Introduction
Leukocyte immunoglobulin-like receptor B2 (LILRB2),
also known as Ig-like transcript (ILT)4 or CD85d, is an
immunosuppressive receptor that is expressed in various types of
cells (1,2). The homologue of LILRB2 in mice is
known as paired immunoglobulin-like receptor B (PirB) (3). Under normal physiological conditions,
LILRB2 is primarily expressed in monocytes and B cells, with lower
expression in endothelial cells, natural killer (NK) cells,
macrophages, placental cells and dendritic cells (DCs) (4). As a critical immune molecule, LILRB2
is closely associated with the activation and differentiation of
immune cells, and plays an important role in innate and adaptive
immunity (2). Furthermore, studies
have demonstrated that LILRB2 has a significant influence on
synaptic plasticity, neurite growth (5) and the proliferation of hematopoietic
stem cells (6).
Structurally, LILRB2 is composed of four
extracellular Ig-like domains, a transmembrane domain and a
cytoplasmic portion containing three immunoreceptor tyrosine-based
switch motifs (ITIMs). ITIMs can regulate cell signal transduction
by recruiting the SH2-containing proteins, tyrosine phosphatase
(SHP)-1 and SHP-2 (7). Overall, two
types of LILRB2 ligands have been discovered to date. The first is
the classical or non-classical major histocompatibility complex
(MHC)-I molecule, which is referred to as human leukocyte antigen
(HLA) in humans. HLA-G has been shown to exhibit the highest
binding ability to LILRB2 among HLA molecules (8). The other type of LILRB2 ligand
includes angiopoietin-like proteins (ANGPTLs), among which ANGPTL2
and ANGPTL5 demonstrate the highest binding ability (9).
Recently, accumulating evidence has suggested that
LILRB2 promotes the occurrence and progression of endometrial
cancer, lung cancer, breast cancer, hepatocellular carcinoma,
colorectal cancer, ovarian cancer, and clear cell renal cell
carcinoma (7,10–17).
Research on LILRB2 in tumors has indicated that LILRB2 can be found
in tumor cells and stromal cells within the tumor microenvironment
(TME) of certain malignant tumors. This enrichment can regulate the
malignant behavior of tumor cells and promote their immune escape
(18). Moreover, LILRB2 is also
positively associated with immunosuppression, tumor cell
proliferation, invasion and metastasis (11). Unconventionally high expression of
LILRB2 has been observed in hematological malignant tumor cells
such as B-cell chronic lymphocytic leukemia, T-cell lymphoma and
acute monocytic leukemia. Increased LILRB2 levels are positively
associated with disease progression (9). The role of LILRB2 in hematological
tumors and related therapies has been adequately studied, but it is
still in the development stage in solid tumors (9). Overall, the findings suggest that
understanding the role of LILRB2 within the TME of solid tumors
presents opportunities for therapeutic interventions aimed at
inhibiting its effects on tumor progression. This suggests that
targeting LILRB2 may offer a new approach for solid tumor targeted
therapy.
Roles and functions of LILRB2 in solid
tumors
LILRB2 and its involvement in human
tumors
Clinical studies have revealed an association
between upregulation of LILRB2 and a diverse number of tumors, such
as endometrial cancer (10),
colorectal cancer (CRC) (15),
non-small cell lung cancer (NSCLC) (12), lung adenocarcinoma (13), hepatocellular carcinoma (14), breast cancer (7), renal cell carcinoma (17) and ovarian cancer (16).
LILRB2 expression has been detected on the tumor
cell membrane, in the cytoplasm or both (7,15), and
it is also present on the surface of CD4+ and
CD8+ T cells in the TME (12). Increased LILRB2 expression level has
been revealed to be associated with a worse patient prognosis.
Bioinformatics analysis revealed that patients with malignant
gliomas with high LILRB2 expression have a 5-year survival rate
that is ~20% lower compared with that in patients with low LILRB2
expression (19). In renal clear
cell carcinoma, the difference is ~10% (20). Furthermore, LILRB2 is upregulated in
the early stages of esophageal cancer (21). Histopathological analysis
demonstrates that higher levels of LILRB2 in breast cancer, CRC,
lung adenocarcinoma and hepatocellular carcinoma tissues are
significantly associated with larger primary tumors, poorer cell
differentiation, lymph node metastasis, reduced T-cell
infiltration, advanced disease stage and shorter overall patient
survival time (7,13–15,22).
The results indicate that LILRB2 may have early diagnostic or
prognostic value in these tumors.
Additionally, analysis of T-cell subsets in patients
with CRC or lung adenocarcinoma reveals that overexpression of
LILRB2 is linked to decreased levels of CD3+ and
CD8+ T cells, and increased levels of FOXP3+
regulatory T (Treg) cells within the TME (13,22).
These results indicate that LILRB2 can promote the tumor to display
a more malignant phenotype and induce the formation of inhibitory
immune microenvironment, thus promoting tumor progression.
Therefore, LILRB2 may become a novel biomarker for predicting the
prognosis of patients with solid tumors.
LILRB2 and experimental tumors
Experimental studies have confirmed an increase in
LILRB2 expression levels in tumor cells compared with corresponding
controls. Furthermore, there is evidence demonstrating that
overexpression of LILRB2 is closely related to the malignancy of
tumor cells or a more malignant immune microenvironment (1,2,10,13,18).
Cell line experiments have confirmed that inhibition
of LILRB2 expression in endometrial cancer and NSCLC cells leads to
a prominent reduction in cell proliferation, colony formation,
migration and invasion (10,12).
In addition, it leads to increased levels of apoptosis and cell
cycle blockage at the G0/G1 phase (10,12,23,24).
Shao et al (25) injected
LILRB2-knockdown or control HC1A endometrial cancer cells into
NOD-SCID mice (non-obese diabetic-severe combined immunodeficiency
mice, which exhibit bone marrow dysfunction, characterized by
deficiencies in T and B cells, and hypoactivity of NK cells) and
revealed that tumor volume and weight in the LILRB2-knockdown group
decreased by more than half compared with that in the control
group. The blockade of LILRB2 has been shown to enhance the effect
of T-cell immune checkpoint inhibitors, reduce the Treg
infiltration in tumor tissue and polarize tumor-infiltrating
myeloid cells toward an inflammatory phenotype in NSCLC tissues,
ultimately promoting antitumor immunity (26). Consistent with this study, LILRB2
overexpression promotes immune tolerance among DCs, resulting in an
inefficient T-cell response for patients with hepatocellular
carcinoma, and suppresses tumor immunity by recruiting M2-type
tumor-associated macrophages (TAMs) and impairing T-cell
proliferation and function (27).
