Introduction
Malignant peritoneal mesothelioma (MPEM) is a rare
aggressive neoplasm that accounts for approximately one-fifth of
all malignant mesothelioma cases (1,2). The
incidence of MPEM has been reported to be 1.7/1,000,000 in men and
1.0 in women, varying widely across different areas (3). Primary diffuse epithelioid MPEM, the
most dominant subtype, has remained to be fully elucidated and no
consensus has been reached on how to treat it (1).
In recent years, immunotherapy has revolutionized
the field of tumor therapy. Rapid progress in immunotherapy showed
promising results in malignant pleural mesothelioma. According to
the current limited clinical evidence, advanced MPEM may also
benefit from immune checkpoint inhibitors (4).
The present study reported a case of MPEM that
showed a favorable response to pembrolizumab. To the best of our
knowledge, this was the first attempt to use single-agent
pembrolizumab as the first-line treatment for a patient with MPEM.
A review of available studies reported to date was also provided,
so that improved immunotherapy strategies could be proposed for the
management of MPEM.
Case presentation
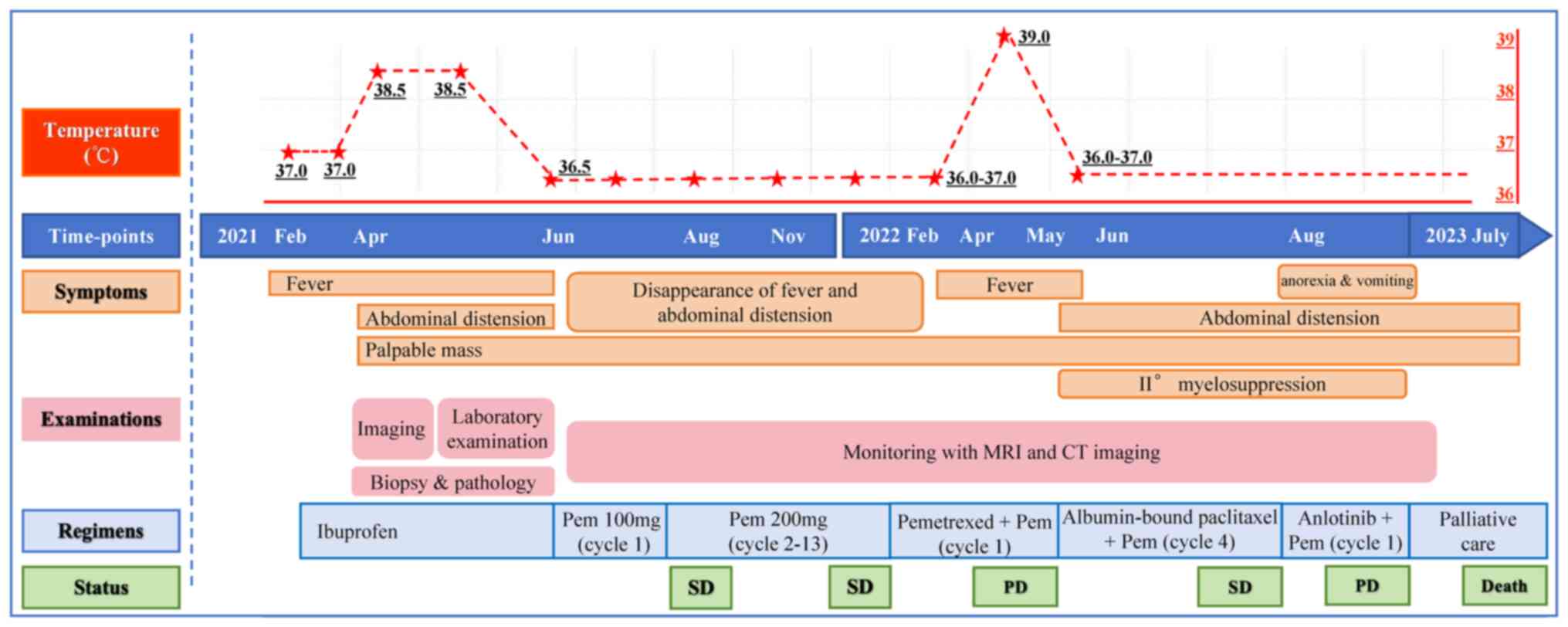
In February 2021, a previously healthy 61-year-old
Chinese woman developed chronic low fever lasting for >4 months
with no obvious predisposing cause. The patient's body temperature
ranged from 36.5 to 37.5°C and became more elevated every
afternoon. After drinking water and resting, the patient's body
temperature returned to normal and it was not treated.
Since April 2021, the body temperature rose to
38.5°C, but the patient's general condition was fair. The patient
took self-administered ibuprofen sustained-release capsules twice a
day to keep the body temperature normal, while there was still
intermittent fever every day. In mid-April, the patient experienced
dressing difficulties due to a palpable abdominal mass and mild
abdominal distension. Therefore, the patient sought medical
treatment. Fig. 1 shows a timeline
of the patient's clinical course. There was no abnormality in the
patient's medical history, such as surgical trauma, allergy,
personal or family history, or known exposure to asbestos.
