Introduction
Ovarian cancer (OC) is a malignant tumor that poses
a significant threat to women's health. Globally, OC is the third
most prevalent gynecological malignancy, yet it is associated with
the highest mortality rate among these types of cancer (1). In 2022, it was estimated that 324,398
new cases of OC would be diagnosed and 206,839 related deaths would
occur worldwide (2). OC is often
diagnosed in its advanced stages, with current screening techniques
unable to facilitate an earlier diagnosis (3); thus, the overall 5-year relative
survival rate for OC is 50% (4).
Delayed clinical presentation and a late diagnosis also contribute
to the high mortality rate associated with this disease, mainly due
to a significant lack of sensitive and specific biomarkers for
early-stage disease (5).
Currently, the standard chemotherapeutic regimen for
OC commonly employs a combination of taxane-based (such as
paclitaxel) and platinum-based drugs (6). Cisplatin was the first platinum
compound used in the primary treatment of OC; however, it is
associated with dose-limiting toxicity, including minor symptoms
such as nausea, severe kidney impairment and peripheral neuropathy.
To overcome the toxicity associated with cisplatin use, organic
platinum analogs of cisplatin, such as carboplatin, have been
developed and used instead of cisplatin in initial OC chemotherapy
(7). In patients with advanced
disease, carboplatin plus paclitaxel chemotherapy is considered an
optimal choice (8). Over time,
chemotherapy for OC has resulted in progressive enhancements in
survival, and the use of platinum/paclitaxel combinations,
particularly in combination with intraperitoneal delivery, has the
potential to double or increase survival time (9). In addition, heat-intraperitoneal
perfusion chemotherapy may improve the disease-free survival of
patients with OC, especially when the residual tumor is ≤1 cm or
invisible, but it may only improve overall survival (OS) in
patients with recurrent disease with a residual tumor of ≤1 cm
(10).
Several challenges remain in the treatment of OC,
despite advances in chemotherapy. For example, all forms of
maintenance therapy have thus far failed to prolong survival.
Intraperitoneal therapy has been shown to improve survival in
small-volume stage III disease but is associated with significant
toxicity (11). Furthermore, a
substantial proportion of patients diagnosed with advanced OC are
at an increased risk of recurrence (12) and drug resistance (13), which presents a significant
challenge for clinicians in terms of developing effective treatment
strategies. It is estimated that 60–80% of patients with epithelial
ovarian carcinoma (EOC) will achieve complete remission following a
combination of surgical and postoperative chemotherapy treatments;
however, ~80% of these patients face the risk of chemotherapeutic
resistance and potential recurrence (14).
With advancements in technology and research,
organoid models have been shown to demonstrate notable improvements
over traditional models in multiple aspects, primarily benefiting
from their ability to more accurately simulate the complexity and
dynamics of biological systems. Traditional two-dimensional (2D)
culture models often overlook the three-dimensional (3D) structure
of cells and the interactions between cells (15), and xenograft models have drawbacks
such as immune rejection and species differences (16). Although patient-derived xenograft
(PDX) models retain the tumor characteristics of patients (17,18),
they are costly and technically complex to establish and maintain
(19). By contrast, organoids,
which are 3D dynamic tumor models that can simulate tumor
environments in vitro, can mimic the disease development
process (20). Organoids can
overcome the limitations of traditional models, and thus provide
new perspectives for OC research and treatment. Thus, there is an
urgent need to develop new in vitro cultivation techniques
that can mimic the development of the disease and produce
organoids, and can be successfully grown from ovarian tumors,
ascites or pleural fluid (21). As
a novel approach to cell culture, organoid technology offers
significant potential for developing individualized treatments and
screening anticancer drugs. This is due to the high degree of tumor
replication, the preservation of tumor heterogeneity, the short
growth cycle and the stable transmission observed in organoids,
which collectively address the limitations of existing preclinical
models, including cell lines and animal models (22). The efficacy and resistance of
chemotherapeutic drugs can be more accurately predicted through
organoid culture, providing a basis for individualized treatment;
therefore, organoids may be considered a convenient and efficient
platform for studying drug resistance mechanisms (23).
The objective of the present review was to present a
thorough overview of the definition, formation and construction of
OC organoids. Additionally, the current review considers the
advancement of OC organoids in chemotherapy, and their
applications, deficiencies and prospects.
Overview of OC organoids
Organoids represent a 3D in vitro cell
culture system (23), which are
derived from cellular or organ progenitors and, through in vitro
differentiation, develop functional cell clusters that display the
essential properties of their organ of origin (24). Furthermore, they can preserve the
heterogeneity of the original tumor and mimic the tumor growth
microenvironment (23); they are
thus considered a powerful model for simulating human disease.
In a 2D cell model, cells are grown as a single cell
layer in a petri dish. Notably, 2D cell culture models cannot
accurately represent the heterogeneity of tumors since they lack
the inherent complexity and the TME of the original tumor (25). In addition, researchers have
transplanted patient-derived subcutaneous or in situ tumor cells
into immunodeficient mice to simulate the heterogeneity of the
original tumors; however, this PDX model is expensive,
time-consuming, and has low transplantation rates (26,27)
and ethical drawbacks (28),
Patient-derived organoids (PDOs) have emerged as a pivotal
preclinical model system in the field of cancer research and
clinical studies (29,30). Organoids retain a high degree of
genomic similarity to their corresponding tumors; moreover, they
can exhibit the inheritance of certain diseases and the ability to
epigenetically alter their phenotype, which may facilitate the
development of specific interventions to promote personalized
medicine (31,32). The primary advantages of in
vitro organoids over other in vitro tumor models are: i)
They retain a high degree of heterogeneity among tumor cells, ii)
they can be cultivated in small samples, and iii) they maintain the
ability for long-term expansion, cryopreservation and genetic
modification (24). The advantages
and disadvantages of in vitro models are shown in Table I.
 | Table I.Advantages and disadvantages of in
vitro models. |
Table I.
Advantages and disadvantages of in
vitro models.
| Model | Advantages | Disadvantages | (Refs.) |
|---|
| 2D cell model | Straightforward and
easy to use; low cost | Lack of tumor
complexity; poor expression of tumor heterogeneity; lack of immune
cells and tumor microenvironment | (19) |
| PDX model | Preservation of
tumor heterogeneity; mimics original tumor characteristics | Time-consuming; low
transplantation rate; ethical issues; cost issues | (20–22) |
| Organoid | Maintenance of high
tumor heterogeneity; high genomic homogeneity; possible to culture
small samples; retention of long-term amplification,
cryopreservation and genetic modification | Technical
limitations; individual differences; standardization issues; cost
issues | (23–25,27,28,88–97) |
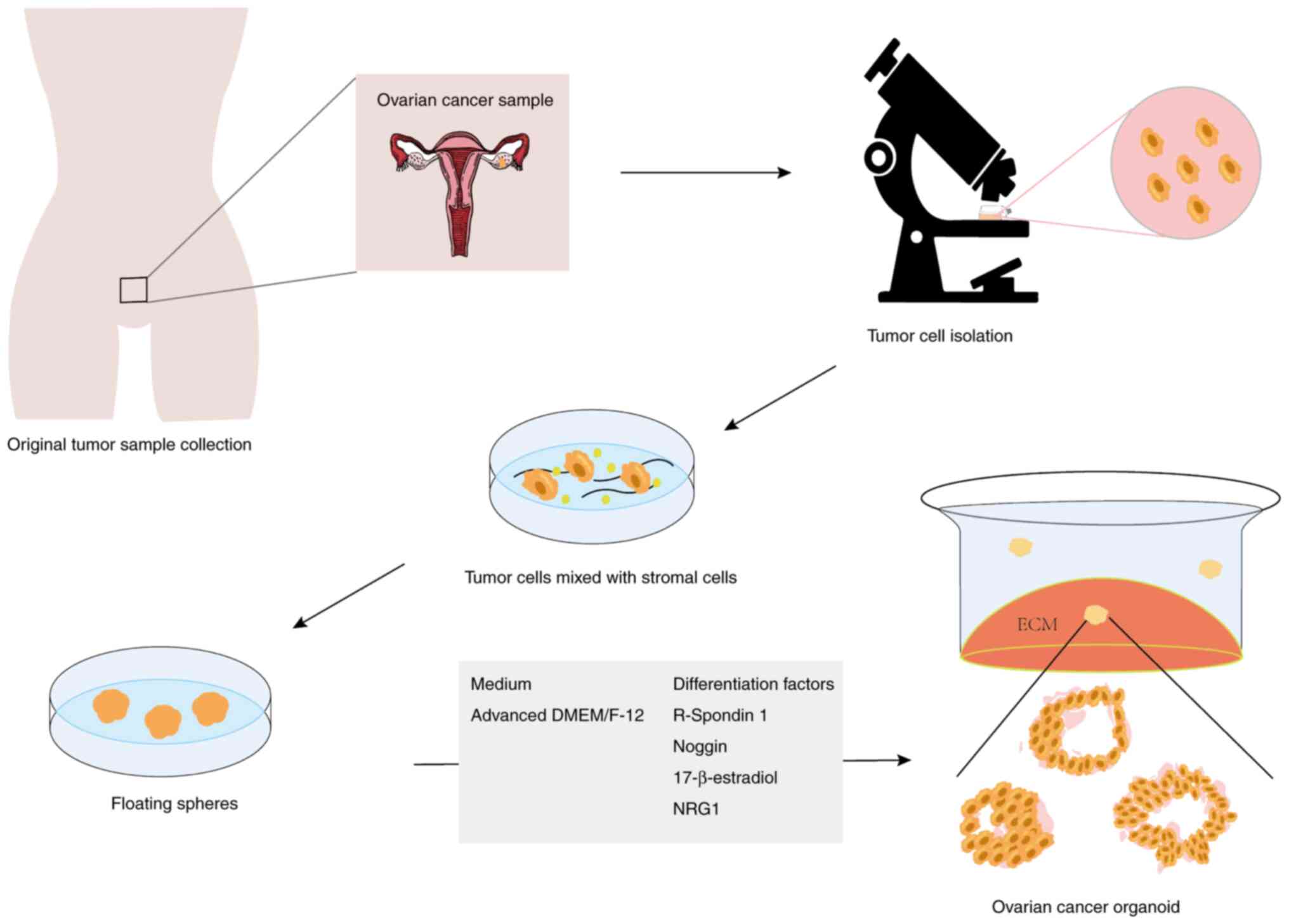
In order to generate organoids, tumor cells isolated
from primary tumor material, such as surgically excised tissues,
biopsies, ascites, pleural fluid or circulating tumor cells, need
to be capsulated in a 3D matrix and cultured in medium containing
variable growth factors and hormones (33) (Fig.
