Introduction
The Notch signaling pathway is a fundamental
mechanism of cellular communication, widely present in animal
cells, which serves essential roles in both organismal development
and cellular homeostasis. Th Notch pathway consists of four
transmembrane receptors: Notch1, Notch2, Notch3 and Notch4, and is
mediated by five ligands expressed by adjacent cells: Delta-like
ligand (DLL)1, DLL3, DLL4, jagged canonical Notch ligand (JAG)1 and
JAG2 (1–3). Among these, DLL3 serves a pivotal role
in Notch signaling, affecting cellular differentiation,
proliferation, survival and apoptosis (4). DLL3 functions as an inhibitory ligand
in the Notch pathway by sequestering itself and occasionally DLL1
within the cell, preventing their localization to the cell surface
and serving as an intrinsic suppressor of Notch signaling. Notably,
DLL3 is upregulated and aberrantly presented on the cell surface in
several advanced solid tumors, including small cell lung cancer
(SCLC), neuroendocrine tumors, pancreatic cancer, melanoma and
liver cancer (5–7).
High DLL3 expression not only promotes tumor cell
growth and proliferation through the Notch signaling pathway, but
also inhibits immune responses within the tumor microenvironment;
this diminishes the effectiveness of antitumor immunity, and
facilitates tumor cell migration and invasion (8). Consequently, elevated DLL3 expression
is strongly associated with poor prognoses in several types of
cancer and serves a key role in sustaining malignant tumor growth.
As a result, DLL3-targeted research and therapeutic strategies have
gained notable global interest, offering the potential to improve
outcomes for patients with DLL3-expressing cancer.
Several DLL3-targeting inhibitors and modulators are
in early clinical development, including the antibody-drug
conjugate rovalpituzumab tesirine (Rova-T), the bispecific T-cell
engager (BiTE) tarlatamab (AMG 757) and the chimeric antigen
receptor T-cell therapy AMG 119 (9). The NCT01901653 study, a pioneering
open-label Phase I clinical trial in the United States of America,
enrolled patients with recurrent or progressive SCLC or large-cell
neuroendocrine carcinoma (NEC). The NCT01901653 study established
the safety, tolerability and maximum tolerated dose of Rova-T, with
notable adverse events (AEs), including thrombocytopenia, pleural
effusion and elevated lipase levels. The maximum tolerated dose was
found to be 0.4 mg/kg, administered every 3 weeks, with a
recommended dose of 0.3 mg/kg every 6 weeks for Phase II trials
(10).
Tarlatamab, a pioneering BiTE molecule with an
extended half-life, binds to DLL3 on tumor cell surfaces and CD3 on
cytotoxic T lymphocytes (CTLs). This binding triggers T-cell
activation, the release of inflammatory cytokines and CTL-mediated
apoptosis of DLL3-expressing tumor cells (11). Results from the Phase I DeLLphi-300
study (12) and the Phase II
DeLLphi-301 study (13)
demonstrated that tarlatamab markedly prolonged progression-free
survival (PFS) and improved the objective response rate (ORR) in
patients with previously treated extensive-stage SCLC, despite the
majority experiencing cytokine release syndrome (CRS). However,
severe AEs of grade ≥3 were rare and reversible. Based on these
promising results, the United States Food and Drug Administration
(FDA) granted expedited approval for tarlatamab to treat patients
with extensive-stage SCLC whose disease had progressed during or
after platinum-based chemotherapy. Other DLL3 inhibitors, including
AMG 119, BI 764532, HPN328, ZL-1310 and QLS31904, have also
advanced to clinical development stages, heralding a new era in
targeted DLL3 inhibition (14–16).
Despite the promising results from Phase I and II
TRINITY clinical studies, which confirmed the antitumor efficacy of
Rova-T, subsequent Phase III trials, namely the TAHOE and MERU
studies (17,18), failed to yield the expected
outcomes. In the TAHOE study, the median overall survival (OS) for
the Rova-T group was 6.3 months and the median PFS was 3.0 months,
both of which did not exceed the results from the second-line
treatment, topotecan (17). As a
result, the development of Rova-T was discontinued in August 2019.
By contrast, tarlatamab has shown higher response rates and
improved PFS in Phase II trials, and its Biologics License
Application is currently under FDA review (13).
To further assess the efficacy and safety of DLL3
inhibitors in patients with DLL3-high-expressing advanced solid
tumors, a systematic review and meta-analysis was performed. The
present review summarizes clinical trial data on ORR, disease
control rate (DCR), median PFS, median OS and the incidence of AEs.
The present analysis aimed to provide a comprehensive understanding
of the potential benefits and limitations of DLL3 inhibitors in
cancer treatment.
