Introduction
Breast cancer (BC) stands as one of the most
commonly diagnosed malignancies worldwide. Mortality rates persist
at a high level, ranking BC as the second leading cause of
cancer-related deaths among females, with ~~290,000 fatalities
annually globally (1). BC is
associated with the disruption of several microbiome compartments,
such as the gut microbiome, breast tissue microbiome and tumor
microbiome, which has been termed oncobiosis (2). The human body harbors diverse
communities of commensal and pathogenic bacteria in both the body
cavity and the body surface, with >90% of the microbes residing
in the gastrointestinal tract. Each individual has a distinct gut
microbial composition, and dysbiosis of the gut microbiota has been
demonstrated to contribute to the pathogenesis of several diseases,
including BC, pancreatic adenocarcinoma, colorectal cancer (CRC),
gastric cancer and hepatocellular carcinoma (3–7). The
notable role of oncobiosis in the pathogenesis of BC is highlighted
by the discovery that antibiotic usage increases susceptibility of
mice to BC (8). Additionally, risk
factors associated with BC, such as high breast density (9), early onset of menstruation, low levels
of physical activity (10),
increased body mass index (11),
advanced age (12) and high alcohol
consumption (10), have also been
associated with alterations in the gut microbiome that contribute
to the oncobiosis associated with BC.
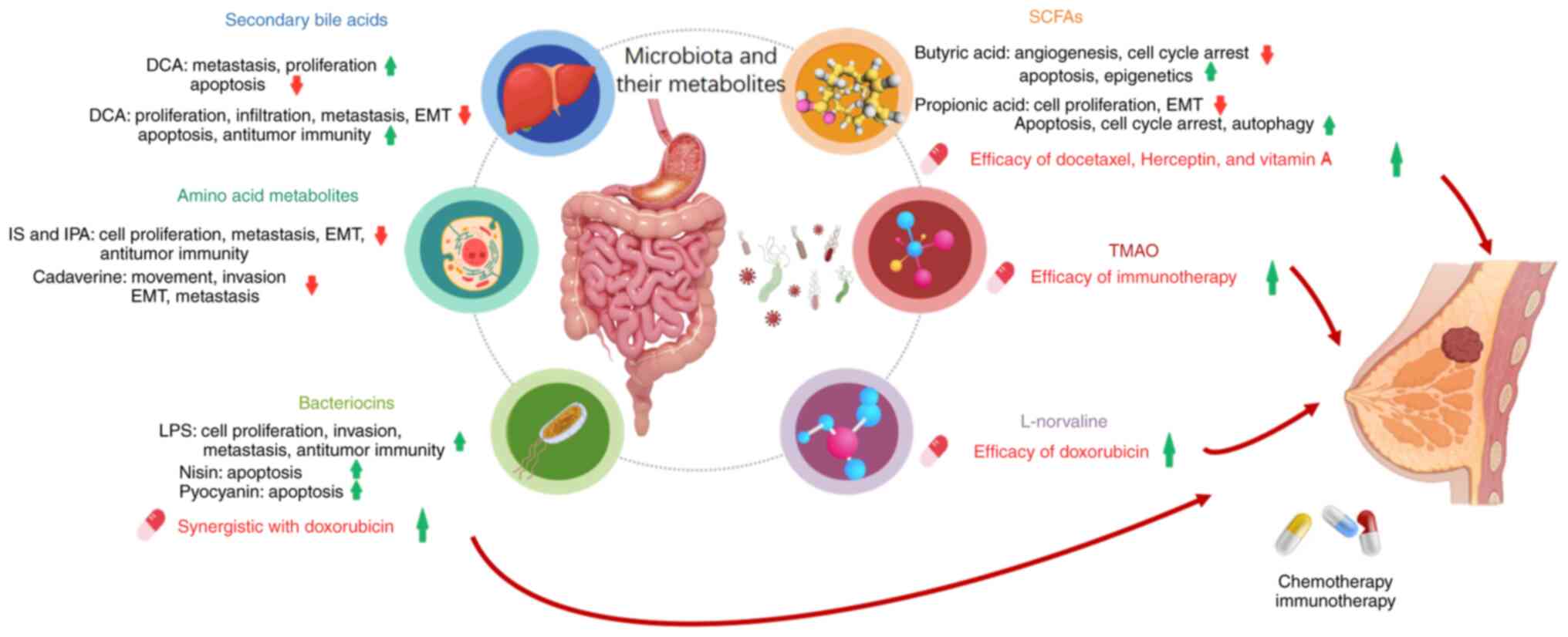
Numerous bacterial metabolites are either products
of microbial metabolism, such as bacteriocins, or compounds derived
from the host that have been modified, such as secondary bile acids
(BAs), short-chain fatty acids (SCFAs) and amino acid metabolites
(13). These metabolites serve as
crucial links in the reciprocal interactions between cancer cells
and their surrounding microenvironment. The disruption of gut
microbial communities, known as dysbiosis, can produce systemic
immune responses due to the breakdown of mucosal barriers and the
translocation of gut microbiome components to breast tissue via the
bloodstream (Fig. 1). Moreover,
microbial metabolites are not solely derived from the gut
microbiota. Human breast tissue also harbors symbiotic bacterial
communities, and these communities and their metabolic products may
influence the occurrence and progression of BC (14). Patients with triple-negative breast
cancer (TNBC) have the least complex microbial signature, whereas
those with luminal BC subtypes have the most complex microbial
signature (15). The microbiota can
influence cancer progression through several mechanisms, including
modulating inflammation, inducing DNA damage and producing
metabolites involved in tumorigenesis or tumor suppression
(16). In summary, both gut-derived
and breast tissue-derived microbiota and metabolites are considered
to influence the development, progression and metastasis of BC, and
its response to treatment.
In particular, previous studies (17–20)
have reported that the efficacy of standard chemotherapeutic,
targeted and immunotherapy drugs is influenced by gut microbial
metabolites, and these metabolites may be implemented as
combination therapies for BC. The impact of different taxa on the
immune checkpoint inhibitor (ICI) response is mediated primarily
through the release of various bacterial metabolites (21); however, there has been limited
research on how these distinct metabolites affect the response to
ICIs. The interaction between these metabolites and the host immune
system is crucial for understanding how the microbiome can affect
ICI efficacy. This also implies that certain metabolites could be
used to predict the response to ICIs (22). Although evidence supports various
gut metabolites having important effects on BC and its drug
treatments, the underlying mechanisms are poorly understood and
warrant further investigation.
Research has shown that the gut microbiota
composition can impact the effectiveness of and adverse reactions
to BC immunotherapy (23,24). However, little is known about the
importance of the interactions between microbiota-induced
metabolites and drug metabolites. In the present review, the
mechanisms and therapeutic potential of several microbial
metabolites in BC are reported and discussed.