1). The composition and concentration of certain substances in
the medium vary depending on the specific experiment or study;
however, advanced Dulbecco's Modified Eagle Medium (DMEM)/Nutrient
Mixture F-12 (F-12) is widely used (34,35).
DMEM and F-12 are often used in combination to obtain higher
concentrations of components of DMEM and a wider variety of
components in the F-12 nutrient mix. DMEM/F-12, however, is free of
growth factors, hormones and lipids; therefore, to achieve optimal
cell proliferation when using DMEM/F-12, it is necessary to add an
appropriate combination of growth factors, hormones, proteins or
peptides, depending on the specific research needs and cell type.
The mixture of growth factors utilized for OC organoid propagation
is not standardized and may vary between studies. For OC organoids,
it has been suggested that R-spondin 1 (an activator of the Wnt
pathway), Noggin (an inhibitor of BMP-dependent differentiation)
and 17β-estradiol are necessary for growth, while neuromodulin-1
(NRG1) may be needed for organoid expansion (36).
The development of OC organoids from multiple stages
and subtypes (56 organoid lineages) has previously been
successfully achieved (37).
Organoid cultivation techniques can be applied to a wide range of
patient samples, including tissues, biopsies, ascites and pleural
effusions (38–40). Most OC organoids generated thus far
have been high-grade serous ovarian carcinoma (HGSOC), but there
are also other types of cancer, such as low-grade serous OC,
mucinous OC, ovarian clear cell carcinoma (OCCC) and ovarian
endometrioid carcinoma (ENOC) (41). Furthermore, the proliferation of
primary ovarian organoids from different histological subtypes,
including HGSOC, OCCC and ENOC, has been reported to have a success
rate of 80% and to retain the original mutation pattern (42). Moreover, OC organoids are suitable
for constructing biobanks to further improve organoid construction
efficiency. For example, a study by Hoffmann et al (43) successfully generated 15 organoids
(with an efficiency of ~30%) from 13–45 patients under conditions
of low Wnt expression for long-term passaging culture. The OC
organoids were cryopreserved for >5 months and passaged every
10–20 days. This previous study showed that there were no
significant changes in the morphology or proliferation capacity of
the organoids even after repeated thawing and freezing cycles.
OC organoid model applications
Application of OC organoid models for
chemotherapeutic drug screening
Drug screening, which refers to testing the effects
of a variety of drugs on several cell lines, is used to identify
and develop medicines (44). In
cancer therapy, drug screening aims to screen numerous compounds or
new compounds for specific physiological activity against tumor
cells. The present review aimed to summarize the differences in
drug screening methods and their application in tumor therapy. For
this purpose, models such as 2D cell cultures, multicellular tumor
spheroids (MTSs) and organ tissues were assessed (Table II).
 | Table II.Multi-model perspective on ovarian
cancer drug screening and sensitivity applications. |
Table II.
Multi-model perspective on ovarian
cancer drug screening and sensitivity applications.
| A, Drug
screening |
|---|
|
|---|
| Model | Key findings | (Refs.) |
|---|
| 2D cell
cultures | Prediction of drug
activity, metabolism and toxicity; only 10% of drugs tested in
vitro are clinically effective and only 5% of anticancer drugs;
only 12 of 900 drugs identified were approved by the Food and Drug
Administration in 2011 | (45,50,52) |
| MTSs | Potential of
screening antitumor drugs; MCTSs demonstrate increased resistance
to chemotherapeutic agents and the ability to screen for active
drug candidates | (61) |
| Organoid | Improvement of
antitumor activity effect; the potential for higher growth rates,
faster drug response and better drug penetration; efficient
screening in a passive microfluidic platform | (62) |
|
| B,
Carboplatin |
|
| Model | Key
findings | (Refs.) |
|
| 2D monolayer, 3D
spheres, 3D tumor ex vivo, mouse xenografts | 3D sphere and mouse
xenograft models best correlate with in vivo response;
Variability in therapeutic response across models | (40) |
| 2D monolayer, 3D
spheres, mouse xenografts | 3D sphere systems
are in best agreement with in vivo models; 2D
super-resistant cell lines are more sensitive in 3D and mouse
models vice versa | (65) |
| Four cell lines,
two PDOs, one PDX | PDX and PDO models
reproduce patient tumor heterogeneity; Resistance to standard
therapeutic drugs; Consistent with the clinic, PDO provides support
for therapeutic decisions | (66) |
|
| C,
Paclitaxel |
|
| Model | Key
findings | (Refs.) |
|
| 2D monolayer versus
3D hydrogel | 3D sphere survival
(40–60%) is better than 2D single layer survival (20%) | (67) |
| 3D spheres
(methylcellulose) | Spheres maintain
cell density, and exhibit higher apoptotic behavior and higher
paclitaxel resistance | (68) |
| PDO | PDO cell viability:
48.7% | (69) |
|
| D, Bevacizumab
(Avastin®) |
|
| Model | Key
findings | (Refs.) |
|
| Mouse xenograft
model versus PDO | Mouse xenograft
model may prolong survival and reduce ascites formation; models
in vivo better reflect actual treatment effects, and PDO may
be used for initial drug sensitivity screening; low effect on cell
viability when used as a single agent; possibility of improvement
of therapeutic effect when used in combination with
chemotherapy | (69,73) |
|
| E,
Olaparib |
|
| Model | Key
findings | (Refs.) |
|
| Monolayer AsPCs
versus 3D AsPCs | Monolayer AsPCs
responded significantly more than 3D AsPCs to PARPis treatment | (41) |
| PDO | Only 2 of 33
organoid cultures tested (6%) were sensitive to olaparib | (41) |
Notably, 2D cell cultures are the mainstay of
fundamental cellular research, and are used to predict drug
activity, metabolism and toxicity in vitro (45). In cancer screening, cytotoxicity
testing is often performed on cultured tumor cell lines in
monolayer culture due to the rapid and uncontrolled growth of these
cell lines (46). By contrast, the
main disadvantage of 2D systems is their inability to reproduce
complex 3D structures or to communicate with the TME (47,48).
Thus, 2D culture models may differ greatly from growing tumors with
regard to cell morphology, proliferation, and gene and protein
expression (49). Therefore, only
10% of the drugs that pass in vitro testing positively
impact the clinic or lead to approval. The proportion of anticancer
drugs that demonstrate clinical efficacy is even lower, at ~5%
(50). Furthermore, a number of
drugs reported to have potent anticancer effects in 2D cell culture
models have failed clinical trials (51). In 2011, ~900 anticancer drugs passed
cell.based tests, but only 12 were approved by the Food and Drug
Administration after clinical testing (52).
The cells in MTS models more closely mimic the cell
shape and environment in vivo than monolayer culture models
(53,54); however, the size of spheroids has a
substantial influence on their survival and reaction to drugs
(55). Spheroids <100 µm do not
reveal the complexity of the tumor or tissue (56). On the other hand, spheroids with
diameters in the range of 400–500 µm can reflect the behavior of
tumors in vivo due to the presence of different cellular
layers: A necrotic core surrounded by a region of quiescent cells
and an outer region composed of proliferating cells. The peripheral
cells may reflect the in vivo tumor situation next to the
capillaries, while the distant internal cells remain quiescent or
die due to apoptosis or necrosis (57). This may also be why a number of
reports (58–60) have shown that antitumor drugs are
less effective against 3D cultured cancer cells than against 2D
cultures.
It has also been shown that the MTS model has the
potential to screen for antitumor drugs. Hirst et al
(61) previously grew a series of
EOC cell lines in 3D cell cultures to form multicellular tumor
spheres (MCTSs). These MCTSs were characterized on the basis of the
molecular and cellular properties of EOC, and were screened against
cells grown in 2D cell culture to identify previously
underappreciated anticancer agents. The MCTSs demonstrated enhanced
resistance to chemotherapy, showed symptoms of senescence and
hypoxia, and expressed numerous stem-cell-related transcripts,
including ALDH1A and CD133 (PROM1). This previous study identified
licofidone as a candidate with more activity in the MCTSs than in
the 2D-cultured cells using a clinically repurposed drug library.
Licofidone has been shown to be synergized with paclitaxel in an
ovarian MCTS model, as well as in a patient-derived tumor xenograft
model. The combination of licofidone and paclitaxel significantly
increased the median survival time (>141 days) in mice compared
with paclitaxel (115 days), licofidone (37 days) or vector (30
days). In addition, the Mantel-Haenszel hazard ratio (HR) was
confirmed to be superior to the vector (HR=0.037) and paclitaxel
(HR=0.017). The results of this previous study identified the
potential use of an underappreciated anti-inflammatory agent in OC
treatment.
