Introduction
TSLP is an IL-7-like cytokine that encounters a
plethora of roles (1). TSLP
receptor (TSLPR) and interleukin 7 receptor-α (IL-7Rα or CD127)
form a high-affinity heteromeric complex, which the human TSLP
binds to fulfill its physiological functions (2). By forming a ternary complex with its
particular receptor, TSLPR, and then with IL-7Rα, TSLP starts
signaling (3–5). TSLP is primarily produced by
epithelial cells, smooth muscle cells, and fibroblasts in the skin,
gut, and lung (6). Furthermore,
TSLP can be produced by immune cells, such as monocytes (7), macrophages (8) and mast cells (9). Likewise, immune and non-immune cell
subsets TSLPR complex expressing are activated by TSLP.
Nevertheless, if we focus on human monocytes of the innate immune
system, few studies have been published. For instance, it has been
shown that the expression of surface antigens is enhanced by TSLP
in CD14+ monocytes/macrophages, in particular increasing
CD80 expression (10). Besides,
human CD14+ monocytes have exclusively been found to
express the TSLPR complex in response to Lipopolysaccharide (LPS)
stimulation, also enriching a functionally discrete subset of
CD14+CD1c+ human monocytes (7). Lastly, TSLP has been demonstrated to
modulate monocyte metabolism by inducing ROS production (11), but again this process remains
unclear and unexplored.
TSLP activated cells lead to various disease models,
including cancer (12). Besides,
TSLP was found to be expressed in various cancer cell types,
including BC (13) that represents
the most accidental cancer in industrialized nations, making it one
of the most aggressive diseases for women globally (14). The development of BC is influenced
by a number of risk variables, including age, gender, and economic
development level, aspects connected to hormones, nutrition,
genetics, and lifestyle (15).
Luminal A, luminal B, HER2+, and triple negative (TNBC)
are among the five molecular subtypes of BC that have been
identified owing to the analysis of gene expression (16).
In BC, the function of TSLP is still debated and
ambiguous. Both a tumor-promoting role and tumor-suppressive role
of TSLP was identified. In detail, it has been discovered that TSLP
is directly produced by human BC cells through the release of IL-1β
by myeloid dendritic cells (DCs), thus causing OX40L expression on
DCs in vitro (17,18). Inter alia, BC metastases in the lung
expressed TSLP (19,20). Demehri et al discovered a
tumor-suppressive role for TSLP in murine models of BC
carcinogenesis, which is in contrast to the results previously
discussed (21). Moreover, it has
been found that TSLP expression was absent from most human tumor
samples under investigation, indicating a lack of TSLP-TSLPR
signaling in BC (22).
Herein, we try to add a new piece on the function of
TSLP in BC. Indeed, we investigated TSLP expression in different
subtypes of BC. Furthermore, we focused on the effect of TSLP to
the immune system to understand which components of innate immunity
were altered by the presence of the protein. Finally, our
investigation also focused on how TSLP might affect the energy
system and metabolism of specific cells of the anticancer
immunity.
Materials and methods
In silico study
A user-friendly and interactive online tool called
Gene Expression Profiling Interactive Analysis (GEPIA2, http://gepia2.cancer-pku.cn/#index, accessed on
25 June 2024) allows users to analyze RNA sequencing expression
data of 9736 tumors and 8587 normal samples from the TCGA and GTEx
projects employing a common handling pipeline (23). Utilizing GEPIA2, the tissue-specific
expression of TSLP was examined in BC tissues compared to normal
tissues, and in BC subtypes (HER2+, luminal A, luminal
B, basal-like) in relation to normal tissues. TCGA normal was
exclusively used for differential analysis and plotting. Beyond
that, the Overall Survival (OS) and Disease Free Survival (DFS)
were calculated using the same tool (23). The UALCAN database (https://ualcan.path.uab.edu/, accessed on 25 June
2024) was used as well for assessing TSLP expression in BC subtypes
tissues (24,25). Finally, TIMER 2.0 (http://timer.cistrome.org/, accessed on 25 June 2024),
a webserver that allows correlation of immune infiltrate abundance
and gene expression using deconvolution algorithms (26).
Cell culture
All cell lines (MCF-10A, MCF-7, BT-474, MDA-MB-231,
THP-1) were obtained from IRCCS Synlab SDN Biobank. In detail, the
BC cell lines were maintained as described below: MCF-7 (luminal A)
in Roswell Park Memorial Institute 1640 Medium (RPMI)
(Gibco-Thermo-Fisher Waltham, MA, USA) supplemented with 20% heat
inactivated (h.i.) fetal bovine serum (FBS), 1% MEM non-essential
amino acids, 1 mM sodium pyruvate and 10 µg/ml of human insulin;
BT-474 (luminal B) in RPMI 1640 supplemented with 20% h.i. FBS, 1%
L-glutamine and 10 µg/ml of human insulin; MDA-MB-231 (TNBC) in
Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Thermo-Fisher
Waltham, MA, USA) supplemented with 10%h.i. FBS and 1% L-glutamine.
Lastly, MCF-10, a human epithelial cell line isolated from the
mammary gland and used as our control condition, was maintained in
Mammary Epithelial Cell Growth Medium bullet kit (MEGM) (Lonza,
Basel, Switzerland) supplemented with 10% h.i. FBS and 1%
L-glutamine. The THP-1 cell line, monocyte cells isolated from an
acute monocytic leukemia patient, was maintained in RPMI 1640
supplemented with 20% h.i. FBS and 1% L-glutamine. All the cell
lines were incubated at 37°C in 5% CO2 and routinely
checked for mycoplasma contamination.
PBMC isolation from whole blood
PBMCs were obtained from five healthy women enrolled
from the active protocol 4/21 who have provided written consent to
participate in the study (Ethical Committee of IRCCS Pascale of
Naples, Italy; approval no. 4/21, 2021). PBMCs were re-covered from
venous blood using density gradient centrifugation
(Pancoll® density 1,077 g/l, PanBiotech, Aidenbach,
Germany) as described previously (27). Briefly, whole blood collected in an
EDTA vacutainer was diluted in 5 ml PBS, layered on 3 ml Pancoll
and centrifuged at 1,200 × g for 10 min at 4°C.
BC conditioned media
BC cells were seeded at 10–20% confluence in tissue
culture plates. Once the cells reached a confluence of 90%, the
cell culture medium was replaced with a serum-free fresh medium.
After 24 h, this CM was harvested, filtered (0.20 µm pore size
filter), and stored at −20°C.
ELISA
In a set of experiments, 1×106 cells/ml
of BC cell lines were seeded in 24-well plates and grown to
confluence for 24 h prior to harvest CM. After treating, the
supernatant was collected while the cellular pellets were lysed in
Tryton X-100 0.1% (Sigma-Aldrich, Saint Louis, MO, USA). After
harvest, both samples were centrifuged at 300 × g at 4°C and then
stored at −80°C for subsequent determination of extracellular and
intracellular mediator content.
TSLP concentrations in supernatant and in cellular
lysates of BC cells were measured using commercially available
ELISA kit (Catalog No. DY1398-05, R&D System, Minneapolis, MN,
USA) according to the manufacturer's instructions. The absorbance
was read at 450 nm using an automatic plate reader (Victor Nivo,
Perkin Elmer, Waltham, MA, USA). These experiments were repeated
five times and data were expressed as pg/ml.