These findings reveal that LILRB2 plays an important
role in maintaining the malignant phenotype of tumor cells while
suppressing tumor immunity. Therefore, the knockdown of LILRB2 may
be an efficient strategy for targeted therapy against solid
tumors.
Mechanisms of LILRB2 in tumor
progression
Studies have demonstrated the overexpression or
inducibility of LILRB2 in solid tumors (2,7,10,13,14),
highlighting its involvement in promoting tumor proliferation and
growth, and invasion and metastasis, as well as maintaining an
immunosuppressive microenvironment through various mechanisms.
Mechanisms of tumor cell-derived
LILRB2
LILRB2 is highly expressed in diverse types of tumor
cells, contributing to their malignancy (2).
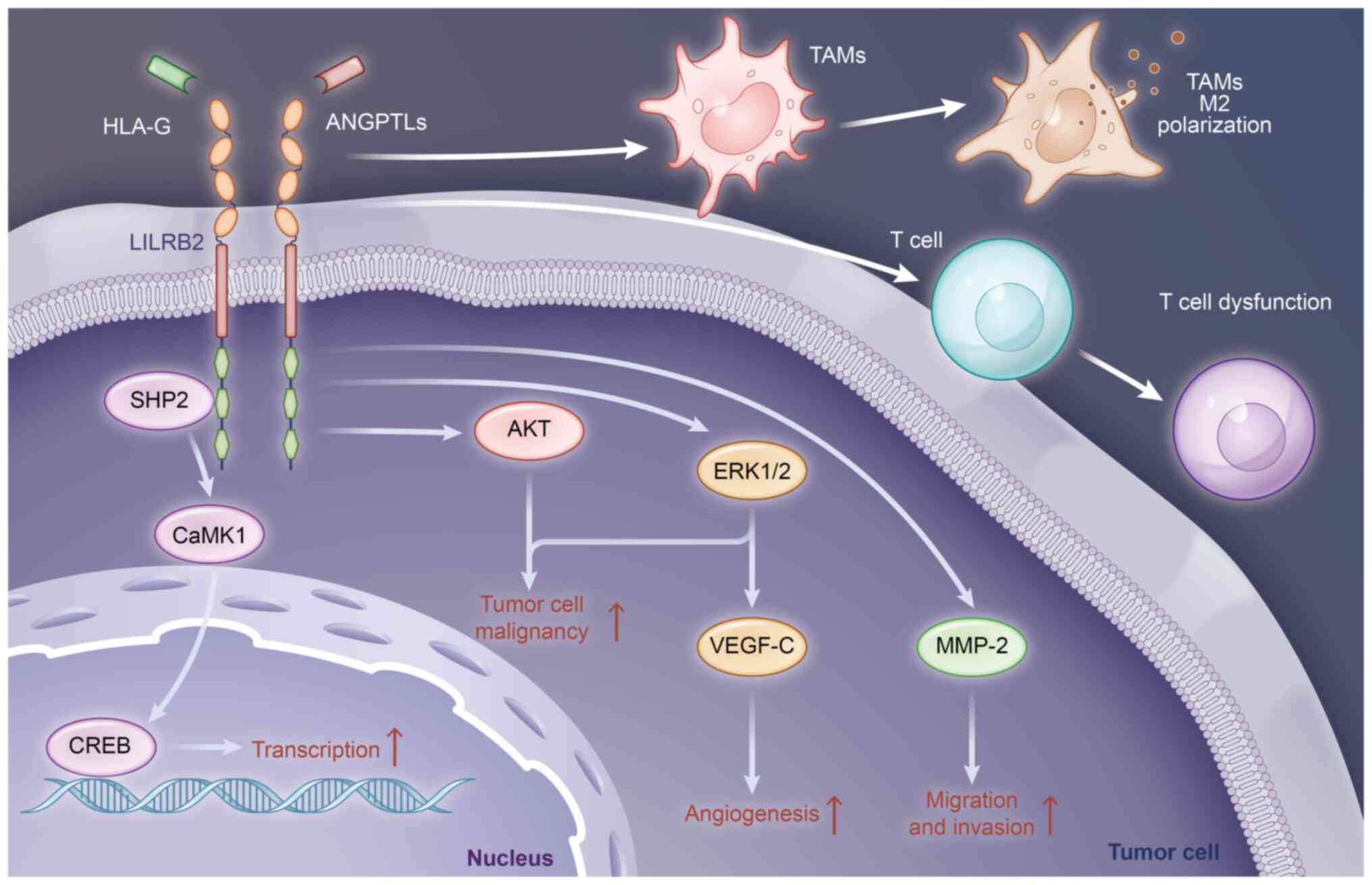
In NSCLC, LILRB2 modulates the proliferation of
NSCLC A549 cells via the SHP-2/calcium/calmodulin-dependent protein
kinase 1 (CaMK1)/cAMP response element-binding protein (CREB) axis
(12). In LILRB2-deficient A549
cells, the phosphorylation of SHP-2 is significantly decreased,
leading to decreased activation of CaMK1 and reduced levels of
phosphorylated-CREB, a target of CaMK1 (12). As a transcription factor, the
activity of CREB can be significantly increased by phosphorylation,
promoting the expression of genes linked to proliferation and
migration, thereby promoting the malignant transformation of tumor
cells (28). Therefore, LILRB2
deficiency can reduce the proliferation of A549 cells through this
pathway. This signaling axis also facilitates the proliferation and
migration of endometrial cancer Ishikawa and HEC-1A cells (25). LILRB2 can also promote the invasion
and migration of NSCLC cells by upregulating MMP-2 expression
(29), whose function is to degrade
the extracellular matrix (30).
Additionally, by interacting with HLA-G, LILRB2 can enhance ERK1/2
phosphorylation and upregulate VEGF-C, thereby promoting NSCLC
progression by activating the classical ERK pathway and increasing
angiogenesis (23,31). Moreover, LILRB2 overexpression
triggered by EGFR activation increases the recruitment of TAMs and
their polarization towards an M2-like phenotype, which further
promotes the T-cell dysfunction induced by TAMs in NSCLC (32).