Although the MTS model has demonstrated its unique
advantages in antitumor drug screening, most identified drugs have
failed in clinical applications due to the differences between MTSs
and the real in vivo environment. Therefore, researchers
have begun to explore OC organoid models. Cavarzerani et al
(62) derived organoids from the
ascites or tissues of patients with OC. In this previous study, the
development of an organoid culture model with a higher growth rate,
a faster drug response and better drug penetration into the
extracellular matrix (ECM) was revealed to be possible in a passive
microfluidic platform that maintained the vital signs of the sample
and collected data for up to 16 medicines on a single plate.
Achieving successful cultivation of organoids originating from
patients with HGSOC was considered feasible in Mimetas Dual Lane
OrganoPlates®. Thus, OC organoids may potentially be
used to assess the antitumor activity of drugs.
OC organoids in chemosensitivity
testing
Organoids can also be applied to chemosensitivity
testing. In vitro susceptibility and resistance tests are
used to determine whether a sample of a patient's tumor tissue
exhibits a response (i.e., a reduction in tumor survival) when
exposed to a selected chemotherapeutic agent under laboratory
conditions (63). Drug response
measurements aim to assess the therapeutic effectiveness of a drug
across a specified concentration range. Wherever possible,
multi-drug response metrics should be used to account for possible
experimental variations during the measurement, initial population
and cell division count, including: Emax (maximal effect of the
drug), EC50 (drug concentration that reaches Emax),
IC50 (median inhibitory concentration), GI50
(concentration that decreases the overall cell growth by 50%),
GR50 (50% inhibition of cell growth rate) and AUC (area
under the dose-response curve, which represents the cumulative
effect of the medicinal product) (64). Therefore, a multi-model perspective
offers a comparative analysis of sensitivity testing in OC
(Table II).
Carboplatin
Carboplatin is the most common first-line
chemotherapy agent in the treatment of gynecological malignancies.
The sensitivity of ovarian epithelial adenocarcinoma cell lines to
carboplatin chemotherapy in vitro varies in 2D or 3D models
(65). Compared with other ex
vivo and in vivo models, the response of the 3D system
to carboplatin has been revealed to be in best agreement with the
in vivo model. To assess the current differences in
treatment response in in vitro and in vivo models,
and to deal with this problem, a comparative study was conducted by
Maru et al (40) to
distinguish the response of four different models to carboplatin
chemotherapy, including 2D single layer, 3D sphere, 3D tumor ex
vivo and mouse xenograft models. Six previously characterized
EOC cell lines with different chemosensitivities were used and
viability tests were carried out on each model. The in vivo
results in the mouse model correlated with the 2D response in 3/6
cell lines, while they correlated with the 3D sphere response in
4/6 cell lines and the 3D ex vivo model in 5/5 cell lines.
These results highlight the variability of treatment responses
across models, and indicated that the response of carboplatin was
best correlated with the response of EOC cell lines grown in a 3D
ex vivo model.
Similarly, Brodeur et al (65) investigated the response of
single-layer and spherical (3D) EOC cells to carboplatin, and then
compared it with the in vivo response (xenografts). Mice
were injected with six ovarian epithelial adenocarcinoma cell lines
that received three different concentrations of carboplatin. Their
responses were evaluated and classified based on tumor volume and
immunofluorescence measurements. The same ovarian epithelial
adenocarcinoma cell lines were seeded onto shallow adhesion plates
to form spheroids and were processed. Flow cytometric analysis was
performed to classify ovarian epithelial adenocarcinoma cell lines
based on IC50; this was compared to previously published
2D IC50 results. The results showed that the 3D system
was in the best agreement with the in vivo model; in
particular, the 2D super-resistant ovarian epithelial
adenocarcinoma cell lines became more sensitive in either the mouse
model or the 3D system. By contrast, the ultrasensitive ovarian
epithelial adenocarcinoma cell line in the 2D system was more
resistant in xenografts and spherical tissues.
Additionally, Thorel et al (66) established seven models (four cell
lines, two PDOs and one PDX), all derived from the same OCCC. In
order to establish their relevance, a comprehensive
characterization was conducted using morphological, histological
and transcriptomic analyses, as well as an evaluation of the
patient's response to treatment, which was compared with the
patient's chemotherapeutic response and showed resistance to drugs,
carboplatin, gemcitabine and doxorubicin. Only PDX and PDO models
derived from tumors could replicate the heterogeneity of patients.
Patients were refractory to carboplatin, doxorubicin and
gemcitabine, whereas cancer cells were susceptible to these agents.
The PDX and PDO models, on the other hand, were resistant to these
three drugs. Transcriptome analysis was consistent with these
results, as models that faithfully reproduced the clinical response
differed from the classical 2D cell culture models. Subsequently,
the researchers examined the potential of previously unused drugs
and determined that the histone deacetylase inhibitor belinostat
was a potential therapeutic agent based on the response of the PDO.
These findings indicated that PDX and PDO models may be the most
relevant; however, only the PDO model offered all of the necessary
preconditions for prediction and could be used in conventionally
refractory types of cancer, especially aggressive ones.
Paclitaxel
Paclitaxel is a commonly used medicine for the
treatment of OC. In a previous study, 3D patient-derived tumor
spheroid structures were tested for paclitaxel susceptibility in
comparison to 2D monolayer cultures. Loessner et al
(67) performed an extensive study
of EOC using hydrogel as a 3D model. The two cell lines (OV-MZ-6
and SKOV-3) were revealed to exhibit similar proliferation in 2D
cultures, but different in 3D cultures. Spherical cell formation
could only be observed in 3D cultures when the cells were embedded
in hydrogels. The proliferation of OV-MZ-6 and SK-OV-3 cells in
polyethylene glycol hydrogel possessing arginyl-glycyl-aspartic
acid functionalized sites with glutamine, a general nutritional
addition to the cell culture process, and matrix metalloproteinase
sensitive sites for 14 days, followed by paclitaxel treatment,
demonstrated a higher survival rate (40–60%) 7 days post-treatment
compared with the single cells (20%) in 2D culture. Thus, 2D
chemosensitivity evaluation does not necessarily reflect patient
pathophysiology.
In order to determine the optimal conditions for
analyzing the drug response of SKOV-3 and OVCAR-3 spheroids with
the expected properties (roundness approaching 1.0; diameter in the
range of 200–500 µm) to paclitaxel, two methods were used to
evaluate the spheroids in two 3D cultures, after the addition of
methylcellulose (0.25 and 0.5%, w/v) to the culture medium.
Compared with 2D culture, SKOV-3 and OVCAR-3 spheroids retained
cell density, increased apoptosis and increased resistance to
paclitaxel treatment. The results indicated that 3D cultures may
provide more reliable drug response results than 2D monolayer
cultures (68). Meanwhile, Bi et
al (69) assessed paired tumors
and adjacent normal tissues from the same patient. At tumor-killing
doses, they reported minimal cell destruction with variable
chemotherapeutic agents. The results indicated that, in comparison
with other drug treatments, the OC organoids of the patient were
more sensitive to paclitaxel, and the survival rate was 48.7% after
exposure to this single agent. The patient OC organoids were also
more sensitive to gemcitabine (65% decrease in viability) and
topotecan (56% decrease in viability) than the previously received
chemotherapy regimen carboplatin + paclitaxel. Thus, organoids were
considered more sensitive to paclitaxel compared to 2D
monolayers.
Other drugs
Bevacizumab (Avastin®) is a recombinant
humanized monoclonal antibody against vascular endothelial growth
factor (VEGF) (70), which binds to
and neutralizes the biological properties of human VEGF by blocking
VEGF interaction with its receptor (71). Bevacizumab is frequently used as an
adjuvant treatment in gynecological tumors (72). In mice bearing HOC22 ×enografts, OS
and complete remission were markedly prolonged when bevacizumab was
used in combination with chemotherapy and continued at the end of
chemotherapy. Bevacizumab, on its own, inhibited the formation of
ascites and had a limited impact on tumor load (73). Bi et al (69) also tested the sensitivity of PDOs to
either bevacizumab alone or in combination with first-line standard
chemotherapy. Overall, bevacizumab as a monotherapy had a minimal
effect on cell viability and, when combined with chemotherapy, had
little additive effect on cell killing
Olaparib was the first approved poly (ADP-ribose)
polymerase inhibitor (PARPi) used to treat patients with advanced
BRCA-mutated OC (70,72,74).
PARPis inhibit PARP in cells with BRCA-mutated tumors; they can
cause ‘synthetic lethality’ by targeting two DNA repair pathways at
the same time and do not affect normal cells (73), Hill et al (41) formulated a 3D spherical functional
evaluation methodology aimed at quantifying the responsiveness of
two PARPi agents, specifically niraparib and olaparib, within
ascites-derived primary cell cultures (AsPCs) derived from patients
diagnosed with HGSOC. An agarose-based protocol for AsPC
preparation enabled efficient isolation and propagation of
monolayer and 3D AsPCs. The results showed a distinct sensitivity
difference to PARPis, with monolayer AsPCs exhibiting 88 and 52%
sensitivity to niraparib and olaparib, respectively, compared with
66 and 38% sensitivity in 3D AsPCs. The latter aligns with prior
homologous recombination deficiency (HRD) estimates in EOC
(40–60%), emphasizing the relevance of 3D models.