Effects of microbial metabolites on BC
development
Secondary BAs
BAs are synthesized from cholesterol in the liver
and released into the small intestine. BAs interfere with glucose,
fatty acid and lipid metabolism mainly by activating the farnesoid
X receptor and the G protein-coupled bile acid receptor (TGR5),
which are crucial for maintaining a healthy gut microbiota,
regulating lipid and carbohydrate metabolism, enhancing insulin
sensitivity and supporting innate immunity (25). Bacteria ultimately mediate the
conversion of BAs into secondary BAs, including deoxycholic acid
(DCA) and lithocholic acid (LCA) (26). A small population of intestinal
species in the genus Clostridium, including Clostridium
scindens, Clostridium hiranonis, Clostridium hylemonae and
Clostridium sordelli, are capable of producing secondary bile
acids (26). Tang et al
(27) and Zhu et al
(28) demonstrated that BAs and
their metabolites can enter breast tumors through BA transporters,
reduce BC aggressiveness and improve BC prognosis. The studies also
showed that BAs may interfere with hormonal pathways in the breast
tissue. The presence of secondary BAs can promote carcinogenesis
through several mechanisms, such as inducing DNA damage, activating
β-catenin signaling and increasing cyclooxygenase-2 activity
(29). Secondary BAs can also
influence tumor development and progression through their
immunosuppressive effects (30),
and have been shown to have anticancer effects and reduce cancer
risk in clinical studies (31).
The contradictory effects of secondary BAs on
carcinogenesis are context-dependent and may arise from the
following factors. Low concentrations of secondary BAs may exert
anticancer effects by activating FXR/TGR5 receptors (25,27,28).
Conversely, chronic or high-dose exposure to secondary BAs can
promote carcinogenesis (29). The
balance between pro- and anti-carcinogenic BAs also depends on
microbial composition. Specific Clostridium species (e.g.,
C. scindens) convert primary BAs to secondary BAs (26). Finaly, receptor signaling dynamics
also contribute to the contradictory actions. For example, TGR5
activation in intestinal L cells stimulates GLP-1 secretion,
improving insulin sensitivity (anticancer), but in macrophages,
chronic TGR5 signaling may drive immunosuppressive M2 polarization
(pro-cancer) (25,30).
DCA is formed by the de-hydroxylation of BAs and
previous studies have shown that DCA increases the number of
metastases generated from 4T1 cell tumors grafted into mouse fat
pads by elevating the expression of vascular endothelial growth
factor receptor 2 (Flk-1) and reducing the ceramide-mediated
apoptosis of BC cells (32). In a
previous in vitro study, physiological levels of DCA
promoted cell proliferation by inducing AKT phosphorylation and
cyclin D1 expression in MCF-7 BC cells, and was cytotoxic at
supraphysiological concentrations; the mechanism of DCA involved
the induction of apoptosis (33).
Cong et al (34) screened a
library of gut microbiota-derived metabolites and identified DCA as
a negative regulator for CD8+ T cell effector function.
The study demonstrated a causal relationship between microbial DCA
metabolism and impaired antitumor CD8+ T cell responses
in CRC, highlighting the immunosuppressive role of DCA in tumor
progression. The dual roles of DCA have important implications for
their therapeutic targeting. On one hand, strategies to inhibit the
production or activity of DCA could restore antitumor immunity and
reduce tumor progression, as suggested by Cong et al
(34). On the other hand,
modulating BA receptor signaling (e.g., FXR/TGR5 agonists) may
offer additional therapeutic avenues (25,27,28).
Furthermore, microbiome-directed interventions, such as probiotics
or dietary modifications, could reshape the BA pool to favor
antitumor effects. However, the precise balance between pro- and
anticancer BAs must be carefully considered, as their effects are
highly context-dependent.
Clostridium in the large intestine is mainly
responsible for the production of LCA (2). In a previous study, patients with
early stage BC had reduced serum levels of LCA, which may be
associated with a decline in the biosynthetic capacity of LCA
within the microbial community (35). Furthermore, serum LCA levels are
negatively associated with the Ki67 labelling index in BC cells
(36). Therefore, promoting the
production of these protective metabolites by the gut microbiota in
women with BC may improve their health (37). Moreover, LCA can reduce BC cell
proliferation by 10–20%, inhibit tumor infiltration and metastasis,
reduce epithelial-mesenchymal transition (EMT) and enhance
antitumor immune responses (35).
Another study revealed a potential therapeutic role of LCA in BC
cells due to its reversal of lipid metabolism dysregulation
(38); LCA induced TGR5 expression
in MCF-7 and MDA-MB-231 BC cells, and had antiproliferative and
proapoptotic effects. Moreover, the expression of the proapoptotic
p53 protein increased and the expression of the antiapoptotic
protein Bcl-2 decreased in MCF-7 cells after LCA treatment.
Furthermore, LCA supplementation reduced vascular endothelial
growth factor (VEGF) production by BC cells in an animal model of
BC (2). In conclusion, LCA may have
a therapeutic effect on BC cells and can be used as an
antiproliferative and proapoptotic drug that targets lipid
metabolism in these cells (38).
SCFAs
Gut microbes influence cancer growth through the
production of SCFAs, such as acetate, butyrate and propionate
(39). SCFAs can directly activate
G protein-coupled receptors (GPCR) and inhibit histone deacetylases
(HDACs) (40), resulting in cell
attachment and differentiation, immune cell migration, cytokine
production, chemotaxis and programmed cell death (41,42).
Although the suppressive function of SCFAs in the development of
CRC has been demonstrated (43),
SCFAs can also affect various organs through the systemic
circulation (29). However, their
role in BC needs further study.
Among the SCFAs, propionic acid (SP), which can be
produced from succinic acid, inhibits tumor growth and EMT, induces
apoptosis in BC cells by binding to the GPCRs, GPR43 and GPR41, and
inhibits BC cell proliferation and apoptosis in a dose-dependent
manner (30). SP also suppresses
the invasion of BC cells overexpressing GPR41 and GPR43 by
activating large tumor suppressor kinase 1 and inhibiting
extracellular signal-regulated kinase 1/2 (44). A previous study demonstrated the
anticancer effects of SP on BC cell growth, programmed cell death,
self-digestion processes and the production of substances that
prevent oxidative damage (45). SP
was shown to inhibit BC cell proliferation and induce apoptosis by
inhibiting STAT3, increasing reactive oxygen species (ROS) levels
and activating p38; however, these effects were not mediated by the
SCFA receptors GPR41 or GPR43. Furthermore, SP suppressed the
proliferation of BC cells by inhibiting the expression or activity
of cell cycle checkpoint proteins, causing
G0/G1 phase arrest and inhibiting DNA
synthesis (45). Collectively,
these results suggest that SP is a candidate therapeutic drug for
BC.
The intestinal concentration of butyrate, the major
protective SCFA, serves a crucial role in cell cycle regulation,
cell proliferation and apoptosis (29). Owing to its anti-inflammatory
properties and ability to induce cell differentiation, trigger
cancer cell apoptosis and promote protective histone
hyperacetylation, butyrate has shown strong antitumor effects in BC
cell lines, including MCF-7, MDA-MB-231 and MDA-MB-453 (42,46),
revealing its potential as an anticancer metabolite. Therefore,
promoting butyrate production may have beneficial implications for
patients with BC or for those at risk of developing BC (37). Previous studies evaluated the
effects of butyrate on the proliferation and ultrastructure of BC
cells (47,48). The study confirmed that the
administration of butyrate resulted in morphological changes to the
ultrastructure of MCF-7 cells, suppressed cell proliferation and
triggered apoptotic cell death; however, the underlying mechanism
of action remains unknown (47,49).