The patient was admitted to the China-Japan
Friendship Hospital (Beijing, China) two months later, with
decreased lymphocytes (17.2%↓, normal range: 20–50%), hemoglobin
(98 g/l↓, normal range: 115–150), platelets (509×109/l↑,
normal range: 125–350), [C-reactive protein (CRP) 17.05 mg/dl↑,
normal range: 0–5.00], coagulation indicators [prothrombin time
(14.2 sec, normal range: 11–15), internal normalized ratio (1.24↑,
normal range: 0.85–1.5), D-dimer (2.50 mg/l, normal range: 0–0.5),
activated partial thromboplastin time (32.8 sec, normal range:
28.0–43.5) and fibrinogen (6.82 g/l, normal range: 2.0–4.0)] and
tissue polypeptide-specific antigen 405.15 u/l↑ (normal range:
0–100.0), and normal tumor markers [CA242, CA125, CA199, CA724,
α-fetoprotein and carcinoembryonic antigen (CEA)]. After two days,
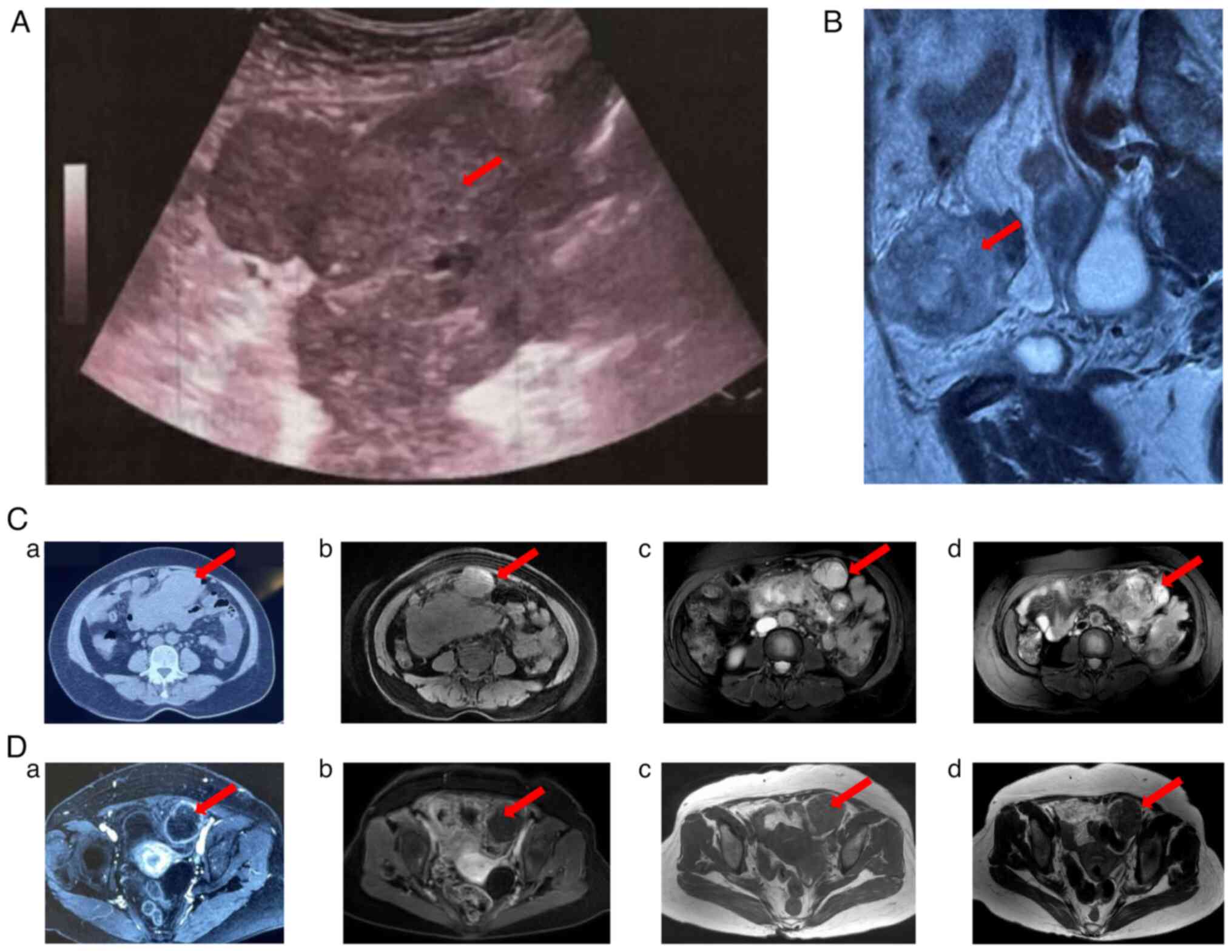
ultrasound-guided biopsy (maximum tumor size, 11.8×7.9 cm) was
performed. The histological results (Fig. S1; protocol available in
supplemental information and antibody details in Table SI) showed that the sample was
epithelioid, with no tumor necrosis or lymphoid infiltrate. The
immunohistochemical results (Fig.
S1; protocol available in supplemental information) were as
follows: D2-40 (2+), Wilms' tumor-1 protein (2+), AE1/AE3 (3+),
Calretinin (3+), caudal-type homeobox transcription factor 2 (−),
paired-box gene 8 (−), human bone marrow endothelial cell marker-1
(−), OCT3/4 (−), special AT-rich sequence-binding protein 2 (−),
P63 (−), P40 (−), Cytokeratin (CK) 5/6 (−), thyroid transcription
factor 1 (−), GATA binding protein 3 (2+), estrogen receptor (−),
human epidermal growth factor receptor 2 (0), progesterone receptor
(−), chromogranin A (−), synaptophysin (−), MELAN-A (−), CEA (−),
CK7 (3+), CK18 (3+), Ki-67 (+, >30%), programmed death ligand 1
(PD-L1) (−), PD-L1 (22C3) (CPS 60%). The pathological diagnosis was
diffuse epithelial MPEM.
According to the peritoneal cancer index (PCI)
scoring system (5) (PCI=33), MPEM
at stage III (T4N0M0) was identified. Surgical resection was not
recommended after careful evaluation by surgical oncologists, as
complete cytoreduction (CC-0/1) could not be achieved. The patient
rejected chemotherapy and was not able to afford a two-drug
combination regimen with nivolumab plus ipilimumab. In addition,
the single-agent pembrolizumab, which was covered by the patient's
insurance, had been approved as a second-line treatment option for
malignant pleural mesothelioma (MPM). Finally, the patient agreed
to this therapy regimen not only due to drug accessibility but also
affordability. Thus, the patient underwent monotherapy with
pembrolizumab (100 mg q21d) as first-line treatment in June 2021.
After the first cycle, the patient's fever completely resolved.
Fortunately, the patient had no discomfort; thus, 2–12 cycles of
standard-dose pembrolizumab (200 mg Q21d) were continued from June
2021 to February 2022. Regular follow-up showed that the mass and
nodular foci in the pelvic cavity had shrunk, with obvious necrosis
in the center of the lesion (Fig. 2C
and D). Until February 2022, the patient still showed lasting
stable disease (SD) to pembrolizumab according to the iRECIST 1.1
criteria (6), and had no adverse
events or new clinical symptoms.