Materials and methods
Study design and population
A meta-analysis was performed following the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
guidelines. The study population consisted of patients with
advanced solid tumors characterized by high DLL3 expression,
including SCLC, NEC, pancreatic cancer, melanoma and hepatocellular
carcinoma. Eligibility criteria included patients who received DLL3
inhibitors (such as Rova-T, tarlatamab, BI 764532, HPN328) in
either monotherapy or combination therapy regimens. The primary
endpoint was median OS, while secondary endpoints included median
PFS, DCR, ORR and treatment-related AEs (trAEs), such as CRS and
thrombocytopenia. The objective of the present analysis was to
provide a comprehensive evaluation of the efficacy and safety
profile of DLL3 inhibitors.
Search strategy
An exhaustive search was performed for clinical
trials evaluating DLL3 inhibitors, such as Rova-T, tarlatamab, AMG
119, BI 764532, HPN328 and other similar agents, across several
prominent databases including PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science
(https://www.webofscience.com), Cochrane
Library (https://www.cochranelibrary.com), EMBASE (https://www.embase.com), Chinese National Knowledge
Infrastructure (CNKI; http://www.cnki.net), Wanfang Data (http://www.wanfangdata.com.cn), Chinese
Biological Medicine Database (https://www.sinomed.ac.cn) and VIP databases
(http://www.cqvip.com) up until October 2024.
Additionally, abstract proceedings and virtual meeting
presentations from major oncology organizations were reviewed,
including the American Society of Clinical Oncology (ASCO;
http://www.asco.org), the American Association
for Cancer Research (AACR; http://www.aacr.org) and the European Society of
Medical Oncology (ESMO; http://www.esmo.org).
Literature selection criteria
The inclusion criteria were as follows: i) Clinical
trials investigating DLL3 inhibitors, whether used alone or in
combination, that reported clinical outcomes, such as ORR, DCR,
PFS, OS and AEs; ii) studies including randomized controlled trials
(RCTs), quasi-RCTs, non-randomized comparative studies, single-arm
trials and trials in which DLL3 inhibitors were used in both
experimental arms; iii) trials in which DLL3 inhibitors were
administered alone or in combination with other chemotherapeutic
agents in the experimental group, with placebo or other
chemotherapeutic agents used in the control group; and iv) trials
that included ≥10 participants. Exclusion criteria encompassed
duplicate publications, review articles, systematic reviews, basic
experimental studies, studies lacking necessary data and studies
with incomplete, inconsistent outcomes or flawed trial designs. Two
investigators independently screened the titles and abstracts of
the identified studies, excluded those deemed irrelevant according
to the inclusion and exclusion criteria, and subsequently assessed
the full texts to confirm eligibility. Ultimately, 21 trials
involving a total of 2,452 patients were included in the final
analysis.
Data extraction and quality
assessment
A customized data extraction form was developed to
collect information regarding study design, participant
characteristics, intervention details and outcome measures. Two
authors independently extracted the relevant data, which included
the first author, publication year, country, study type, type of
DLL3 inhibitor, number of participants, patient demographics (age,
sex), tumor type, treatment regimen and clinical outcomes, such as
ORR, DCR, PFS, OS and AEs. The methodological quality of the
included studies was evaluated using the methodological index for
non-randomized studies criteria (Table
I) (19). All studies met the
predefined inclusion criteria and displayed a high level of
methodological rigor, thereby ensuring the reliability of the
findings presented in the current analysis.
 | Table I.Methodological index for
non-randomized studies. |
Table I.
Methodological index for
non-randomized studies.
| First author,
year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Scorea | (Refs.) |
|---|
| Mansfield et
al, 2021 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 | (31) |
| Blackhall et
al, 2021 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 | (17) |
| Morgensztern et
al, 2019 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 | (20) |
| Spigel et
al, 2017 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | (32) |
| Calvo et al,
2021 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 | (33) |
| Borghaei et
al, 2022 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | (34) |
| Udagawa et
al, 2019 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 13 | (35) |
| Johnson et
al, 2021 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 | (18) |
| Paz-Ares et
al, 2023 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | (36) |
| Paz-Ares et
al, 2023 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | (37) |
| Pietanza et
al, 2015 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | (38) |
| Malhotra et
al, 2021 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 13 | (39) |
| Ahn et al,
2023 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 | (13) |
| Choudhury et
al, 2023 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | (14) |
| Owonikoko et
al, 2021 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | (40) |
| Rudin et al,
2017 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 | (10) |
| Champiat et
al, 2022 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 13 | (41) |
| Champiat et
al, 2023 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | (12) |
| Owonikoko et
al, 2021 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 1 | 12 | (15) |
| Wermke et
al, 2023 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 | (16) |
| Kuboki et
al, 2023 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 | (42) |
Statistical analysis
Meta-analysis was performed using Stata statistical
software (version 16; StataCorp LP). The overall clinical
percentages for the primary outcomes and the total number of
participants were entered into Stata, which calculated the standard
errors for these quasi-normal distribution ‘rates’. Based on these
rates and their standard errors, the 95% confidence intervals (CIs)
for the lower and upper bounds were determined. Pooled effect sizes
(ES), represented by median rates with 95% CIs, were also derived.