Total RNA extraction and reverse
transcription-quantitative (RT-qPCR)
Total RNA was extracted by TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. Subsequently, it was quantified
employing Nanophotometer NP-80 spectrophotometer (Implen, Munich,
Germany). 1 µg of total RNA was reverted in cDNA exploiting Xpert
cDNA Synthesis SuperMix (Grisp, Porto, Portugal).
qPCR was performed by means of IQ SYBR Green
Supermix (Bio-Rad Laboratories, Hercules, CA, USA) on a CFX384
Real-time detection system (Bio-Rad Laboratories, Hercules, CA,
USA). GAPDH was used as endogenous controls to normalize Cq (cycle
quantification) values applying the 2−ΔCq formula
(28). Each cDNA sample was
analyzed in triplicate and the corresponding no-RT mRNA sample was
included as a negative control. The following human primers were
used in this study: GAPDH forward, 5′-GTCCACTGGCGTCTTCAC-3′,
reverse, 5′-CTTGAGGCTGTTGTCATACTTC-3′; ACTINB forward,
5′-CAAGAGATGGCCACG-3′, reverse, 5′-TCCTTCTGCATCCTG-3′; TSLP
forward, 5′-CACCGTCTCTTGTAGCAATCG-3′, reverse,
5′-TAGCCTGGGCACCAGATAGC-3′.
Flow cytometric analysis
Fresh PBMC samples obtained as described above were
stimulated with BC CM or with TSLP. Specifically, 1×105
PBMCs were pelleted and resuspended with 100 µl BC CM or treated
with different concentrations of TSLP (10, 50, and 100 ng/ml) in
96-well U-bottom plates, and then incubated for 24 h at 37°C in 5%
CO2. The following monoclonal antibodies from Beckman
Coulter (Brea, CA, USA) were used: CD3-PC5 (6607010), CD25-PC7
(A52882), CD14-APC 700 (A99020), CD45-KrO (B36294, CD16-APC 750
(B00845), and Myeloid activation antibody cocktail composed by
CD169-PE, HLA-DR-APC and CD64-PB (C63854), CD31-FITC (IM1431U),
CD335-PE (IM3711), HLA-DR-ECD (B92438), CD19-PC5 (A07771), CD45-PC7
(IM3548), CD56-APC (IM2474), CD3-APC 700 (B10823), CD9-APC-750
(B13649), CD4-PB (B49197) and CD8-KrO (B00067). Each antibody were
purchased by Beckman Coulter (Beckman Coulter, Brea, CA, USA) and
were prepared in a 1:10 dilution and mixed throughout. Flow
cytometry experiments were conducted using a minimum of 10,000
recorded events using the Cytoflex V2-B4-R2 instrument. At last,
data were analyzed through the Kaluza Analysis Software 2.1
(Beckman Coulter, Brea, CA, USA). Doublets and debris (identified
based on forward- and side-scatter properties) were excluded from
the analysis.
ROS production
To quantitatively assess the reactive oxygen species
(ROS) production, the DCFDA/H2DCFDA-Cellular ROS Assay Kit
(ab113851) purchased from Abcam (Cam-bridge, UK) was used. In
detail, THP-1 cells were seeded in a 96-well plate at the density
of 1×105 cells/well in FBS-supplemented medium for 1 h.
Then, cells were incubated with TSLP 100 ng/ml in combination with
DCFDA (10 µM) for 30 min protected from light, washed with Buffer
1X (provided in the kit), and analyzed on a microplate reader
(Victor Nivo, Perkin Elmer, Waltham, MA, USA) with the excitation
at 485 nm and the emission at 535 nm according to manufacturer's
instructions. ROS production was measured immediately, after 30 min
and 1 h. The unlabeled THP-1 cells were analyzed and used as
negative controls. Duplicates of the experiments were
conducted.
Fluorescent calcium measurement
The calcium-sensitive fluorescent single wavelength
dye, Fluo-4 AM (Invitrogen, Thermo-Fisher Waltham, MA, USA) was
used to measure the intracellular calcium modulation post TSLP
stimulation. THP-1 cells (1×105 cells in 100 µl/well)
were loaded with 10 µM of Fluo-4 AM at 37 C for 30 min, and
subsequently treated with TSLP 100 ng/ml for starting time (time
zero), 30 min and 1 h. The fluorescent activity of Fluo-4 was
acquired using a fluorescence microplate reader (Victor Nivo,
Perkin Elmer, Waltham, MA, USA), setting the excitation at 485 nm
and the emission at 535 nm, according to manufacturer's
instructions.
Intracellular ATP measurement
The ATPlite Luminescence Assay System (Catalog No.
6016943), purchased from PerkinElmer (Waltham, MA, USA), was used
to measure the Adenosine TriPhosphate (ATP), according to the
manufacturer's instructions. Specifically, to monitor the effects
of TSLP, THP-1 cells were plated in 96-well plates (100 µl/well) at
the concentration of 1×105, and treated with 100 ng/ml
of TSLP. Adding the lysis buffer provided by the kit, the
luminescence was measured immediately, after 30 min and 1 h using
the luminescence plate, OPTIPLATE (Catalog No. 6005290,
PerkinElmer, Waltham, MA, USA), loaded on microplate reader (Victor
Nivo, Perkin Elmer, Waltham, MA, USA). The calibration curve was
prepared by ATP standard reconstituted in double-distilled water to
a concentration of 10×106 pM. The dilution series were
drawn up in the range 1×106−1 pM as a base for an
ATP-standard curve. Double-distilled water was used as a blank.
Cell Counting Kit-8 assay
To measure cell viability, the Cell Counting Kit-8
(CCK-8) assay (Catalog No. 96992, purchased from Merck KGaA,
Darmstadt, Germany) was used. 1×103 THP-1 cells were
plated (100 µl/well) in 96-well plates and were treated with TSLP
for 24 h at 37°C in 5% CO2. After each treatment time,
10 µl of CCK-8 solution was added to each well and incubated for 3
h. The absorbance was read at 450 nm with a microplate reader
(Victor Nivo, Perkin Elmer, Waltham, MA, USA). Experiments were
performed in triplicates.
Statistical analysis
For the comparison between normal and BC subtype
tissues, the GEPIA2 tool used the previously reported statistical
method (23) and the UALCAN tool
used the previously described statistical method (24,25).
For the prognostic study (OS and DFS), Kaplan-Meier
analyses were determined using GEPIA2. GEPIA2 employs the
Mantel-Cox test and quartile was applied to determine the
expression threshold for splitting the high-expression and
low-expression cohorts.
In order to evaluate immune infiltrates, TIMER 2.0
uses partial Spearman's correlation applied on tumor purity to
perform the correlation between immune infiltrates estimation value
and TSLP expression.