In February 2022, MRI showed that the targeted
lesion in the upper abdominal cavity was slightly enlarged;
however, the patient still achieved an SD response. One month
later, MRI showed shrunken pelvic lesions. Finally, the patient
achieved an SD response to pembrolizumab, with a progression-free
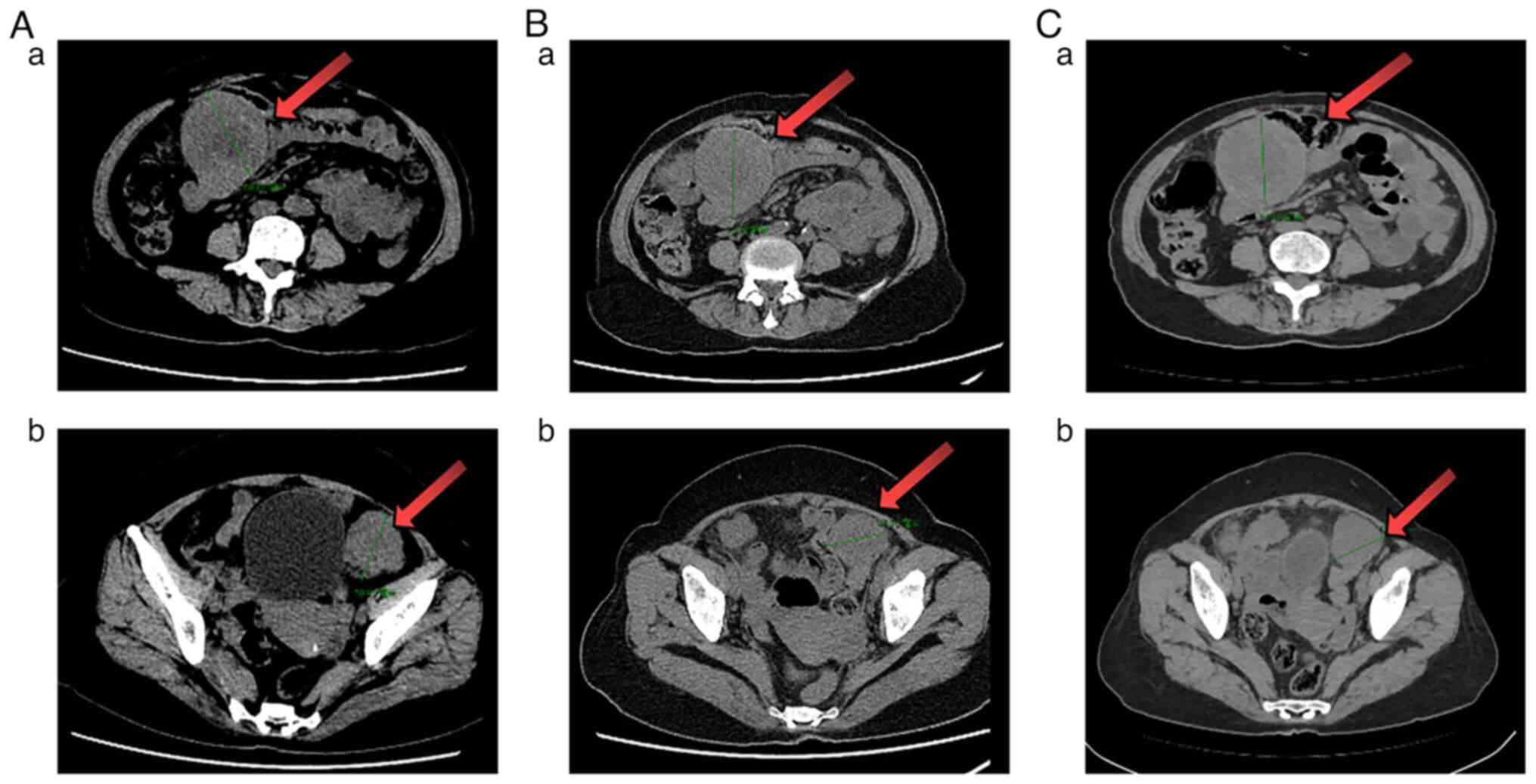
survival (PFS) of 10.0 months. Subsequently, the patient received
two cycles of pemetrexed based on the first-line regimen, but no
clinical or imaging benefits (Fig.
3A) were obtained. The patient was afebrile again (with a body
temperature of 39°C) but achieved symptom remission from the
third-line regimen (four cycles albumin-bound paclitaxel plus
pembrolizumab). Radiographic assessment (Fig. 3B) showed slight shrinkage, but the
patient could not tolerate the II° myelosuppression and lasting
abdominal distension. The regimen was changed to anlotinib and
pembrolizumab (fourth-line treatment). However, the patient could
not tolerate the following severe adverse events (including new
symptoms such as anorexia and vomiting). The CT results showed a
slight increase in the abdominal mass after one cycle of
fourth-line therapy (Fig. 3C).
Subsequently, the patient refused further treatment after the
fourth-line regimen due to financial reasons. Follow-up was
continued every month and it was found that the patient had died in
July 2023. The overall survival (OS) of the patient was calculated
as 26.2 months.
Discussion
MPEM is a rare, aggressive neoplasm that arises from
the peritoneal lining, resulting in limited data summarizing its
clinical characteristics. Most MPEMs are asymptomatic or have a
nonspecific insidious onset in the early stages. Typical symptoms
of MPEM include abdominal bloating, abdominal pain, nausea,
vomiting, and bowel obstruction (7). The patient presented with a lasting
fever as the first symptom, which is much less common. Except for a
higher CRP value and low-grade fever, there were few signs of
infection in this patient. According to previous observational
studies, fever, as a prognostic indicator, may have a short
survival (8). Thrombocytosis and
anemia are common signs of hematologic paraneoplastic syndromes
associated with MPEM. A paraneoplastic syndrome should also be
considered. Thus, the literature on neoplastic fever and
hematologic system paraneoplastic syndromes associated with MPEM
was reviewed (Table I). A total of
six cases with various hematologic system paraneoplastic syndrome
and three with fever had been published. It seemed that these
symptoms are not associated with gender or pathological patterns.
Previous studies revealed that patients with MPEM and
paraneoplastic syndrome appear to have a short OS and unfavorable
outcomes (9). It was one reminder
that more attention needs to be paid to patients suffering from
lasting fever and other hematologic signs.
 | Table I.Literature on neoplastic fever and
hematologic system paraneoplastic syndrome associated with
MPEM. |
Table I.