Moreover, butyrate can induce calcium influx into MCF-7 cells
(42), which activates caspases and
other pro-apoptotic pathways, leading to cell death.
Compared with healthy premenopausal women,
premenopausal patients with BC were determined to have markedly
lower levels of SCFA-producing bacteria in the gut, as well as
lower levels of key SCFA-producing enzymes; therefore, these SCFA
receptors may be new targets for the treatment of premenopausal
patients with BC (50). Overall,
SCFAs are promising for BC treatment. Future studies should
evaluate the effects of SCFAs on BC to understand their molecular
mechanisms of action, as well as how SCFAs affect the efficacy and
safety of standard BC drug therapies and BC prognosis.
Amino acid metabolites
Similar to SCFAs and LCA, certain amino acid
metabolites play important roles in BC. Several bacterial taxa,
such as Clostridium, Bacillus, Lactobacillus, Streptococcus
and Proteobacteria species, efficiently metabolize proteins
(51). Tryptophan catabolism is
inhibited in BC cells and reduced tryptophan catabolism is
associated with decreased survival in patients with BC, whereas
elevated levels of extracellular tryptophan are linked to worse BC
prognosis (52). Tryptophan
metabolites may stimulate the immune system by interacting with the
aryl hydrocarbon receptor (AHR) to impact human health and disease
(52,53).
The microbiome contributes to 4–6% of the overall
tryptophan catabolism and generates indole derivatives, including
indole propionic acid (IPA) and indoxyl sulfate (IS), both of which
have cytostatic effects on BC (54,55).
Similar to other indole derivatives, IPA and IS act through the
pregnane-X receptor and AHR. The expression of these receptors
decreases as BC progresses, and in patients with a poor prognosis,
the expression of these receptors is lower. In addition, indole
derivatives have strong immunostimulatory effects, and can
stimulate an antitumor immune response in BC and alter the
microbiome composition (54,55).
The enzyme lysine decarboxylase catalyzes the
decarboxylation of lysine to produce cadaverine (CAD), which is
also produced by numerous bacterial species (e.g. E. coli,
Vibrio sp. and Lactobacillus sp.) in the human
microbiome (56). Reduced CAD
biosynthesis in the gut has been reported in patients with early
BC, leading to reduced production of anticancer bacterial
metabolites and resulting in increased BC invasion (39). Kovács et al (56) reported that CAD inhibited BC
metastasis and reduced the aggressiveness of the primary tumor.
Moreover, CAD amine treatment of BC cell lines reversed EMT,
inhibited cell motility and invasion, and rendered cells less stem
cell-like by reducing mitochondrial oxidation over the range of
serum concentrations tested (100–800 nM). CAD is thus expected to
contribute to the development of tumor therapies; however, the
mechanism of CAD in BC requires further investigation.
Lipopolysaccharide (LPS)
Metabolites such as LPS, glycans and endotoxins are
present in the outer membranes of gram-negative bacteria. The
inherent microbiome of the breast and the gut is enriched in
gram-negative bacteria. Bacterial LPS was found in
CD45+/CD68− cells of a highly inflamed breast
tumor, indicating that the colonizing bacteria tune and activate
the immune system (57). LPS is
closely linked to human immunity. Toll-like receptors (TLRs), which
respond to LPS stimulation, have gained significant attention in
cancer research due to their role in tumor progression (58). TLRs serve essential roles in the
initiation of inflammation and the activation of host innate immune
responses against invading microorganisms through their recognition
of pathogen-associated molecular patterns (PAMPs). LPS is a type of
PAMP that has been reported to enhance the invasiveness of BC cells
(2) and stimulate BC cell
proliferation (59). The metastatic
potential of BC cells is enhanced upon TLR4 and NF-κB activation,
making these proteins promising therapeutic targets for metastasis
prevention (60). In a previous
study, the LPS-mediated activation of TLR4 markedly increased the
mRNA expression of matrix metalloproteinases, MMP-2 and MMP-9, and
VEGF. The subsequent triggering of the TLR4 downstream protein,
myeloid differentiation factor 88, resulting in increased
production of interleukins, IL-6 and IL-10, in human BC cells. TLR4
was demonstrated to be overexpressed in human BC tissues and has
been associated with lymph node metastasis (61). A study by Li et al (59) revealed that in MCF-7 and MDA-MB-231
BC cells, LPS-stimulated TLR4 pathway activation triggered
β-catenin signaling via PI3K/Akt/GSK3β to promote downstream
β-catenin target gene transcription during BC metastasis. These
findings suggest that TLR4 may be involved in the progression and
metastasis of human BC and may be a new therapeutic target.
Nisin
Nisin, the most abundantly produced bacteriocin in
the gut, also has important effects on BC and has been demonstrated
to be capable of modulating the innate immune system by inducing
chemokine secretion and inhibiting LPS-stimulated cytokines in
vitro and in vivo (62).
The precise mechanism of the anticancer effect of nisin remains
unclear. However, it is hypothesized that nisin induces apoptotic
cell death by initiating cell cycle arrest and altering
intracellular ion levels (e.g., calcium) through membrane
disruption and pore formation. This alters the transmembrane
potential (42,62) and increases the permeability of the
phospholipid bilayer (63).
Previous studies have demonstrated the antitumor
potential of nisin in head and neck squamous cell carcinoma in
vitro and in vivo (64).
In addition, Ahmadi et al (65) demonstrated that nisin induced the
intrinsic apoptosis pathway in colon cancer cells. While there is
less research on nisin in BC, the in vitro experiments of
Avand et al (62) reported
for the first time the potent toxicity of nisin to MCF-7 BC cells.
Compared with that in normal human umbilical vein endothelial cells
(HUVECs), nisin exhibited selective toxicity to cancer cells
(62). Overall, nisin is expected
to be an anticancer agent; however, its precise anticancer
mechanism needs further study.
Pyocyanin
Pyocyanin is a unique extracellular secondary
metabolite pigment produced by Pseudomonas aeruginosa that
exhibits redox activity and is toxic to mammalian cells (66). Previous research has demonstrated
the apoptotic effects of pyocyanin on cancer cell lines, including
rhabdomyosarcoma, hepatocellular carcinoma (HepG2) and human
pancreatic cancer cell lines (67,68).
Abdelaziz et al (69) were
the first to report the toxic effects of pyocyanin to MCF-7 human
breast adenocarcinoma cells. The study demonstrates that purified
pyocyanin may be used in treatment strategies of human breast
adenocarcinoma (MCF-7), which results in decreasing the viability
of cells by the induction of necrosis and accelerating apoptosis
via caspase-3 activation. Pyocyanin has a low molecular weight (210
Da) and exists as a zwitterion that readily diffuses across cell
membranes under aerobic conditions, increasing the intracellular
ROS levels (66). Excessive ROS
leads to caspase-3 activation, which promotes cancer cell death via
apoptosis. Therefore, pyocyanin is considered to have potential to
be a new and effective alternative treatment for cancer.