P<0.05 was considered to indicate statistical significance.
Hypotheses were made only when the 95% CIs of some
data were missing. The statistical method relied on was that the
CI=[sample mean-Z × (standard deviation/√n), sample mean + Z ×
(standard deviation/√n)], where Z was the Z-value at the 95%
confidence level (usually 1.96).
To account for potential variability across studies,
random-effects models were used for all pooled ES. This approach
was chosen because it accommodates study-level differences without
requiring a detailed examination of heterogeneity. Given the
inherent variability in non-comparative studies, heterogeneity was
assessed using the I2 statistic, although it was not
involved in decision-making. Meta-regression analysis was also
performed to explore the relationship between study-level
characteristics and effect sizes, helping to identify sources of
heterogeneity. Sensitivity analyses were performed to assess the
robustness and consistency of the combined results. Finally,
Egger's test was performed to examine potential publication bias.
To address any potential publication bias, the trim-and-fill method
was also applied. This method trims the asymmetrical portion of the
funnel plot and imputes missing studies to restore symmetry,
providing a more accurate estimate of the overall effect size. The
leave-one-out method involves iteratively excluding one study at a
time from the analysis and recalculating the results. This helps
assess the impact of individual studies on the overall findings and
ensures result robustness.
Results
Overview of included studies
A thorough search was performed across multiple
databases, including PubMed, Embase, Web of Science, Sinomed,
WanFang, VIP, CNKI and Cochrane Library, up to October 2024. A
total of 1,153 records were initially retrieved: Sinomed (n=30),
WanFang (n=468), VIP (n=84), CNKI (n=33), Embase (n=300), Cochrane
Library (n=28), PubMed (n=163) and Web of Science (n=47). After
excluding 215 duplicate records, 681 articles were excluded based
on irrelevant content and 207 articles were excluded due to their
classification as systematic reviews, animal studies or case
reports. Full-text articles were further excluded for the following
reasons: Inconsistent outcomes (19 articles) and unpublished
results (10 articles). After a thorough review of the remaining 50
articles, 29 studies were excluded based on the inclusion and
exclusion criteria [inconsistent outcomes (n=19 articles) and
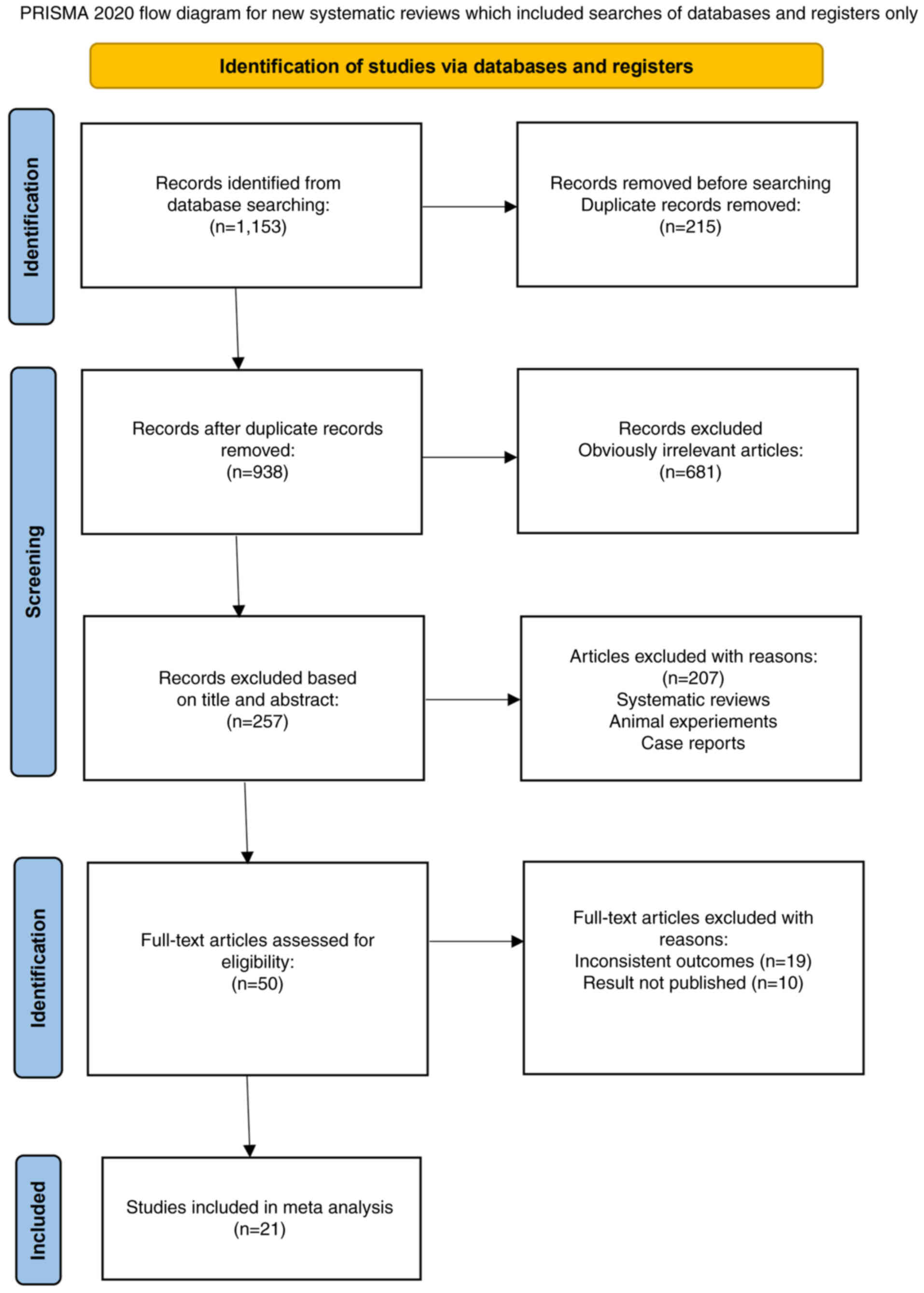
unpublished results (n=10 articles)]. The flowchart of the
literature screening process is shown in Fig. 1.
Ultimately, 21 trials involving 2,452 patients were
included in the analysis. Among these, 5 studies were published
before 2021, while 16 were published thereafter. The included
studies comprised 2 RCTs, 8 multi-arm studies and 11 single-arm
studies. Excluding 2 large-scale Phase III trials, the remaining
studies were Phase I or II clinical trials. Most participants in
the included studies had SCLC. In addition, 10 studies utilized