Literature on neoplastic fever and
hematologic system paraneoplastic syndrome associated with
MPEM.
| Author, year | Sex | Pathological
pattern | Paraneoplastic
syndrome | Outcomes | (Refs.) |
|---|
| Selleslag,
1989 | M | NA | Autoimmune
hemolytic anemia | OS 3 m | (33) |
| Kimura, 2005 | M | Decidual | Marked
leukocytosis, thrombocytosis and elevated serum levels of
C-reactive protein, granulocyte colony-stimulating factor and
IL-6 | OS 1 year | (34) |
| Banayan, 2006 | F | NA | Weakness, anemia,
recurrent jugular thrombosis/inflammatory biological syndrome | NA | (35) |
| Socola, 2012 | M | NA | Recurrent
thrombotic thrombocytopenic purpura syndrome | NA | (36) |
| Thakral, 2020 | F | Decidual | Leukemoid
reaction | NA | (37) |
| Su, 2022 | F | Epithelioid | Thrombocytosis,
moderate anemia | OS 15 m | (9) |
| Chen, 2011 | F | NA | Persistent high
fever | NA | (38) |
| Hermann, 2013 | M | Epithelioid | Fever of unknown
origin | OS 2 m | (39) |
| Ishizuka, 2022 | M | NA | Fever | OS 2 m | (40) |
In the present case, pathological analysis supported
the diagnosis of diffuse epithelioid MPEM, rather than benign
cystic mesothelioma. Ki-67 protein is present during all active
phases of the cell cycle (G1, S, G2 and mitosis) but is absent in
resting cells (G0). Therefore, Ki-67 protein is regarded
as an excellent marker of cellular proliferation and provides an
inference of tumor aggressiveness. Immunohistochemical analysis
indicated that the Ki-67 index was as high as 30%. Several studies
have found that Ki-67 >9 or >10% is an independent indicator
of poor OS benefits in malignant peritoneal mesothelioma. Ki-67
>30% suggests that the tumor grows faster than in indolent
disease (10,11).
The multidisciplinary team did not recommend
surgical resection for three relative contraindications (inability
to achieve CC-0/1, PCI>17 and Ki-67 >9%). According to one
clinical study (10), patients with
Ki-67 >9% are unlikely to benefit from the procedure and should
be considered for other treatment protocols. Due to limitations of
clinical technology and medical equipment, hyperthermic
intraperitoneal chemotherapy was not available at our hospital at
the time. The absence of standard recommendations and the patient's
financial situation made the therapeutic plan even more
challenging. A literature review of the PubMed database was
performed for eligible studies on immunotherapy in patients with
MPEM, which was updated until July 2024. Key words and Medical
Subject Headings terms pertinent to the intervention of interest,
such as ‘peritoneal mesothelioma’ and ‘immunotherapy’ were used.
Further details of the eligible studies are presented in Table II. Immunotherapy was previously
applied for >1 line treatment of advanced mesothelioma. As a
first-line regimen, Rizzolo et al (12) reported the first case using
nivolumab combined with ipilimumab for MPEM in 2022 with PFS
>8.0 m. In July 2023, Tang et al (13) reported the first OS data (23.0
months) of first-line single agent nivolumab for MPEM. In addition,
two small-cohort retrospective trials and two case reports
supported the possibility of pembrolizumab used to treat MPEM,
while no accurate OS data of pembrolizumab as first-line treatment
are currently available (11–14).
 | Table II.Studies on immunotherapy for
peritoneal mesothelioma. |
Table II.
Studies on immunotherapy for
peritoneal mesothelioma.
| Identification | Author, year | Disease | Trial type | Numbera | Phase | ICI | ICI targets | Line | Outcomes | (Refs.) |
|---|
| NCT01649024 | Calabrò, 2013 | Mesothelioma | Single-arm
trial | 1/25 | 2 | Tremelimumab | CTLA-4 | >1 | Possible
encouraging effect | (41) |
| MESOT | Calabrò, 2015 | Chemotherapy- | Single-arm
trial | 1/29 | 2 | Tremelimumab | CTLA-4 | 2 | ORR 13.8% | (42) |
| TREM-2012 |
| resistant |
|
|
|
|
|
|
|
|
|
|
| mesothelioma |
|
|
|
|
|
|
|
|
| DETERMINE | Maio, 2017 | Relapsed | RCT | 26/382 | 2b | Tremelimumab | CTLA-4 | 2/3 | Not significantly
prolonged | (43) |
|
|
| mesothelioma |
|
|
|
|
|
| OS |
|
| NCT02399371 | Desai, 2019 | Advanced | Prospective
trial | 8/64 | 2 | Pembrolizumab | PD-1 | >1 | mPFS 4.5 m; mOS
11.5 m | (44) |
|
|
| mesothelioma |
|
|
|
|
|
|
|
|
| JAVELIN | Hassan, 2019 | Advanced | Single-arm
trial | N/53 | 1b | Avelumab | PD-L1 | >1 | Possible durable
disease | (45) |
| NCT01772004 |
| mesothelioma |
|
|
|
|
|
| control |
|
| NIBIT-MESO-1 | Calabrò, 2021 | Advanced | Single-arm
trial | 2/40 | 2 | Tremelimumab | CTLA-4 | 1/2 | PFS 8·0 m; | (46) |
|
|
| mesothelioma |
|
|
| + durvalumab | +PD-L1 |
| OS 16.6 m |
|
| - | Ikushima, 2020 | MPEM | Case report | 1/1 | - | Nivolumab | PD-1 | 2 | Hyper-progressive
disease | (47) |
| - | Tanaka, 2020 | MPEM | Case report | 1/1 | - | Nivolumab | PD-1 | 2 | Remission of
