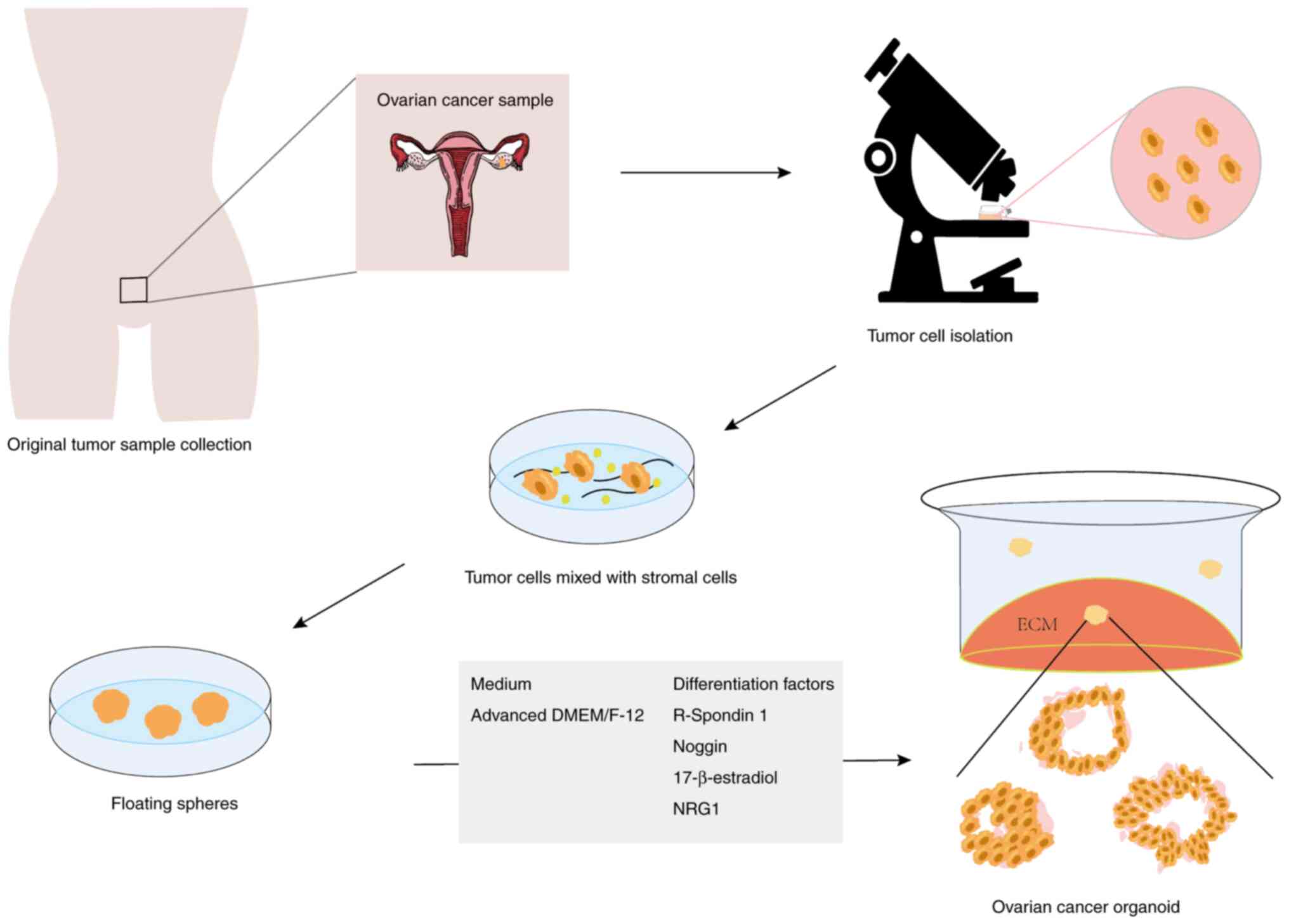
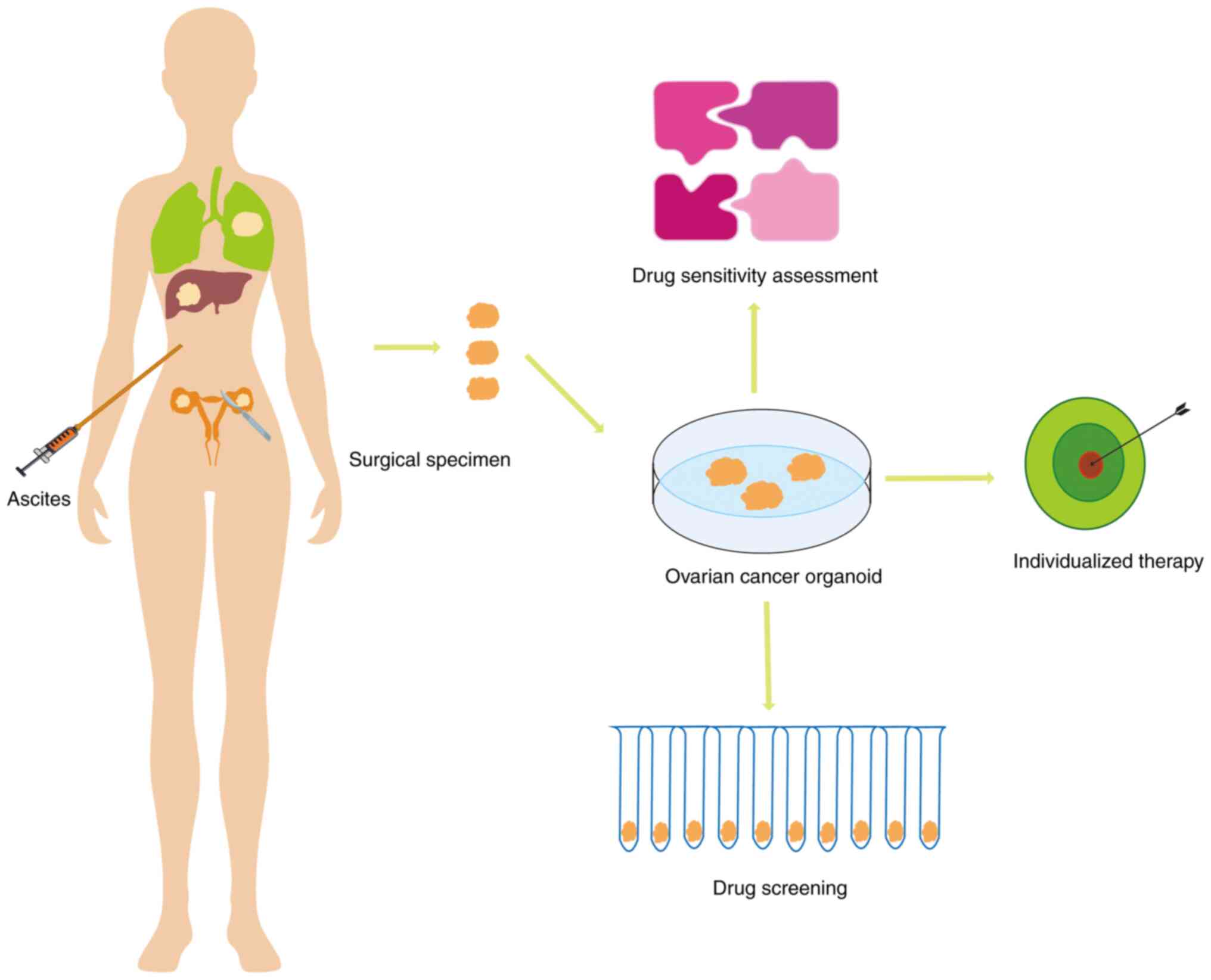
|
1
|
Kuroki L and Guntupalli SR: Treatment of
epithelial ovarian cancer. BMJ. 371:m37732020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American Cancer Society: Cancer Facts and
Figures 2023. American Cancer Society; Atlanta, GA: 2023
|
|
5
|
Liberto JM, Chen SY, Shih IM, Wang TH,
Wang TL and Pisanic TR II: Current and emerging methods for ovarian
cancer screening and diagnostics: A comprehensive review. Cancers
(Basel). 14:28852022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kikuchi Y, Kita T, Takano M, Kudoh K and
Yamamoto K: Treatment options in the management of ovarian cancer.