Rova-T as the DLL3 inhibitor, 8 used tarlatamab (AMG 757), 1 used
HPN 328 and 2 used BI 764532. The choice of DLL3 inhibitors was
closely associated with the publication year, with studies
published prior to 2021 primarily using Rova-T. A detailed summary
of the characteristics of each study is presented in Tables II and III. These comprised 2 RCTs and 19
non-RCTs, assessing the efficacy and safety of DLL3 inhibitors for
the treatment of solid tumors.
 | Table II.Characteristics of clinical trials
included in the single-arm meta-analysis. |
Table II.
Characteristics of clinical trials
included in the single-arm meta-analysis.
| First author,
year | Phase | Design | Type of tumors | Type of
inhibitors | Total samples | Age, years
(range) | Male sex, n
(%) | (Refs.) |
|---|
| Mansfield et
al, 2021 | I/II | Prospective | NEC, MTC, GBM, MM,
other solid | Rova-T | 145 | 61 (28–84) | 126 (63) | (31) |
| Blackhall et
al, 2021 | III | RCT | SCLC | Rova-T | 287/129 | 63 (36–85)/64 (32–85) | 191 (65)/86
(58) | (17) |
| Morgensztern et
al, 2019 | II | Prospective | SCLC | Rova-T | 339 | 62 (24–86) | 170 (50) | (20) |
| Spigel et
al, 2017 | I | Prospective | SCLC | Rova-T | 60 | NA | NA | (32) |
| Calvo et al,
2021 | I | Prospective | SCLC | Rova-T | 31 | 62 (40–77) | 13 (42) | (33) |
| Borghaei et
al, 2022 | I | Prospective | SCLC | Tarlatamab (AMG
757) | 102 | 63 (32–80) | NA | (34) |
| Udagawa et
al, 2019 | I | Prospective | SCLC | Rova-T | 29 | 68 (47–86) | 22 (76) | (35) |
| Johnson et
al, 2021 | III | RCT | SCLC | Rova-T | 372/376 | 64 (39–94)/64 (38–85) | 258 (69)/239
(64) | (18) |
| Paz-Ares et
al, 2023 | II | Prospective | SCLC | Tarlatamab (AMG
757) | 100/88 | NA | NA | (36) |
| Paz-Ares et
al, 2023 | I | Prospective | SCLC | Tarlatamab (AMG
757) | 107 | 63 (32–80) | 61 (57) | (37) |
| Pietanza et
al, 2015 | I | Prospective | SCLC | Rova-T | 79 | 62 (44–81) | NA | (38) |
| Malhotra et
al, 2021 | I/II | Prospective | SCLC | Rova-T | 30/12 | 61.5 (48–79)/62
(25–72) | 16 (53)/7 (58) | (39) |
| Ahn et al,
2023 | II | Prospective | SCLC | Tarlatamab (AMG
757) | 100/88 | 64 (35–82)/62 (34–80) | 72 (72)/62
(70) | (13) |
| Choudhury et
al, 2023 | I/II | Prospective | NEPC, other NEC,
SCLC | HPN 328 | 44 | NA | NA | (14) |
| Owonikoko et
al, 2021 | I | Prospective | SCLC | Tarlatamab (AMG
757) | 40 | 64 (44–80) | NA | (40) |
| Rudin et al,
2017 | I | Prospective | SCLC | Rova-T | 74 | 61 (55–69) | 42 (57) | (10) |
| Champiat et
al, 2022 | I | Prospective | SCLC | Tarlatamab (AMG
757) | 106 | NA | NA | (41) |
| Champiat et
al, 2023 | I | Prospective | SCLC | Tarlatamab (AMG
757) | 46/136 | 62 (32–80)/62 (32–80) | NA | (12) |
| Owonikoko et
al, 2021 | I | Prospective | SCLC | Tarlatamab (AMG
757) | 64 | 64 (32–80) | NA | (15) |
| Wermke et
al, 2023 | I | Prospective | SCLC | BI 764532 | 90 | 60 (32–78) | NA | (16) |
| Kuboki et
al, 2023 | I | Prospective | epNEC, LCNEC, SCLC,
other solid | BI 764532 | 107 | 60 (32–79) | 57 (53) | (42) |
 | Table III.Original data extracted from included
clinical trials. |
Table III.