symptoms | (24) |
| - | Marmarelis, | MPEM | Retrospective
trial | 13/13 | - | Pembrolizumab | PD-1 | >1 | DCR 81%; mPFS 5.7
m; | (23) |
|
| 2020 |
|
|
|
|
|
|
| mOS 20.9 m |
|
| - | Kitadai, 2021 | MPEM | Retrospective
trial | 3/26 | - | Nivolumab | PD-1 | 2 | OS 36.7 m; PFS 8.1
m | (48) |
| NCT03074513 | Raghav, 2021 | MPEM | Prospective
trial | 20/20 | 2 | Atezolizumab | PD-L1 | >1 | ORR 40%; 1-year PFS
61%; | (49) |
|
|
|
|
|
|
|
|
|
| 1-year OS 85%, PFS
17.6 m, |
|
|
|
|
|
|
|
|
|
|
| OS not reached |
|
| - | Raghav, 2021 | MPEM | Retrospective
trial | 29 | - | Ipilimumab+ | CTLA-4 | >1 | DCR 65.4%; mPFS 5.5
m, | (50) |
|
|
|
|
|
|
| nivolumab or | +PD-1 or |
| mOS 19.2 m |
|
|
|
|
|
|
|
| other single- | single- |
|
|
|
|
|
|
|
|
|
| agent ICIs | agent ICIs |
|
|
|
| CONFIRM | Fennell, 2021 | Relapsed | RCT | 10/221 | 3 | Nivolumab | PD-1 | >1 | mPFS 3.0 m; 1-year
PFS | (51) |
|
|
| MPEM |
|
|
|
|
|
| 14%; mOS 19.8 m;
1-year |
|
|
|
|
|
|
|
|
|
|
| OS 68% |
|
| - | Huang, 2022 | MPEM | Case report | 1/1 | - | Unknown | Unknown | >1 | OS 2.0 m | (52) |
| NCT02141347 | Fujiwara, 2022 | Mesothelioma | Phase I trial | 11/11 | 1 | Tremelimumab | CTLA-4 ± | >2 | - | (53) |
|
|
| and other
solid |
|
|
| ± durvalumab | PD-L1 |
|
|
|
|
|
| tumor |
|
|
|
|
|
|
|
|
|
| Rizzolo, 2022 | MPEM | Case report | 1/1 | - | Ipilimumab+ | CTLA-4 | 1 | PFS >8.0 m | (12) |
|
|
|
|
|
|
| nivolumab | +PD-1 |
|
|
|
| - | Peng, 2023 | MPEM | Case report | 1/1 | - | Zimberelimab | PD-1 | 1 | PFS 7.0 m | (54) |
|
| Sugarbaker,
2023 | MPEM | Case report | 1/1 | - | Nivolumab | PD-1 | 2 | DFS 5 years | (55) |
| - | Lal, 2023 | MPEM with | Case report | 1/1 | - | Nivolumab + | PD-1 + | 2 | PFS 21 m | (56) |
|
|
| brain
metastases |
|
|
| ipilimumab | CTLA-4 |
|
|
|
| - | Tang, 2023 | Double primary | Case report | 1/1 | - | Nivolumab | PD-1 | 1 | PFS 12 m, OS 23
m | (13) |
|
|
| malignant
tumors |
|
|
|
|
|
|
|
|
|
|
| with MPEM and |
|
|
|
|
|
|
|
|
|
|
| nasopharyngeal |
|
|
|
|
|
|
|
|
|
|
| carcinoma |
|
|
|
|
|
|
|
|
| - | Marmarelis,
2023 | MPEM | Retrospective
trial | 24/24 | - | Pembrolizumab | PD-1 | All | PR rate 21.0%, mOS
20.9 m | (27) |
| - | Deng, 2024 | MPEM | Case report | 1/1 | - | Pembrolizumab | PD-1 | 1 | DFS 33 m | (14) |
| - | Bairos Menezes,
2024 | MPEM | Case report | 1/1 | - | Pembrolizumab | PD-1 | 2 | DFS 4 years | (17) |
In general, MPEM and MPM are frequently studied
together to assess the therapeutic regimens (15). Since MPEM and pleural mesothelioma
are both rare lethal cancers, there are few reports on the
immunological and molecular differences between them (16). Notably, only a few case reports and
cohort studies have focused on MPEM, thus providing insufficient
evidence for clinical guidance (11,15–17).
However, differences were observed in their clinical features. The
lack of prospective randomized controlled trials (RCTs) established
guidelines and consensus on MPEM management poses challenges in
standardizing treatments for advanced non-resectable cases.
According to previous reports, MPEM usually occurs in younger
women, with less emphasis on asbestos exposure as a risk factor and
a lower mean age at death (18).
Furthermore, mesothelioma from the pleura and peritoneum have
distinct genomic profiles, and thus, various dysregulated pathways
(19,20). Despite these differences, no
recommendations have been proposed specifically for the treatment
of MPEM at the time of diagnosis. Although approved by the Food and
Drug Administration, Chinese patients were unable to obtain
tremelimumab from any hospital or drug store at that time. The
single-agent PD-1 inhibitor pembrolizumab has been proven to be an
active agent and has been added to the National Comprehensive
Cancer Network (NCCN) and Chinese Society of Clinical Oncology
guidelines as a second-line treatment option for MPM (21,22).
More importantly, there have been few successful attempts to obtain
preliminary results on pembrolizumab for MPEM in a small-sample
retrospective trial (23). In
addition, the patient agreed to this therapy regimen because of not
only drug accessibility, but also affordability.
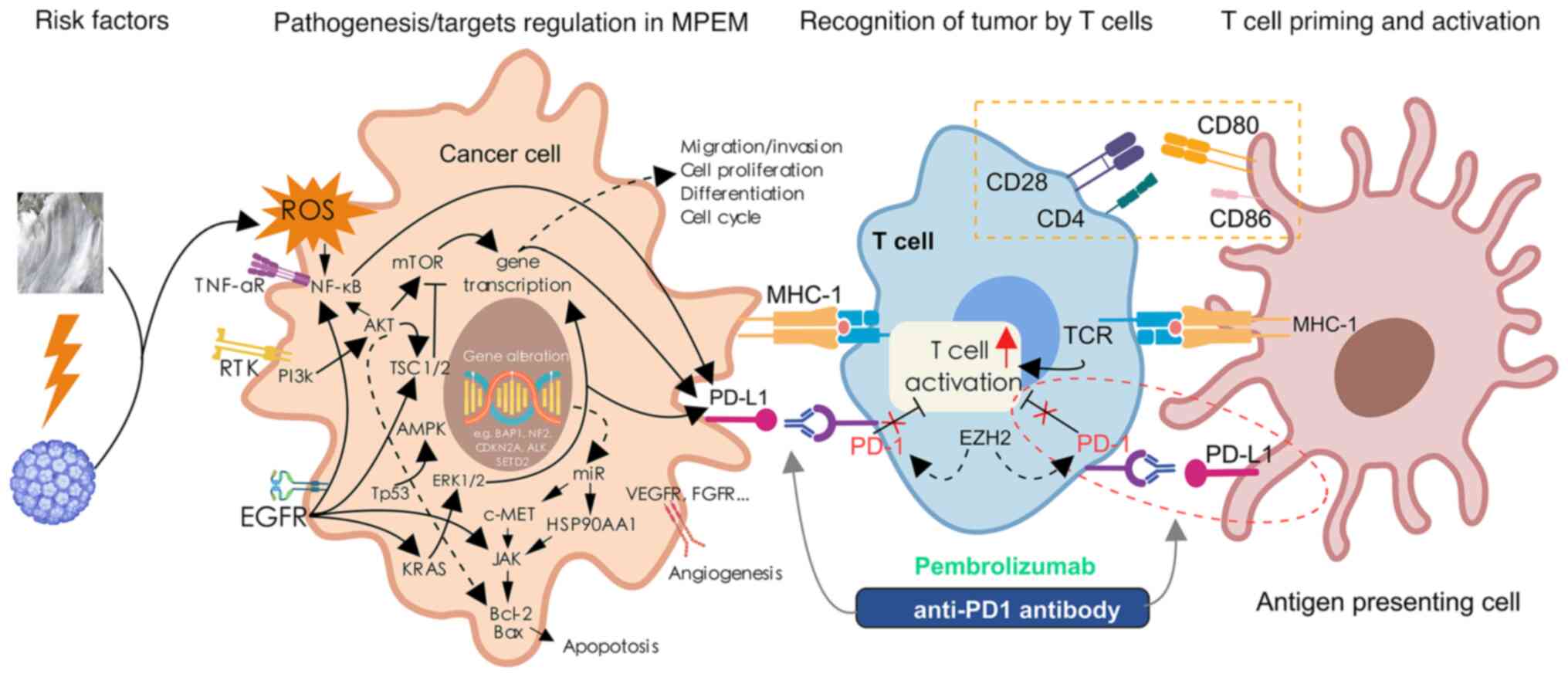
An increasing number of studies, including genomic
sequencing analyses, have revealed some of the possible molecular
mechanisms underlying pathogenic/target regulation in MPEM
(Table SII). PD-L1 expression and
the PD-1 checkpoint pathway in cancer are promising processes that
involve crucial targets, such as EGFR, PI3K, AKT and mTOR. The
current understanding of immunotherapy in MPEM is limited by the
small number of patients treated in clinical studies. A possible
mechanism of pembrolizumab treatment for MPEM is summarized in
Fig. 4. The anti-PD-1 agent
pembrolizumab could reactivate T cells, enhancing their anti-tumor