For the in vitro study, Graphpad Prism 9
(Graphpad Software, Graphpad Holdings, LLC, CA, USA) was employed
for all statistical analysis. Statistical analysis was performed by
unpaired two-tailed Mann-Whitney U test when two groups were
compared. The non-parametric Kruskal-Wallis test followed by Dunn's
test was performed for comparing means in a situation where there
are more than two groups. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Evaluation of TSLP expression through
an in silico study
To evaluate TSLP expression in BC, we first
investigated the gene expression levels of TSLP in BC tissues. As
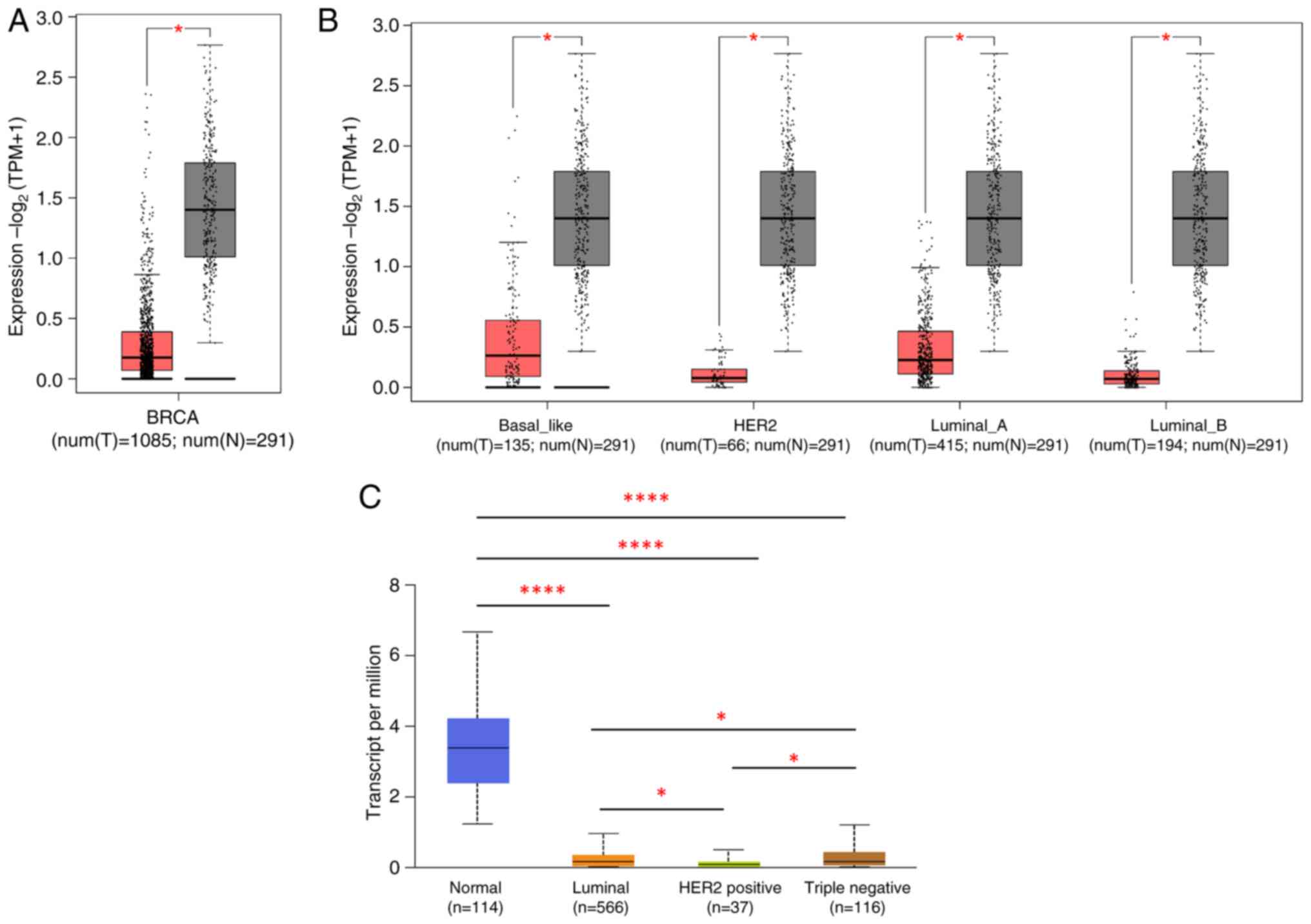
illustrated in Fig. 1A, the
expression level of TSLP was significantly lower in BC tissues (red
boxplot) compared to the normal tissues (gray boxplot). Given this
result, we next analyzed the expression of TSLP in distinct BC
patients subtypes. As shown in Fig.
1B, TSLP mRNA was found to be lowly expressed in every BC
subtypes compared with normal tissues. These data were obtained
through the GEPIA tool.
Furthermore, using the UALCAN tool it emerged that
TSLP expression was significantly higher in TNBC in comparison to
the Luminal subtypes and HER2+ (Fig. 1C).
Evaluation of prognostic value of TSLP
mRNA expression levels in BC patients
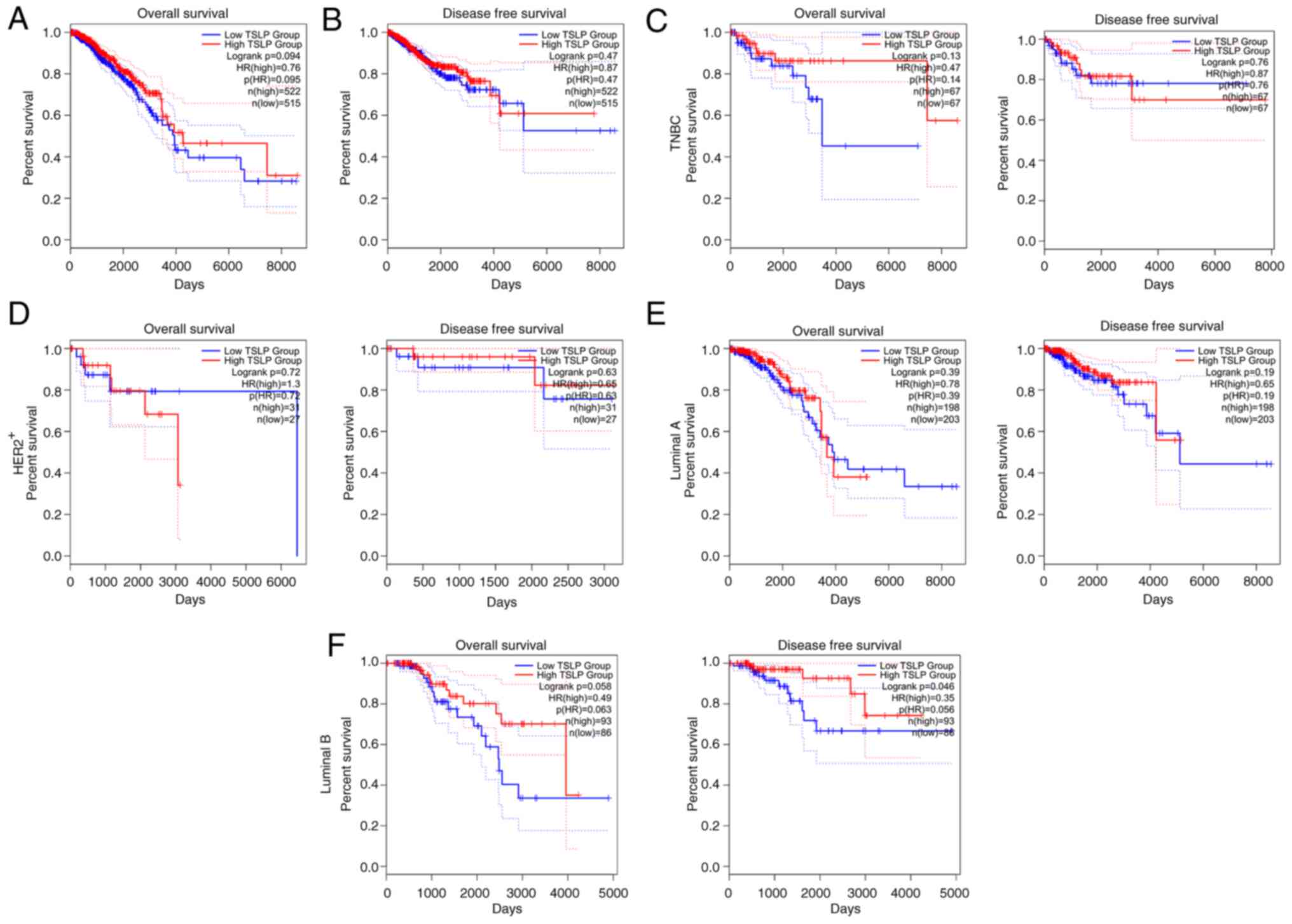
Using the ‘Survival Map’ module of GEPIA, the
prognostic significance of TSLP in BC was examined. We studied the
survival impact of TSLP in terms of OS and DFS in this manner.