Original data extracted from included
clinical trials.
| First author,
year | Total samples | Intervention | Objective response
rate, n (%) | Disease control
rate, n (%) | Median
progression-free survival, months (95% CI) | Median overall
survival, months (95% CI) | (Refs.) |
|---|
| Mansfield et
al, 2021 | 145 | Rova-T 0.3 mg/kg,
Q6w | 15 (10.3) | NA | 4.1 (2.8–4.8) | 7.1 (5.6–9.7) | (31) |
| Blackhall et
al, 2021 | 287/129 | Rova-T 0.3 mg/kg,
d1 ivgtt, Q42d vs. topotecan | 42 (14.6)/ | 103 (35.8)/ | 3.0 (2.9–3.6)/ | 6.3 (5.6–7.3)/ | (17) |
|
|
| 1.5
mg/m2, d1-5 ivgtt, Q21d | 27 (20.9) | 56 (43.4) | 4.3 (3.8–5.4) | 8.6 (7.7–10.1) |
|
| Morgensztern et
al, 2019 | 339 | Rova-T 0.3 mg/kg,
Q6w | 42 (12.4) | 236 (69.6) | 3.5 (3.0–3.9) | 5.6 (4.9–6.1) | (20) |
| Spigel et
al, 2017 | 60 | Rova-T 0.2–0.4
mg/kg, Q3w or Q6w | 11 (18.3) | 41 (68.3) | NA | NA | (32) |
| Calvo et al,
2021 | 31 | Rova-T 0.3 mg/kg,
Q6W DEX 8 mg bid po | 7 (24.1) | 22 (70.9) | 3.5 (2.4–4.7) | NA | (33) |
| Borghaei et
al, 2022 | 102 | AMG757 0.003–100
mg, Q2w | 25 (24.5) | 52 (50.9) | 3.5 (2.1–4.6) | 12.3 (7.2-NE) | (34) |
| Udagawa et
al, 2019 | 29 | Rova-T 0.2 or 0.3
mg/kg, Q6w, DEX 8 mg bid po | 3 (10.3) | 16 (55.1) | 2.2 (1.2–3.0) | 5.8 (4.1–9.2) | (35) |
| Johnson et
al, 2021 | 372/376 | Rova-T 0.3 mg/kg,
Q6w, DEX 8 mg | 28 (7.5)/ | NA | 3.7 (2.9–4.0)/ | 8.8
(7.95–9.53)/ | (18) |
|
|
| bid po vs. placebo,
Q6w, DEX 8 mg bid po | 14 (3.7) |
| 1.4 (1.4–1.5) | 9.9 (8.6–11) |
|
| Paz-Ares et
al, 2023 | 100/88 | AMG 757 10 mg, Q2w
vs. AMG 757 10 mg, Q2w | 40 (40)/ | 70 (70)/55 | 4.9 (2.9–6.7)/ | 14.3
(10.8-NE)/ | (36) |
|
|
|
| 28 (31.8) | (62.5) | 3.9 (2.6–4.4) | NE (12.4-NE) |
|
| Paz-Ares et
al, 2023 | 107 | AMG 757 0.003,
0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100 mg, Q2w | 27 (25.2) | 55 (51.4) | 3.7 (2.1–5.4) | 13.2 (10.5-NE) | (37) |
| Pietanza et
al, 2015 | 79 | Rova-T 0.05, 0.1,