immune activity and exerting a strong anti-tumor effect. According
to pathological data, PD-L1 is expressed in ~40–60% of
mesotheliomas (24–26). A high frequency of PD-L1 expression
is associated with more aggressive tumor biology and is also a
biomarker for patients with MPEM that may benefit from
immunotherapy (27–30). In the patient of the present study,
immunotherapy may have been a priority, since PD-L1 expression was
60%. Finally, surprisingly, the patient of the present study
benefited greatly from the therapy regimen covered by the
insurance. Of note, the patient's fever and other unpleasant
symptoms disappeared. Furthermore, an abdominal lump shrinkage was
observed. The patient achieved maintained SD for up to 10 months
and a long-term survival benefit of 26.2 months. In addition, this
case sets a new record in the OS data for MPEM with paraneoplastic
syndromes. This finding is encouraging in rare disease settings in
which treatment options are limited.
However, individual factors cannot be ignored. The
latest NCCN guidelines for MPEM published on December 22nd, 2021,
recommended combination chemotherapy regimens of pemetrexed plus
cisplatin/carboplatin, with a median PFS of 7.3 months; as well as
pemetrexed and cisplatin/carboplatin plus bevacizumab, with a
median PFS of 9.2 months (31), or
immunotherapy regimens (nivolumab plus ipilimumab; median PFS, 6.8
months) (32) as first-line
treatment. Although pembrolizumab may be a new first-line treatment
option, the best treatment for MPEM requires verification in more
clinical cases. Further multicenter, large-sample RCTs are
essential to establish standard MPEM treatment strategies.
In conclusion, pembrolizumab may be an effective and
well-tolerated method for treating MPEM. More proof is needed from
further extensive clinical trials, resulting in better
individualized treatment plans and outcomes for such patients.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Figure 4 was created
using MedPeer (https://www.medpeer.cn/).
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 82004151), Capital's Funds for
Health Improvement and Research (grant no. 2024-4-40614) and the
Noncommunicable Chronic Diseases-National Science and Technology
Major Project (grant no. 2023ZD0502803).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CX, ZD and KT participated in the study design and
wrote the original manuscript; CX, JZ and HC analyzed pathological
images and made the diagnosis; ZD, XZ and YY obtained medical
images and analyzed patient data; HC, JZ and SW were involved in
drafting the manuscript, revising it critically for important
intellectual content, and were responsible for managing this
research project; SW contributed to the follow-up; JZ and SW
provided grant support; CX and ZD confirm the authenticity of all
the raw data. All authors have read and agreed to the published
version of the manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
principles of the Declaration of Helsinki. Institutional Review
Board approval is not required at our institution for case-report
studies.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of potentially identifiable images and
data included in this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim J, Bhagwandin S and Labow DM:
Malignant peritoneal mesothelioma: A review. Ann Transl Med.
5:2362017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moolgavkar SH, Meza R and Turim J: Pleural
and peritoneal mesotheliomas in SEER: Age effects and temporal
trends, 1973–2005. Cancer Causes Control. 20:935–944. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boffetta P: Epidemiology of peritoneal
mesothelioma: A review. Ann Oncol. 18:985–990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gray SG and Mutti L: Immunotherapy for
mesothelioma: A critical review of current clinical trials and
future perspectives. Transl Lung Cancer Res. 9 (Suppl 1):S100–S119.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jacquet P and Sugarbaker PH: Clinical
research methodologies in diagnosis and staging of patients with
peritoneal carcinomatosis. Cancer Treat Res. 82:359–374. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shavelle R, Vavra-Musser K, Lee J and
Brooks J: Life expectancy in pleural and peritoneal mesothelioma.
Lung Cancer Int. 2017:27825902017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Acherman YI, Welch LS, Bromley CM and
Sugarbaker PH: Clinical presentation of peritoneal mesothelioma.
Tumori. 89:269–273. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su Y, Liu G, Du X and Li Y: Diffuse
malignant peritoneal mesothelioma with paraneoplastic syndrome: A
case report and literature review. Cancer Res Prev Treat.
49:246–250. 2022.