Effects of microbial metabolites on BC
therapy
There is growing evidence that the gut microbiota
influences the effectiveness of cancer therapies and their
associated side effects. In the present section, the roles and
underlying mechanisms of gut microbial metabolites on the efficacy
and side effects of several anticancer treatments are summarized.
Understanding these interactions may lead to the development of
effective adjuvant therapies to improve the efficacy of anticancer
treatments.
SCFAs
Although butyrate has shown notable anticancer
effects as a stand-alone therapy, the synergistic effects of this
compound combined with conventional anticancer medications have
been documented in previous years (70–72).
By inhibiting HDACs, butyrate has the potential to enhance the
clinical efficacy and mitigate the toxicity associated with
standard chemotherapeutic agents (42). A previous study evaluated the
antitumor potential of Herceptin in combination with butyrate
against HER2-overexpressing BC cells (73). The combination of butyrate and
Herceptin markedly increased the growth-inhibitory effect on SKBR3
BC cells compared with the effects of butyrate and Herceptin alone
(73). The potential synergistic
effect of butyrate with vitamin A on MCF-7 BC cells was
investigated in another in vitro study, which revealed that
vitamin A enhanced the anti-proliferative effects of butyrate. Cell
proliferation inhibition was 34, 10 and 46% following treatment
with butyrate, vitamin A and their combination, respectively,
suggesting that vitamin A potentiated the inhibitory activities of
butyrate (74). Thus, the available
evidence suggests that butyrate may offer notable benefits as an
adjuvant therapy alongside standard anticancer drugs and has
potential far-reaching clinical implications for the management of
BC (42).
A clinical cohort study was performed to assess the
fecal and plasma concentrations of SCFAs in patients with primary
cancer undergoing treatment with the ICIs nivolumab or
pembrolizumab. The results indicated that there may be an
association between fecal SCFA levels and ICI efficacy. High
concentrations of fecal or plasma SCFAs were associated with a
response to ICI treatment and longer progression-free survival,
suggesting that SCFAs could act as mediators between the gut
microbiome and immunotherapy (75).
Another study indicated that gut microbiota diversity was notably
associated with the response to ICIs therapy in patients with
non-small cell lung cancer. Responders (Rs) presented with a marked
increase in the abundance of Faecalibacterium in their gut
microbiota, along with increased levels of SCFAs, particularly
butyrate, acetate and hexanoate. Furthermore, fecal microbiota
transplantation from Rs to non-responders enhanced the anticancer
effects of ICIs in mice and reduced Ki-67 expression in tumor cells
(76). These studies provide
evidence regarding how SCFAs affect ICIs therapy efficacy. SCFAs
may modulate the response to ICIs by affecting the functions of
immune cells and the tumor microenvironment (77). High blood SCFAs levels are
associated with resistance to CTLA-4 blockade and a higher
proportion of Treg cells (78).
SCFAs, particularly butyrate, enhance the differentiation and
function of cytotoxic CD8+ T cells by inhibiting HDACs,
leading to increased expression of effector molecules such as IFN-γ
and granzyme B (42). This promotes
antitumor immunity and synergizes with ICIs. The specific mechanism
of action needs further investigation.
Trimethylamine N-oxide (TMAO)
TMAO, a gut microbiota metabolite, is derived from
phosphatidylcholine, choline, betaine, and L-carnitine. In previous
years, TMAO has received increasing attention due to its possible
carcinogenic effects (79,80). However, a recent study (81) revealed that TMAO triggers
endoplasmic reticulum stress via eukaryotic translation initiation
factor 2α kinase 3, which activates caspase 3 and gasdermin E,
leading to pyroptosis in tumor cells and enhancing CD8+
T-cell-mediated immunity against TNBC. In addition, high plasma
TMAO levels are associated with improved immunotherapy outcomes in
patients with advanced TNBC, indicating that TMAO is a potential
biomarker for the response to immunotherapy (81). In mice, TMAO combined with
anti-programmed cell death protein 1 antibodies inhibited tumor
growth to a markedly greater extent than antibody treatment alone,
suggesting that TMAO can increase the efficacy of immunotherapy
(81). Therefore, as a driver of
antitumor immunity, TMAO may enhance the antitumor immune response
in BC. However, the effect of TMAO on BC therapy needs further
investigation.
Zhou et al (79) demonstrated that TMAO promotes the
proliferation and migration of hepatocellular carcinoma cells
through the MAPK pathway, which is involved in regulating cell
proliferation, differentiation and apoptosis. Given the role of the
MAPK pathway in EMT, it is possible that TMAO may also influence
EMT in hepatocellular carcinoma cells, but further research is
needed to confirm this. Another study revealed that TMAO serves a
carcinogenic role in CRC by promoting cell proliferation and
angiogenesis (82). Although some
studies have reported its carcinogenic effects, a growing body of
research has recently focused on its role as a driver of antitumor
immunity. TMAO is a significant inducer of inflammatory effects,
reconfiguring tumor immune infiltrates to an immune-activated
phenotype. Jalandra et al (80) also discussed the possible anticancer
mechanisms of TMAO, which include inflammation, oxidative stress,
DNA damage and protein misfolding. These studies provide evidence
of the potential role of TMAO in immunotherapy efficacy.
The dual role of TMAO in cancer development suggests
that its effects are context-dependent and may vary depending on
the type of cancer and the specific microenvironment. Therefore,
the use of TMAO as a BC treatment option requires careful
consideration of its potential pro- and anticancer effects. Further
research is needed to fully understand the mechanisms underlying
these dual effects and to determine the most effective and safe
ways to utilize TMAO in BC treatment.
Pyocyanin
Pyocyanin, a toxin produced and secreted by P.
aeruginosa, can induce cancer cell apoptosis and suppress
lymphocyte function. Pyocyanin is considered to promote cancer cell
death (83) and enhance tumor
proliferation inhibition by doxorubicin (84). Pyocyanin has redox activity and is
toxic to mammalian cells, making it a potential new anticancer
drug. A previous study reported that purified pyocyanin has marked
toxicity to MCF-7 BC cells by inhibiting cell growth via the
induction of necrosis and accelerating apoptosis induced by
caspase-3 activation (69). When
used in combination, pyocyanin can enhance the cytotoxic effects of
doxorubicin chemotherapy, especially in MDA-MB-231 and MCF-7 BC
cells. Compared with the use of doxorubicin alone, the addition of
10% P. aeruginosa culture supernatant markedly increased
doxorubicin-induced caspase-7 protein cleavage, indicating that
pyocyanin enhances chemotherapy-induced apoptosis (85).
Additionally, since pyocyanin is a potent inducer of
ROS, it is reasonable to hypothesize that pyocyanin enhances
chemotherapy efficacy via a ROS-dependent mechanism. Compared with
normal cells, cancer cells are more sensitive to increases in the
ROS content, as cancer cells have a higher baseline level of ROS
(86). The aforementioned studies
have demonstrated that pyocyanin may be a promising anticancer drug
candidate.
