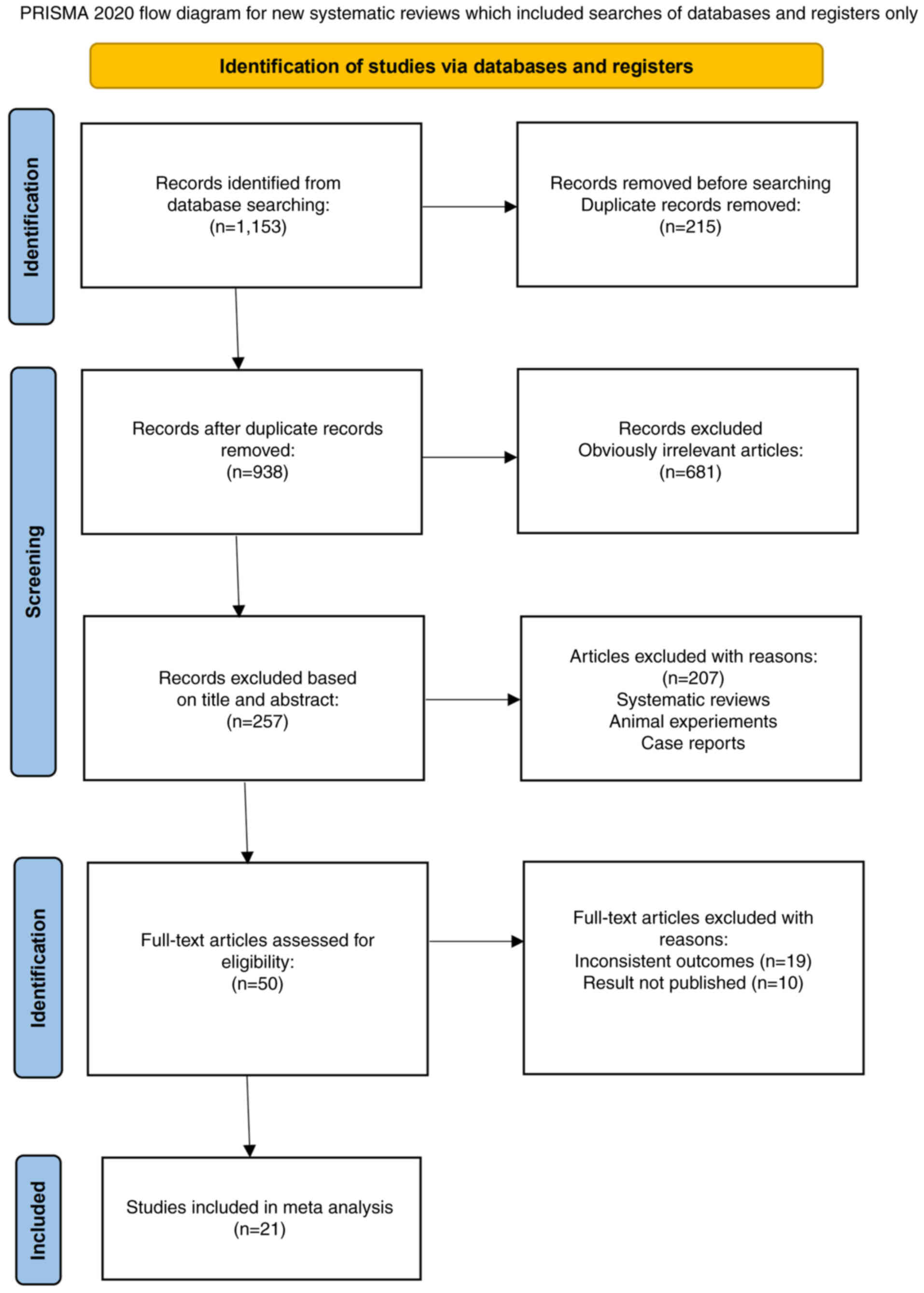
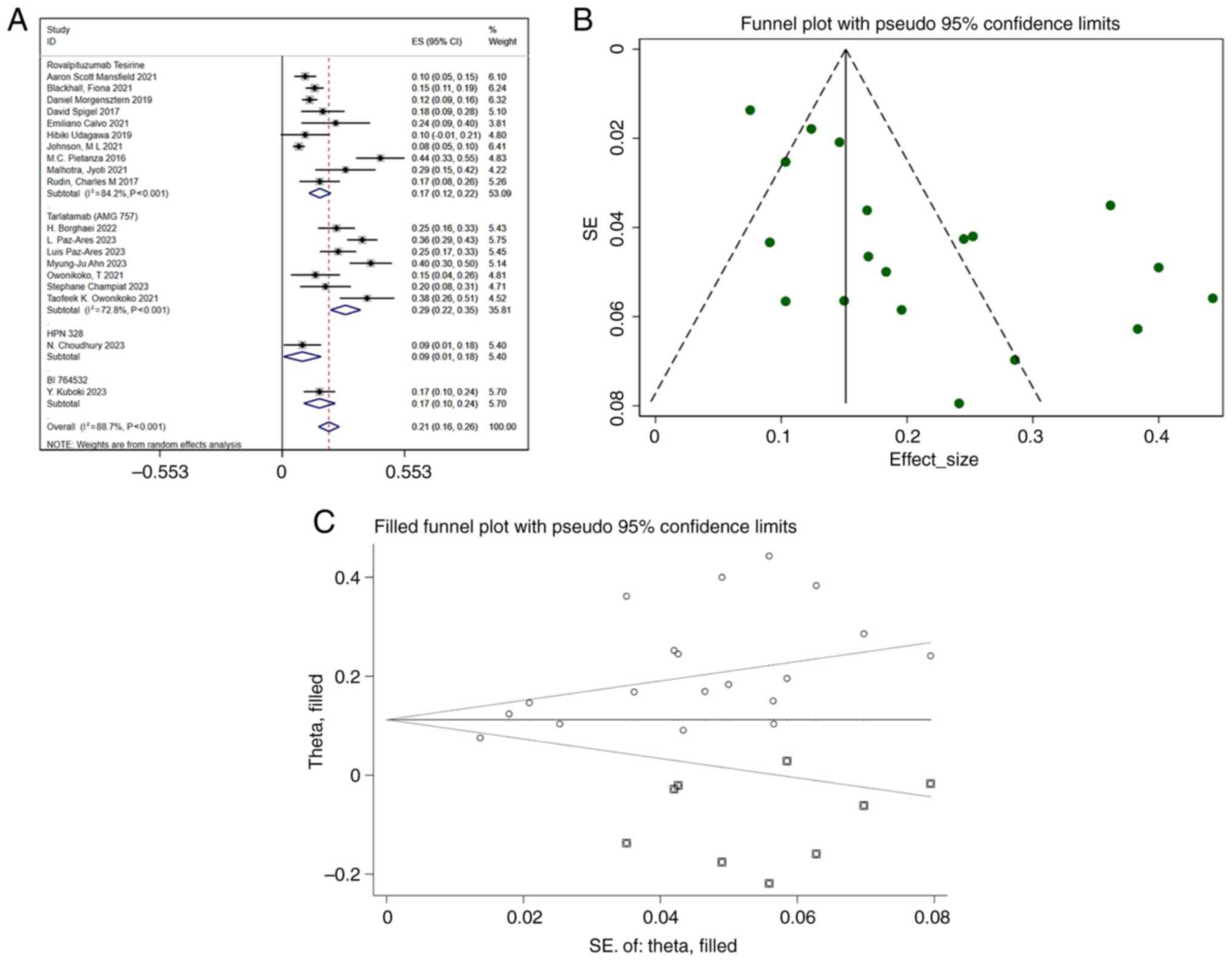
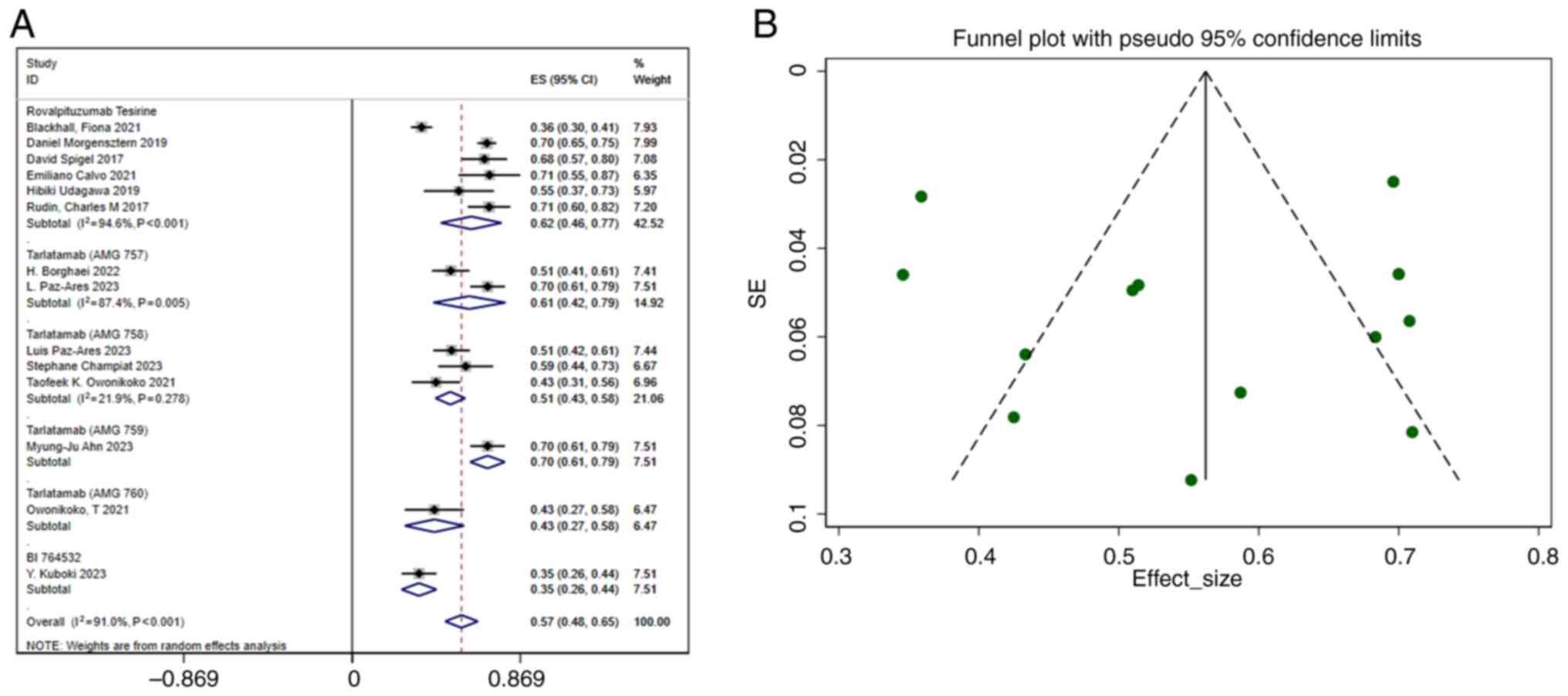
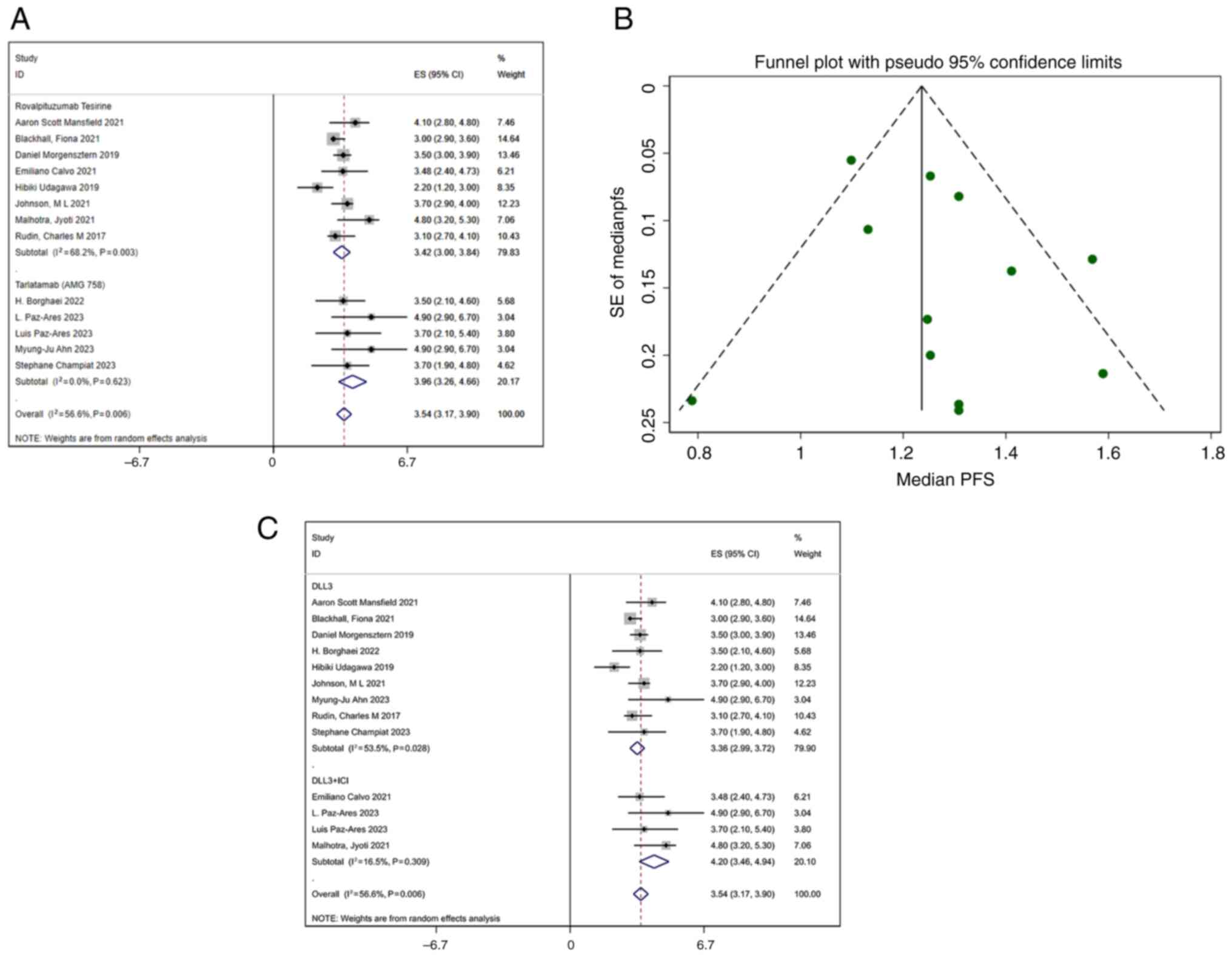
|
1
|
Guruharsha KG, Kankel MW and
Artavanis-Tsakonas S: The Notch signalling system: Recent insights
into the complexity of a conserved pathway. Nat Rev Genet.
13:654–666. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray SJ: Notch signalling in context. Nat
Rev Mol Cell Bio. 17:722–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meurette O and Mehlen P: Notch signaling
in the tumor microenvironment. Cancer Cell. 34:536–548. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Radtke F and Raj K: The role of Notch in
tumorigenesis: Oncogene or tumour suppressor? Nat Rev Cancer.
3:756–767. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chapman G, Sparrow DB, Kremmer E and
Dunwoodie SL: Notch inhibition by the ligand DELTA-LIKE 3 defines
the mechanism of abnormal vertebral segmentation in spondylocostal
dysostosis. Hum Mol Genet. 20:905–916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabari JK, Lok BH, Laird JH, Poirier JT
and Rudin CM: Unravelling the biology of SCLC: Implications for
therapy. Nat Rev Clin Oncol. 14:549–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saunders LR, Bankovich AJ, Anderson WC,
Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang
A, et al: A DLL3-targeted antibody-drug conjugate eradicates
high-grade pulmonary neuroendocrine tumor-initiating cells in vivo.