Expert Opin Pharmacother. 6:743–754. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bookman MA: First-line chemotherapy in
epithelial ovarian cancer. Clin Obstet Gynecol. 55:96–113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Phase III trial of carboplatin and paclitaxel
compared with cisplatin and paclitaxel in patients with optimally
resected stage III ovarian cancer: A gynecologic oncology group
study. J Clin Oncol. 41:4077–4083. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kyrgiou M, Salanti G, Pavlidis N,
Paraskevaidis E and Ioannidis JP: Survival benefits with diverse
chemotherapy regimens for ovarian cancer: Meta-analysis of multiple
treatments. J Natl Cancer Inst. 98:1655–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SI, Cho J, Lee EJ, Park S, Park SJ,
Seol A, Lee N, Yim GW, Lee M, Lim W, et al: Selection of patients
with ovarian cancer who may show survival benefit from hyperthermic
intraperitoneal chemotherapy: A systematic review and
meta-analysis. Medicine (Baltimore). 98:e183552019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ozols RF: Challenges for chemotherapy in
ovarian cancer. Ann Oncol. 17 (Suppl 5):v181–v187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fung-Kee-Fung M, Oliver T, Elit L, Oza A,
Hirte HW and Bryson P: Optimal chemotherapy treatment for women
with recurrent ovarian cancer. Curr Oncol. 14:195–208. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pokhriyal R, Hariprasad R, Kumar L and
Hariprasad G: Chemotherapy resistance in advanced ovarian cancer
patients. Biomark Cancer. 11:1179299×198608152019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cornelison R, Llaneza DC and Landen CN:
Emerging therapeutics to overcome chemoresistance in epithelial
ovarian cancer: A mini-review. Int J Mol Sci. 18:21712017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baker BM and Chen CS: Deconstructing the
third dimension: How 3D culture microenvironments alter cellular
cues. J Cell Sci. 125:3015–3024. 2012.PubMed/NCBI
|
|
16
|
Zhang Z, Bédard E, Luo Y, Wang H, Deng S,
Kelvin D and Zhong R: Animal models in xenotransplantation. Expert
Opin Investig Drugs. 9:2051–2068. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bertotti A, Migliardi G, Galimi F, Sassi
F, Torti D, Isella C, Corà D, Di Nicolantonio F, Buscarino M, Petti
C, et al: A molecularly annotated platform of patient-derived
xenografts (‘xenopatients’) identifies HER2 as an effective
therapeutic target in cetuximab-resistant colorectal cancer. Cancer
Discov. 1:508–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
DeRose YS, Wang G, Lin YC, Bernard PS,
Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, et al:
Tumor grafts derived from women with breast cancer authentically
reflect tumor pathology, growth, metastasis and disease outcomes.
Nat Med. 17:1514–1520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sachs N, de Ligt J, Kopper O, Gogola E,
Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H,
et al: A living biobank of breast cancer organoids captures disease
heterogeneity. Cell. 172:373–386.e310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zanoni M, Cortesi M, Zamagni A, Arienti C,
Pignatta S and Tesei A: Modeling neoplastic disease with spheroids
and organoids. J Hematol Oncol. 13:972020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Graham O, Rodriguez J, van Biljon L,
Fashemi B, Graham E, Fuh K, Khabele D and Mullen M: Generation and
culturing of high-grade serous ovarian cancer patient-derived
organoids. J Vis Exp. 6:1912023.
|
|
22
|
Yani W, Qi J, Yuchen Z and Haiyan Z:
Application of organoids technology in drug sensitivity test of
ovarian cancer. J Int Obstet Gynecol. 49:181–185. 2022.
|
|
23
|
Yujie S, Hong Y, Jia L and Ying X:
Application prospects on organoid culture system in drug screening
and treatment target for ovarian cancer. J Chin Oncol.
28:1042–1045. 2022.(In Chinese).
|
|
24
|
Jianjun G, Wei Q, Hao W and Xiangyu Z:
Application and prospect of organoid technique in cancer research.
Chin J Tissue Engineering Res. 23:1136–1141. 2019.(In Chinese).
|
|
25
|
Aihara A, Abe N, Saruhashi K, Kanaki T and
Nishino T: Novel 3-D cell culture system for in vitro evaluation of
anticancer drugs under anchorage-independent conditions. Cancer
Sci. 107:1858–1866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ben-David U, Ha G, Tseng YY, Greenwald NF,
Oh C, Shih J, McFarland JM, Wong B, Boehm JS, Beroukhim R and Golub
TR: Patient-derived xenografts undergo mouse-specific tumor
evolution. Nat Genet. 49:1567–1575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Byrne AT, Alférez DG, Amant F, Annibali D,
Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, et
al: Interrogating open issues in cancer medicine with
patient-derived xenografts. Nat Rev Cancer. 17:6322017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sachs N and Clevers H: Organoid cultures
the analysis of cancer phenotypes. Curr Opin Genet Dev. 24:68–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bleijs M, van de Wetering M, Clevers H and
Drost J: Xenograft and organoid model systems in cancer research.