|
|
10
|
Kusamura S, Torres Mesa PA, Cabras A,
Baratti D and Deraco M: The role of Ki-67 and pre-cytoreduction
parameters in selecting diffuse malignant peritoneal mesothelioma
(DMPM) patients for cytoreductive surgery (CRS) and hyperthermic
intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol. 23:1468–1473.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Zheng G, Yang D, Guo X, Tian L,
Song H and Liang Y: Osteopontin, GLUT1 and Ki-67 expression in
malignant peritoneal mesothelioma: Prognostic implications. Intern
Med J. 51:896–904. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizzolo A, Ah-Lan KC, Nu TNT and Alcindor
T: Response to ipilimumab and nivolumab in a patient with malignant
peritoneal mesothelioma. Clin Colorectal Cancer. 21:371–374. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang LK, Li ZK, Xiang YL, Ma DY and Du GB:
Metachronous double primary malignant tumors with nasopharyngeal
carcinoma and diffuse malignant peritoneal mesothelioma accompanied
with paraneoplastic syndromes treated with nivolumab: A case
report. Medicine (Baltimore). 102:e343492023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng M, Zhang X, Xu C, Luo R, Chen L, Zhou
Y and Hou Y: Clinical and pathological observation of conversion
therapy for malignant peritoneal mesothelioma: A case report and
literature review. Pathol Oncol Res. 29:16115772024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hung YP, Dong F, Watkins JC, Nardi V,
Bueno R, Dal Cin P, Godleski JJ, Crum CP and Chirieac LR:
Identification of ALK rearrangements in malignant peritoneal
mesothelioma. JAMA Oncol. 4:235–238. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chapel DB, Stewart R, Furtado LV, Husain
AN, Krausz T and Deftereos G: Tumor PD-L1 expression in malignant
pleural and peritoneal mesothelioma by Dako PD-L1 22C3 pharmDx and
Dako PD-L1 28-8 pharmDx assays. Hum Pathol. 87:11–17. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bairos Menezes M, Pedroso de Lima R,
Dunões I, Inácio M and Dinis R: A complete response to
pembrolizumab in malignant peritoneal mesothelioma: A case report.
Cureus. 16:e527162024.PubMed/NCBI
|
|
18
|
Mensi C, Mendola M, Dallari B, Sokooti M,
Tabibi R, Riboldi L and Consonni D: Differences between peritoneal
and pleural mesothelioma in Lombardy, Italy. Cancer Epidemiol.
51:68–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borczuk AC, Cappellini GC, Kim HK,
Hesdorffer M, Taub RN and Powell CA: Molecular profiling of
malignant peritoneal mesothelioma identifies the
ubiquitin-proteasome pathway as a therapeutic target in poor
prognosis tumors. Oncogene. 26:610–617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
López-Ríos F, Chuai S, Flores R, Shimizu
S, Ohno T, Wakahara K, Illei PB, Hussain S, Krug L, Zakowski MF, et
al: Global gene expression profiling of pleural mesotheliomas:
overexpression of aurora kinases and P16/CDKN2A deletion as
prognostic factors and critical evaluation of microarray-based
prognostic prediction. Cancer Res. 66:2970–2979. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Xu C, Wang W, Zhang Y, Li Z, Song
Z, Wang J, Yu J, Liu J, Zhang S, et al: Chinese expert consensus on
the diagnosis and treatment of malignant pleural mesothelioma.
Thorac Cancer. 14:2715–2731. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Comprehensive Cancer Network
(NCCN), . Mesothelioma. Peritoneal, Version 1. NCCN; Plymouth
Meeting, PA: 2021
|
|
23
|
Marmarelis ME, Wang X, Roshkovan L, Walker
S, McNulty S, Ciunci CA, Muira J, Katz SI, Cengel KA, Karakousis G
and Langer CJ: Real-world outcomes of pembrolizumab in peritoneal
mesothelioma. J Clin Oncol. 38 (15-suppl):e210942020. View Article : Google Scholar
|
|
24
|
Tanaka T, Miyamoto Y, Sakai A and Fujimoto
N: Nivolumab for malignant peritoneal mesothelioma. BMJ Case Rep.
13:e2377212020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khanna S, Thomas A, Abate-Daga D, Zhang J,
Morrow B, Steinberg SM, Orlandi A, Ferroni P, Schlom J, Guadagni F
and Hassan R: Malignant mesothelioma effusions are infiltrated by
CD3+ T cells highly expressing PD-L1 and the PD-L1+ tumor cells
within these effusions are susceptible to ADCC by the Anti-PD-L1
antibody avelumab. J Thorac Oncol. 11:1993–2005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mansfield AS, Roden AC, Peikert T, Sheinin
YM, Harrington SM, Krco CJ, Dong H and Kwon ED: B7-H1 expression in
malignant pleural mesothelioma is associated with sarcomatoid
histology and poor prognosis. J Thorac Oncol. 9:1036–1040. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marmarelis ME, Wang X, Roshkovan L, Grady
CB, Miura JT, Ginsberg MS, Ciunci CA, Egger J, Walker S, Cercek A,
et al: Clinical outcomes associated with pembrolizumab monotherapy
among adults with diffuse malignant peritoneal mesothelioma. JAMA
Netw Open. 6:e2325262023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gazivoda VP, Kangas-Dick AW, Greenbaum AA,
Roshal J, Chen C, Moore DF, Langan RC, Kennedy TJ, Minerowicz C and
Alexander HR: Expression of PD-L1 in patients with malignant
peritoneal mesothelioma: A pilot study. J Surg Res. 277:131–137.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valmary-Degano S, Colpart P, Villeneuve L,
Monnien F, M'Hamdi L, Lang Averous G, Capovilla M, Bibeau F,
Laverriere MH, Verriele-Beurrier V, et al: Immunohistochemical
evaluation of two antibodies against PD-L1 and prognostic
significance of PD-L1 expression in epithelioid peritoneal
malignant mesothelioma: A RENAPE study. Eur J Surg Oncol.
43:1915–1923. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
White MG, Schulte JJ, Xue L, Berger Y,
Schuitevoerder D, Vining CC, Kindler HL, Husain A, Turaga KK and
Eng OS: Heterogeneity in PD-L1 expression in malignant peritoneal
mesothelioma with systemic or intraperitoneal chemotherapy. Br J
Cancer. 124:564–566. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zalcman G, Mazieres J, Margery J,
Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre
R, Monnet I, Gounant V, et al: Bevacizumab for newly diagnosed
pleural mesothelioma in the Mesothelioma Avastin cisplatin
pemetrexed study (MAPS): A randomised, controlled, open-label,
phase 3 trial. Lancet. 387:1405–1414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baas P, Scherpereel A, Nowak AK, Fujimoto
N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, et
al: First-line nivolumab plus ipilimumab in unresectable malignant
pleural mesothelioma (CheckMate 743): A multicentre, randomised,
open-label, phase 3 trial. Lancet. 397:375–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Selleslag DL, Geraghty RJ, Ganesan TS,
Slcvin ML, Wrigley PF and Brown R: Autoimmune haemolytic anaemia
associated with malignant peritoneal mesothelioma. Acta Clin Belg.
44:199–201. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kimura N, Ogasawara T, Asonuma S, Hama H,
Sawai T and Toyota T: Granulocyte-colony stimulating factor- and
interleukin 6-producing diffuse deciduoid peritoneal mesothelioma.