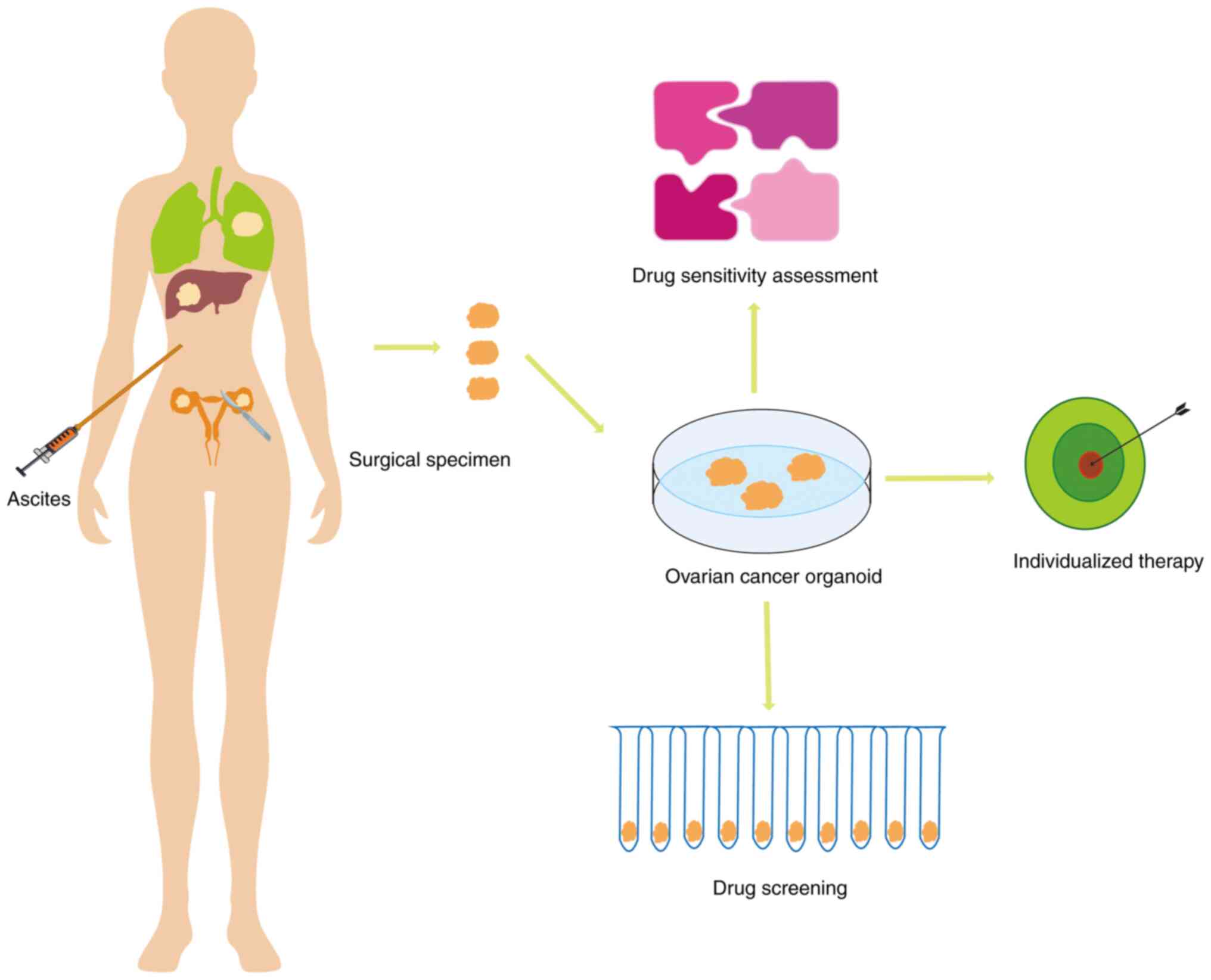
Application of OC organoid models in
individualized therapy
The application of organoids in personalized
medicine has also begun to be implemented (74,75).
The development of personalized medicine is about preventing,
diagnosing and treating pathological conditions based on individual
characteristics (38). The
characteristics of the individual include the entire body, tissues
and cells, including genetic, epigenetic, transcriptomic, proteomic
and metabolomic markers (76). In
addition, gene expression profiles can be studied and changes
associated with tumors can be identified (77). Personalized medicine obtains
patient-specific disease information to select drugs and the
correct dosage, and to optimize therapeutic procedures (78). Personalized medicine also influences
preclinical studies of drugs, not just regarding efficacy and
safety, but also to reduce drug development costs (38). OC encompasses a diverse array of
histotypes, each characterized by distinct origins, underlying
genetic and epigenetic alterations, and varying survival outcomes.
Moreover, the differing prevalence of these histotypes across
various ethnicities adds another layer of intricacy (79). In light of these multifaceted
variables, the use of traditional 2D cell lines, albeit
immortalized and homogeneous, as in vitro models of OC, are
inherently limited in their capacity to capture the complexity of
the disease at any single time point or in a microenvironment that
accurately mirrors the in vivo scenario. Consequently,
organoid technology has emerged as a promising avenue for advancing
cancer research and facilitating the development of tailored
therapeutic strategies (80). In
clinical practice, OC organoid models have demonstrated their
unique value in personalized therapy. For example, Lui et al
(81) provided prospective and
retrospective evidence from ≥18 cases, which indicated that
organoid drug screening can accurately predict clinical response to
chemotherapy and targeted therapy. This previous study also
reported on a patient with platinum-resistant plasma OC who
responded to ibrutinib therapy after screening, with this drug
identified as having an excellent response in their organoid. After
3 months of treatment, the CA125 levels of the patient had
decreased from 250 to 125 U/ml.
Preclinical studies
It is becoming increasingly apparent that standard
treatment does not apply to every patient and that it would be
helpful to use pretreatments to provide a more individualized
regimen. Drug testing of PDOs can be executed in a timely manner;
in addition, PDOs have the potential to identify the most
efficacious drug in each case, and to offer a promising preclinical
platform for tailoring personalized cancer treatment strategies in
patients with gynecological malignancies (69).
For example, in 2019, Phan et al (82) pioneered the use of PDOs from HGSOC
and ovarian sarcoma, using an automated screening system to assess
the unique responses of these organoids to a panel of 240 kinase
inhibitors. The high-throughput methodology not only identified
sensitive signaling pathways but also facilitated the selection of
the most efficacious drugs within specific molecular classes. This
previous study presented a scalable and functional precision
medicine platform titled Drug Efficacy Testing in 3D Culture, which
can quantify the responsiveness of patient-derived cells to various
drugs and drug combinations through live-cell imaging. Employing
this platform, 27 tailored drug combinations were evaluated,
achieving results within 10 days post-surgery. Notably, the
findings revealed that the combination of carboplatin and a Bcl-xL
inhibitor exhibited synergistic effects in 4 out of 8 OC samples
tested, whereas afatinib and the Bcl-xL inhibitor A-1331852
demonstrated synergism in 5 of 7 OC models. These results suggested
that combining the Bcl-xL inhibitor A-1331852 with either afatinib
or carboplatin may hold promise as a potential future therapeutic
strategy for patients with OC (83).
Biomarkers
Predictive biomarkers of therapeutic response are
vital for assessing clinical benefit in patients with OC, yet they
remain an unmet need (84).
Notably, the potential of RAD51 foci as predictive biomarkers in
HGSOC warrants validation in clinical trials (85). RAD51 has emerged as a prime
candidate biomarker for such assessments due to several reasons.
Firstly, upon the occurrence of DNA double-strand breaks, the
kinase ATM swiftly phosphorylates histone H2AX (ᵧH2AX), initiating
a cascade that leads to the formation of single-stranded DNA with
3′ projections. These, in turn, engage replication protein A, which
RAD51 subsequently displaces via BRCA1 and BRCA2, facilitating
homologous recombination (HRec). Given the multitude of upstream
events required for RAD51 binding and its central role in HRec,
RAD51 provides a holistic readout of the intricate steps of HRec.
Secondly, RAD51 binding results in the formation of nucleoprotein
filaments that can be visualized as foci under a microscope, with
RAD51 focus deficiency serving as a functional marker for HRD
(86–88). Thirdly, RAD51 foci detection has
been shown to possess predictive value for platinum chemotherapy
and PARPi responses in OC xenografts (89–91),
correlating with in vitro platinum responses in established
and primary OC cell lines (Pearson r=0.96; P=0.01). Tumor tissue
cells that did not respond to platinum have been reported to have
significantly higher RAD51 scores than those that responded to
platinum (P<0.001). Notably, tumors with low RAD51 scores
exhibited heightened platinum sensitivity, with a significantly
higher likelihood of achieving pathologic complete response
[relative risk (RR) 5.28; P<0.001] and platinum sensitivity (RR,
∞; P=0.05). Furthermore, the RAD51 score could accurately predict
chemotherapy response (AUC, 0.90; 95% CI, 0.78.1.0; P<0.001),
with an automated quantification system mirroring manual test
results at 92% accuracy. In the validation cohort, RAD51-low tumors
displayed enhanced platinum sensitivity (RR, ∞; P<0.001), a 100%
positive predictive value for platinum sensitivity, and improved
progression-free survival (HR, 0.53; 95% CI, 0.33.0.85; P<0.001)
and OS (HR, 0.43; 95% CI, 0.25.0.75; P=0.003) compared with
RAD51-high tumors (85).
Advancements have focused on assessing HRec proficiency through
RAD51 foci formation at DNA damage sites, exemplified by the repair
capability (RECAP) test (92).
Initially devised for breast cancer HRD assessment, this test has
been validated using tumor sections and has demonstrated
applicability in OC (93),
underlining the versatility of tumor sections and
diagnostic/therapeutic biomarker potential. Additionally,
functional HRec status in PDOs has been evaluated using the RECAP
test, correlating with PDO drug sensitivity (including platinum
chemotherapy and PARPis) (37,42)
and clinical response (41).
However, as evidenced by in vivo studies, HRD alone does not
guarantee a response to platinum or PARPis (41), pointing to a more intricate
landscape requiring further exploration.