Nisin
Nisin has been reported to have specific anticancer
activity against MCF-7 breast adenocarcinoma cells and potential
synergy with doxorubicin (62).
Nisin exhibits highly selective toxicity to MCF-7 cells, with an
IC50 value of 5 µM, but is not toxic to noncancerous
HUVECs. The combination of nisin and doxorubicin at subinhibitory
concentrations resulted in increased cytotoxic activity compared
with either agent alone, indicating potential synergistic effects
(62). This observation was further
supported by another in vitro study demonstrating that the
coadministration of nisin and doxorubicin improved treatment
outcomes in patients with skin cancer (87). The potential synergistic
interactions between nisin and standard chemotherapeutics may lead
to improved clinical outcomes for patients with cancer (88). Thus, nisin used alone or in
combination with other chemotherapeutic agents could be a potential
treatment option for patients with BC. Future research on nisin
should prioritize in vitro studies, using multiple BC cell
lines and animal studies to understand its potential synergy with
standard anticancer therapies and provide more evidence for
clinical trials.
L-norvaline
Metabolic profiling has revealed that L-norvaline, a
gut-derived metabolite, is key in the diversity of the gut
microbiota and has the capacity to modulate disease progression
through interactions with the microbiome (89). Notably, Lautropia, Rothia,
Centipeda, Corynebacterium and Actinomyces species have
exhibited notable associations with L-norvaline biosynthesis
(28). As an arginase 1 (ARG1)
inhibitor, L-norvaline impedes cancer development by interacting
with doxorubicin (28,90,91).
ARG1 facilitates the conversion of L-arginine to L-ornithine and
urea within M2-type macrophages (92,93).
The generated L-ornithine is subsequently metabolized into
polyamines by ornithine decarboxylase, a process that can increase
cancer cell proliferation and differentiation. Recent research
demonstrated that the coadministration of L-norvaline and
doxorubicin in an M2 macrophage and BC cell coculture system
markedly inhibited cancer cell proliferation (28). This combined therapy has shown
superior efficacy over monotherapy with either agent alone, further
reducing the proliferative activity of BC cells (28). The potential of bacteriocins to
exert anticarcinogenic effects on BC cells highlights their utility
as adjuvants in standard BC treatment regimens. Collectively, these
discoveries provide novel perspectives for the development of
innovative BC therapeutic strategies.
Conclusions and future perspectives
This review examines the types and sources of common
metabolites from intestinal and tissue-resident microbiota, their
impact on BC development, and their role in modulating tumor
therapy responses. The potential roles of secondary BAs, SCFAs,
amino acid metabolites and bacteriocins (such as LPS, nisin and
pyocyanin) in BC carcinogenesis, progression and metastasis were
explored. The impact of bacterial metabolites on the efficacy of BC
drug treatments, including chemotherapy, targeted therapy and
immunotherapy was also discussed.
Pretreatment analysis of the microbiota provides
oncologists with insights into tumor aggressiveness and
chemotherapy sensitivity to guide treatment modifications.
Currently, breast microbiota research is primarily preclinical and
has focused on comparing the microbiota of breast tumor tissue with
that of healthy tissue to identify tumor-specific characteristics.
Most studies have been performed in vitro, with limited
in vivo data and no clinical trials documented. However,
both in vitro and in vivo studies are crucial for
understanding the complex interactions between the gut metabolites
and cancer cells within the host.
Diet, probiotics and prebiotics may markedly impact
BC, suggesting they have potential as adjuvants in standard BC
treatments (94). Certain
Lactobacillus strains have been shown to have effects
against BC (95). Prebiotics, which
are indigestible fibers, foster the growth of beneficial bacterial
in the gut, which can metabolize the conversion of these fibers
into phytoestrogens and SCFAs (96). These metabolites, along with others,
possess tumor suppressive properties, and exhibit antiestrogenic
and antiproliferative effects, which reduce the risk of BC.
Investigating the synergistic effects of gut metabolites combined
with chemotherapies and immunotherapies could enhance their
clinical efficacy and safety.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81700745) and the Shanghai Natural
Science Foundation (grant no. 20ZR1456600).
Availability of data and materials
Not applicable.
Authors' contributions
YG and WD wrote the first draft of the manuscript.
YG contributed to the study conception, and reviewing and editing
of the manuscript. YG, WD, DS, XZ, ZH, CL and YS contributed to
manuscript revision. Data authentication is not applicable. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kovacs T, Miko E, Ujlaki G, Yousef H,
Csontos V, Uray K and Bai P: The involvement of oncobiosis and
bacterial metabolite signaling in metastasis formation in breast
cancer. Cancer Metastasis Rev. 40:1223–1249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mikó E, Vida A and Bai P: Translational
aspects of the microbiome-to be exploited. Cell Biol Toxicol.
32:153–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mikó E, Kovács T, Sebő É, Tóth J, Csonka
T, Ujlaki G, Sipos A, Szabó J, Méhes G and Bai P:
Microbiome-microbial metabolome-cancer cell interactions in breast
cancer-familiar, but unexplored. Cells. 8:2932019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kiss B, Mikó E, Sebő É, Toth J, Ujlaki G,
Szabó J, Uray K, Bai P and Árkosy P: Oncobiosis and microbial
metabolite signaling in pancreatic adenocarcinoma. Cancers.
12:10682020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuo WT, Lee TC and Yu LC: Eritoran
suppresses colon cancer by altering a functional balance in
toll-like receptors that bind lipopolysaccharide. Cancer Res.
76:4684–4695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen MC, Chen YL, Wang TW, Hsu HP and Lai
MD: Membrane bile acid receptor TGR5 predicts good prognosis in
ampullary adenocarcinoma patients with hyperbilirubinemia. Oncol
Rep. 36:1997–2008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McKee AM, Kirkup BM, Madgwick M, Fowler
WJ, Price CA, Dreger SA, Ansorge R, Makin KA, Caim S, Le Gall G, et
al: Antibiotic-induced disturbances of the gut microbiota result in
accelerated breast tumor growth. iScience. 24:1030122021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones GS, Feigelson HS, Falk RT, Hua X,
Ravel J, Yu G, Flores R, Gail MH, Shi J, Xu X and Goedert JJ:
Mammographic breast density and its association with urinary
estrogens and the fecal microbiota in postmenopausal women. PLoS
One. 14:e02161142019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu AH, Tseng C, Vigen C, Yu Y, Cozen W,
Garcia AA and Spicer D: Gut microbiome associations with breast
cancer risk factors and tumor characteristics: A pilot study.
Breast Cancer Res Treat. 182:451–463. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frugé AD, Van Der Pol W, Rogers LQ, Morrow
CD, Tsuruta Y and Demark-Wahnefried W: Fecal Akkermansia
muciniphila is associated with body composition and microbiota
diversity in overweight and obese women with breast cancer
participating in a presurgical weight loss trial. J Acad Nutr Diet.