0.2, 0.4, 0.8 mg/kg, Q2w | 35 (44.3) | NA | NA | NA | (38) |
| Malhotra et
al, 2021 | 30/12 | Rova-T 0.3 mg/kg,
Q6w, DEX 8 mg bid po + 4th w | 8 (26.6)/ | NA | 4.8 (3.2–5.3)/ | 7.4 (5.0–9.1)/ | (39) |
|
|
| nivolumab 360 mg,
Q3w + 10th w nivolumab 480 mg, Q4w vs. Rova-T 0.3 mg/kg, Q6w, DEX 8
mg bid po + 4th w nivolumab 360 mg, Q3w + 10th w nivolumab 480 mg,
Q4w | 4 (33.3) |
| 4.1 (1.3–6.0) | 11.0
(2.3–17.0) |
|
| Ahn et al,
2023 | 100/88 | AMG 757 10 mg, Q2w
vs. AMG 757 10 mg, Q2w | 40 (40)/ | 70 (70)/ | 4.9 (2.9–6.7)/ | 14.3
(10.8-NE)/ | (13) |
|
|
|
| 28 (31.8) | 55 (62.5) | 3.9 (2.6–4.4) | NE (12.4-NE) |
|
| Choudhury et
al, 2023 | 44 | HPN328 0.015–24 mg,
Q1w | 4 (9.1) | NA | NA | NA | (14) |
| Owonikoko et
al, 2021 | 40 | AMG 757 0.003–100
mg, Q2w | 6 (15) | 17 (42.5) | NA | NA | (40) |
| Rudin et al,
2017 | 74 | Rova-T dosage:
0.05–0.8 mg/kg, administered every 3 weeks (Q3w) or 6 weeks (Q6w).
Follow-up doses: 0.3 mg/kg or 0.4 mg/kg, administered every 6 weeks
(Q6w); 0.2 mg/kg or 0.4 mg/kg, administered every3 weeks
(Q3w). | 11 (16.9) | 46 (70.7) | 3.1 (2.7–4.1) | 4.6 (3.9–7.1) | (10) |
| Champiat et
al, 2022 | 106 | AMG 757 0.003–100
mg, Q2w | NA | NA | NA | NA | (41) |
| Champiat et
al, 2023 | 46/136 | AMG 757 0.003–100
mg, Q2w (BM) vs. AMG 757 0.003–100 mg, Q2w (not BM) | 9 (19.5)/34
(25) | 27 (58.6)/68
(50) | 3.7 (1.9–4.8)/3.7
(1.9–5.3) | 13.2/15.5 | (12) |
| Owonikoko et
al, 2021 | 64 | AMG 757 0.003–100
mg, Q2w | 23 (38.3) | 26 (43.3) | NA | NA | (15) |
| Wermke et
al, 2023 | 90 | BI 764532 plan A,
fixed dose Q3w; plan B1, fixed dose Q1w (days 1, 8, 15 of a 3-week
cycle); plan B2, initial intervention dose, then fixed dose
Q1w | NA | NA | NA | NA | (16) |
| Kuboki et
al, 2023 | 107 | BI 764532 plan A,
fixed dose Q3w; plan B1, fixed dose Q1w (D1, 8, 15 of 3-week
cycle); plan B2, initial dose, then Q1w | 18 (16.8) | 37 (34.5) | NA | NA | (42) |
ORR
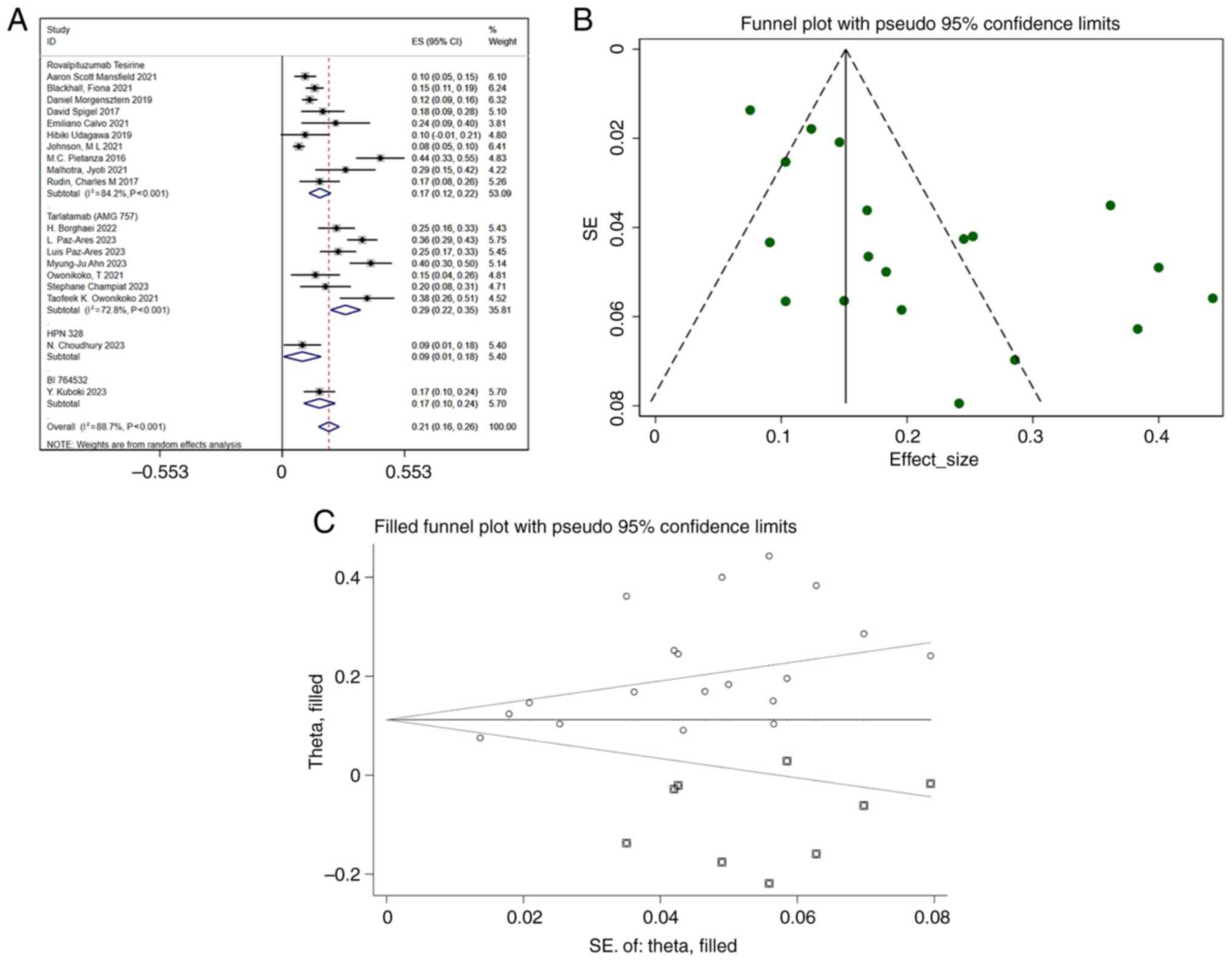