In CRC, LILRB2 promotes the proliferation, invasion
and migration of CRC HT29 and SW480 cells, while enhancing the
expression of HLA-G, one of its ligands. Furthermore, the HLA-G
fusion protein notably increases the expression of LILRB2 in a
dose-dependent manner. This interaction facilitates the progression
of CRC HT29 cells by activating the AKT and ERK signaling pathways
(24). Additionally, a study using
a mouse model showed that when CRC MC-38 cells overexpressing PirB
(the mouse homologue of LILRB2) are injected into mice, it induces
Treg infiltration and reduces production of interferon (IFN)-γ in
tumor-infiltrating lymphocytes compared to mice injected with
control cell (22).
In pancreatic ductal carcinoma, the autocrine
signaling between LILRB2 and ANGPTL2 plays an important role in
sustaining cell metastasis during epithelial-mesenchymal transition
and in early pancreatic cancer precursors. Blocking LILRB2 reduces
ANGPTL2-induced cell proliferation and invasion. Serial KRAS
activation, HER2 expression and p16/p14-silencing are sufficient to
enhance ANGPTL2 secretion and LILRB2 expression (33).
Additionally, Gao et al (18) revealed that LILRB2/PirB from NSCLC,
prostate cancer and breast cancer cells can enhance fatty acid
synthesis and lipid accumulation in these cells by activating the
ERK1/2 signaling pathway through research on human cells and breast
cancer and melanoma mouse tumor models. By contrast, in the tumor
cell lines A549, H1299, ZR751, M628 and PC-3, LILRB2 knockdown
notably decreases the expression of two limiting enzymes,
acetyl-CoA carboxylases 1 and fatty acid synthase, thus inhibiting
fatty acid synthesis and lipid accumulation. Therefore, high levels
of LILRB2 in tumors can lead to fatty acid and lipid accumulation
that ultimately leads to effector T-cell senescence and tumor
progression (18).
In summary, tumor cell-derived LILRB2 can promote
cancer malignancy by activating classical or non-classical pathways
by itself or by interacting with its ligands, promoting
angiogenesis and EMT, reducing the secretion of tumor-killing
factors, and promoting the accumulation of fatty acids and lipids.
The main mechanisms of action of tumor cell-derived LILRB2 are
summarized in Fig. 1.
Mechanisms of immune cell-derived
LILRB2
LILRB2 is expressed in various immune cells and is
involved in regulating their state and function.
Macrophages are phagocytic cells that perform a
pivotal role in eliminating foreign particles, aging or damaged
cells, killing tumor cells and participating in the immune response
(34). Macrophages can be
classified as M1 (pro-inflammatory) and M2 (anti-inflammatory)
types, which are associated with NF-κB/STAT1 or STAT6 activation,
respectively (35). In the presence
of macrophage-stimulating factor lipopolysaccharide (LPS) or IFN-γ,
the LILRB2 antagonism causes macrophages to produce an inflammatory
phenotype, and increase the phosphorylation of NF-κB, ERK1/2, p38
and STAT1 (just in response to IFN-γ); the reason for these changes
is the interruption of SHP-1 activation signal and the inhibition
of the PI3K/AKT pathway caused by LILRB2 blockade (26). Additionally, antagonizing LILRB2
increases macrophage resistance to IL-4-mediated humoral
cytokine-dependent activation of STAT6, thereby alleviating
macrophage inhibition of T-cell proliferation (26).
DCs are important in both innate and acquired
immunity due to their ability to uptake and present antigens
(36); their functions can be
modulated by inhibitory receptors, including LILRB2, which is
linked to the tolerogenic phenotype of DCs (37,38).
HLA-G inhibits the maturation and differentiation of
LILRB2-positive DCs by recruiting SHP-1 and SHP-2, resulting in
increased IL-6 expression and STAT3 activation (39). In addition, IFN-γ, TNF-α and IL-10
can induce LILRB2 expression upregulation in DCs, promoting a
pro-tolerogenic state of DCs (37,40,41).
Furthermore, the presence of LILRB2 on DC surfaces can influence T
cells through various pathways. Tryptophan deprivation induces
tolerogenic DCs expressing a high level of LILRB2 and LILRB4
through a GCN2-mediated stress response pathway, which induces the
production of CD4+CD25+ Tregs (42). A subset of DCs with high expression
of LILRB2 and HLA-G can secrete abundant IL-10 and induce adaptive
type 1 Treg cells through IL-10-related pathways (43).
In mouse models, PirB has been detected in T-cell
progenitors, but rarely in mature T cells; and after antigen or
allogeneic stimulation, PirB combined with MHC-I inhibits proximal
T-cell receptor signaling, leading to reduced T-helper type 1
response in peripheral T cells that ectopically express PirB
(44). Thus, PirB regulates the
development of early T lymphocytes.
In summary, LILRB2 mainly affects macrophages and
DCs. Low LILRB2 levels can promote the function of the immune
system, whereas high LILRB2 levels have the opposite effect.
Impact of LILRB2 on drug response
Recent research indicates that LILRB2 can enhance
the tolerance of cells in the TME to certain drugs, potentially
leading to the reduced efficacy of tumor drug therapy.
Cyclosporine A
Cyclosporine A is a classical non-cytotoxic
immunosuppressant that is involved in the treatment of inflammation
and autoimmune diseases (45).
Cyclosporine A has also shown potential for prostate cancer and
renal cell carcinoma treatment, particularly in reversing multidrug
resistance in tumors and enhancing the therapeutic effect of
chemotherapy drugs (46,47).
Si et al (48) exposed NK cells to different doses of
cyclosporine A and observed a significant increase in LILRB2
expression on the NK cells in a dose- and time-dependent manner.
After treatment with cyclosporine A, the proliferation of NK cells
decreased and the cytotoxic activity of NK cells against the human
gastric cancer BGC-823 cell line and choriocarcinoma JEG-3 cell
line was reduced. The results suggest that cyclosporine A
upregulates LILRB2 expression on NK cells, thereby resulting in the
inhibition of the antitumor activity of NK cells. Patients
receiving cyclosporine A treatment for a long time may have
decreased NK cell activity due to the upregulation of LILRB2, and
thus decreased immune function (48). These findings highlight that LILRB2
overexpression in NK cells impairs the facilitating effect that
cyclosporine A exerts on the anti-tumor immune response. Inhibition
of LILRB2 expression may be an effective method to enhance the
activity of NK cells and thus the function of the immune
system.