120:650–659. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Yang Y, Su J, Zheng X, Wang C,
Chen S, Liu J, Lv Y, Fan S, Zhao A, et al: Age-related
compositional changes and correlations of gut microbiome, serum
metabolome, and immune factor in rats. Geroscience. 43:709–725.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rahman S, O'Connor AL, Becker SL, Patel
RK, Martindale RG and Tsikitis VL: Gut microbial metabolites and
its impact on human health. Ann Gastroenterol. 36:360–368.
2023.PubMed/NCBI
|
|
14
|
Neagoe CX, Ionica M, Neagoe OC and Trifa
AP: The Influence of microbiota on breast cancer: A review.
Cancers. 16:34682024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banerjee S, Tian T, Wei Z, Shih N, Feldman
MD, Peck KN, DeMichele AM, Alwine JC and Robertson ES: Distinct
microbial signatures associated with different breast cancer types.
Front Microbiol. 9:9512018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhatt AP, Redinbo MR and Bultman SJ: The
role of the microbiome in cancer development and therapy. CA Cancer
J Clin. 67:326–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernandes MR, Aggarwal P, Costa RGF, Cole
AM and Trinchieri G: Targeting the gut microbiota for cancer
therapy. Nat Rev Cancer. 22:703–722. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang L, Li A, Wang Y and Zhang Y:
Intratumoral microbiota: Roles in cancer initiation, development
and therapeutic efficacy. Signal Transduct Target Ther. 8:352023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cullin N, Antunes CA, Straussman R,
Stein-Thoeringer CK and Elinav E: Microbiome and cancer. Cancer
Cell. 39:1317–1341. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rao Malla R, Marni R, Kumari S,
Chakraborty A and Lalitha P: Microbiome assisted tumor
microenvironment: Emerging target of breast cancer. Clin Breast
Cancer. 22:200–211. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kovtonyuk LV and McCoy KD: Microbial
metabolites and immunotherapy: Basic rationale and clinical
indications. Semin Immunol. 67:1017552023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han J, Zhang S, Xu Y, Pang Y, Zhang X, Hu
Y, Chen H, Chen W, Zhang J and He W: Beneficial effect of
antibiotics and microbial metabolites on expanded Vδ2Vγ9 T cells in
hepatocellular carcinoma immunotherapy. Front Immunol. 11:13802020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo C, Kong L, Xiao L, Liu K, Cui H, Xin
Q, Gu X, Jiang C and Wu J: The impact of the gut microbiome on
tumor immunotherapy: From mechanism to application strategies. Cell
Biosci. 13:1882023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vitorino M, Baptista de Almeida S, Alpuim
Costa D, Faria A, Calhau C and Azambuja Braga S: Human microbiota
and immunotherapy in breast cancer-a review of recent developments.
Front Oncol. 11:8157722021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jia W, Xie G and Jia W: Bile
acid-microbiota crosstalk in gastrointestinal inflammation and
carcinogenesis. Nat Rev Gastroenterol Hepatol. 15:111–128. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ridlon JM, Kang DJ, Hylemon PB and Bajaj
JS: Bile acids and the gut microbiome. Curr Opin Gastroenterol.
30:332–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang W, Putluri V, Ambati CR, Dorsey TH,
Putluri N and Ambs S: Liver- and microbiome-derived bile acids
accumulate in human breast tumors and inhibit growth and improve
patient survival. Clin Cancer Res. 25:5972–5983. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Q, Zai H, Zhang K, Zhang X, Luo N, Li
X, Hu Y and Wu Y: L-norvaline affects the proliferation of breast
cancer cells based on the microbiome and metabolome analysis. J
Appl Microbiol. 133:1014–1026. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsvetikova SA and Koshel EI: Microbiota
and cancer: Host cellular mechanisms activated by gut microbial
metabolites. Int J Med Microbiol. 310:1514252020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di Modica M, Arlotta V, Sfondrini L,
Tagliabue E and Triulzi T: The link between the microbiota and
HER2+ breast cancer: The new challenge of precision medicine. Front
Oncol. 12:9471882022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jaye K, Li CG, Chang D and Bhuyan DJ: The
role of key gut microbial metabolites in the development and
treatment of cancer. Gut Microbes. 14:20388652022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krishnamurthy K, Wang G, Rokhfeld D and
Bieberich E: Deoxycholate promotes survival of breast cancer cells
by reducing the level of pro-apoptotic ceramide. Breast Cancer Res.
10:R1062008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gándola YB, Fontana C, Bojorge MA,
Luschnat TT, Moretton MA, Chiapetta DA, Verstraeten SV and González
L: Concentration-dependent effects of sodium cholate and
deoxycholate bile salts on breast cancer cells proliferation and
survival. Mol Biol Rep. 47:3521–3539. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cong J, Liu P, Han Z, Ying W, Li C, Yang
Y, Wang S, Yang J, Cao F, Shen J, et al: Bile acids modified by the
intestinal microbiota promote colorectal cancer growth by
suppressing CD8+ T cell effector functions. Immunity. 57:876–889.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mikó E, Vida A, Kovács T, Ujlaki G,
Trencsényi G, Márton J, Sári Z, Kovács P, Boratkó A, Hujber Z, et
al: Lithocholic acid, a bacterial metabolite reduces breast cancer
cell proliferation and aggressiveness. Biochim Biophys Acta
Bioenerg. 1859:958–974. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang X, Lin CC, Spasojevic I, Iversen ES,
Chi JT and Marks JR: A joint analysis of metabolomics and genetics
of breast cancer. Breast Cancer Res. 16:4152014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sampsell K, Hao D and Reimer RA: The gut
microbiota: A potential gateway to improved health outcomes in
breast cancer treatment and survivorship. Int J Mol Sci.
21:92392020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luu TH, Bard JM, Carbonnelle D, Chaillou
C, Huvelin JM, Bobin-Dubigeon C and Nazih H: Lithocholic bile acid
inhibits lipogenesis and induces apoptosis in breast cancer cells.
Cell Oncol (Dordr). 41:13–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eslami SZ, Majidzadeh AK, Halvaei S,
Babapirali F and Esmaeili R: Microbiome and breast cancer: New role
for an ancient population. Front Oncol. 10:1202020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hou H, Chen D, Zhang K, Zhang W, Liu T,
Wang S, Dai X, Wang B, Zhong W and Cao H: Gut microbiota-derived
short-chain fatty acids and colorectal cancer: Ready for clinical
translation? Cancer Lett. 526:225–235. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mirzaei R, Afaghi A, Babakhani S, Sohrabi
MR, Hosseini-Fard SR, Babolhavaeji K, Khani Ali Akbari S,
Yousefimashouf R and Karampoor S: Role of microbiota-derived
short-chain fatty acids in cancer development and prevention.
Biomed Pharmacother. 139:1116192021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jaye K, Chang D, Li CG and Bhuyan DJ: Gut
metabolites and breast cancer: The continuum of dysbiosis, breast
cancer risk, and potential breast cancer therapy. Int J Mol Sci.