Results revealed that lower TSLP mRNA expression levels in BC
patients was associated with a worst prognosis (Fig. 2A). Nevertheless, DFS was not
significant (Fig. 2B).
In light of this finding, we proceeded to examine
the prognostic impact of TSLP in each BC subgroups. As shown in
Fig. 2, the OS and DFS did not
change in the TNBC (Fig. 2C),
HER2+ (Fig. 2D), and
luminal A (Fig. 2E) groups.
Instead, it is interesting to note that lower TSLP expression
levels in the luminal B group were substantially linked to shorter
OS and significantly adverse impacts on DFS (Fig. 2F).
Correlation analysis between TSLP
expression and immune cell infiltration
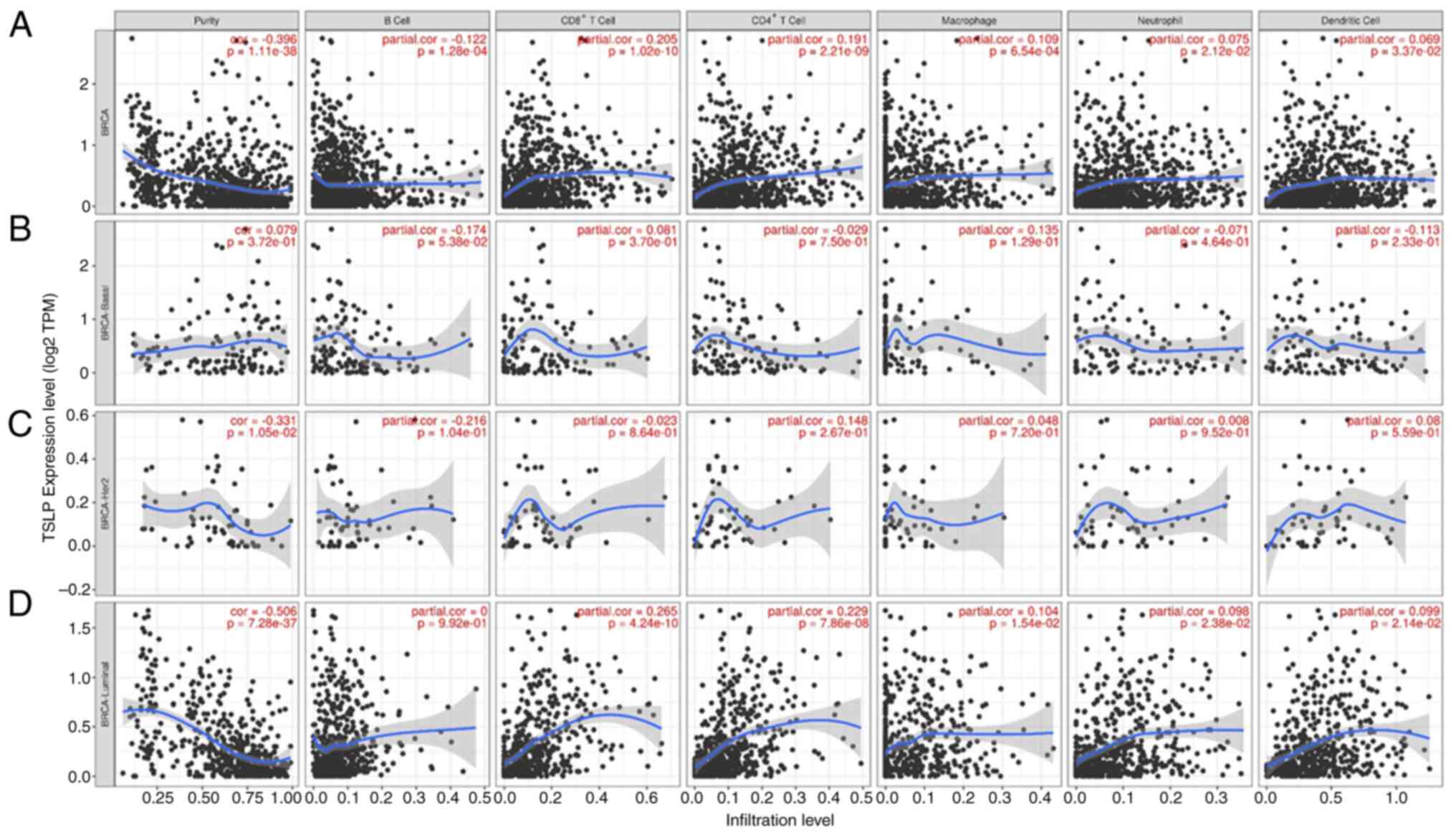
Using the TIMER 2.0, the immune infiltration status
was evaluated. In BC, infiltration levels of B Cells was found to
be negatively correlated with the TSLP expression. Infiltration
level of T cells (CD4+ and CD8+), and
macrophages had a positive correlation with the TSLP expression. No
significant correlation was observed between neutrophils, dendritic
cells and TSLP expression (Fig.
3A).
Next, we evaluated immune infiltration status in
each BC subtypes. In basal-like and in HER2+, no
significant correlation was observed between all immune cells
tested and TSLP expression (Fig. 3B and
C). In Luminal, infiltration levels of T cells (CD4+
and CD8+) had a positive correlation with the TSLP
expression, whereas no significant correlation was discovered
between macrophages, B Cells, neutrophils, Dendritic Cells and TSLP
expression (Fig. 3D).