The analysis included 19 studies reporting the ORR.
The pooled ORR was 0.21 (95% CI, 0.16–0.26), with substantial
heterogeneity (I2=88.7%; P<0.001) (Fig. 2A). Funnel plot analysis (Fig. 2B) and Egger's test (Table IV) suggested potential publication
bias, highlighting the need for additional studies to confirm these
results. Further analysis with the trim-and-fill method indicated
that studies reporting lower ORR values may have been
underreported, and the adjusted pooled ORR was slightly reduced
following correction (Fig. 2C). To
ensure the robustness of the present findings, future studies with
lower ORR values should be incorporated. A meta-regression analysis
was performed to explore potential sources of heterogeneity,
considering eight covariates: DLL3 inhibitor type, year, region,
age, sex, combination with immune checkpoint inhibitors,
combination with dexamethasone and study design. The results of the
meta-regression did not reveal any statistically significant
differences among the variables (Table
V). While substantial heterogeneity (I2=88.7%) was
observed, the subgroup analyses did not identify any specific
sources of variation. This unexplained heterogeneity may stem from
unmeasured factors, such as variations in study design or
population characteristics. Nevertheless, sensitivity analyses
confirmed the robustness of the overall findings, suggesting that
further studies are needed to elucidate the potential moderators of
the ORR.
 | Table IV.Egger's test summary. |
Table IV.
Egger's test summary.
| Study
identifier | Slope
(coefficient)a | Standard
errorb |
t-scorec |
P-valued |
|---|
| Objective response
rate | 0.040962 | 1.043596 | 3.81 | 0.001 |
| Disease control
rate | 0.5545577 | 2.477987 | 0.07 | 0.945 |
| Overall
survival | 6.518415 | 2.521691 | 0.04 | 0.972 |
| Progression-free
survival | 2.897503 | 0.7562294 | 1.94 | 0.078 |
 | Table V.P-value associated with the
meta-regression. |
Table V.