Sci Transl Med. 7:302ra1362015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao J, Bergsland E, Aggarwal R, Aparicio
A, Beltran H, Crabtree JS, Hann CL, Ibrahim T, Byers LA, Sasano H,
et al: DLL3 as an emerging target for the treatment of
neuroendocrine neoplasms. Oncologist. 27:940–951. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Owen DH, Giffin MJ, Bailis JM, Smit MD,
Carbone DP and He K: DLL3: An emerging target in small cell lung
cancer. J Hematol Oncol. 12:612019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rudin CM, Pietanza MC, Bauer TM, Ready N,
Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA III,
Robert F, et al: Rovalpituzumab tesirine, a DLL3-targeted
antibody-drug conjugate, in recurrent small-cell lung cancer: A
first-in-human, first-in-class, open-label, phase 1 study. Lancet
Oncol. 18:42–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giffin MJ, Cooke K, Lobenhofer EK, Estrada
J, Zhan J, Deegen P, Thomas M, Murawsky CM, Werner J, Liu S, et al:
AMG 757, a half-life extended, DLL3-targeted bispecific T-cell
engager, shows high potency and sensitivity in preclinical models
of small-cell lung cancer. Clin Cancer Res. 27:1526–1537. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Champiat S, Boyer MJ, Govindan R, Paz-Ares
LG, Owonikoko TK, Borghaei H, Izumi H, Steeghs N, Helen Blackhall
F, Terbuch A, et al: Tarlatamab in small cell lung cancer (SCLC):
Safety and efficacy analyzed by baseline brain metastasis. J Clin
Oncol. 41 (16 Suppl):S85822023. View Article : Google Scholar
|
|
13
|
Ahn MJ, Cho BC, Felip E, Korantzis I,
Ohashi K, Majem M, Juan-Vidal O, Handzhiev S, Izumi H, Lee JS, et
al: Tarlatamab for patients with previously treated small-cell lung
cancer. New Engl J Med. 389:2063–2075. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choudhury N, Jain P, Dowlati A, Thompson
J, Johnson ML, Mamdani H, Sanborn RE, Schenk EL, Aggarwal R, Sankar
K, et al: 698P Interim results from a phase I/II study of HPN328, a
tri-specific, half-life (T1/2) extended DLL3-targeting T cell
engager in patients (pts) with small cell lung cancer (SCLC) and
other neuroendocrine neoplasms (NEN). Ann Oncol. 34 (Suppl
2):S4862023. View Article : Google Scholar
|
|
15
|
Owonikoko TK, Champiat S, Johnson ML,
Govindan R, Izumi H, Lai WVV, Borghaei H, Boyer MJ, Boosman RJ,
Hummel HD, et al: Updated results from a phase 1 study of AMG 757,
a half-life extended bispecific T-cell engager (BiTE)
immuno-oncology therapy against delta-like ligand 3 (DLL3), in
small cell lung cancer (SCLC). J Clin Oncol. 39 (15
Suppl):S85102021. View Article : Google Scholar
|
|
16
|
Wermke M, Kuboki Y, Felip E, Alese OB,
Morgensztern D, Sayehli C, Arriola E, Sanmamed MF, Hamed ZO, Song
E, et al: OA01.05 Phase I dose escalation trial of the DLL3/CD3
Igg-like T cell engager BI 764532 in patients with DLL3+ tumors:
Focus on SCLC. J Thorac Oncol. 18 (Suppl):S45–S46. 2023. View Article : Google Scholar
|
|
17
|
Blackhall F, Jao K, Greillier L, Cho BC,
Penkov K, Reguart N, Majem M, Nackaerts K, Syrigos K, Hansen K, et
al: Efficacy and safety of rovalpituzumab tesirine compared with
topotecan as second-line therapy in DLL3-high SCLC: Results from
the phase 3 TAHOE study. J Thorac Oncol. 16:1547–1558. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson ML, Zvirbule Z, Laktionov K,
Helland A, Cho BC, Gutierrez V, Colinet B, Lena H, Wolf M,
Gottfried M, et al: Rovalpituzumab tesirine as a maintenance
therapy after first-line platinum-based chemotherapy in patients
with extensive-stage-SCLC: Results from the phase 3 MERU study. J
Thorac Oncol. 16:1570–1581. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morgensztern D, Besse B, Greillier L,
Santana-Davila R, Ready N, Hann CL, Glisson BS, Farago AF, Dowlati
A, Rudin CM, et al: Efficacy and safety of rovalpituzumab tesirine
in third-line and beyond patients with DLL3-expressing,
relapsed/refractory small-cell lung cancer: Results from the phase
II TRINITY study. Clin Cancer Res. 25:6958–6966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lovely B and Hollasch M: DLL3-targeting
agents are poised to fill unmet needs in SCLC. Oncol
Live®. 24:2023.