TSLP mRNA expression and
concentrations in human BC cell lines
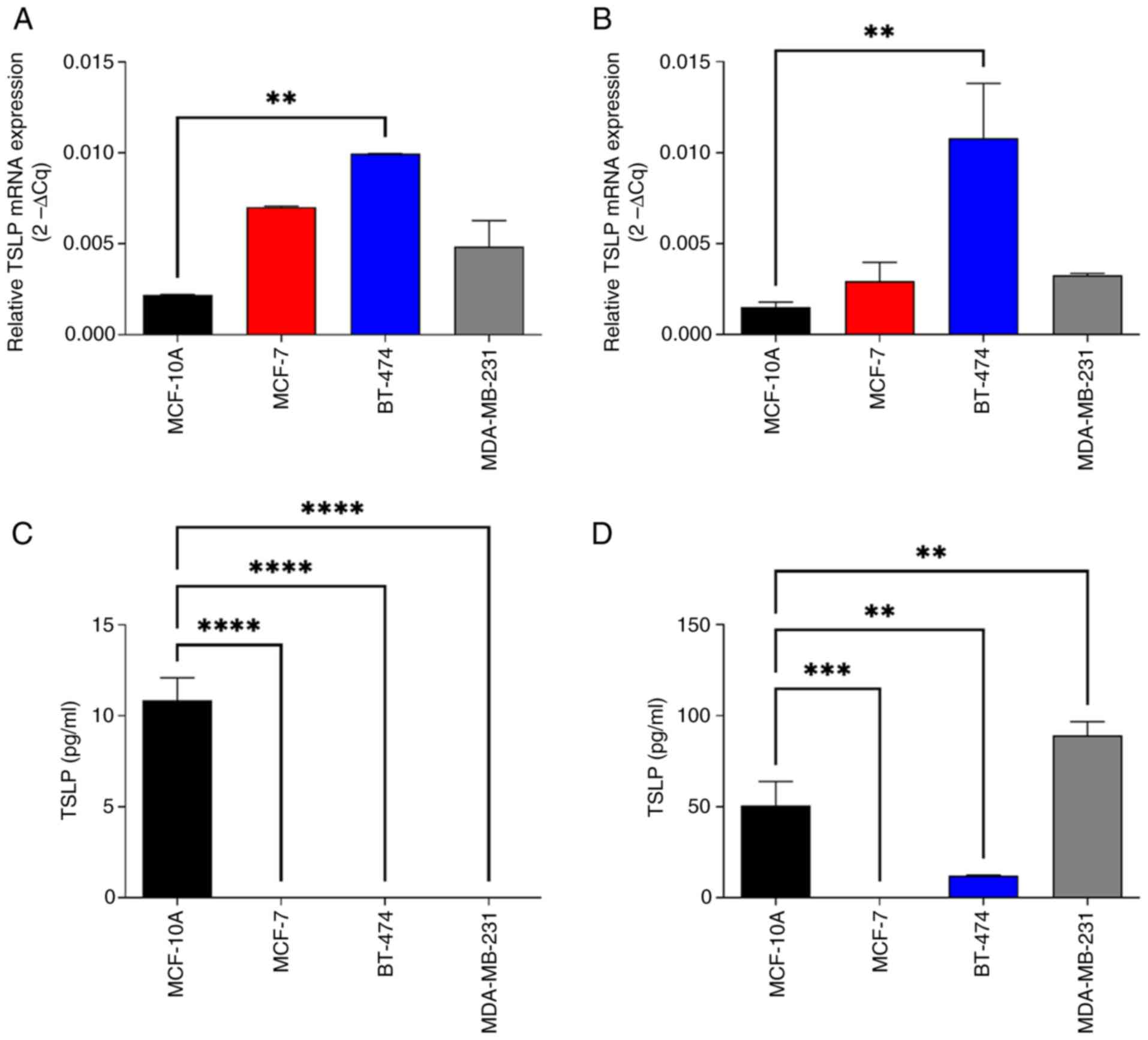
As our analysis revealed that mRNA expression levels
of TSLP were reduced in BC tissues compared to control tissues, we
examined the mRNA and protein expression level of TSLP in different
BC cell lines (Fig. 4).
To this end, cell lines that represent models for
luminal A (MCF-7), luminal B (BT-474), and TNBC (MDA-MB-231) BC
subtypes, and non-tumorigenic breast epithelial cell line (MCF-10A)
used as control, were employed. Having demonstrated in
silico that the HER2+ subtype had the lowest
expression of TSLP, we decided not to perform an in vitro
study for this tumor type.
As indicated in Fig. 4A
and B, TSLP mRNA expression seemed to be higher in BC cell line
models in comparison to the control cells, using two different
reference genes (GAPDH, Fig. 4A and
ACTINB, Fig. 4B). In particular,
TSLP expression was significantly higher in luminal B cells
compared to the control cells, without finding any significant
difference between the various subtypes analyzed.
Interestingly, the scenario completely changed when
we evaluated protein expression. As shown in Fig. 4B, intracellular TSLP protein was
significantly overexpressed in normal cells (MCF10A) compared to
the luminal A and luminal B, (MCF7 and BT474), whereas it was
reduced in normaloid cell line respect to the TNBC model
(MDA-MB-231). Lastly, TSLP vanished from the tumor cell lines
entirely appearing exclusively in the control cell line, according
to an analysis of the secreted proteins (Fig. 4C), while in the total cell extracts
the protein was also present in the other cell lines (Fig. 4D)
BC conditioned media and TSLP affect
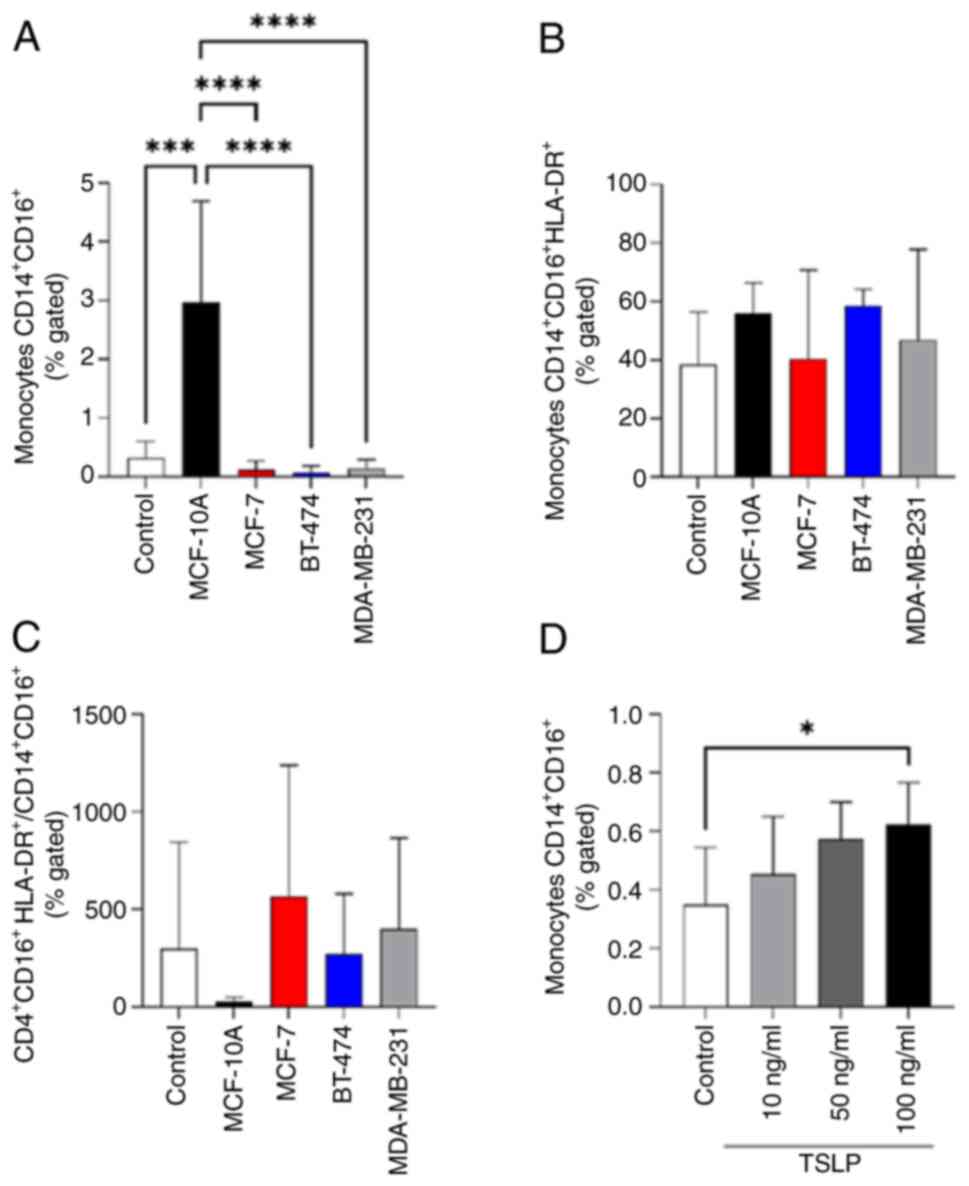
monocytes CD14+CD16+ expansion
Subsequently, to investigate the role of TSLP on
anti-cancer immunity we treated PBMCs derived from healthy
volunteers for 24 h with CM derived from MCF-10A, MCF-7, BT-474 and
MDA-MB-231. BC CM cell line models does not affect the percentage
of all lymphocytes population (Fig.