Genomics
Precision healthcare represents a strategy that
tailors therapeutic interventions by comprehensively assessing the
interplay of an individual's genetic makeup, environmental factors
and lifestyle patterns. Since genomics alone is insufficient to
determine treatment options for most patients with advanced
diseases, the creation of live biological specimens of tumor-like
organs can facilitate the integration of genomic profiles and drug
screening within an iterative framework, enabling the
identification of personalized therapeutic strategies tailored to
each patient (38). In comparison
to traditional 2D cultures, 3D organoid cultures exhibit a broader
spectrum of drug responses that more accurately mirror genomic
variations (39), rendering them an
ideal platform for drug sensitivity assessments in translational
medicine and precision healthcare endeavors. PDX models of OC also
reflect the heterogeneity of tumor genomic profiles and serve as a
valuable tool for drug evaluation (94,95).
Nevertheless, organoids emerge as a superior 3D culture system,
surpassing PDX in terms of i) reduced tumor sample requirements,
ii) expedited engraftment timelines and iii) enhanced engraftment
success rates (96). Notably, de
Witte et al (97) conducted
in vitro drug screening utilizing 36 genome-wide profiled
PDOs from 23 patients with OC, demonstrating the ability of OC PDOs
to faithfully recapitulate individual patient responses to
first-line chemotherapeutics. Notably, variations in sensitivity
were observed, with low responsiveness to carboplatin/paclitaxel,
PARPis and specific tyrosine kinase inhibitors (afatinib,
adavotinib), contrasted by high responsiveness to gemcitabine,
flavopiridol (a cell cycle-dependent kinase inhibitor) and
vimofenib (a BRAF V600E kinase inhibitor). Therefore, OC organoid
models may be mainly applied for drug screening, sensitivity and
individualized therapy (Fig.
2).
Deficiencies and prospects of OC organoid
models in chemotherapy
Deficiencies
Technical limitations
PDO cultures may not be representative of tumor
settings because of the absence of stroma, vasculature and immune
cells (35); therefore, the
organoid model cannot mimic the response to immunotherapy and
antiangiogenic therapy (98).
Heterogeneity within PDO culture tumors is also incomplete
(97). The ability of organoids to
maintain biological properties and functions similar to those of
the primary tumors in an in vitro environment holds
paramount significance for developing clinical applications.
In vivo, tissue development is constrained by
external stimulation delivered in an exact space and time sequence.
Usually, this does not occur in conventional 3D culture systems,
where cells are embedded in an isotropic matrix and uniformly
flooded with biochemical and ecological niche signals. This
limitation can be overcome by a 3D culture matrix that can release
or present biomolecules under spatiotemporal control (99,100).
Achieving accurate control of the growth and differentiation of
organoids is a critical task in organic research, which carries
profound significance across diverse areas, such as disease
modeling, pharmaceutical screening and regenerative medical
research.
Individual differences
Because of the significant individual differences in
patients with OC and the wide variety of tumor mutant types (e.g.
Trp53, Brca1, KRAS, BRAF, PTEN, PIK3CA), it is not possible to
fully simulate each patient's individual conditions. This may
result in some bias in the screening and evaluation of
chemotherapeutic drugs, and also increases the difficulty and cost
of organoid culture.
Tumor characteristics and physiological barriers,
such as the blood-brain barrier (BBB), vary from patient to
patient, affecting drug penetration and metabolism in cancer
tissues and thus altering therapeutic efficacy. Therefore, the use
of human equivalent dose (the in vivo concentration of a
drug in tumor tissue) is recommended in cancer organ tissue
therapeutic trials to more accurately assess drug efficacy. The
development of microfluidics and an artificial BBB based on
microchip organ technology may advance this field (101,102).
Cost
Compared with monolayer cell cultures, organoid
cultures are more costly because of the need for specific media,
including ECM (e.g., Matrigel) and cofactors (e.g., growth factors
and hormones) (103,104). In addition, the existence of
individual differences and the difficulty of standardization
further increases the cost of experiments, as different
laboratories and research groups may need to use different methods
and materials to conduct experiments, leading to higher costs of
duplicating experiments and validation.
The key to improving the efficacy and comparability
of organoid techniques is standardization; however, there are
currently notable differences in organoid culture protocols from
different investigators (34,103),
which may lead to inconsistent results. To achieve standardization,
key factors such as tissue manipulation procedures, culture media
and growth factors need to be harmonized (103). However, standardization is
challenged by individual differences, unknown composition of
animal-derived scaffolds and poor tunability (105). In addition, the animal-derived
Engelbreth-Holm-Swarm substrates used in organoid cultures vary
widely between batches and contain unknown contaminants (106), which also limits standardization.
Next-generation organoid models, such as co-culture models that
incorporate different cells, may partially address these issues but
have variable success rates and have also not been standardized
(107).
Prospects
3D bioprinting
3D bioprinting is a promising method for the
construction of heterogeneous and reproducible cancer models with
controlled shape and structure. The 3D-bioprinted structure can be
used to simulate the TME. Different cell layers can be produced
using bioprinting, including normal tissue-specific cells,
connective tissue and cancer cells (108). This technology can accurately
replicate the complex structure and microenvironment of cancer
cells, creating organoid models that are more similar to the
real-world situation of the tumor (109). Baka et al (110) described a 3D-bioprinted ovarian
carcinoma model that combined cancer cells (SKOV-3) and
cancer-associated fibroblasts (CAFs). The resulting tumor models
demonstrated their ability to maintain cell viability and
proliferation, and cells were observed to self-assemble in
heterogeneous aggregates. Furthermore, it was observed that CAFs
were recruited and multiplied in the surrounding tumor cells in an
in vivo process occurring in the TME. Notably, this approach
has also demonstrated an ability to produce a large number of
reproducible tumor models, which can undergo conventional
experimental methods (cell viability and metabolism assays,
histology and immunology, and real-time imaging). 3D bioprinting of
tumor constructs as personalized in vitro models may be a
useful tool for screening for anticancer drugs and establishing
accurate treatment regimens. As an example, MRC-5 fibroblasts and
human OC cells (OVCAR-5) were previously printed on Matrigel by
means of droplet-based bioprinting to form high-throughput,
reproducible multi-cellular structures in a controlled space. This
approach was shown to provide a platform for minimizing the size of
3D culture models at the macro scale; this could be considered an
innovative platform for high-throughput and robust personalized
drug screening, including tumor and stromal cell microenvironments
(111).
Gene editing technologies
Gene editing techniques, such as CRISPR-Cas9, have
the potential to introduce or modify mutations that mimic
tumor-forming processes, and potentially identify and modify driver
genes in cancer organoids (112).
Through CRISPR-Cas9, organoids can be artificially altered by
mutation of function or loss of function to induce malignancy
(113). Notably, these gene
editing techniques may be used to modify specific genes in organoid
models to investigate the role of these genes in the occurrence,
progression and treatment of OC. This could help to improve the
understanding of the mechanisms of OC and to develop new
therapeutic agents. Lõhmussaar et al (114) previously introduced mutations into
common HGSOC genes, including Trp53, Brca1, Nf1 and Pten, using the
genome editing method of CRISPR-Cas9. Organoids have also been
artificially modified by gain-of-function or loss-of-function
mutations to induce malignancy via CRISPR-Cas9 (115). Furthermore, Kopper et al
(37) demonstrated the feasibility
of this approach in OC using normal Fallopian tube organoids from
high-risk OC donors that could be efficiently genome-edited via
CRISPR-Cas9 and clonally amplified. In addition, in this previous
study, CRISPR-Cas9 genome editing was used to introduce common
HGSOC gene defects into mouse oviducts and the ovarian surface
epithelium cell-like organs, showing that both organ types are
potentially carcinogenic.
Genomics
Single-cell sequencing has emerged as an advanced
biotechnological technique capable of decoding the OC landscape at
single-cell resolution. It works at the gene, transcriptome,
protein, epigenome and metabolism levels, and provides detailed
information that differs from bulk sequencing methods, which only
provide average data, representing the overall or average
characteristics of the cell population in the entire sample or
lesion area on specific lesions. Single-cell sequencing techniques
provide detailed insights into the immune and molecular mechanisms
that underlie tumorigenesis, progression, resistance and immune
escape. These insights can be used to develop innovative diagnostic
markers, treatment strategies and prognostic indicators (116). Wan et al (117) reported that HGSOC-like organoid
co-cultures were treated with bispecific anti-PD-1 or PD-L1
antibodies, and then underwent single-cell RNA-sequencing. The
results showed that this treatment reprogrammed inert natural
killer cells and T cells into highly active cytotoxic states, thus
indicating the potential benefit of bispecific antibody treatment
in HGSOC. Gonzalez et al (118) performed an in-depth single-cell
phenotypic characterization of HGSOC by multiparametric mass
cytometry. The features of HGSOC biology were examined using a
cytometry antibody panel and rare tumor subtypes of HGSOC were
identified. It was shown that one of these rare subtypes was
enriched in epithelial-mesenchymal transition signaling and was
associated with increased tumor metastasis.
Conclusion
In summary, OC organoids maintain high tumor cell
heterogeneity and are cultured in small samples, which can be
maintained for long-term expansion and can be cryopreserved. OC
organoids have been successfully developed from multiple stages and
subtypes (56 organoid lines) (37).
Thus far, OC organoid models have been widely used in drug
screening, biomarker identification and personalized therapy;
however, there are challenges to the use of these organoid models,
including technical limitations, individual differences, cost
issues and issues of standardization. To overcome these
shortcomings, a further exploration into the application of
technologies, such as 3D bioprinting, gene editing and genomics, is
recommended in the optimization of organoid models to improve the
future stability and reproducibility of these models. The
development of these technologies is expected to make organoid
models more stable and reproducible, which will better simulate the
in vivo environment, and improve the accuracy and efficiency
of drug screening. Therefore, the OC organoid model has a broad
potential for application in chemotherapy. Along with the
development of the technique, it is hoped that this model will be a
more precise and efficient method to predict the prognosis of OC,
and to improve therapeutic effects and the quality of life in
patients with OC. In addition, research in this area may provide
valuable information for other cancer treatments.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation
General Project of Hubei Provincial (grant no. 2024AFB1044), the
Development Fund Project of Yangtze University (grant no.