23:94902022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu P, Wang Y, Yang G, Zhang Q, Meng L,
Xin Y and Jiang X: The role of short-chain fatty acids in
intestinal barrier function, inflammation, oxidative stress, and
colonic carcinogenesis. Pharmacol Res. 165:1054202021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thirunavukkarasan M, Wang C, Rao A, Hind
T, Teo YR, Siddiquee AA, Goghari MAI, Kumar AP and Herr DR:
Short-chain fatty acid receptors inhibit invasive phenotypes in
breast cancer cells. PLoS One. 12:e01863342017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park HS, Han JH, Park JW, Lee DH, Jang KW,
Lee M, Heo KS and Myung CS: Sodium propionate exerts anticancer
effect in mice bearing breast cancer cell xenograft by regulating
JAK2/STAT3/ROS/p38 MAPK signaling. Acta Pharmacol Sin.
42:1311–1323. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen J, Zhao KN and Vitetta L: Effects of
intestinal microbial-elaborated butyrate on oncogenic signaling
pathways. Nutrients. 11:10262019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Hu PC, Ma YB, Fan R, Gao FF, Zhang
JW and Wei L: Sodium butyrate-induced apoptosis and ultrastructural
changes in MCF-7 breast cancer cells. Ultrastruct Pathol.
40:200–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mandal M and Kumar R: Bcl-2 expression
regulates sodium butyrate-induced apoptosis in human MCF-7 breast
cancer cells. Cell Growth Differ. 7:311–318. 1996.PubMed/NCBI
|
|
49
|
Chopin V, Toillon RA, Jouy N and Le
Bourhis X: Sodium butyrate induces P53-independent, Fas-mediated
apoptosis in MCF-7 human breast cancer cells. Br J Pharmacol.
135:79–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He C, Liu Y, Ye S, Yin S and Gu J: Changes
of intestinal microflora of breast cancer in premenopausal women.
Eur J Clin Microbiol Infect Dis. 40:503–513. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dai ZL, Wu G and Zhu WY: Amino acid
metabolism in intestinal bacteria: Links between gut ecology and
host health. Front Biosci (Landmark Ed). 16:1768–1786. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Roager HM and Licht TR: Microbial
tryptophan catabolites in health and disease. Nat Commun.
9:32942018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Renga G, Nunzi E, Pariano M, Puccetti M,
Bellet MM, Pieraccini G, D'Onofrio F, Santarelli I, Stincardini C,
Aversa F, et al: Optimizing therapeutic outcomes of immune
checkpoint blockade by a microbial tryptophan metabolite. J
Immunother Cancer. 10:e0037252022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sári Z, Mikó E, Kovács T, Boratkó A,
Ujlaki G, Jankó L, Kiss B, Uray K and Bai P: Indoxylsulfate, a
metabolite of the microbiome, has cytostatic effects in breast
cancer via activation of AHR and PXR receptors and induction of
oxidative stress. Cancers. 12:29152020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sári Z, Mikó E, Kovács T, Jankó L, Csonka
T, Lente G, Sebő É, Tóth J, Tóth D, Árkosy P, et al:
Indolepropionic acid, a metabolite of the microbiome, has
cytostatic properties in breast cancer by activating AHR and PXR
receptors and inducing oxidative stress. Cancers (Basel).
12:24112020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kovács T, Mikó E, Vida A, Sebő É, Toth J,
Csonka T, Boratkó A, Ujlaki G, Lente G, Kovács P, et al:
Cadaverine, a metabolite of the microbiome, reduces breast cancer
aggressiveness through trace amino acid receptors. Sci Rep.
9:13002019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nejman D, Livyatan I, Fuks G, Gavert N,
Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E,
et al: The human tumor microbiome is composed of tumor
type-specific intracellular bacteria. Science. 368:973–980. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shcheblyakov DV, Logunov DY, Tukhvatulin
AI, Shmarov MM, Naroditsky BS and Gintsburg AL: Toll-like receptors
(TLRs): The role in tumor progression. Acta Naturae. 2:21–29. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li J, Yin J, Shen W, Gao R, Liu Y, Chen Y,
Li X, Liu C, Xiang R and Luo N: TLR4 promotes breast cancer
metastasis via Akt/GSK3β/β-catenin pathway upon LPS stimulation.
Anat Rec (Hoboken). 300:1219–1229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liao SJ, Zhou YH, Yuan Y, Li D, Wu FH,
Wang Q, Zhu JH, Yan B, Wei JJ, Zhang GM and Feng ZH: Triggering of
toll-like receptor 4 on metastatic breast cancer cells promotes
αvβ3-mediated adhesion and invasive migration. Breast Cancer Res
Treat. 133:853–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang H, Wang B, Wang T, Xu L, He C, Wen H,
Yan J, Su H and Zhu X: Toll-like receptor 4 prompts human breast
cancer cells invasiveness via lipopolysaccharide stimulation and is
overexpressed in patients with lymph node metastasis. PLoS One.
9:e1099802014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Avand A, Akbari V and Shafizadegan S: In
vitro cytotoxic activity of a Lactococcus lactis antimicrobial
peptide against breast cancer cells. Iran J Biotechnol.
16:e18672018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Paiva AD, De Oliveira MD, De Paula SO,
Baracat-Pereira MC, Breukink E and Mantovani HC: Toxicity of
bovicin HC5 against mammalian cell lines and the role of
cholesterol in bacteriocin activity. Microbiology (Reading).
158:2851–2858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kamarajan P, Hayami T, Matte B, Liu Y,
Danciu T, Ramamoorthy A, Worden F, Kapila S and Kapila Y: Nisin ZP,
a bacteriocin and food preservative, inhibits head and neck cancer
tumorigenesis and prolongs survival. PLoS One. 10:e01310082015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ahmadi S, Ghollasi M and Hosseini HM: The
apoptotic impact of nisin as a potent bacteriocin on the colon
cancer cells. Microb Pathog. 111:193–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hall S, McDermott C, Anoopkumar-Dukie S,
McFarland AJ, Forbes A, Perkins AV, Davey AK, Chess-Williams R,
Kiefel MJ, Arora D and Grant GD: Cellular effects of pyocyanin, a
secreted virulence factor of Pseudomonas aeruginosa. Toxins
(Basel). 8:2362016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhao J, Wu Y, Alfred AT, Wei P and Yang S:
Anticancer effects of pyocyanin on HepG2 human hepatoma cells. Lett
Appl Microbiol. 58:541–548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Moayedi A, Nowroozi J and Sepahy AA:
Cytotoxic effect of pyocyanin on human pancreatic cancer cell line
(Panc-1). Iran J Basic Med Sci. 21:794–799. 2018.PubMed/NCBI
|
|
69
|
Abdelaziz AA, Kamer AMA, Al-Monofy KB and
Al-Madboly LA: A purified and lyophilized Pseudomonas
aeruginosa derived pyocyanin induces promising apoptotic and
necrotic activities against MCF-7 human breast adenocarcinoma.
Microb Cell Fact. 21:2622022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Geng HW, Yin FY, Zhang ZF, Gong X and Yang
Y: Butyrate suppresses glucose metabolism of colorectal cancer
cells via GPR109a-AKT signaling pathway and enhances chemotherapy.