Resveratrol
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) is an
inartificial polyphenolic compound that is widely found in plants;
it possesses various pharmacological properties, such as protection
against cardiovascular ischemic injury, regulation of lipid
metabolism, and anti-inflammatory and antitumor effects (49). Resveratrol has been demonstrated to
inhibit tumor development and progression, and shows promising
efficacy in the clinical treatment of colorectal and prostate
cancer (50,51).
Resveratrol can induce DC tolerance, particularly
during differentiation. In a previous study, costimulatory
molecules CD40/80/86 and MHC-II were downregulated in tolerogenic
DCs, while LILRB2 and ILT3 were induced. LILRB2 in DCs was not
upregulated after treatment with resveratrol on day 5 prior to
stimulation. However, when resveratrol was present during the whole
process of DC differentiation, LILRB2 was significantly
upregulated. Furthermore, DCs treated with resveratrol did not
produce the antitumor factor IL-12p70 but instead produced more
immunosuppressive factor IL-10. Thus, LILRB2 may act as a vital
influencing factor in the tolerance of DCs induced by resveratrol
and thereby affect the impact of DCs on tumors (52).
Niflumic acid
Niflumic acid is a commonly used non-steroidal
anti-inflammatory drug, primarily functioning by inhibiting the
activity of cyclooxygenase 2 (COX-2), and is predominantly
prescribed for the treatment of rheumatoid arthritis and to
alleviate inflammatory pain (53,54).
Additionally, niflumic acid has demonstrated antitumor effects by
promoting apoptosis in breast cancer, colon cancer and liver cancer
cells and complexes of niflumic acid with metals such as Ni(II) and
Co(II) showed better anti-tumor effects (55,56).
Svajger et al (57) revealed that niflumic acid can
upregulate LILRB2 expression in LPS-induced mature monocyte-derived
DCs. LILRB2 expression level was positively related to the
concentration of niflumic acid administered, and negatively related
to expression level of co-stimulatory molecules CD80/86, indicating
that LILRB2 influences LPS-induced tolerance in mature DCs treated
with niflumic acid, which may impact their effectiveness against
tumors.
Prostaglandin E2 (PGE2)
PGE2 is a small molecule derived from arachidonic
acid, and its synthesis is catalyzed by COX-1, COX-2 and PGE
synthetase. PGE2 is widely distributed in animals and plays a role
in the expansion and contraction of blood vessels, the control of
blood pressure, the regulation of inflammation, and other
physiological activities (58).
However, in tumors, PGE2 is expressed at a high level (59).
The expression of LILRB2 significantly increases
after the addition of PGE2 to monocytic-myeloid-derived suppressor
cells (M-MDSCs) induced by granulocyte-macrophage
colony-stimulating factor (GM-CSF) and IL-6; and after blocking
LILRB2, M-MDSCs induced by GM-CSF/IL-6 stimulate a low percentage
of type 1 Treg cells (60). PGE2
promotes tumorigenesis by increasing the expression of LILRB2,
which further promotes the development of M-MDSC-induced type 1
Treg cells, adversely affecting antitumor immunity (60). Therefore, targeting LILRB2 can
reduce the tumor-promoting effect of PGE2.
IFNs
IFNs are a group of active proteins primarily
produced by monocytes and lymphocytes, with a variety of functions,
including antiviral activity, inhibition of cell proliferation,
regulation of immunity and antitumor effects. IFNs can be mainly
categorized into IFN-α, IFN-β and IFN-γ, among which IFN-α and
IFN-γ play pivotal roles in antitumor immunity (61). Clinical application of IFN in tumor
treatment has shown efficacy in inhibiting tumor growth, and its
combination with other therapies can also improve antitumor
treatment outcomes (62).
LILRB2 is notable in the induction and maintenance
of tolerogenic DCs. The expression level of LILRB2 on immature DCs
is significantly upregulated after treatment with IFN-α (1,000
U/ml) or high doses of IFN-γ (>500 U/ml), while the high level
of LILRB2 is a universal feature of tolerogenic DCs (37,41).
Furthermore, tolerogenic DCs can lead to tumor progression and are
associated with poor patient outcomes (63). In addition, the stimulatory activity
of DCs treated with IL-10 and IFN-α is low (37). Accordingly, LILRB2 may adversely
affect the efficacy of IFN in tumor treatment.
In summary, the aforementioned drugs have been
indicated to cause upregulated expression of LILRB2 on immune cells
(mainly DCs, NK cells and M-MDSCs) within the TME, which is not
conducive to the antitumor function of the immune system.
Therefore, therapies targeting LILRB2 may improve the efficacy of
these drugs.
Impact of LILRB2 on radiotherapy
Radiation therapy, which destroys the chromosomes of
cells through radiation, is one of the common therapies for tumors
(64). However, for a variety of
reasons, tumors develop resistance to radiation therapy, resulting
in treatment failure (65).
It has been demonstrated that LILRB2 can resist the
effect of radiotherapy. In patients with lung adenocarcinoma,
bioinformatics analysis has revealed that stereotactic body
radiotherapy upregulates the expression of LILRB2 in
tumor-infiltrating lymphocytes (66). Analogously, in patients with NSCLC,
radiotherapy promotes LILRB2 expression, which increases M2-TAM
migration by activating the NF-κB pathway and the secretion of
chemokine CXCL1 (67). Radiation
enhances LILRB2 expression in several NSCLC cell lines in a
time-dependent manner, and knockdown of LILRB2 promotes the
radiosensitivity of NSCLC cells (68). In addition, radiation can also
facilitate NSCLC cellular senescence and the senescence-associated
secretory phenotype, whereas blocking LILRB2 expression decreases
these effects by suppressing the JAK2/STAT3 pathway (68). Thus, targeting LILRB2 can enhance
tumor radiosensitivity.