WJ2019-22) and the Start Fund of First Affiliated Hospital of
Yangtze University (grant no. 2022DIF01).
Availability of data and materials
Not applicable.
Authors' contributions
WZ designed and conceived the study, wrote the
manuscript and acquired funding. YD designed and conceived the
study and wrote the manuscript. HH and KC conducted the literature
analysis. QZ and XC were involved in visualization. HZ and YX
revised the manuscript. Data authentication is not applicable. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OC
|
ovarian cancer
|
|
2D
|
two-dimensional
|
|
3D
|
three-dimensional
|
|
PDX
|
patient-derived xenograft
|
|
OS
|
overall survival
|
|
HGSOC
|
high-grade serous ovarian
carcinoma
|
|
TME
|
tumor microenvironment
|
|
ECM
|
extracellular matrix
|
|
PDOs
|
patient-derived organoids
|
|
OCCC
|
ovarian clear cell carcinoma
|
|
ENOC
|
ovarian endometrioid carcinoma
|
|
MTSs
|
multicellular tumor spheroids
|
|
MCTSs
|
multicellular tumor spheres
|
|
EOC
|
epithelial ovarian carcinoma
|
|
IC50
|
median inhibitory concentration
|
|
VEGF
|
vascular endothelial growth
factor
|
|
PARPi
|
poly(ADP-ribose) polymerase
inhibitor
|
|
AsPCs
|
ascites-derived primary cell
cultures
|
|
HRec
|
homologous recombination
|
|
BBB
|
blood-brain barrier
|
|
CAFs
|
cancer-associated fibroblasts
|
|
HR
|
hazard ratio
|
|
HRD
|
homologous recombination
deficiency
|
References
|
1
|
Kuroki L and Guntupalli SR: Treatment of
epithelial ovarian cancer. BMJ. 371:m37732020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American Cancer Society: Cancer Facts and
Figures 2023. American Cancer Society; Atlanta, GA: 2023
|
|
5
|
Liberto JM, Chen SY, Shih IM, Wang TH,
Wang TL and Pisanic TR II: Current and emerging methods for ovarian
cancer screening and diagnostics: A comprehensive review. Cancers
(Basel). 14:28852022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kikuchi Y, Kita T, Takano M, Kudoh K and
Yamamoto K: Treatment options in the management of ovarian cancer.
Expert Opin Pharmacother. 6:743–754. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bookman MA: First-line chemotherapy in
epithelial ovarian cancer. Clin Obstet Gynecol. 55:96–113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Phase III trial of carboplatin and paclitaxel
compared with cisplatin and paclitaxel in patients with optimally
resected stage III ovarian cancer: A gynecologic oncology group
study. J Clin Oncol. 41:4077–4083. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kyrgiou M, Salanti G, Pavlidis N,
Paraskevaidis E and Ioannidis JP: Survival benefits with diverse
chemotherapy regimens for ovarian cancer: Meta-analysis of multiple
treatments. J Natl Cancer Inst. 98:1655–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SI, Cho J, Lee EJ, Park S, Park SJ,
Seol A, Lee N, Yim GW, Lee M, Lim W, et al: Selection of patients
with ovarian cancer who may show survival benefit from hyperthermic
intraperitoneal chemotherapy: A systematic review and
meta-analysis. Medicine (Baltimore). 98:e183552019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ozols RF: Challenges for chemotherapy in
ovarian cancer. Ann Oncol. 17 (Suppl 5):v181–v187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fung-Kee-Fung M, Oliver T, Elit L, Oza A,
Hirte HW and Bryson P: Optimal chemotherapy treatment for women
with recurrent ovarian cancer. Curr Oncol. 14:195–208. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pokhriyal R, Hariprasad R, Kumar L and
Hariprasad G: Chemotherapy resistance in advanced ovarian cancer
patients. Biomark Cancer. 11:1179299×198608152019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cornelison R, Llaneza DC and Landen CN:
Emerging therapeutics to overcome chemoresistance in epithelial
ovarian cancer: A mini-review. Int J Mol Sci. 18:21712017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baker BM and Chen CS: Deconstructing the
third dimension: How 3D culture microenvironments alter cellular
cues. J Cell Sci. 125:3015–3024. 2012.PubMed/NCBI
|
|
16
|
Zhang Z, Bédard E, Luo Y, Wang H, Deng S,
Kelvin D and Zhong R: Animal models in xenotransplantation. Expert
Opin Investig Drugs. 9:2051–2068. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bertotti A, Migliardi G, Galimi F, Sassi
F, Torti D, Isella C, Corà D, Di Nicolantonio F, Buscarino M, Petti
C, et al: A molecularly annotated platform of patient-derived
xenografts (‘xenopatients’) identifies HER2 as an effective
therapeutic target in cetuximab-resistant colorectal cancer. Cancer
Discov. 1:508–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
DeRose YS, Wang G, Lin YC, Bernard PS,
Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, et al:
Tumor grafts derived from women with breast cancer authentically
reflect tumor pathology, growth, metastasis and disease outcomes.
Nat Med. 17:1514–1520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sachs N, de Ligt J, Kopper O, Gogola E,
Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H,
et al: A living biobank of breast cancer organoids captures disease
heterogeneity. Cell. 172:373–386.e310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zanoni M, Cortesi M, Zamagni A, Arienti C,
Pignatta S and Tesei A: Modeling neoplastic disease with spheroids
and organoids. J Hematol Oncol. 13:972020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Graham O, Rodriguez J, van Biljon L,
Fashemi B, Graham E, Fuh K, Khabele D and Mullen M: Generation and
culturing of high-grade serous ovarian cancer patient-derived
organoids. J Vis Exp. 6:1912023.
|
|
22
|
Yani W, Qi J, Yuchen Z and Haiyan Z:
Application of organoids technology in drug sensitivity test of
ovarian cancer. J Int Obstet Gynecol. 49:181–185. 2022.
|
|
23
|
Yujie S, Hong Y, Jia L and Ying X:
Application prospects on organoid culture system in drug screening
and treatment target for ovarian cancer. J Chin Oncol.
28:1042–1045. 2022.(In Chinese).
|
|
24
|
Jianjun G, Wei Q, Hao W and Xiangyu Z:
Application and prospect of organoid technique in cancer research.
Chin J Tissue Engineering Res. 23:1136–1141. 2019.(In Chinese).
|
|
25
|
Aihara A, Abe N, Saruhashi K, Kanaki T and
Nishino T: Novel 3-D cell culture system for in vitro evaluation of
anticancer drugs under anchorage-independent conditions. Cancer
Sci. 107:1858–1866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ben-David U, Ha G, Tseng YY, Greenwald NF,
Oh C, Shih J, McFarland JM, Wong B, Boehm JS, Beroukhim R and Golub
TR: Patient-derived xenografts undergo mouse-specific tumor
evolution. Nat Genet. 49:1567–1575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Byrne AT, Alférez DG, Amant F, Annibali D,
Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, et
al: Interrogating open issues in cancer medicine with
patient-derived xenografts. Nat Rev Cancer. 17:6322017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sachs N and Clevers H: Organoid cultures
the analysis of cancer phenotypes. Curr Opin Genet Dev. 24:68–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bleijs M, van de Wetering M, Clevers H and
Drost J: Xenograft and organoid model systems in cancer research.
EMBO J. 38:e1016542019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wensink GE, Elias SG, Mullenders J,
Koopman M, Boj SF, Kranenburg OW and Roodhart JML: Patient-derived
organoids as a predictive biomarker for treatment response in
cancer patients. NPJ Precis Oncol. 5:302021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perkhofer L, Frappart PO, Müller M and
Kleger A: Importance of organoids for personalized medicine. Per
Med. 15:461–465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rossi G, Manfrin A and Lutolf MP: Progress
and potential in organoid research. Nat Rev Genet. 19:671–687.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsang SI, Hassan AA, To SKY and Wong AST:
Experimental models for ovarian cancer research. Exp Cell Res.
416:1131502022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Huang S, Cheng S, Jin Y, Zhang N
and Wang Y: Application of ovarian cancer organoids in precision
medicine: Key challenges and current opportunities. Front Cell Dev
Biol. 9:7014292021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aboulkheyr Es H, Montazeri L, Aref AR,
Vosough M and Baharvand H: Personalized cancer medicine: An
organoid approach. Trends Biotechnol. 36:358–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maenhoudt N, Defraye C, Boretto M, Jan Z,
Heremans R, Boeckx B, Hermans F, Arijs I, Cox B, Van Nieuwenhuysen
E, et al: developing organoids from ovarian cancer as experimental
and preclinical models. Stem Cell Reports. 14:717–729. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kopper O, de Witte CJ, Lõhmussaar K,
Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost
N, Begthel H, et al: An organoid platform for ovarian cancer
captures intra- and interpatient heterogeneity. Nat Med.
25:838–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pauli C, Hopkins BD, Prandi D, Shaw R,
Fedrizzi T, Sboner A, Sailer V, Augello M, Puca L, Rosati R, et al:
Personalized in vitro and in vivo cancer models to guide precision
medicine. Cancer Discov. 7:462–477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jabs J, Zickgraf FM, Park J, Wagner S,
Jiang X, Jechow K, Kleinheinz K, Toprak UH, Schneider MA, Meister
M, et al: Screening drug effects in patient-derived cancer cells
links organoid responses to genome alterations. Mol Syst Biol.