EMBO J. 38:e1016542019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wensink GE, Elias SG, Mullenders J,
Koopman M, Boj SF, Kranenburg OW and Roodhart JML: Patient-derived
organoids as a predictive biomarker for treatment response in
cancer patients. NPJ Precis Oncol. 5:302021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perkhofer L, Frappart PO, Müller M and
Kleger A: Importance of organoids for personalized medicine. Per
Med. 15:461–465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rossi G, Manfrin A and Lutolf MP: Progress
and potential in organoid research. Nat Rev Genet. 19:671–687.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsang SI, Hassan AA, To SKY and Wong AST:
Experimental models for ovarian cancer research. Exp Cell Res.
416:1131502022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Huang S, Cheng S, Jin Y, Zhang N
and Wang Y: Application of ovarian cancer organoids in precision
medicine: Key challenges and current opportunities. Front Cell Dev
Biol. 9:7014292021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aboulkheyr Es H, Montazeri L, Aref AR,
Vosough M and Baharvand H: Personalized cancer medicine: An
organoid approach. Trends Biotechnol. 36:358–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maenhoudt N, Defraye C, Boretto M, Jan Z,
Heremans R, Boeckx B, Hermans F, Arijs I, Cox B, Van Nieuwenhuysen
E, et al: developing organoids from ovarian cancer as experimental
and preclinical models. Stem Cell Reports. 14:717–729. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kopper O, de Witte CJ, Lõhmussaar K,
Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost
N, Begthel H, et al: An organoid platform for ovarian cancer
captures intra- and interpatient heterogeneity. Nat Med.
25:838–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pauli C, Hopkins BD, Prandi D, Shaw R,
Fedrizzi T, Sboner A, Sailer V, Augello M, Puca L, Rosati R, et al:
Personalized in vitro and in vivo cancer models to guide precision
medicine. Cancer Discov. 7:462–477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jabs J, Zickgraf FM, Park J, Wagner S,
Jiang X, Jechow K, Kleinheinz K, Toprak UH, Schneider MA, Meister
M, et al: Screening drug effects in patient-derived cancer cells
links organoid responses to genome alterations. Mol Syst Biol.
13:9552017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maru Y, Tanaka N, Itami M and Hippo Y:
Efficient use of patient-derived organoids as a preclinical model
for gynecologic tumors. Gynecol Oncol. 154:189–198. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hill SJ, Decker B, Roberts EA, Horowitz
NS, Muto MG, Worley MJ Jr, Feltmate CM, Nucci MR, Swisher EM,
Nguyen H, et al: Prediction of DNA repair inhibitor response in
short-term patient-derived ovarian cancer organoids. Cancer Discov.
8:1404–1421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nanki Y, Chiyoda T, Hirasawa A, Ookubo A,
Itoh M, Ueno M, Akahane T, Kameyama K, Yamagami W, Kataoka F and
Aoki D: Patient-derived ovarian cancer organoids capture the
genomic profiles of primary tumours applicable for drug sensitivity
and resistance testing. Sci Rep. 10:125812020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hoffmann K, Berger H, Kulbe H,
Thillainadarasan S, Mollenkopf HJ, Zemojtel T, Taube E,
Darb-Esfahani S, Mangler M, Sehouli J, et al: Stable expansion of
high-grade serous ovarian cancer organoids requires a low-Wnt
environment. EMBO J. 39:e1040132020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kondo J and Inoue M: application of cancer
organoid model for drug screening and personalized therapy. Cells.
8:4702019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Antoni D, Burckel H, Josset E and Noel G:
Three-dimensional cell culture: A breakthrough in vivo. Int J Mol
Sci. 16:5517–5527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kimlin LC, Casagrande G and Virador VM: In
vitro three-dimensional (3D) models in cancer research: An update.
Mol Carcinog. 52:167–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shoemaker RH: The NCI60 human tumour cell
line anticancer drug screen. Nat Rev Cancer. 6:813–823. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rizvanov AA, Yalvaç ME, Shafigullina AK,
Salafutdinov II, Blatt NL, Sahin F, Kiyasov AP and Palotás A:
Interaction and self-organization of human mesenchymal stem cells
and neuro-blastoma SH-SY5Y cells under co-culture conditions: A
novel system for modeling cancer cell micro-environment. Eur J
Pharm Biopharm. 76:253–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Enmon RM Jr, O'Connor KC, Lacks DJ,
Schwartz DK and Dotson RS: Dynamics of spheroid self-assembly in
liquid-overlay culture of DU 145 human prostate cancer cells.
