: Nobiletin and derivatives: Functional compounds from citrus fruit peel for colon cancer chemoprevention. Cancers (Basel). 11:8672019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moon JY, Manh Hung LV, Unno T and Cho SK: Nobiletin enhances chemosensitivity to adriamycin through modulation of the Akt/GSK3β/β-catenin/MYCN/MRP1 signaling pathway in A549 human non-small-cell lung cancer cells. Nutrients. 10:18292018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang H, Chen H, Jin C, Mo J and Wang H: Nobiletin flavone inhibits the growth and metastasis of human pancreatic cancer cells via induction of autophagy, G0/G1 cell cycle arrest and inhibition of NF-kB signalling pathway. J BUON. 25:1070–1075. 2020.PubMed/NCBI
|
|
26
|
Yang J, Yang Y, Wang L, Jin Q and Pan M: Nobiletin selectively inhibits oral cancer cell growth by promoting apoptosis and DNA damage in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol. 130:419–427. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Q, Gu Y, Tan C, Sundararajan B, Li Z, Wang D and Zhou Z: Comparative effects of five polymethoxyflavones purified from Citrus tangerina on inflammation and cancer. Front Nutr. 9:9636622022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyata Y, Sato T, Imada K, Dobashi A, Yano M and Ito A: A citrus polymethoxyflavonoid, nobiletin, is a novel MEK inhibitor that exhibits antitumor metastasis in human fibrosarcoma HT-1080 cells. Biochem Biophys Res Commun. 366:168–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Da C, Liu Y, Zhan Y, Liu K and Wang R: Nobiletin inhibits epithelial-mesenchymal transition of human non-small cell lung cancer cells by antagonizing the TGF-β1/Smad3 signaling pathway. Oncol Rep. 35:2767–2774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao XJ, Liu JW, Zhang QG, Zhang JJ, Xu HT and Liu HJ: Nobiletin inhibited hypoxia-induced epithelial-mesenchymal transition of lung cancer cells by inactivating of Notch-1 signa ling and switching on miR-200b. Pharmazie. 70:256–262. 2015.PubMed/NCBI
|
|
31
|
Keshtkar S, Kaviani M, Jabbarpour Z, Geramizadeh B, Motevaseli E, Nikeghbalian S, Shamsaeefar A, Motazedian N, Al-Abdullah IH, Ghahremani MH and Azarpira N: Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci Rep. 9:117012019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Z, Wu D, Huang L, Jiang C, Pan T, Kang X and Pan J: Nobiletin inhibits IL-1β-induced inflammation in chondrocytes via suppression of NF-κB signaling and attenuates osteoarthritis in mice. Front Pharmacol. 10:5702019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Youn K, Lee S and Jun M: Discovery of nobiletin from citrus peel as a potent inhibitor of β-amyloid peptide toxicity. Nutrients. 11:26482019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuboi T, Lu R, Yonezawa T, Watanabe A, Woo JT, Abe-Dohmae S and Yokoyama S: Molecular mechanism for nobiletin to enhance ABCA1/G1 expression in mouse macrophages. Atherosclerosis. 297:32–39. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang BF, Jiang H, Ch J, Guo X, Li Y, Hu Q and Yang S: Nobiletin ameliorates myocardial ischemia and reperfusion injury by attenuating endoplasmic reticulum stress-associated apoptosis through regulation of the PI3K/AKT signal pathway. Int Immunopharmacol. 73:98–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuk T, Kim Y, Yang J, Sung J, Jeong HS and Lee J: Nobiletin inhibits hepatic lipogenesis via activation of AMP-activated protein kinase. Evid Based Complement Altern Med. 2018:74202652018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li S, Pan MH, Lai CS, Lo CY, Dushenkov S and Ho CT: Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines. Bioorg Med Chem. 15:3381–3389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiao H, Yang CS, Li S, Jin H, Ho CT and Patel T: Monodemethylated polymethoxyflavones from sweet orange (Citrus sinensis) peel inhibit growth of human lung cancer cells by apoptosis. Mol Nutr Food Res. 53:398–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Asakawa T, Sagara H, Kanakogi M, Hiza A, Tsukaguchi Y, Ogawa T, Nakayama M, Ouchi H, Inai M and Kan T: Practical synthesis of polymethylated flavones: Nobiletin and its desmethyl derivatives. Org Process Res Dev. 23:595–602. 2019. View Article : Google Scholar
|
|
40
|
Song M, Lan Y, Wu X, Han Y, Wang M, Zheng J, Li Z, Li F, Zhou J, Xiao J, et al: The chemopreventive effect of 5-demethylnobiletin, a unique citrus flavonoid, on colitis-driven colorectal carcinogenesis in mice is associated with its colonic metabolites. Food Funct. 11:4940–4952. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song M, Wu X, Charoensinphon N, Wang M, Zheng J, Gao Z, Xu F, Li Z, Li F, Zhou J and Xiao H: Dietary 5-demethylnobiletin inhibits cigarette carcinogen NNK-induced lung tumorigenesis in mice. Food Funct. 8:954–963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nemoto K, Ikeda A, Yoshida C, Kimura J, Mori J, Fujiwara H, Yokosuka A, Mimaki Y, Ohizumi Y and Degawa M: Characteristics of nobiletin-mediated alteration of gene expression in cultured cell lines. Biochem Biophys Res Commun. 431:530–534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang X, Zheng K, Li C, Zhao Y, Li H, Liu X, Long Y and Yao J: Nobiletin inhibits invasion via inhibiting AKT/GSK3β/β-catenin signaling pathway in Slug-expressing glioma cells. Oncol Rep. 37:2847–2856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Andugulapati SB, Gourishetti K, Tirunavalli SK, Shaikh TB and Sistla R: Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-β mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems. Phytomedicine. 78:1532982020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma X, Xie Y, Gong Y, Hu C, Qiu K, Yang Y, Shen H, Zhou X, Long C and Lin X: Silibinin prevents TGFβ-induced EMT of RPE in proliferative vitreoretinopathy by inhibiting Stat3 and Smad3 phosphorylation. Invest Ophthalmol Vis Sci. 64:472023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peng L, Wen L, Shi QF, Gao F, Huang B, Meng J, Hu CP and Wang CM: Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 11:9782020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu F, Zhang S, Yin M, Guo L, Xu M and Wang Y: Nobiletin inhibits hypoxia-induced epithelial-mesenchymal transition in renal cell carcinoma cells. J Cell Biochem. 120:2039–2046. 2019. View Article : Google Scholar : PubMed/NCBI
|






