13:9552017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maru Y, Tanaka N, Itami M and Hippo Y:
Efficient use of patient-derived organoids as a preclinical model
for gynecologic tumors. Gynecol Oncol. 154:189–198. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hill SJ, Decker B, Roberts EA, Horowitz
NS, Muto MG, Worley MJ Jr, Feltmate CM, Nucci MR, Swisher EM,
Nguyen H, et al: Prediction of DNA repair inhibitor response in
short-term patient-derived ovarian cancer organoids. Cancer Discov.
8:1404–1421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nanki Y, Chiyoda T, Hirasawa A, Ookubo A,
Itoh M, Ueno M, Akahane T, Kameyama K, Yamagami W, Kataoka F and
Aoki D: Patient-derived ovarian cancer organoids capture the
genomic profiles of primary tumours applicable for drug sensitivity
and resistance testing. Sci Rep. 10:125812020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hoffmann K, Berger H, Kulbe H,
Thillainadarasan S, Mollenkopf HJ, Zemojtel T, Taube E,
Darb-Esfahani S, Mangler M, Sehouli J, et al: Stable expansion of
high-grade serous ovarian cancer organoids requires a low-Wnt
environment. EMBO J. 39:e1040132020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kondo J and Inoue M: application of cancer
organoid model for drug screening and personalized therapy. Cells.
8:4702019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Antoni D, Burckel H, Josset E and Noel G:
Three-dimensional cell culture: A breakthrough in vivo. Int J Mol
Sci. 16:5517–5527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kimlin LC, Casagrande G and Virador VM: In
vitro three-dimensional (3D) models in cancer research: An update.
Mol Carcinog. 52:167–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shoemaker RH: The NCI60 human tumour cell
line anticancer drug screen. Nat Rev Cancer. 6:813–823. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rizvanov AA, Yalvaç ME, Shafigullina AK,
Salafutdinov II, Blatt NL, Sahin F, Kiyasov AP and Palotás A:
Interaction and self-organization of human mesenchymal stem cells
and neuro-blastoma SH-SY5Y cells under co-culture conditions: A
novel system for modeling cancer cell micro-environment. Eur J
Pharm Biopharm. 76:253–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Enmon RM Jr, O'Connor KC, Lacks DJ,
Schwartz DK and Dotson RS: Dynamics of spheroid self-assembly in
liquid-overlay culture of DU 145 human prostate cancer cells.
Biotechnol Bioeng. 72:579–591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Westhouse RA: Safety assessment
considerations and strategies for targeted small molecule cancer
therapeutics in drug discovery. Toxicol Pathol. 38:165–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wong CC, Cheng KW and Rigas B: Preclinical
predictors of anticancer drug efficacy: Critical assessment with
emphasis on whether nanomolar potency should be required of
candidate agents. J Pharmacol Exp Ther. 341:572–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ravi M, Paramesh V, Kaviya SR, Anuradha E
and Solomon FD: 3D cell culture systems: Advantages and
applications. J Cell Physiol. 230:16–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jamieson LE, Harrison DJ and Campbell CJ:
Chemical analysis of multicellular tumour spheroids. Analyst.
140:3910–3920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Beningo KA, Dembo M and Wang Yl: Responses
of fibroblasts to anchorage of dorsal extracellular matrix
receptors. Proc Natl Acad Sci USA. 101:18024–18029. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sambale F, Lavrentieva A, Stahl F, Blume
C, Stiesch M, Kasper C, Bahnemann D and Scheper T: Three
dimensional spheroid cell culture for nanoparticle safety testing.
J Biotechnol. 205:120–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jarockyte G, Dapkute D, Karabanovas V,
Daugmaudis JV, Ivanauskas F and Rotomskis R: 3D cellular spheroids
as tools for understanding carboxylated quantum dot behavior in
tumors. Biochim Biophys Acta Gen Subj. 1862:914–923. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mehta G, Hsiao AY, Ingram M, Luker GD and
Takayama S: Opportunities and challenges for use of tumor spheroids
as models to test drug delivery and efficacy. J Control Release.
164:192–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Souza AG, Silva IBB, Campos-Fernandez E,
Barcelos LS, Souza JB, Marangoni K, Goulart LR and Alonso-Goulart
V: Comparative assay of 2D and 3D cell culture models:
Proliferation, gene expression and anticancer drug response. Curr
Pharm Des. 24:1689–1694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Breslin S and O'Driscoll L: The relevance
of using 3D cell cultures, in addition to 2D monolayer cultures,
when evaluating breast cancer drug sensitivity and resistance.
Oncotarget. 7:45745–45756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Verjans ET, Doijen J, Luyten W, Landuyt B
and Schoofs L: Three-dimensional cell culture models for anticancer
drug screening: Worth the effort? J Cell Physiol. 233:2993–3003.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hirst J, Pathak HB, Hyter S, Pessetto ZY,
Ly T, Graw S, Koestler DC, Krieg AJ, Roby KF and Godwin AK:
Licofelone enhances the efficacy of paclitaxel in ovarian cancer by
reversing drug resistance and tumor stem-like properties. Cancer
Res. 78:4370–4385. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cavarzerani E, Caligiuri I, Bartoletti M,
Canzonieri V and Rizzolio F: 3D dynamic cultures of HGSOC organoids
to model innovative and standard therapies. Front Bioeng
Biotechnol. 11:11353742023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Samson DJ, Seidenfeld J, Ziegler K and
Aronson N: Chemotherapy sensitivity and resistance assays: A
systematic review. J Clin Oncol. 22:3618–3630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Brooks EA, Galarza S, Gencoglu MF,
Cornelison RC, Munson JM and Peyton SR: Applicability of drug
response metrics for cancer studies using biomaterials. Philos
Trans R Soc Lond B Biol Sci. 374:201802262019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Brodeur MN, Simeone K, Leclerc-Deslauniers
K, Fleury H, Carmona E, Provencher DM and Mes-Masson AM:
Carboplatin response in preclinical models for ovarian cancer:
Comparison of 2D monolayers, spheroids, ex vivo tumors and in vivo
models. Sci Rep. 11:181832021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Thorel L, Morice PM, Paysant H, Florent R,
Babin G, Thomine C, Perréard M, Abeilard E, Giffard F, Brotin E, et
al: Comparative analysis of response to treatments and molecular
features of tumor-derived organoids versus cell lines and PDX
derived from the same ovarian clear cell carcinoma. J Exp Clin
Cancer Res. 42:2602023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Loessner D, Stok KS, Lutolf MP, Hutmacher
DW, Clements JA and Rizzi SC: Bioengineered 3D platform to explore
cell-ECM interactions and drug resistance of epithelial ovarian
cancer cells. Biomaterials. 31:8494–8506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tofani LB, Abriata JP, Luiz MT, Marchetti
JM and Swiech K: Establishment and characterization of an in vitro
3D ovarian cancer model for drug screening assays. Biotechnol Prog.
36:e30342020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bi J, Newtson AM, Zhang Y, Devor EJ,
Samuelson MI, Thiel KW and Leslie KK: Successful patient-derived
organoid culture of gynecologic cancers for disease modeling and
drug sensitivity testing. Cancers (Basel). 13:29012021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Garcia J, Hurwitz HI, Sandler AB, Miles D,
Coleman RL, Deurloo R and Chinot OL: Bevacizumab
(Avastin®) in cancer treatment: A review of 15 years of
clinical experience and future outlook. Cancer Treat Rev.
86:1020172020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cohen MH, Gootenberg J, Keegan P and
Pazdur R: FDA drug approval summary: Bevacizumab plus FOLFOX4 as
second-line treatment of colorectal cancer. Oncologist. 12:356–361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Govindaraju S and Yun K: Synthesis of gold
nanomaterials and their cancer-related biomedical applications: An
update 3. Biotech. 8:1132018.PubMed/NCBI
|
|
73
|
Oliva P, Decio A, Castiglioni V, Bassi A,
Pesenti E, Cesca M, Scanziani E, Belotti D and Giavazzi R:
Cisplatin plus paclitaxel and maintenance of bevacizumab on tumour
progression, dissemination, and survival of ovarian carcinoma
xenograft models. Br J Cancer. 107:360–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang H, Wang Y and Wang P, Zhang N and
Wang P: Tumor organoids for cancer research and personalized
medicine. Cancer Biol Med. 19:319–332. 2021.PubMed/NCBI
|
|
75
|
Seidlitz T, Koo BK and Stange DE: Gastric
organoids-an in vitro model system for the study of gastric
development and road to personalized medicine. Cell Death Differ.
28:68–83. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rivenbark AG, O'Connor SM and Coleman WB:
Molecular and cellular heterogeneity in breast cancer: Challenges
for personalized medicine. Am J Pathol. 183:1113–1124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Verma M: Personalized medicine and cancer.
J Pers Med. 2:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Offit K: Personalized medicine: New
genomics, old lessons. Hum Genet. 130:3–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Peres LC, Risch H, Terry KL, Webb PM,
Goodman MT, Wu AH, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy
ML, et al: Racial/ethnic differences in the epidemiology of ovarian
cancer: A pooled analysis of 12 case-control studies. Int J
Epidemiol. 47:10112018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nero C, Vizzielli G, Lorusso D, Cesari E,
Daniele G, Loverro M, Scambia G and Sette C: Patient-derived
organoids and high grade serous ovarian cancer: From disease
modeling to personalized medicine. J Exp Clin Cancer Res.
40:1162021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lui G, Richardson A, Chatterjee P,
Pollastro M, Lints M, Peretti D, Rosati R, Appleyard L, Durenberger
G, Diaz R, et al: Functional drug screening of organoids from
ovarian cancer patients demonstrates clinical and genomic
concordance and identifies novel therapeutic vulnerabilities.
Cancer Res. 81:534. 2021. View Article : Google Scholar
|
|
82
|
Phan N, Hong JJ, Tofig B, Mapua M,
Elashoff D, Moatamed NA, Huang J, Memarzadeh S, Damoiseaux R and
Soragni A: A simple high-throughput approach identifies actionable
drug sensitivities in patient-derived tumor organoids. Commun Biol.