Novel drugs targeting LILRB2
Currently, antibody drugs targeting LILRB2 have been
developed and are being tested in clinical trials. Therapies
involving LILRB2 antibody drugs primarily focus on enhancing the
activity of immune cells in the TME, ultimately promoting T
cell-mediated killing of tumors. These drugs have demonstrated
prospective therapeutic effects when utilized alone or in
combination with other treatments.
JTX-8064
JTX-8064 is a humanized monoclonal antibody that
specifically targets LILRB2 and acts as an antagonist by inhibiting
the interaction between LILRB2 and MHC-I (69,70).
An in vitro human tumor culture model
obtained from lung, kidney and head and neck cancer revealed that
the pharmacodynamic response induced by JTX-8064 was significantly
higher compared with that of the isotype control (71). This is due to the transformation of
human macrophages and DCs to immunostimulated inflammatory
phenotypes after stimulation with JTX-8064, resulting in increased
antigen presentation and enhanced T-cell activation (69–72).
Furthermore, JTX-8064 can also improve the effectiveness of
programmed cell death protein 1 (PD-1) inhibitors in treating
tumors. The combination of JTX-8064 and anti-PD-1 can approximately
double the expression level of IFN-γ obtained with treatment with
anti-PD-1 alone (69).
Overall, these results provide evidence for the
efficacy of targeting LILRB2 as a single drug or as an adjunct
approach to cancer treatment.
MK-4830
MK-4830 is an IgG4 monoclonal antibody that binds to
myeloid-specific LILRB2, whose functions include mitigating
myelosuppression, facilitating TAMs reprogramming and increasing T
cell function (73).
MK-4830 has been evaluated in a clinical trial
(NCT03564691) as a monotherapy or in combination with pembrolizumab
for the treatment of advanced melanoma, NSCLC, colorectal cancer
and renal cell carcinoma. Relevant studies have revealed that
MK-4830 demonstrates favorable tolerability, safety and antitumor
activities in the treatment of advanced tumors, and its target
binding ability is dose-dependent (73–75).
Of 84 patients, 50 received MK4830 monotherapy, 34 received MK4830
combined with pembrolizumab, preliminary efficacy data show 11
objective responses. Among these, one of the patients received
MK-4830 monotherapy and 5 patients did not respond to anti-PD-L1
therapy but improved with the combination of MK-4830. In addition,
some patients experienced fatigue, nausea, decreased appetite or
diarrhea during treatment (73).
Patients who received MK-4830 and pembrolizumab simultaneously
demonstrated a higher sensitivity to T-cell inflammation than
expected compared with the response to pembrolizumab monotherapy
(74).
These studies provide evidence for the potential
value of MK-4830 as a novel immunotherapy or adjuvant therapeutic
agent for tumors.
Conclusion
LILRB2 shows an increased expression level in the
microenvironment of diverse solid tumors, promoting proliferation,
colony formation, and the migration and invasion of tumor cells,
and shifting the TME in an inhibitory direction, thereby
facilitating tumorigenesis and progression. Consequently, LILRB2
may represent a novel target for tumor-targeted therapy.
Researchers have developed new drugs, JTX-8064 and MK-4830, to
target LILRB2, which have exhibited positive results in clinical
trials either as monotherapy or in combination with other drugs.
Further research may reveal that targeting LILRB2 constitutes a
more effective strategy for targeted therapy of solid tumors in
future.
Acknowledgements
Not applicable..
Funding
This study was funded by the National Natural Science Foundation
of China (grant no. 82203692), the Natural Science Basic Research
Program of Shaanxi Province (grant nos. 2021JQ-777 and
2023-JC-QN-0863), the Scientific Research Plan of Shaanxi
Provincial Education Department (grant no. 21JS040), the Young
Talent Fund of Association for Science and Technology in Shaanxi
(grant no. 20220610) and the Innovation Team of Xi'an Medical
University (grant no. 2021TD05 and 2021TD-48).
Availability of data and materials
Not applicable.
Authors' contributions
MC wrote the manuscript. JL and HL collected the
literature, designed the figure and edited the manuscript. CZ and
ZZ reviewed and revised the manuscript. NG drafted the manuscript
and offered writing guidance. All authors have read and approved
the final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LILRB2
|
leukocyte immunoglobulin-like receptor
B2
|
|
TME
|
tumor microenvironment
|
|
PirB
|
paired immunoglobulin-like receptor
B
|
|
NK
|
natural killer
|
|
DCs
|
dendritic cells
|
|
ITIMs
|
immunoreceptor tyrosine-based switch
motifs
|
|
SHP-1
|
tyrosine phosphatase-1
|
|
MHC
|
major histocompatibility complex
|
|
HLA
|
human leukocyte antigen
|
|
ANGPTLs
|
angiopoietin-like proteins
|
|
NSCLC
|
non-small cell lung cancer
|
|
Treg
|
regulatory T cells
|
|
CaMK1
|
calcium/calmodulin-dependent protein
kinase 1
|
|
TAM
|
tumor-associated macrophage
|
|
CRC
|
colorectal cancer
|
|
LPS
|
lipopolysaccharide
|
|
PGE2
|
prostaglandin E2
|
|
COX
|
cyclooxygenase
|
|
IFN
|
interferon
|
References
|
1
|
Zhang P, Yu S, Li H, Liu C, Li J, Lin W,
Gao A, Wang L, Gao W and Sun Y: ILT4 drives B7-H3 expression via
PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates
with poor prognosis in non-small cell lung cancer. FEBS Lett.
589:2248–2256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao A, Sun Y and Peng G: ILT4 functions as
a potential checkpoint molecule for tumor immunotherapy. Biochim
Biophys Acta Rev Cancer. 1869:278–285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yue J, Zhang C, Shi X, Wei Y, Liu L, Liu S
and Yang H: Activation of leukocyte immunoglobulin-like receptor B2
signaling pathway in cortical lesions of pediatric patients with
focal cortical dysplasia type IIb and tuberous sclerosis complex.