S1) and classical monocytes CD14+ (Figs. S2 and S3). Interestingly, the CM derived from
normal cells (MCF-10A) significantly increased the number of
CD14+CD16+ monocytes compared to untreated
PBMCs (control) and CM derived from the other BC types used
(Figs. 5A and S3). By contrast no effects on activated
monocytes CD14+CD16+HLA-DR+ were
found (Fig. 5B and C).
To validate these data, the next step was to
stimulate PBMCs with increasing concentration of TSLP to verify
whether it was able to affect monocytes
CD14+CD16+ expansion. In Fig. 5D, the stimulation with 100 ng/ml of
TSLP induced a significant increase of
CD14+CD16+ monocytes proliferation.
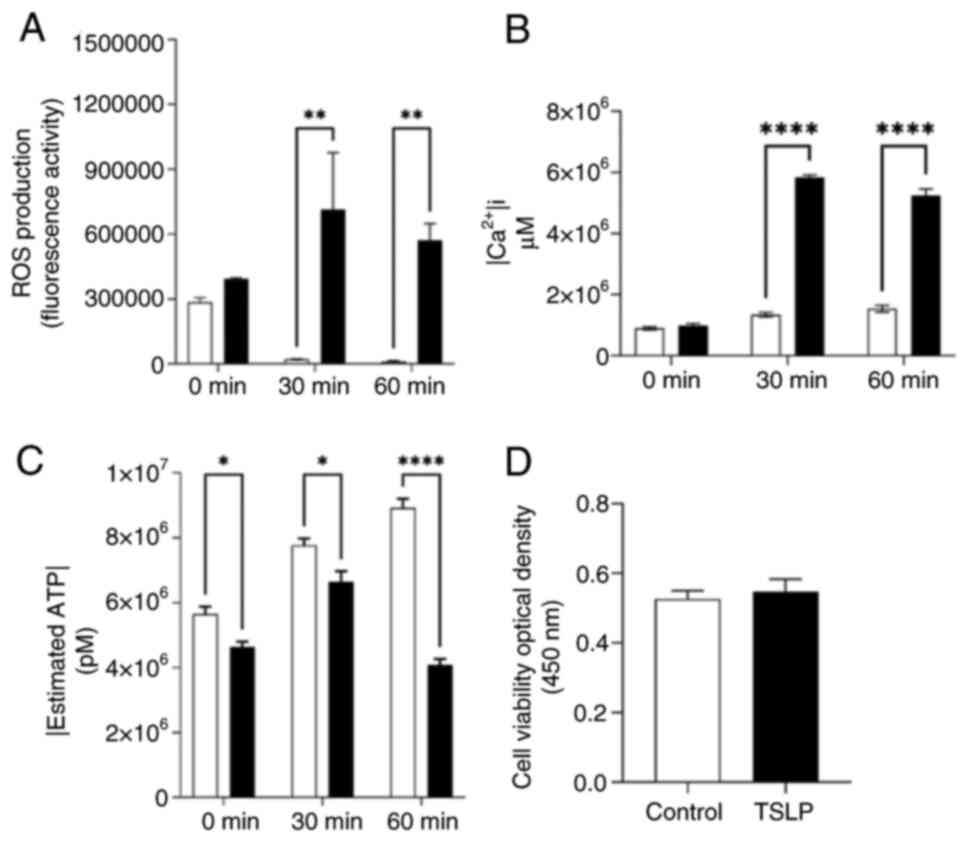
Effects of TSLP on cellular metabolism
of CD14+CD16+
Since it has been shown that TSLP increases
mitochondrial ROS production causing anti-inflammatory monocytes
phenotype (11), we investigated
whether TSLP could affect cellular metabolism. To evaluate this
mechanism, we used a monocyte cellular model, THP-1 cell line as
these cells are positive for CD14 and CD16 markers on the surface
(29).
THP-1 cells were treated with best concentration
TSLP (100 ng/ml) having an expansion effect on PMBCs for 24 h.
Post-treatment, we evaluated ROS production and intracellular
calcium signaling via fluorescence, and ATP concentration via
luminescence.
In Fig. 6A,
time-course experiment revealed that TSLP within 1 h progressively
induced the production of ROS in THP-1 cells treated with 10 µM of
DCFDA. Considering ROS signaling pathways interact with other
biological signaling systems, including calcium, we investigated
whether TSLP also had an effect on intracellular calcium. To this
end, 10 µM of Fluo 4-AM was used to determine intracellular calcium
in TSLP-treated-THP-1 cells. As shown in Fig. 6B, these cells enhanced the
intracellular calcium levels compared to the untreated THP-1 in a
time-dependent manner.
Next, we studied whether TSLP influenced cellular
energy. To this end, an ATPlite assay was performed to measure the
intracellular ATP concentration. THP-1 cells treated with TSLP
caused a reduction of intracellular ATP levels compared to control
cells (Fig. 6C). Finally, to
understand whether TSLP caused an effect also on monocytes
viability, a CCK-8 assay was conducted. As shown in Fig. 6D, CCK-8 assay highlight that TSLP
did not affect THP-1 viability.
Discussion
Over the last ten years, TSLP involvement in a
number of cancers has been amply demonstrated. Since its function
in BC is still unclear, the role of TSLP continues to be discussed
nowadays. In this study, we showed how TSLP could affect the BC
microenvironment also playing a defensive function by the immune
system.
Using firstly an in silico tool, we
discovered a lower expression of TSLP in tissues of various classes
of BC subtypes compared to healthy tissues, showing also a poor
prognosis for these BC patients. Taken together, these findings
suggested a tumor-suppressing role of TSLP in BC, as already shown
in other cancer types, such as colon (30).