|
|
22
|
Lin S, Zhang Y, Yao J, Yang J, Qiu Y, Zhu
Z and Hua H: DB-1314, a novel DLL3-targeting ADC with DNA
topoisomerase I inhibitor, exhibits promising safety profile and
therapeutic efficacy in preclinical small cell lung cancer models.
J Transl Med. 22:7662024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sands JM, Champiat S, Hummel H, Paulson
KG, Borghaei H, Bustamante Alvarez J, Carbone DP, Carlisle JW,
Choudhury NJ, Clarke JM, et al: Practical management of adverse
events in patients receiving tarlatamab, a DLL3-targeted bispecific
T-cell engager immunotherapy, for previously treated small cell
lung cancer. medRxiv. 2024.
|
|
24
|
von Pawel J, Schiller JH, Shepherd FA,
Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer
MC, Depierre A, et al: Topotecan versus cyclophosphamide,
doxorubicin, and vincristine for the treatment of recurrent
small-cell lung cancer. J Clin Oncol. 17:658–6567. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armstrong D and O'Reilly S: Clinical
guidelines for managing topotecan-related hematologic toxicity.
Oncologist. 3:4–10. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding J and Yeong C: Advances in
DLL3-targeted therapies for small cell lung cancer: Challenges,
opportunities, and future directions. Front Oncol. 14:15041392024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ogawa H, Sakai Y, Nishio W, Fujibayashi Y,
Nishikubo M, Nishioka Y, Tane S, Kitamura Y, Sudo T, Sakuma T and
Yoshimura M: DLL3 expression is a predictive marker of sensitivity
to adjuvant chemotherapy for pulmonary LCNEC. Thorac Cancer.
11:2561–2569. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tully KM, Tendler S, Carter LM, Sharma SK,
Samuels ZV, Mandleywala K, Korsen JA, Delos Reyes AM, Piersigilli
A, Travis WD, et al: Radioimmunotherapy targeting delta-like ligand
3 in small cell lung cancer exhibits antitumor efficacy with low
toxicity. Clin Cancer Res. 28:1391–1401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aggarwal RR, Rottey S, Bernard-Tessier A,
Mellado-Gonzalez B, Kosaka T, Stadler WM, Sandhu S, Yu B, Shaw C,
Ju CH, et al: Phase 1b study of tarlatamab in de novo or
treatment-emergent neuroendocrine prostate cancer (NEPC). J Clin
Oncol. 42 (16 Suppl):S50122024. View Article : Google Scholar
|
|
30
|
Ataee MH, Mirhosseini SA, Mirnejad R,
Rezaie E, Hosseini HM and Amani J: Design of two immunotoxins based
rovalpituzumab antibody against DLL3 receptor; a promising
potential opportunity. Res Pharm Sci. 17:428–444. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mansfield AS, Hong DS, Hann CL, Farago AF,
Beltran H, Waqar SN, Hendifar AE, Anthony LB, Taylor MH, Bryce AH,
et al: A phase I/II study of rovalpituzumab tesirine in delta-like
3-expressing, advanced solid tumors. NPJ Precis Oncol. 5:742021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spigel D, Pietanza MC, Bauer T, Ready N,
Morgensztern D, Glisson BS, Byers LA, Johnson M, Burris H, Robert
F, et al: OA05.03 Single-agent rovalpituzumab tesirine, a
delta-like protein 3 (DLL3)-targeted antibody-drug conjugate (ADC),
in small-cell lung cancer (SCLC). J Thorac Oncol. 12
(Suppl):S260–S261. 2017. View Article : Google Scholar
|
|
33
|
Calvo E, Spira A, Miguel MD, Kondo S,
Gazzah A, Millward M, Prenen H, Rottey S, Warburton L, Alanko T, et
al: Safety, pharmacokinetics, and efficacy of budigalimab with
rovalpituzumab tesirine in patients with small cell lung cancer.