Brain Dev. 41:829–838. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borges L and Cosman D: LIRs/ILTs/MIRs,
inhibitory and stimulatory Ig-superfamily receptors expressed in
myeloid and lymphoid cells. Cytokine Growth Factor Rev. 11:209–217.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yue J, Li W, Liang C, Chen B, Chen X, Wang
L, Zang Z, Yu S, Liu S, Li S and Yang H: Activation of LILRB2
signal pathway in temporal lobe epilepsy patients and in a
pilocarpine induced epilepsy model. Exp Neurol. 285:51–60. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng M, Lu Z, Zheng J, Wan X, Chen X,
Hirayasu K, Sun H, Lam Y, Chen L, Wang Q, et al: A motif in LILRB2
critical for Angptl2 binding and activation. Blood. 124:924–935.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Wang L, Gao W, Li L, Cui X, Yang H,
Lin W, Dang Q, Zhang N and Sun Y: Inhibitory receptor
immunoglobulin-like transcript 4 was highly expressed in primary
ductal and lobular breast cancer and significantly correlated with
IL-10. Diagn Pathol. 9:852014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carosella ED, Gregori S and Tronik-Le Roux
D: HLA-G/LILRBs: A cancer immunotherapy challenge. Trends Cancer.
7:389–392. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng J, Umikawa M, Cui C, Li J, Chen X,
Zhang C, Huynh H, Kang X, Silvany R, Wan X, et al: Inhibitory
receptors bind ANGPTLs and support blood stem cells and leukaemia
development. Nature. 485:656–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao H, Ma L, Jin F, Zhou Y, Tao M and
Teng Y: Immune inhibitory receptor LILRB2 is critical for the
endometrial cancer progression. Biochem Biophys Res Commun.
506:243–250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y, Liu J, Gao P, Wang Y and Liu C:
Expression of Ig-like transcript 4 inhibitory receptor in human
non-small cell lung cancer. Chest. 134:783–788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Yu X, Xie J, Zhan M, Yu Z, Xie L,
Zeng H, Zhang F, Chen G, Yi X and Zheng J: ANGPTL2/LILRB2 signaling
promotes the propagation of lung cancer cells. Oncotarget.
6:21004–21015. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q, Li J, Wang S, Wang J, Chen X, Zhou
D, Fang Y, Gao A and Sun Y: Overexpressed immunoglobulin-like
transcript (ILT) 4 in lung adenocarcinoma is correlated with
immunosuppressive T cell subset infiltration and poor patient
outcomes. Biomark Res. 8:112020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Wei X, Xu H, Sha Z, Gao A, Sun Y, Li
J and Xu L: Expression of leukocyte immunoglobulin-like receptor B2
in hepatocellular carcinoma and its clinical significance. J Cancer
Res Ther. 14:1655–1659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He J, Xu J, Yu X, Zhu H, Zeng Y, Fan D and
Yi X: Overexpression of ANGPTL2 and LILRB2 as predictive and
therapeutic biomarkers for metastasis and prognosis in colorectal
cancer. Int J Clin Exp Pathol. 11:2281–2294. 2018.PubMed/NCBI
|
|
16
|
Kun L, Yunyan P, Xiangshan Y, Hongxin N
and Junyuan Y: Relationship between HPV 16/18 infection in ovarian
cancer patients and expression of ILT4. Chin J Nosocomiol.
24:3901–3903. 2014.
|
|
17
|
García M, Palma MB, Verine J, Miriuka S,
Inda AM, Errecalde AL, Desgrandchamps F, Carosella ED and Tronik-Le
Roux D: The immune-checkpoint HLA-G/ILT4 is involved in the
regulation of VEGF expression in clear cell renal cell carcinoma.
BMC Cancer. 20:6242020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao A, Liu X, Lin W, Wang J, Wang S, Si F,
Huang L, Zhao Y, Sun Y and Peng G: Tumor-derived ILT4 induces T
cell senescence and suppresses tumor immunity. J Immunother Cancer.
9:e0015362021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Deng G, Qi Y, Zhang H, Gao L, Jiang
H, Ye Z, Liu B and Chen Q: Bioinformatic profiling of
Prognosis-related genes in malignant glioma microenvironment. Med
Sci Monit. 26:e9240542020.PubMed/NCBI
|
|
20
|
Chalbatani GM, Momeni SA, Mohammadi Hadloo
MH, Karimi Z, Hadizadeh M, Jalali SA, Miri SR, Memari F and Hamblin
MR: Comprehensive analysis of ceRNA networks to determine genes
related to prognosis, overall survival, and immune infiltration in
clear cell renal carcinoma. Comput Biol Med. 141:1050432022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Warnecke-Eberz U, Metzger R, Hölscher AH,
Drebber U and Bollschweiler E: Diagnostic marker signature for
esophageal cancer from transcriptome analysis. Tumour Biol.
37:6349–6358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Z, Gao A, Shi W, Wang J, Zhang X, Xu
Z, Xu T, Zheng Y, Sun Y and Yang F: ILT4 in colorectal cancer cells
induces suppressive T cell contexture and disease progression. Onco
Targets Ther. 14:4239–4254. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang P, Guo X, Li J, Yu S, Wang L, Jiang
G, Yang D, Wei Z, Zhang N, Liu J and Sun Y: Immunoglobulin-like
transcript 4 promotes tumor progression and metastasis and
up-regulates VEGF-C expression via ERK signaling pathway in
non-small cell lung cancer. Oncotarget. 6:13550–13563. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai Z, Wang L, Han Y, Gao W, Wei X, Gong
R, Zhu M, Sun Y and Yu S: Immunoglobulin-like transcript 4 and
human leukocyte antigen-G interaction promotes the progression of
human colorectal cancer. Int J Oncol. 54:1943–1954. 2019.PubMed/NCBI
|
|
25
|
Shao H, Ma L, Jin F, Zhou Y, Tao M and
Teng Y: Immune inhibitory receptor LILRB2 is critical for the
endometrial cancer progression. Biochem Biophys Res Commun.
506:243–250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen HM, van der Touw W, Wang YS, Kang K,
Mai S, Zhang J, Alsina-Beauchamp D, Duty JA, Mungamuri SK, Zhang B,
et al: Blocking immunoinhibitory receptor LILRB2 reprograms
tumor-associated myeloid cells and promotes antitumor immunity. J
Clin Invest. 128:5647–5662. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan J, Han J, Li J, Gu A, Yin D, Song F,
Wang L and Yi Y: The expression and function of immunoglobulin-like
transcript 4 in dendritic cells from patients with hepatocellular
carcinoma. Hum Immunol. 81:714–725. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho EC, Mitton B and Sakamoto KM: CREB and
leukemogenesis. Crit Rev Oncog. 16:37–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu JF, Li J, Yan P, Gong WJ and Sun YP:
Silencing of ILT4 suppresses migration and invasion of non-small
cell lung cancer cells by inhibiting MMP-2. Int J Clin Exp Med.