As an immunogenic tumor, BC appears to exhibit a
significant association between immune cell infiltration and
clinical outcomes (31). In this
context, the role of TSLP on immune cells has been the subject of
several studies, with contradictory findings. For instance, one
study found that TSLP is produced directly by BC cells, promoting a
TH2 microenvironment and tumor progression (17).
In our study, the expression of TSLP correlated with
the infiltration of different immune cells. Particularly, TSLP
expression was associated mainly with T cells (CD4+ and
CD8+), B cells and macrophages. In addition, we
discovered in Luminal subtype, infiltration level of T cell
(CD4+ and CD8+) had a positive correlation
with the TSLP expression. Hence, we hypothesized that TSLP might
regulate BC tumor immunity through multiple immune cell
populations, mainly via adaptative immunity.
To support these results, we set up in vitro
experiments. Although we found a statistical increase or trend
regarding TSLP mRNA in the various BC subtypes compared to
normaloid cells, evaluating its levels in BC cell pellets and in
their supernatant, we discovered that the expression of TSLP was
reduced and even undetectable, respectively. This difference
between mRNA and protein levels could be related to
posttranscriptional changes that drastically alter TSLP protein
levels in BC cells compared with normaloid cells. Future studies
will be conducted to try to clarify what the reasons are for this
difference between mRNA and TSLP protein levels in BC cells and
normaloid cells.
Next, we demonstrated that secretome derived from
healthy cells could have a protective role against cancer. As
suggested by The Protein Atlas Database, TSLP has a cellular
localization in the Golgi apparatus or vesicles, suggesting its
secretion through extracellular vesicles (https://www.proteinatlas.org/ENSG00000145777-TSLP). As
a matter of fact, stimulating PBMCs with MCF-10A CM (normaloid
cells) increased the percentage of CD14+CD16+
monocytes population compared to PBMCs with BC CM subtypes.
CD14+CD16+ monocytes, also
called intermediate monocytes or patrolling monocytes, exert
phagocytic and anti-inflammatory activities (32,33),
exhibiting also enhanced cytotoxic and cytostatic (34). However, their protective value is
still not clear.
To determine whether TSLP could be involved in the
expansion of CD14+CD16+ monocytes, we treated
PBMCs with different concentrations of TSLP proving that this
cytokine affected CD14+CD16+ monocytes in a
dose-dependent manner. Since TSLP induced M2-like effects in THP-1
cells (11), we investigated
whether our concentration of TSLP could increase ROS production.
The results showed that within 1 h of treatment there is a strong
increase in reactive species. ROS are produced from a wide range of
sources, including multiple extramitochondrial enzymes and the
activity of the mitochondrial respiratory chain (35–37).
Notably, calcium has the ability to regulate many of these
processes. One may conceptualize ROS and calcium signaling as
having a reciprocal interaction, in which ROS can regulate cellular
calcium signaling and calcium signaling is required for the
production of ROS (37,38).
In this study, we found that TSLP induced an
increase in intracellular calcium in THP-1 cells, suggesting that
it may be responsible for the significant ROS generation detected.
Calcium stimulates the generation of ATP at several levels inside
the organelle and is a crucial regulator of mitochondrial function
(39). Here we demonstrated a
reduction in intracellular ATP levels following stimulation with
TSLP. These results can be explained by the fact that as
intracellular calcium increases, there is an increased hydrolysis
of ATP into ADP (40,41). Reduced respiratory chain activity,
which results in decreased ATP levels, and higher AMP stimulate
AMPK.
It is already established that TSLP promotes AMPK
activation, which modulates mitochondrial biogenesis and stimulates
protein signaling associated with mitophagy (11). Our data provide further confirmation
of what has already been demonstrated. Due to this energetic and
metabolic change of the TSLP-treated cell, we hypothesized that
proliferation may also be modulated by this cytokine. After 24 h of
treatment, no change in growth was detected, thus modulating
exclusively the percentage expansion of
CD14+CD16+ without alternating the cell
cycle.
Collectively, these findings provide new insights
into the mechanism by which TSLP is involved in BC, and raise the
possibility that TSLP acts as anti-tumor mediator by promoting
CD14+CD16+ monocyte expansion, alternating
its energetic and metabolic function. In addition, the reduction in
TSLP expression could be associated with unfavorable prognosis in
breast cancer. High levels of TSLP expression in normal breast
tissue compared to low ones in different biological subtypes of
breast cancer, lowest in TNBC, could be associated with a key role
of this chemokine in cell differentiation or the lack of regulation
and dedifferentiation of cancer cells (42). However, further studies are needed
to determine other TSLP roles in the BC contest.
In conclusion, we aimed to investigate the role of
TSLP in BC with an emphasis on its involvement in immune system
activation. Since both the morbidity and mortality rates of BC have
significantly increased over the past decades, it is an urgent need
to acquire new understandings in mechanisms that affect BC
progression. So far, TSLP was found to be lower in BC tissues and
in subtypes of BC cell cultures respect to healthy counterparts. In
addition, we demonstrated that TSLP could be able to increase the
number of CD14+CD16+ monocytes also by
enhancing ROS and Ca2+ concentration levels and
modulating their energetic metabolism. Hence, the ongoing
investigation of the primary molecular processes behind
immune-cancer interaction has added a new piece to the
understanding of the role TSLP in BC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank SDN Biobank, partner
of BBMRI.it, the Italian node of BBMRI-ERIC (Biobanking and
BioMolecular Research Infrastructure-European Research
Infrastructure Consortium) for their permission for the cell lines
used for experiments.
Funding
This study was funded by Progetti di Ricerca Corrente of the
Italian Ministry of Health.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GS, MB, AS and SM conceptualized the study. AMG and
MB evaluated the accuracy of the data. GS, MB, AMG and SM collected
the data and assessed the scientific correctness of the data. MB
and SM performed the experiments. GS supervised the work in its
entirety. AS, MB, SM, AMG and GS wrote and reviewed the original
draft. All authors read and approved the final version of the
manuscript. GS, MB, SM and AMG confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The procedures followed in this study, in line with
The Declaration of Helsinki, have been approved by the local
ethical committees using the active protocol 4/21 (Ethical
Committee of IRCCS Pascale Naples, Italy; approval no. 4/21, 2021).
Written informed consent to participate was obtained from all
subjects involved in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ziegler SF: The role of thymic stromal
lymphopoietin (TSLP) in allergic disorders. Curr Opin Immunol.
22:795–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu X, Li H and Ren X: Signaling cascades
initiated by TSLP-mediated signals in different cell types. Cell
Immunol. 279:174–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corren J and Ziegler SF: TSLP: From
allergy to cancer. Nat Immunol. 20:1603–1609. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verstraete K, Peelman F, Braun H, Lopez J,
Van Rompaey D, Dansercoer A, Vandenberghe I, Pauwels K, Tavernier
J, Lambrecht BN, et al: Structure and antagonism of the receptor
complex mediated by human TSLP in allergy and asthma. Nat Commun.