Cancer Treat Res Commun. 28:1004052021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borghaei H, Paz-Ares L, Johnson M,
Champiat S, Owonikoko T, Lai V, Boyer M, Hummel HD, Govindan R,
Steeghs N, et al: OA12.05 phase 1 updated exploration and first
expansion data for DLL3-targeted T-cell engager tarlatamab in small
cell lung cancer. J Thorac Oncol. 17 (Suppl):S332022. View Article : Google Scholar
|
|
35
|
Udagawa H, Akamatsu H, Tanaka K, Takeda M,
Kanda S, Kirita K, Teraoka S, Nakagawa K, Fujiwara Y, Yasuda I, et
al: Phase I safety and pharmacokinetics study of rovalpituzumab
tesirine in Japanese patients with advanced, recurrent small cell
lung cancer. Lung Cancer. 135:145–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paz-Ares L, Ahn M, Felip E, Handzhiev S,
Korantzis I, Izumi H, Ohashi K, Tarruella MM, Wolf J, Reck M, et
al: 508MO Tarlatamab for patients (pts) with previously treated
small cell lung cancer (SCLC): Primary analysis of the phase II
DeLLphi-301 study. Ann Oncol. 34:S1664–S1665. 2023. View Article : Google Scholar
|
|
37
|
Paz-Ares L, Champiat S, Lai WV, Izumi H,
Govindan R, Boyer M, Hummel HD, Borghaei H, Johnson ML, Steeghs N,
et al: Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell
engager, in recurrent small-cell lung cancer: An open-label, phase
I study. J Clin Oncol. 41:2893–2903. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pietanza MC, Spigel D, Bauer TM, Ready NE,
Glisson BS, Morgensztern D, Robert F, Salgia R, Kochendorfer M,
Patel M, et al: 7LBA Safety, activity, and response durability
assessment of single agent rovalpituzumab tesirine, a delta-like
protein 3 (DLL3)-targeted antibody drug conjugate (ADC), in small
cell lung cancer (SCLC). Eur J Cancer. 51:S7122015. View Article : Google Scholar
|
|
39
|
Malhotra J, Nikolinakos P, Leal T, Lehman
J, Morgensztern D, Patel JD, Wrangle JM, Curigliano G, Greillier L,
Johnson ML, et al: A phase 1–2 study of rovalpituzumab tesirine in
combination with nivolumab plus or minus ipilimumab in patients
with previously treated extensive-stage SCLC. J Thorac Oncol.
16:1559–1569. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Owonikoko T, Boyer M, Johnson M, Govindan
R, Rodrigues L, Blackhall F, Boosman R, Champiat S, Hummel HD, Lai
WV, et al: OA11.03 A phase 1 study of AMG 757, half-life extended
bispecific T-cell engager (BiTE®)Immune therapy against
DLL3, in SCLC. J Thorac Oncol. 16:S1262021. View Article : Google Scholar
|
|
41
|
Champiat S, Boyer M, Paz-Ares L,
Schoenfeld A, Izumi H, Govindan R, Carlisle J, Borghaei H, Johnson
ML, Steeghs N, et al: 147P Characterizing CRS in phase I study of
DLL3-targeted T cell engager tarlatamab in small cell lung cancer.
Immun Oncol Technol. 16 (Suppl 1):S1002592022. View Article : Google Scholar
|
|
42
|
Kuboki Y, Gambardella V, Capdevila
Castillon J, Alese OB, Morgensztern D, Sayehli CM, Sanmamed MF,
Arriola E, Wolf J, Owonikoko TK, et al: 75MO Phase I trial of the
DLL3/CD3 IgG-like T cell engager BI 764532 in patients (pts) with
DLL3+ tumors: Focus on Asian pts. Ann Oncol. 34 (Suppl
4):S14952023. View Article : Google Scholar
|