Biotechnol Bioeng. 72:579–591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Westhouse RA: Safety assessment
considerations and strategies for targeted small molecule cancer
therapeutics in drug discovery. Toxicol Pathol. 38:165–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wong CC, Cheng KW and Rigas B: Preclinical
predictors of anticancer drug efficacy: Critical assessment with
emphasis on whether nanomolar potency should be required of
candidate agents. J Pharmacol Exp Ther. 341:572–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ravi M, Paramesh V, Kaviya SR, Anuradha E
and Solomon FD: 3D cell culture systems: Advantages and
applications. J Cell Physiol. 230:16–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jamieson LE, Harrison DJ and Campbell CJ:
Chemical analysis of multicellular tumour spheroids. Analyst.
140:3910–3920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Beningo KA, Dembo M and Wang Yl: Responses
of fibroblasts to anchorage of dorsal extracellular matrix
receptors. Proc Natl Acad Sci USA. 101:18024–18029. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sambale F, Lavrentieva A, Stahl F, Blume
C, Stiesch M, Kasper C, Bahnemann D and Scheper T: Three
dimensional spheroid cell culture for nanoparticle safety testing.
J Biotechnol. 205:120–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jarockyte G, Dapkute D, Karabanovas V,
Daugmaudis JV, Ivanauskas F and Rotomskis R: 3D cellular spheroids
as tools for understanding carboxylated quantum dot behavior in
tumors. Biochim Biophys Acta Gen Subj. 1862:914–923. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mehta G, Hsiao AY, Ingram M, Luker GD and
Takayama S: Opportunities and challenges for use of tumor spheroids
as models to test drug delivery and efficacy. J Control Release.
164:192–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Souza AG, Silva IBB, Campos-Fernandez E,
Barcelos LS, Souza JB, Marangoni K, Goulart LR and Alonso-Goulart
V: Comparative assay of 2D and 3D cell culture models:
Proliferation, gene expression and anticancer drug response. Curr
Pharm Des. 24:1689–1694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Breslin S and O'Driscoll L: The relevance
of using 3D cell cultures, in addition to 2D monolayer cultures,
when evaluating breast cancer drug sensitivity and resistance.
Oncotarget. 7:45745–45756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Verjans ET, Doijen J, Luyten W, Landuyt B
and Schoofs L: Three-dimensional cell culture models for anticancer
drug screening: Worth the effort? J Cell Physiol. 233:2993–3003.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hirst J, Pathak HB, Hyter S, Pessetto ZY,
Ly T, Graw S, Koestler DC, Krieg AJ, Roby KF and Godwin AK:
Licofelone enhances the efficacy of paclitaxel in ovarian cancer by
reversing drug resistance and tumor stem-like properties. Cancer
Res. 78:4370–4385. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cavarzerani E, Caligiuri I, Bartoletti M,
Canzonieri V and Rizzolio F: 3D dynamic cultures of HGSOC organoids
to model innovative and standard therapies. Front Bioeng
Biotechnol. 11:11353742023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Samson DJ, Seidenfeld J, Ziegler K and
Aronson N: Chemotherapy sensitivity and resistance assays: A
systematic review. J Clin Oncol. 22:3618–3630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Brooks EA, Galarza S, Gencoglu MF,
Cornelison RC, Munson JM and Peyton SR: Applicability of drug
response metrics for cancer studies using biomaterials. Philos
Trans R Soc Lond B Biol Sci. 374:201802262019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Brodeur MN, Simeone K, Leclerc-Deslauniers
K, Fleury H, Carmona E, Provencher DM and Mes-Masson AM:
Carboplatin response in preclinical models for ovarian cancer:
Comparison of 2D monolayers, spheroids, ex vivo tumors and in vivo
models. Sci Rep. 11:181832021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Thorel L, Morice PM, Paysant H, Florent R,
Babin G, Thomine C, Perréard M, Abeilard E, Giffard F, Brotin E, et
al: Comparative analysis of response to treatments and molecular
features of tumor-derived organoids versus cell lines and PDX
derived from the same ovarian clear cell carcinoma. J Exp Clin
Cancer Res. 42:2602023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Loessner D, Stok KS, Lutolf MP, Hutmacher
DW, Clements JA and Rizzi SC: Bioengineered 3D platform to explore
cell-ECM interactions and drug resistance of epithelial ovarian
cancer cells. Biomaterials. 31:8494–8506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tofani LB, Abriata JP, Luiz MT, Marchetti
JM and Swiech K: Establishment and characterization of an in vitro
3D ovarian cancer model for drug screening assays. Biotechnol Prog.