12:5306–5314. 2019.
|
|
30
|
Cabral-Pacheco GA, Garza-Veloz I,
Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA,
Guerrero-Rodriguez JF, Martinez-Avila N and Martinez-Fierro ML: The
roles of matrix metalloproteinases and their inhibitors in human
diseases. Int J Mol Sci. 21:97392020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Zhao J, Qiu L, Zhang P, Li J,
Yang D, Wei X, Han Y, Nie S and Sun Y: Co-expression of ILT4/HLA-G
in human non-small cell lung cancer correlates with poor prognosis
and ILT4-HLA-G interaction activates ERK signaling. Tumour Biol.
37:11187–11198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Gao A, Zhang F, Yang Z, Wang S,
Fang Y, Li J, Wang J, Shi W, Wang L, et al: ILT4 inhibition
prevents TAM- and dysfunctional T cell-mediated immunosuppression
and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR
activation. Theranostics. 11:3392–3416. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carbone C, Piro G, Fassan M, Tamburrino A,
Mina MM, Zanotto M, Chiao PJ, Bassi C, Scarpa A, Tortora G and
Melisi D: An angiopoietin-like protein 2 autocrine signaling
promotes EMT during pancreatic ductal carcinogenesis. Oncotarget.
6:13822–13834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment.
F1000Prime Rep. 6:132014. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gardner A, de Mingo Pulido Á and Ruffell
B: Dendritic cells and their role in immunotherapy. Front Immunol.
11:9242020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manavalan JS, Rossi PC, Vlad G, Piazza F,
Yarilina A, Cortesini R, Mancini D and Suciu-Foca N: High
expression of ILT3 and ILT4 is a general feature of tolerogenic
dendritic cells. Transpl Immunol. 11:245–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guerra-de Blas Pdel C, Villaseñor-Talavera
YS, Cruz-González Dde J, Baranda L, Doníz-Padilla L, Abud-Mendoza
C, González-Amaro R and Monsiváis-Urenda AE: Analysis of the
expression and function of Immunoglobulin-like transcript 4 (ILT4,
LILRB2) in dendritic cells from patients with systemic lupus
erythematosus. J Immunol Res. 2016:41630942016.PubMed/NCBI
|
|
39
|
Liang S, Ristich V, Arase H, Dausset J,
Carosella ED and Horuzsko A: Modulation of dendritic cell
differentiation by HLA-G and ILT4 requires the IL-6-STAT3 signaling
pathway. Proc Natl Acad Sci USA. 105:8357–8362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Trojandt S, Bellinghausen I, Reske-Kunz AB
and Bros M: Tumor-derived immuno-modulators induce overlapping
pro-tolerogenic gene expression signatures in human dendritic
cells. Hum Immunol. 77:1223–1231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Svajger U, Obermajer N and Jeras M:
IFN-γ-rich environment programs dendritic cells toward silencing of
cytotoxic immune responses. J Leukoc Biol. 95:33–46. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brenk M, Scheler M, Koch S, Neumann J,
Takikawa O, Häcker G, Bieber T and von Bubnoff D: Tryptophan
deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic
cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory
cells. J Immunol. 183:145–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gregori S, Magnani CF and Roncarolo MG:
Role of human leukocyte antigen-G in the induction of adaptive type
1 regulatory T cells. Hum Immunol. 70:966–969. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Imada M, Masuda K, Satoh R, Ito Y, Goto Y,
Matsuoka T, Endo S, Nakamura A, Kawamoto H and Takai T: Ectopically
expressed PIR-B on T cells constitutively binds to MHC class I and
attenuates T helper type 1 responses. Int Immunol. 21:1151–1161.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Patocka J, Nepovimova E, Kuca K and Wu W:
Cyclosporine A: Chemistry and Toxicity-A review. Curr Med Chem.
28:3925–3934. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qadir M, O'Loughlin KL, Fricke SM,
Williamson NA, Greco WR, Minderman H and Baer MR: Cyclosporin A is
a broad-spectrum multidrug resistance modulator. Clin Cancer Res.
11:2320–2326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Z, Jiang L, Li Y, Xie B, Xie J, Wang
Z, Zhou X, Jiang H, Fang Y, Pan H and Han W: Cyclosporine A
sensitizes lung cancer cells to crizotinib through inhibition of
the Ca2+/calcineurin/Erk pathway. EBioMedicine. 42:326–339. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Si YQ, Bian XK, Lu N, Jia YF, Hou ZH and
Zhang Y: Cyclosporine induces up-regulation of immunoglobulin-like
transcripts 3 and 4 expression on and activity of NKL cells.
Transplant Proc. 44:1407–1411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Malaguarnera L: Influence of resveratrol
on the immune response. Nutrients. 11:9462019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ko JH, Sethi G, Um JY, Shanmugam MK,
Arfuso F, Kumar AP, Bishayee A and Ahn KS: The role of resveratrol
in cancer therapy. Int J Mol Sci. 18:25892017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ren B, Kwah MX, Liu C, Ma Z, Shanmugam MK,
Ding L, Xiang X, Ho PC, Wang L, Ong PS and Goh BC: Resveratrol for
cancer therapy: Challenges and future perspectives. Cancer Lett.
515:63–72. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Svajger U, Obermajer N and Jeras M:
Dendritic cells treated with resveratrol during differentiation
from monocytes gain substantial tolerogenic properties upon
activation. Immunology. 129:525–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Acebedo-Martínez FJ, Alarcón-Payer C,
Frontera A, Barbas R, Prohens R, Di Crisci M, Domínguez-Martín A,
Gómez-Morales J and Choquesillo-Lazarte D: Novel polymorphic
cocrystals of the Non-steroidal anti-inflammatory drug niflumic
acid: Expanding the pharmaceutical landscape. Pharmaceutics.
13:21402021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jin LH, Kim BH, Lee JH, Lee K, Kwack K and
Yim SV: Screening study for genetic polymorphisms affecting
pharmacokinetics of talniflumate. Transl Clin Pharmacol.