Mod Pathol. 18:446–450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Banayan S, Hot A, Janier M, Ninet J,
Zurlinden O and Billotey C: Malignant mesothelioma of the
peritoneum as the cause of a paraneoplastic syndrome: Detection by
18F-FDG PET. Eur J Nucl Med Mol Imaging. 33:7512006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Socola F, Loaiza-Bonilla A,
Bustinza-Linares E, Correa R and Rosenblatt JD: Recurrent
thrombotic thrombocytopenic purpura-like syndrome as a
paraneoplastic phenomenon in malignant peritoneal mesothelioma: A
case report and review of the literature. Case Rep Oncol Med.
2012:6193482012.PubMed/NCBI
|
|
37
|
Thakral B and Loghavi S: Marked
paraneoplastic leukemoid reaction in a patient with mesothelioma
mimicking a myeloid neoplasm. Blood. 135:4572020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen LY, Huang LX, Wang J, Qian Y and Fang
LZ: Malignant peritoneal mesothelioma presenting with persistent
high fever. J Zhejiang Univ Sci B. 12:381–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hermann J, Bajko G, Stajgis M, Szmeja J,
Kościński T and Drews M: Fever of unknown origin: A clinical mask
of malignant peritoneal mesothelioma. Contemp Oncol (Pozn).
16:596–599. 2012.PubMed/NCBI
|
|
40
|
Ishizuka K, Uehara T, Arai M, Ikeda J,
Hirose Y and Ikusaka M: Medical-type peritoneal mesothelioma
leading to death two months after onset of fever of unknown origin.
Radiol Case Rep. 17:540–543. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Calabrò L, Morra A, Fonsatti E, Cutaia O,
Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M,
Mutti L and Maio M: Tremelimumab for patients with
chemotherapy-resistant advanced malignant mesothelioma: An
open-label, single-arm, phase 2 trial. Lancet Oncol. 14:1104–1111.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Calabrò L, Morra A, Fonsatti E, Cutaia O,
Fazio C, Annesi D, Lenoci M, Amato G, Danielli R, Altomonte M, et
al: Efficacy and safety of an intensified schedule of tremelimumab
for chemotherapy-resistant malignant mesothelioma: an open-label,
single-arm, phase 2 study. Lancet Respir Med. 3:301–309. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maio M, Scherpereel A, Calabrò L, Aerts J,
Perez SC, Bearz A, Nackaerts K, Fennell DA, Kowalski D, Tsao AS, et
al: Tremelimumab as second-line or third-line treatment in relapsed
malignant mesothelioma (DETERMINE): A multicentre, international,
randomised, double-blind, placebo-controlled phase 2b trial. Lancet
Oncol. 18:1261–1273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Desai A, Karrison T, Rose B, Pemberton E,
Hill B, Straus CM, Tan YHC, Seiwert TY and Kindler HL: Phase II
trial of pembrolizumab (P) in patients (pts) with
previously-treated mesothelioma (MM). J Clin Oncol. 36
(15-suppl):S85652018. View Article : Google Scholar
|
|
45
|
Hassan R, Thomas A, Nemunaitis JJ, Patel
MR, Bennouna J, Chen FL, Delord JP, Dowlati A, Kochuparambil ST,
Taylor MH, et al: Efficacy and safety of avelumab treatment in
patients with advanced unresectable mesothelioma: Phase 1b results
from the JAVELIN solid tumor trial. JAMA Oncol. 5:351–357. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Calabrò L, Rossi G, Morra A, Rosati C,
Cutaia O, Daffinà MG, Altomonte M, Di Giacomo AM, Casula M, Fazio
C, et al: Tremelimumab plus durvalumab retreatment and 4-year
outcomes in patients with mesothelioma: A follow-up of the open
label, non-randomised, phase 2 NIBIT-MESO-1 study. Lancet Respir
Med. 9:969–976. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ikushima H, Sakatani T, Ohara S, Takeshima
H, Horiuchi H, Morikawa T and Usui K: Cisplatin plus pemetrexed
therapy and subsequent immune checkpoint inhibitor administration
for malignant peritoneal mesothelioma without pleural lesions: Case
report. Medicine (Baltimore). 99:e199562020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kitadai R, Shimoi T, Sudo K, Noguchi E,
Nagata Y, Sawada R, Takashima A, Boku N and Yonemori K: Efficacy of
second-line treatment and prognostic factors in patients with
advanced malignant peritoneal mesothelioma: A retrospective study.
BMC Cancer. 21:2942021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Raghav K, Liu S, Overman MJ, Willett AF,
Knafl M, Fu SC, Malpica A, Prasad S, Royal RE, Scally CP, et al:
Efficacy, safety, and biomarker analysis of combined PD-L1
(Atezolizumab) and VEGF (Bevacizumab) blockade in advanced
malignant peritoneal mesothelioma. Cancer Discov. 11:2738–2747.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Raghav K, Liu S, Overman M, Morani A,
Willette A, Fournier K and Varadhachary G: Clinical efficacy of
immune checkpoint inhibitors in patients with advanced malignant
peritoneal mesothelioma. JAMA Netw Open. 4:e21199342021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fennell DA, Ewings S, Ottensmeier C,
Califano R, Hanna GG, Hill K, Danson S, Steele N, Nye M, Johnson L,
et al: Nivolumab versus placebo in patients with relapsed malignant
mesothelioma (CONFIRM): A multicentre, double-blind, randomised,
phase 3 trial. Lancet Oncol. 22:1530–1540. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang X, Hong Y, Xie SY, Liao HL, Huang
HM, Liu JH and Long WJ: Malignant peritoneal mesothelioma with
massive ascites as the first symptom: A case report. World J Clin
Cases. 10:10317–10325. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fujiwara Y, Takahashi Y, Okada M,
Kishimoto T, Kondo S, Fujikawa K, Hayama M, Sugeno M, Ueda S,
Komuro K, et al: Phase I study of tremelimumab monotherapy or in
combination with durvalumab in japanese patients with advanced
solid tumors or malignant mesothelioma. Oncologist. 27:e703–e722.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Peng XD, You ZY, He LX and Deng Q:
Zimberelimab plus chemotherapy as the first-line treatment of
malignant peritoneal mesothelioma: A case report and review of
literature. World J Clin Cases. 11:5296–5302. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sugarbaker PH: Response to Nivolumab
followed by complete cytoreductive surgery with HIPEC resulted in
long-term survival in a patient with sarcomatoid-predominant
biphasic peritoneal mesothelioma. A case report. Int J Surg Case
Rep. 107:1083592023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lal D, Misiura M, Adeel W and Tariq R:
Brain metastases with malignant peritoneal mesothelioma: Never
reported before. Cureus. 15:e437442023.PubMed/NCBI
|