36:e30342020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bi J, Newtson AM, Zhang Y, Devor EJ,
Samuelson MI, Thiel KW and Leslie KK: Successful patient-derived
organoid culture of gynecologic cancers for disease modeling and
drug sensitivity testing. Cancers (Basel). 13:29012021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Garcia J, Hurwitz HI, Sandler AB, Miles D,
Coleman RL, Deurloo R and Chinot OL: Bevacizumab
(Avastin®) in cancer treatment: A review of 15 years of
clinical experience and future outlook. Cancer Treat Rev.
86:1020172020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cohen MH, Gootenberg J, Keegan P and
Pazdur R: FDA drug approval summary: Bevacizumab plus FOLFOX4 as
second-line treatment of colorectal cancer. Oncologist. 12:356–361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Govindaraju S and Yun K: Synthesis of gold
nanomaterials and their cancer-related biomedical applications: An
update 3. Biotech. 8:1132018.PubMed/NCBI
|
|
73
|
Oliva P, Decio A, Castiglioni V, Bassi A,
Pesenti E, Cesca M, Scanziani E, Belotti D and Giavazzi R:
Cisplatin plus paclitaxel and maintenance of bevacizumab on tumour
progression, dissemination, and survival of ovarian carcinoma
xenograft models. Br J Cancer. 107:360–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang H, Wang Y and Wang P, Zhang N and
Wang P: Tumor organoids for cancer research and personalized
medicine. Cancer Biol Med. 19:319–332. 2021.PubMed/NCBI
|
|
75
|
Seidlitz T, Koo BK and Stange DE: Gastric
organoids-an in vitro model system for the study of gastric
development and road to personalized medicine. Cell Death Differ.
28:68–83. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rivenbark AG, O'Connor SM and Coleman WB:
Molecular and cellular heterogeneity in breast cancer: Challenges
for personalized medicine. Am J Pathol. 183:1113–1124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Verma M: Personalized medicine and cancer.
J Pers Med. 2:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Offit K: Personalized medicine: New
genomics, old lessons. Hum Genet. 130:3–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Peres LC, Risch H, Terry KL, Webb PM,
Goodman MT, Wu AH, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy
ML, et al: Racial/ethnic differences in the epidemiology of ovarian
cancer: A pooled analysis of 12 case-control studies. Int J
Epidemiol. 47:10112018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nero C, Vizzielli G, Lorusso D, Cesari E,
Daniele G, Loverro M, Scambia G and Sette C: Patient-derived
organoids and high grade serous ovarian cancer: From disease
modeling to personalized medicine. J Exp Clin Cancer Res.
40:1162021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lui G, Richardson A, Chatterjee P,
Pollastro M, Lints M, Peretti D, Rosati R, Appleyard L, Durenberger
G, Diaz R, et al: Functional drug screening of organoids from
ovarian cancer patients demonstrates clinical and genomic
concordance and identifies novel therapeutic vulnerabilities.
Cancer Res. 81:534. 2021. View Article : Google Scholar
|
|
82
|
Phan N, Hong JJ, Tofig B, Mapua M,
Elashoff D, Moatamed NA, Huang J, Memarzadeh S, Damoiseaux R and
Soragni A: A simple high-throughput approach identifies actionable
drug sensitivities in patient-derived tumor organoids. Commun Biol.
2:782019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Åkerlund E, Gudoityte G,
Moussaud-Lamodière E, Lind O, Bwanika HC, Lehti K, Salehi S,
Carlson J, Wallin E, Fernebro J, et al: The drug efficacy testing
in 3D cultures platform identifies effective drugs for ovarian
cancer patients. NPJ Precis Oncol. 7:1112023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Clark J, Fotopoulou C, Cunnea P and Krell
J: Novel ex vivo models of epithelial ovarian cancer: The future of
biomarker and therapeutic research. Front Oncol. 12:8372332022.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Compadre AJ, van Biljon LN, Valentine MC,
Llop-Guevara A, Graham E, Fashemi B, Herencia-Ropero A, Kotnik EN,
Cooper I, Harrington SP, et al: RAD51 foci as a biomarker
predictive of platinum chemotherapy response in ovarian cancer.