25:166–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Altay A, Caglar S and Caglar B: Silver(I)
complexes containing diclofenac and niflumic acid induce apoptosis
in human-derived cancer cell lines. Arch Physiol Biochem.
128:69–79. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Caglar S, Altay A, Kuzucu M and Caglar B:
In vitro anticancer activity of novel co(II) and Ni(II) complexes
of Non-steroidal Anti-inflammatory drug niflumic acid against human
breast adenocarcinoma MCF-7 cells. Cell Biochem Biophys.
79:729–746. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Svajger U, Vidmar A and Jeras M: Niflumic
acid renders dendritic cells tolerogenic and up-regulates
inhibitory molecules ILT3 and ILT4. Int Immunopharmacol.
8:997–1005. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sakata D, Yao C and Narumiya S:
Prostaglandin E2, an immunoactivator. J Pharmacol Sci. 112:1–5.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Santiso A, Heinemann A and Kargl J:
Prostaglandin E2 in the tumor microenvironment, a convoluted affair
mediated by EP receptors 2 and 4. Pharmacol Rev. 76:388–413. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tomić S, Joksimović B, Bekić M, Vasiljević
M, Milanović M, Čolić M and Vučević D: Prostaglanin-E2 potentiates
the suppressive functions of human mononuclear Myeloid-derived
suppressor cells and increases their capacity to expand
IL-10-Producing regulatory T cell subsets. Front Immunol.
10:4752019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dunn GP, Koebel CM and Schreiber RD:
Interferons, immunity and cancer immunoediting. Nat Rev Immunol.
6:836–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Saleiro D and Platanias LC: Interferon
signaling in cancer. Non-canonical pathways and control of
intracellular immune checkpoints. Semin Immunol. 43:1012992019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Plesca I, Müller L, Böttcher JP, Medyouf
H, Wehner R and Schmitz M: Tumor-associated human dendritic cell
subsets: Phenotype, functional orientation, and clinical relevance.
Eur J Immunol. 52:1750–1758. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Alamilla-Presuel JC, Burgos-Molina AM,
González-Vidal A, Sendra-Portero F and Ruiz-Gómez MJ: Factors and
molecular mechanisms of radiation resistance in cancer cells. Int J
Radiat Biol. 98:1301–1315. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sun L, Zhou H, Wu C and Peng Y: Molecular
markers that predict response to combined radiotherapy and
immunotherapy in patients with lung adenocarcinoma: A
bioinformatics analysis. Transl Cancer Res. 12:2646–2659. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen X, Wang M, Wu F, Lu J, Xiao C, Wu M,
Yu J and Chen D: Overcoming Radio-immunotherapy treatment
resistance through ILT4 blockade and reversal of HFRT induced
CXCL1-CXCR2 axis activation and Tumor-associated macrophage
immunosuppression. Int J Radiat Oncol Biol Phys. 117
(Suppl):S72–S73. 2023. View Article : Google Scholar
|
|
68
|
Chen X, Yuan M, Zhong T, Wang M, Wu F, Lu
J, Sun D, Xiao C, Sun Y, Hu Y, et al: LILRB2 inhibition enhances
radiation sensitivity in Non-small cell lung cancer by attenuating
Radiation-induced senescence. Cancer Lett. 593:2169302024.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Umiker B, Hashambhoy-Ramsay Y, Smith J,
Rahman T, Mueller A, Davidson R, Meyer C, Patankar G, Alam MM,
Jaffe S, et al: Inhibition of LILRB2 by a novel blocking antibody
designed to reprogram immunosuppressive macrophages to drive T-cell
activation in tumors. Mol Cancer Ther. 22:471–484. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Papadopoulos KP, Lakhani NJ, Yap TA,
Naumovski Al, Brown KS, Umiker B, McGrath L, Zhang W, Stack E,
Riley G, et al: Phase 1, first-in-human trial of JTX-8064, an
anti-LILRB2/ILT4 monoclonal antibody, as monotherapy and in
combination with anti-PD-1 in adult patients with advanced solid
tumors (INNATE). J Clin Oncol. 39:TPS26722021. View Article : Google Scholar
|
|
71
|
Hashambhoy-Ramsay Y, Spaulding V, Priess
M, O'Malley K, Gostissa M, Stack E, Smith J, Willer M, Umiker B and
Shaffer D: 217 Evaluating biomarkers of JTX-8064 (anti-LILRB2/ILT4
monoclonal antibody) in an ex vivo human tumor histoculture system
to inform clinical development. J Immunother Cancer. 82020.
|
|
72
|
Cohen H, Hashambhoy-Ramsay Y, Pepper LR,
Smith JY, Willer M, Guay K, Spaulding V, O'Malley K, Gostissa M,
Dhaneshwar A, et al: Preclinical evaluation of JTX-8064, an
anti-LILRB2 antagonist antibody, for reprogramming tumor-associated
macrophages. Cancer Res. 79:50072019. View Article : Google Scholar
|
|
73
|
Siu LL, Wang D, Hilton J, Geva R, Rasco D,
Abraham AK, Markensohn JF, Suttner L, Siddiqi S, Altura AR and
Maurice-Dror C: Initial results of a phase I study of MK-4830, a
first-in-class anti-immunoglobulin-like transcript 4 (ILT4)
myeloid-specific Antibody in patients (pts) with advanced solid
tumours. Ann Oncol. 31:S462. 2020. View Article : Google Scholar
|
|
74
|
Siu LL, Wang D, Hilton J, Geva R, Rasco D,
Perets R, Abraham AK, Wilson DC, Markensohn JF, Lunceford J, et al:
Correction: First-in-class Anti-immunoglobulin-like Transcript 4
Myeloid-Specific Antibody MK-4830 Abrogates a PD-1 Resistance
Mechanism in Patients with Advanced Solid Tumors. Clin Cancer Res.
28:17342022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cho BC, Hilton J, Rodriguez CP, Bonomi M,
Siu LL, Gil-Martin M, Siddiqi S, Myer NM, Suttner L, Wilson D, et
al: Abstract CT114: Phase 1 study of the anti-immunoglobulin-like
transcript 4 (ILT4) monoclonal antibody MK-4830 plus pembrolizumab
in patients with previously untreated advanced head and neck
squamous cell carcinoma (HNSCC) or non-small cell lung cancer
(NSCLC). Cancer Res. 84:CT1142024. View Article : Google Scholar
|