Front Mol Biosci. 8:6348742021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen M, Jiang W, Xiao C, Yang W, Qin Q,
Mao A, Tan Q, Lian B and Wei C: Sodium butyrate combined with
docetaxel for the treatment of lung adenocarcinoma A549 cells by
targeting Gli1. Onco Targets Ther. 13:8861–8875. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lajkó E, Spring S, Hegedüs R, Biri-Kovács
B, Ingebrandt S, Mező G and Kőhidai L: Comparative cell biological
study of in vitro antitumor and antimetastatic activity on melanoma
cells of GnRH-III-containing conjugates modified with short-chain
fatty acids. Beilstein J Org Chem. 14:2495–2509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen W, Wei F, Xu J, Wang Y, Chen L, Wang
J and Guan X: Trastuzumab enhances the anti-tumor effects of the
histone deacetylase inhibitor sodium butyrate on a
HER2-overexpressing breast cancer cell line. Int J Mol Med.
28:985–991. 2011.PubMed/NCBI
|
|
74
|
Andrade FO, Nagamine MK, Conti AD, Chaible
LM, Fontelles CC, Jordão Junior AA, Vannucchi H, Dagli ML, Bassoli
BK, Moreno FS and Ong TP: Efficacy of the dietary histone
deacetylase inhibitor butyrate alone or in combination with vitamin
A against proliferation of MCF-7 human breast cancer cells. Braz J
Med Biol Res. 45:841–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nomura M, Nagatomo R, Doi K, Shimizu J,
Baba K, Saito T, Matsumoto S, Inoue K and Muto M: Association of
short-chain fatty acids in the gut microbiome with clinical
response to treatment with nivolumab or pembrolizumab in patients
with solid cancer tumors. JAMA Netw Open. 3:e2028952020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ren S, Feng L, Liu H, Mao Y and Yu Z: Gut
microbiome affects the response to immunotherapy in non-small cell
lung cancer. Thorac Cancer. 15:1149–1163. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Muradas TC, Freitas RD, Goncalves JI,
Xavier FA and Marinowic DR: Potential antitumor effects of
short-chain fatty acids in breast cancer models. Am J Cancer Res.
14:1999–2019. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Coutzac C, Jouniaux JM, Paci A, Schmidt J,
Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix
L, et al: Systemic short chain fatty acids limit antitumor effect
of CTLA-4 blockade in hosts with cancer. Nat Commun. 11:21682020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhou C, Basnet R, Zhen C, Ma S, Guo X,
Wang Z and Yuan Y: Trimethylamine N-oxide promotes the
proliferation and migration of hepatocellular carcinoma cell
through the MAPK pathway. Discov Oncol. 15:3462024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jalandra R, Dalal N, Yadav AK, Verma D,
Sharma M, Singh R, Khosla A, Kumar A and Solanki PR: Emerging role
of trimethylamine-N-oxide (TMAO) in colorectal cancer. Appl
Microbiol Biotechnol. 105:7651–7660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang H, Rong X, Zhao G, Zhou Y, Xiao Y, Ma
D, Jin X, Wu Y, Yan Y, Yang H, et al: The microbial metabolite
trimethylamine N-oxide promotes antitumor immunity in
triple-negative breast cancer. Cell Metab. 34:581–594.e8. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yang S, Dai H, Lu Y, Li R, Gao C and Pan
S: Trimethylamine N-oxide promotes cell proliferation and
angiogenesis in colorectal cancer. J Immunol Res. 2022:70438562022.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chiba A, Bawaneh A, Velazquez C, Clear
KYJ, Wilson AS, Howard-McNatt M, Levine EA, Levi-Polyachenko N,
Yates-Alston SA, Diggle SP, et al: Neoadjuvant chemotherapy shifts
breast tumor microbiota populations to regulate drug responsiveness
and the development of metastasis. Mol Cancer Res. 18:130–139.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Groizeleau J, Rybtke M, Andersen JB,
Berthelsen J, Liu Y, Yang L, Nielsen TE, Kaever V, Givskov M and
Tolker-Nielsen T: The anti-cancerous drug doxorubicin decreases the
c-di-GMP content in Pseudomonas aeruginosa but promotes
biofilm formation. Microbiology (Reading). 162:1797–1807. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Abdelaziz AA, Kamer AMA, Al-Monofy KB and
Al-Madboly LA: Pseudomonas aeruginosa's greenish-blue
pigment pyocyanin: Its production and biological activities. Microb
Cell Fact. 22:1102023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chiba A, Bawaneh A, Velazquez C, Clear
KYJ, Wilson AS, Howard-McNatt M, Levine EA, Levi-Polyachenko N,
Yates-Alston SA, Diggle SP, et al: Neoadjuvant Chemotherapy shifts
breast tumor microbiota populations to regulate drug responsiveness
and the development of metastasis. Mol Cancer Res. 18:130–139.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Baindara P and Mandal SM: Bacteria and
bacterial anticancer agents as a promising alternative for cancer
therapeutics. Biochimie. 177:164–189. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rana K, Sharma R and Preet S: Augmented
therapeutic efficacy of 5-fluorouracil in conjunction with
lantibiotic nisin against skin cancer. Biochem Biophys Res Commun.
520:551–559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Coker OO, Liu C, Wu WKK, Wong SH, Jia W,
Sung JJY and Yu J: Altered gut metabolites and microbiota
interactions are implicated in colorectal carcinogenesis and can be
non-invasive diagnostic biomarkers. Microbiome. 10:352022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gao L, Zhang JH, Chen XX, Ren HL, Feng XL,
Wang JL and Xiao JH: Combination of L-Arginine and L-Norvaline
protects against pulmonary fibrosis progression induced by
bleomycin in mice. Biomed Pharmacother. 113:1087682019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ren X, Wang N, Zhou Y, Song A, Jin G, Li Z
and Luan Y: An injectable hydrogel using an immunomodulating
gelator for amplified tumor immunotherapy by blocking the arginase
pathway. Acta Biomater. 124:179–190. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Arlauckas SP, Garren SB, Garris CS, Kohler
RH, Oh J, Pittet MJ and Weissleder R: Arg1 expression defines
immunosuppressive subsets of tumor-associated macrophages.
Theranostics. 8:5842–5854. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yurdagul AJ, Subramanian M, Wang X, Crown
SB, Ilkayeva OR, Darville L, Kolluru GK, Rymond CC, Gerlach BD,
Zheng Z, et al: Macrophage metabolism of apoptotic cell-derived
arginine promotes continual efferocytosis and resolution of injury.
Cell Metab. 31:518–533.e10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wieërs G, Belkhir L, Enaud R, Leclercq S,
Philippart de Foy JM, Dequenne I, de Timary P and Cani PD: How
probiotics affect the microbiota. Front Cell Infect Microbiol.
9:4542020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
German R, Marino N, Hemmerich C, Podicheti
R, Rusch DB, Stiemsma LT, Gao H, Xuei X, Rockey P and Storniolo AM:
Exploring breast tissue microbial composition and the association
with breast cancer risk factors. Breast Cancer Res. 25:822023.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Davani-Davari D, Negahdaripour M,
Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A and
Ghasemi Y: Prebiotics: Definition, types, sources, mechanisms, and
clinical applications. Foods. 8:922019. View Article : Google Scholar : PubMed/NCBI
|