Clin Cancer Res. 29:2466–2479. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ceccaldi R, Rondinelli B and D'Andrea AD:
Repair pathway choices and consequences at the double-strand break.
Trends Cell Biol. 26:52–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ito K, Murayama Y, Kurokawa Y, Kanamaru S,
Kokabu Y, Maki T, Mikawa T, Argunhan B, Tsubouchi H, Ikeguchi M, et
al: Real-time tracking reveals catalytic roles for the two DNA
binding sites of Rad51. Nat Commun. 11:29502020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wilson AJ, Stubbs M, Liu P, Ruggeri B and
Khabele D: The BET inhibitor INCB054329 reduces homologous
recombination efficiency and augments PARP inhibitor activity in
ovarian cancer. Gynecol Oncol. 149:575–584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Pellegrino B, Herencia-Ropero A,
Llop-Guevara A, Pedretti F, Moles-Fernández A, Viaplana C,
Villacampa G, Guzmán M, Rodríguez O, Grueso J, et al: Preclinical
in vivo validation of the RAD51 test for identification of
homologous recombination-deficient tumors and patient
stratification. Cancer Res. 82:1646–1657. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mukhopadhyay A, Elattar A, Cerbinskaite A,
Wilkinson SJ, Drew Y, Kyle S, Los G, Hostomsky Z, Edmondson RJ and
Curtin NJ: Development of a functional assay for homologous
recombination status in primary cultures of epithelial ovarian
tumor and correlation with sensitivity to poly(ADP-ribose)
polymerase inhibitors. Clin Cancer Res. 16:2344–2351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shah MM, Dobbin ZC, Nowsheen S, Wielgos M,
Katre AA, Alvarez RD, Konstantinopoulos PA, Yang ES and Landen CN:
An ex vivo assay of XRT-induced Rad51 foci formation predicts
response to PARP-inhibition in ovarian cancer. Gynecol Oncol.
134:331–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Meijer TG, Verkaik NS, Sieuwerts AM, van
Riet J, Naipal KAT, van Deurzen CHM, den Bakker MA, Sleddens HFBM,
Dubbink HJ, den Toom TD, et al: Functional ex vivo assay reveals
homologous recombination deficiency in breast cancer beyond BRCA
gene defects. Clin Cancer Res. 24:6277–6287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
van Wijk LM, Vermeulen S, Meijers M, van
Diest MF, Ter Haar NT, de Jonge MM, Solleveld-Westerink N, van
Wezel T, van Gent DC, Kroep JR, et al: The RECAP test rapidly and
reliably identifies homologous recombination-deficient ovarian
carcinomas. Cancers (Basel). 12:28052020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu JF, Palakurthi S, Zeng Q, Zhou S,
Ivanova E, Huang W, Zervantonakis IK, Selfors LM, Shen Y, Pritchard
CC, et al: Establishment of patient-derived tumor xenograft models
of epithelial ovarian cancer for preclinical evaluation of novel
therapeutics. Clin Cancer Res. 23:1263–1273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cybulska P, Stewart JM, Sayad A, Virtanen
C, Shaw PA, Clarke B, Stickle N, Bernardini MQ and Neel B: A
genomically characterized collection of high-grade serous ovarian
cancer xenografts for preclinical testing. Am J Pathol.
188:1120–1131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hidalgo M, Amant F, Biankin AV, Budinská
E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo
GM, et al: Patient-derived xenograft models: An emerging platform
for translational cancer research. Cancer Discov. 4:998–1013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
de Witte CJ, Valle-Inclan JE, Hami N,
Lõhmussaar K, Kopper O, Vreuls CPH, Jonges GN, van Diest P, Nguyen
L, Clevers H, et al: Patient-Derived ovarian cancer organoids mimic
clinical response and exhibit heterogeneous inter- and intrapatient
drug responses. Cell Rep. 31:1077622020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhou Z, Cong L and Cong X: Patient-Derived
organoids in precision medicine: Drug screening, organoid-on-a-chip
and living organoid biobank. Front Oncol. 11:7621842021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Narita Y, Kitazoe Y, Kurihara Y, Okuhara
Y, Takamatsu K, Saito N and Doi Y: Increase or decrease of
HDL-cholesterol concentrations during pravastatin treatment
depending on the pre-treatment HDL cholesterol levels. Eur J Clin
Pharmacol. 52:461–463. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Mosiewicz KA, Kolb L, van der Vlies AJ,
Martino MM, Lienemann PS, Hubbell JA, Ehrbar M and Lutolf MP: In
situ cell manipulation through enzymatic hydrogel photopatterning.