2:782019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Åkerlund E, Gudoityte G,
Moussaud-Lamodière E, Lind O, Bwanika HC, Lehti K, Salehi S,
Carlson J, Wallin E, Fernebro J, et al: The drug efficacy testing
in 3D cultures platform identifies effective drugs for ovarian
cancer patients. NPJ Precis Oncol. 7:1112023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Clark J, Fotopoulou C, Cunnea P and Krell
J: Novel ex vivo models of epithelial ovarian cancer: The future of
biomarker and therapeutic research. Front Oncol. 12:8372332022.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Compadre AJ, van Biljon LN, Valentine MC,
Llop-Guevara A, Graham E, Fashemi B, Herencia-Ropero A, Kotnik EN,
Cooper I, Harrington SP, et al: RAD51 foci as a biomarker
predictive of platinum chemotherapy response in ovarian cancer.
Clin Cancer Res. 29:2466–2479. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ceccaldi R, Rondinelli B and D'Andrea AD:
Repair pathway choices and consequences at the double-strand break.
Trends Cell Biol. 26:52–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ito K, Murayama Y, Kurokawa Y, Kanamaru S,
Kokabu Y, Maki T, Mikawa T, Argunhan B, Tsubouchi H, Ikeguchi M, et
al: Real-time tracking reveals catalytic roles for the two DNA
binding sites of Rad51. Nat Commun. 11:29502020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wilson AJ, Stubbs M, Liu P, Ruggeri B and
Khabele D: The BET inhibitor INCB054329 reduces homologous
recombination efficiency and augments PARP inhibitor activity in
ovarian cancer. Gynecol Oncol. 149:575–584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Pellegrino B, Herencia-Ropero A,
Llop-Guevara A, Pedretti F, Moles-Fernández A, Viaplana C,
Villacampa G, Guzmán M, Rodríguez O, Grueso J, et al: Preclinical
in vivo validation of the RAD51 test for identification of
homologous recombination-deficient tumors and patient
stratification. Cancer Res. 82:1646–1657. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mukhopadhyay A, Elattar A, Cerbinskaite A,
Wilkinson SJ, Drew Y, Kyle S, Los G, Hostomsky Z, Edmondson RJ and
Curtin NJ: Development of a functional assay for homologous
recombination status in primary cultures of epithelial ovarian
tumor and correlation with sensitivity to poly(ADP-ribose)
polymerase inhibitors. Clin Cancer Res. 16:2344–2351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shah MM, Dobbin ZC, Nowsheen S, Wielgos M,
Katre AA, Alvarez RD, Konstantinopoulos PA, Yang ES and Landen CN:
An ex vivo assay of XRT-induced Rad51 foci formation predicts
response to PARP-inhibition in ovarian cancer. Gynecol Oncol.
134:331–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Meijer TG, Verkaik NS, Sieuwerts AM, van
Riet J, Naipal KAT, van Deurzen CHM, den Bakker MA, Sleddens HFBM,
Dubbink HJ, den Toom TD, et al: Functional ex vivo assay reveals
homologous recombination deficiency in breast cancer beyond BRCA
gene defects. Clin Cancer Res. 24:6277–6287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
van Wijk LM, Vermeulen S, Meijers M, van
Diest MF, Ter Haar NT, de Jonge MM, Solleveld-Westerink N, van
Wezel T, van Gent DC, Kroep JR, et al: The RECAP test rapidly and
reliably identifies homologous recombination-deficient ovarian
carcinomas. Cancers (Basel). 12:28052020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu JF, Palakurthi S, Zeng Q, Zhou S,
Ivanova E, Huang W, Zervantonakis IK, Selfors LM, Shen Y, Pritchard
CC, et al: Establishment of patient-derived tumor xenograft models
of epithelial ovarian cancer for preclinical evaluation of novel
therapeutics. Clin Cancer Res. 23:1263–1273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cybulska P, Stewart JM, Sayad A, Virtanen
C, Shaw PA, Clarke B, Stickle N, Bernardini MQ and Neel B: A
genomically characterized collection of high-grade serous ovarian
cancer xenografts for preclinical testing. Am J Pathol.
188:1120–1131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hidalgo M, Amant F, Biankin AV, Budinská
E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo
GM, et al: Patient-derived xenograft models: An emerging platform
for translational cancer research. Cancer Discov. 4:998–1013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
de Witte CJ, Valle-Inclan JE, Hami N,
Lõhmussaar K, Kopper O, Vreuls CPH, Jonges GN, van Diest P, Nguyen
L, Clevers H, et al: Patient-Derived ovarian cancer organoids mimic
clinical response and exhibit heterogeneous inter- and intrapatient
drug responses. Cell Rep. 31:1077622020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhou Z, Cong L and Cong X: Patient-Derived
organoids in precision medicine: Drug screening, organoid-on-a-chip
and living organoid biobank. Front Oncol. 11:7621842021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Narita Y, Kitazoe Y, Kurihara Y, Okuhara
Y, Takamatsu K, Saito N and Doi Y: Increase or decrease of
HDL-cholesterol concentrations during pravastatin treatment
depending on the pre-treatment HDL cholesterol levels. Eur J Clin
Pharmacol. 52:461–463. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Mosiewicz KA, Kolb L, van der Vlies AJ,
Martino MM, Lienemann PS, Hubbell JA, Ehrbar M and Lutolf MP: In
situ cell manipulation through enzymatic hydrogel photopatterning.
Nat Mater. 12:1072–1078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Verduin M, Hoeben A, De Ruysscher D and
Vooijs M: Patient-derived cancer organoids as predictors of
treatment response. Front Oncol. 11:6419802021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ahn SI, Sei YJ, Park HJ, Kim J, Ryu Y,
Choi JJ, Sung HJ, MacDonald TJ, Levey AI and Kim YT:
Microengineered human blood-brain barrier platform for
understanding nanoparticle transport mechanisms. Nat Commun.
11:1752020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Nagle PW, Plukker JTM, Muijs CT, van Luijk
P and Coppes RP: Patient-derived tumor organoids for prediction of
cancer treatment response. Semin Cancer Biol. 53:258–264. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Psilopatis I, Sykaras AG, Mandrakis G,
Vrettou K and Theocharis S: Patient-derived organoids: The
beginning of a new era in ovarian cancer disease modeling and drug
sensitivity testing. Biomedicines. 11:12022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kenny PA, Lee GY, Myers CA, Neve RM,
Semeiks JR, Spellman PT, Lorenz K, Lee EH, Barcellos-Hoff MH,
Petersen OW, et al: The morphologies of breast cancer cell lines in
three-dimensional assays correlate with their profiles of gene
expression. Mol Oncol. 1:84–96. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hughes CS, Postovit LM and Lajoie GA:
Matrigel: A complex protein mixture required for optimal growth of
cell culture. Proteomics. 10:1886–1890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chan WS, Mo X, Ip PPC and Tse KY:
Patient-derived organoid culture in epithelial ovarian
cancers-Techniques, applications, and future perspectives. Cancer
Med. 12:19714–19731. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Mandrycky C, Wang Z, Kim K and Kim DH: 3D
bioprinting for engineering complex tissues. Biotechnol Adv.
34:422–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Annett S, Moore G, Short A, Marshall A,
McCrudden C, Yakkundi A, Das S, McCluggage WG, Nelson L, Harley I,
et al: FKBPL-based peptide, ALM201, targets angiogenesis and cancer
stem cells in ovarian cancer. Br J Cancer. 122:361–371. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Baka Z, Godier C, Lamy L, Mallick A,
Gribova V, Figarol A, Bezdetnaya L, Chateau A, Magne Z, Stiefel M,
et al: A coculture based, 3D bioprinted ovarian tumor model
combining cancer cells and cancer associated fibroblasts. Macromol
Biosci. 23:e22004342023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xu F, Celli J, Rizvi I, Moon S, Hasan T
and Demirci U: A three-dimensional in vitro ovarian cancer
coculture model using a high-throughput cell patterning platform.
Biotechnol J. 6:204–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Driehuis E and Clevers H: CRISPR/Cas 9
genome editing and its applications in organoids. Am J Physiol
Gastrointest Liver Physiol. 312:G257–G265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Neal JT and Kuo CJ: Organoids as models
for neoplastic transformation. Ann Rev Pathol. 11:199–220. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lõhmussaar K, Kopper O, Korving J, Begthel
H, Vreuls CPH, van Es JH and Clevers H: Assessing the origin of
high-grade serous ovarian cancer using CRISPR-modification of mouse
organoids. Nat Commun. 11:26602020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dumont S, Jan Z, Heremans R, Van Gorp T,
Vergote I and Timmerman D: Organoids of epithelial ovarian cancer
as an emerging preclinical in vitro tool: A review. J Ovarian Res.
12:1052019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li Z, Gu H, Xu X, Tian Y, Huang X and Du
Y: Unveiling the novel immune and molecular signatures of ovarian
cancer: Insights and innovations from single-cell sequencing. Front
Immunol. 14:12880272023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wan C, Keany MP, Dong H, Al-Alem LF,
Pandya UM, Lazo S, Boehnke K, Lynch KN, Xu R, Zarrella DT, et al:
Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint
blockade in high-grade serous ovarian cancer. Cancer Res.
81:158–173. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gonzalez VD, Samusik N, Chen TJ, Savig ES,
Aghaeepour N, Quigley DA, Huang YW, Giangarrà V, Borowsky AD,
Hubbard NE, et al: Commonly occurring cell subsets in high-grade
serous ovarian tumors identified by single-cell mass cytometry.
Cell Rep. 22:1875–1888. 2018. View Article : Google Scholar : PubMed/NCBI
|