8:149372017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marković I and Savvides SN: Modulation of
signaling mediated by TSLP and IL-7 in inflammation, autoimmune
diseases, and cancer. Front Immunol. 11:15572020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varricchi G, Pecoraro A and Marone G,
Criscuolo G, Spadaro G, Genovese A and Marone G: Thymic stromal
lymphopoietin isoforms, inflammatory disorders, and cancer. Front
Immunol. 9:15952018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borriello F, Iannone R, Di Somma S,
Vastolo V, Petrosino G, Visconte F, Raia M, Scalia G, Loffredo S,
Varricchi G, et al: Lipopolysaccharide-Elicited TSLPR expression
enriches a functionally discrete subset of human CD14+ CD1c+
monocytes. J Immunol. 198:3426–3435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Braile M, Fiorelli A, Sorriento D, Di
Crescenzo RM, Galdiero MR, Marone G, Santini M, Varricchi G and
Loffredo S: Human lung-resident macrophages express and are targets
of thymic stromal lymphopoietin in the tumor microenvironment.
Cells. 10:20122021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marcella S, Petraroli A, Canè L, Ferrara
AL, Poto R, Parente R, Palestra F, Cristinziano L, Modestino L,
Galdiero MR, et al: Thymic stromal lymphopoietin (TSLP) is a
substrate for tryptase in patients with mastocytosis. Eur J Intern
Med. 117:111–118. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirano R, Hasegawa S, Hashimoto K, Haneda
Y, Ohsaki A and Ichiyama T: Human thymic stromal lymphopoietin
enhances expression of CD80 in human CD14+ monocytes/macrophages.
Inflamm Res. 60:605–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin YC, Lin YC, Tsai ML, Liao WT and Hung
CH: TSLP regulates mitochondrial ROS-induced mitophagy via histone
modification in human monocytes. Cell Biosci. 12:322022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ebina-Shibuya R and Leonard WJ: Role of
thymic stromal lymphopoietin in allergy and beyond. Nat Rev
Immunol. 23:24–37. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boieri M, Marchese E, Pham QM, Azin M,
Steidl LE, Malishkevich A and Demehri S: Thymic stromal
lymphopoietin-stimulated CD4+ T cells induce senescence in advanced
breast cancer. Front Cell Dev Biol. 10:10026922022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smolarz B, Nowak AZ and Romanowicz H:
Breast Cancer-epidemiology, classification, pathogenesis and
treatment (review of literature). Cancers (Basel). 14:25692022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Momenimovahed Z and Salehiniya H:
Epidemiological characteristics of and risk factors for breast
cancer in the world. Breast Cancer (Dove Med Press). 11:151–164.
2019.PubMed/NCBI
|
|
16
|
Mehrgou A and Akouchekian M: The
importance of BRCA1 and BRCA2 genes mutations in breast cancer
development. Med J Islam Repub Iran. 30:3692016.PubMed/NCBI
|
|
17
|
Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C,
Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T, et
al: Thymic stromal lymphopoietin fosters human breast tumor growth
by promoting type 2 inflammation. J Exp Med. 208:479–490. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu TC, Xu K, Martinek J, Young RR,
Banchereau R, George J, Turner J, Kim KI, Zurawski S, Wang X, et
al: IL1 receptor antagonist controls transcriptional signature of
inflammation in patients with metastatic breast cancer. Cancer Res.
78:5243–5258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olkhanud PB, Rochman Y, Bodogai M,
Malchinkhuu E, Wejksza K, Xu M, Gress RE, Hesdorffer C, Leonard WJ
and Biragyn A: Thymic stromal lymphopoietin is a key mediator of
breast cancer progression. J Immunol. 186:5656–5662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olkhanud PB, Baatar D, Bodogai M, Hakim F,
Gress R, Anderson RL, Deng J, Xu M, Briest S and Biragyn A: Breast
cancer lung metastasis requires expression of chemokine receptor
CCR4 and regulatory T cells. Cancer Res. 69:5996–6004. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Demehri S, Cunningham TJ, Manivasagam S,
Ngo KH, Moradi Tuchayi S, Reddy R, Meyers MA, DeNardo DG and
Yokoyama WM: Thymic stromal lymphopoietin blocks early stages of
breast carcinogenesis. J Clin Invest. 126:1458–1470. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghirelli C, Sadacca B, Reyal F, Zollinger
R, Michea P, Sirven P, Pattarini L, Martínez-Cingolani C,
Guillot-Delost M, Nicolas A, et al: No evidence for TSLP pathway
activity in human breast cancer. Oncoimmunology. 5:e11784382016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Z, Li C, Kang B, Gao G, Li Cc and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smaldone G, Coppola L, Incoronato M,
Parasole R, Ripaldi M, Vitagliano L, Mirabelli P and Salvatore M:
KCTD15 protein expression in peripheral blood and acute myeloid
leukemia. Diagnostics (Basel). 10:3712020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sasserath T, Rumsey JW, McAleer CW,
Bridges LR, Long CJ, Elbrecht D, Schuler F, Roth A,
Bertinetti-LaPatki C, Shuler ML and Hickman JJ: Differential
monocyte actuation in a Three-organ functional innate immune
System-on-a-Chip. Adv Sci (Weinh). 7:20003232020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yue W, Lin Y, Yang X, Li B, Liu J and He
R: Thymic stromal lymphopoietin (TSLP) inhibits human colon tumor
growth by promoting apoptosis of tumor cells. Oncotarget.
7:16840–16854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dieci MV, Miglietta F and Guarneri V:
Immune infiltrates in breast cancer: Recent updates and clinical
implications. Cells. 10:2232021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SJ, Yoon BR, Kim HY, Yoo SJ, Kang SW
and Lee WW: Activated platelets convert CD14+CD16-Into CD14+CD16+
monocytes with enhanced FcγR-mediated phagocytosis and skewed M2
polarization. Front Immunol. 11:6111332020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cassetta L and Pollard JW: Cancer
immunosurveillance: Role of patrolling monocytes. Cell Res. 26:3–4.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szaflarska A, Baj-Krzyworzeka M, Siedlar
M, Weglarczyk K, Ruggiero I, Hajto B and Zembala M: Antitumor
response of CD14+/CD16+ monocyte subpopulation. Exp Hematol.
32:748–755. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sauer H, Wartenberg M and Hescheler J:
Reactive oxygen species as intracellular messengers during cell
growth and differentiation. Cell Physiol Biochem. 11:173–186. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holmström KM and Finkel T: Cellular
mechanisms and physiological consequences of redox-dependent
signalling. Nat Rev Mol Cell Biol. 15:411–4121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Görlach A, Bertram K, Hudecova S and
Krizanova O: Calcium and ROS: A mutual interplay. Redox Biol.
6:260–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gordeeva AV, Zvyagilskaya RA and Labas YA:
Cross-talk between reactive oxygen species and calcium in living
cells. Biochemistry (Mosc). 68:1077–1080. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brookes PS, Yoon Y, Robotham JL, Anders MW
and Sheu SS: Calcium, ATP, and ROS: A mitochondrial love-hate
triangle. Am J Physiol Cell Physiol. 287:C817–C833. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu H and Van Remmen H: The
SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: A potential
target for intervention in aging and skeletal muscle pathologies.
Skelet Muscle. 11:252021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Doblado L, Lueck C, Rey C, Samhan-Arias
AK, Prieto I, Stacchiotti A and Monsalve M: Mitophagy in human
diseases. Int J Mol Sci. 22:39032021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li J and Stanger BZ: How tumor cell
dedifferentiation drives immune evasion and resistance to
immunotherapy. Cancer Res. 80:4037–4041. 2020. View Article : Google Scholar : PubMed/NCBI
|