Nat Mater. 12:1072–1078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Verduin M, Hoeben A, De Ruysscher D and
Vooijs M: Patient-derived cancer organoids as predictors of
treatment response. Front Oncol. 11:6419802021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ahn SI, Sei YJ, Park HJ, Kim J, Ryu Y,
Choi JJ, Sung HJ, MacDonald TJ, Levey AI and Kim YT:
Microengineered human blood-brain barrier platform for
understanding nanoparticle transport mechanisms. Nat Commun.
11:1752020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Nagle PW, Plukker JTM, Muijs CT, van Luijk
P and Coppes RP: Patient-derived tumor organoids for prediction of
cancer treatment response. Semin Cancer Biol. 53:258–264. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Psilopatis I, Sykaras AG, Mandrakis G,
Vrettou K and Theocharis S: Patient-derived organoids: The
beginning of a new era in ovarian cancer disease modeling and drug
sensitivity testing. Biomedicines. 11:12022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kenny PA, Lee GY, Myers CA, Neve RM,
Semeiks JR, Spellman PT, Lorenz K, Lee EH, Barcellos-Hoff MH,
Petersen OW, et al: The morphologies of breast cancer cell lines in
three-dimensional assays correlate with their profiles of gene
expression. Mol Oncol. 1:84–96. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hughes CS, Postovit LM and Lajoie GA:
Matrigel: A complex protein mixture required for optimal growth of
cell culture. Proteomics. 10:1886–1890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chan WS, Mo X, Ip PPC and Tse KY:
Patient-derived organoid culture in epithelial ovarian
cancers-Techniques, applications, and future perspectives. Cancer
Med. 12:19714–19731. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Mandrycky C, Wang Z, Kim K and Kim DH: 3D
bioprinting for engineering complex tissues. Biotechnol Adv.
34:422–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Annett S, Moore G, Short A, Marshall A,
McCrudden C, Yakkundi A, Das S, McCluggage WG, Nelson L, Harley I,
et al: FKBPL-based peptide, ALM201, targets angiogenesis and cancer
stem cells in ovarian cancer. Br J Cancer. 122:361–371. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Baka Z, Godier C, Lamy L, Mallick A,
Gribova V, Figarol A, Bezdetnaya L, Chateau A, Magne Z, Stiefel M,
et al: A coculture based, 3D bioprinted ovarian tumor model
combining cancer cells and cancer associated fibroblasts. Macromol
Biosci. 23:e22004342023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xu F, Celli J, Rizvi I, Moon S, Hasan T
and Demirci U: A three-dimensional in vitro ovarian cancer
coculture model using a high-throughput cell patterning platform.
Biotechnol J. 6:204–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Driehuis E and Clevers H: CRISPR/Cas 9
genome editing and its applications in organoids. Am J Physiol
Gastrointest Liver Physiol. 312:G257–G265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Neal JT and Kuo CJ: Organoids as models
for neoplastic transformation. Ann Rev Pathol. 11:199–220. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lõhmussaar K, Kopper O, Korving J, Begthel
H, Vreuls CPH, van Es JH and Clevers H: Assessing the origin of
high-grade serous ovarian cancer using CRISPR-modification of mouse
organoids. Nat Commun. 11:26602020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dumont S, Jan Z, Heremans R, Van Gorp T,
Vergote I and Timmerman D: Organoids of epithelial ovarian cancer
as an emerging preclinical in vitro tool: A review. J Ovarian Res.
12:1052019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li Z, Gu H, Xu X, Tian Y, Huang X and Du
Y: Unveiling the novel immune and molecular signatures of ovarian
cancer: Insights and innovations from single-cell sequencing. Front
Immunol. 14:12880272023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wan C, Keany MP, Dong H, Al-Alem LF,
Pandya UM, Lazo S, Boehnke K, Lynch KN, Xu R, Zarrella DT, et al:
Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint
blockade in high-grade serous ovarian cancer. Cancer Res.
81:158–173. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gonzalez VD, Samusik N, Chen TJ, Savig ES,
Aghaeepour N, Quigley DA, Huang YW, Giangarrà V, Borowsky AD,
Hubbard NE, et al: Commonly occurring cell subsets in high-grade
serous ovarian tumors identified by single-cell mass cytometry.
Cell Rep. 22:1875–1888. 2018. View Article : Google Scholar : PubMed/NCBI
|