P-value associated with the
meta-regression.
| Outcome | Inhibitor type | Year | Study design | Age | Male | Immunotherapy
inhibitor | Dexamethasone | Country |
|---|
| Overall
survival | NA | 0.043 | 0.576 | 0.889 | 0.237 | 0.577 | 0.527 | 0.258 |
| Progression-free
survival | 0.186 | 0.186 | 0.429 | 0.12 | 0.16 | 0.099 | 0.29 | 0.109 |
| Disease control
rate | 0.633 | 0.419 | 0.218 | 0.966 | 0.658 | 0.582 | 0.97 | 0.72 |
| Objective response
rate | 0.232 | 0.879 | 0.309 | 0.897 | 0.963 | 0.306 | 0.281 | 0.814 |
| Treatment-related
adverse events | 0.925 | 0.943 | 0.993 | 0.979 | 0.865 | 0.769 | 0.98 | 0.914 |
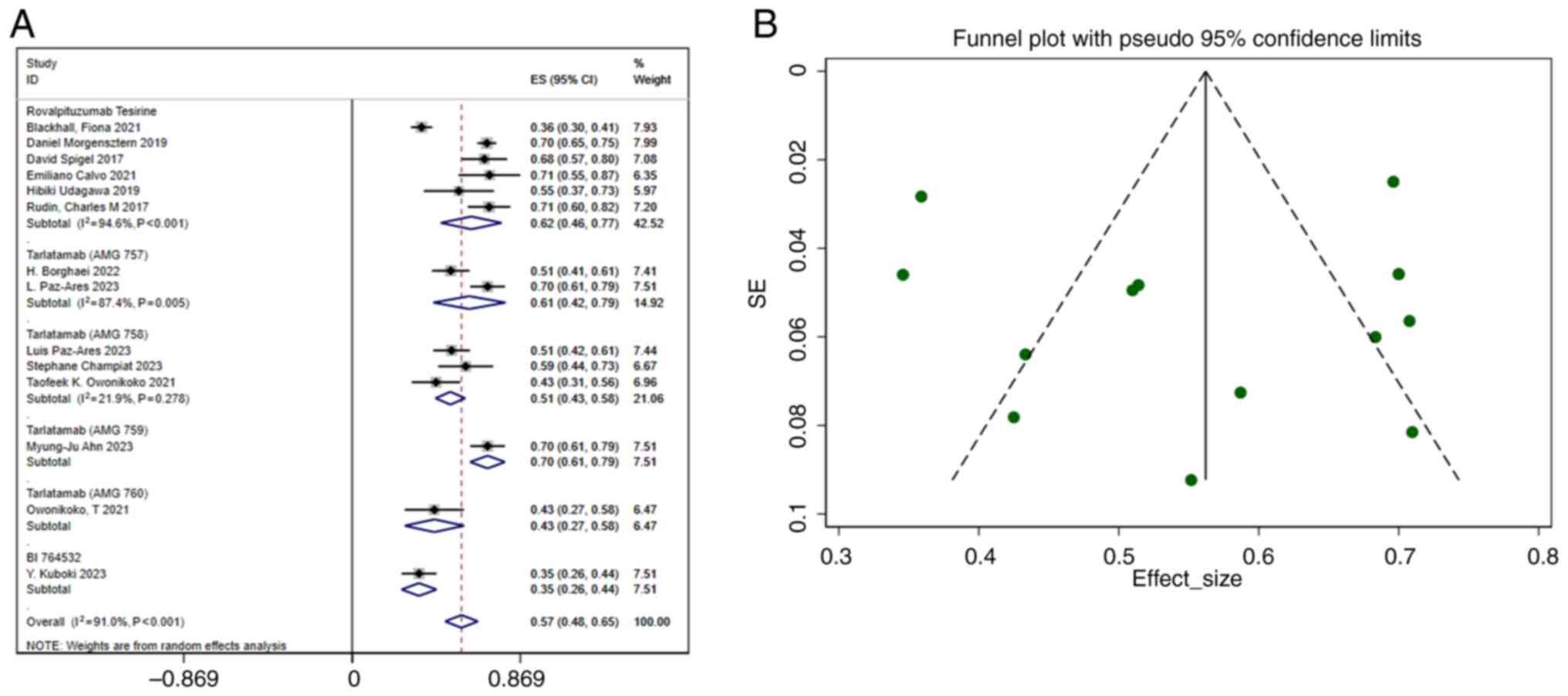
DCR
A total of 14 studies reported the DCR. The pooled
DCR was 0.57 (95% CI, 0.48–0.65), with considerable heterogeneity
(I2 =91.0%; P<0.001) (Fig. 3A). The funnel plot (Fig. 3B) and Egger's regression analysis
(Table IV) indicated no
publication bias, reinforcing the reliability of the findings. To
explore potential sources of heterogeneity, eight variables (DLL3
inhibitor type, year, region, age, sex, combination with immune
checkpoint inhibitors, combination with dexamethasone and study
design) were included in a meta-regression analysis. The results
revealed no statistically significant differences in the P-values
of these variables (Table V).
Heterogeneity was observed across studies (I2=91.0%),
but the subgroup analyses failed to identify any notable sources.
This unexplained heterogeneity may stem from unmeasured factors,
such as variations in study design or population characteristics.
Nevertheless, sensitivity analyses confirmed that the overall
findings were robust. Therefore, further studies are needed to
explore the potential moderators of the DCR.
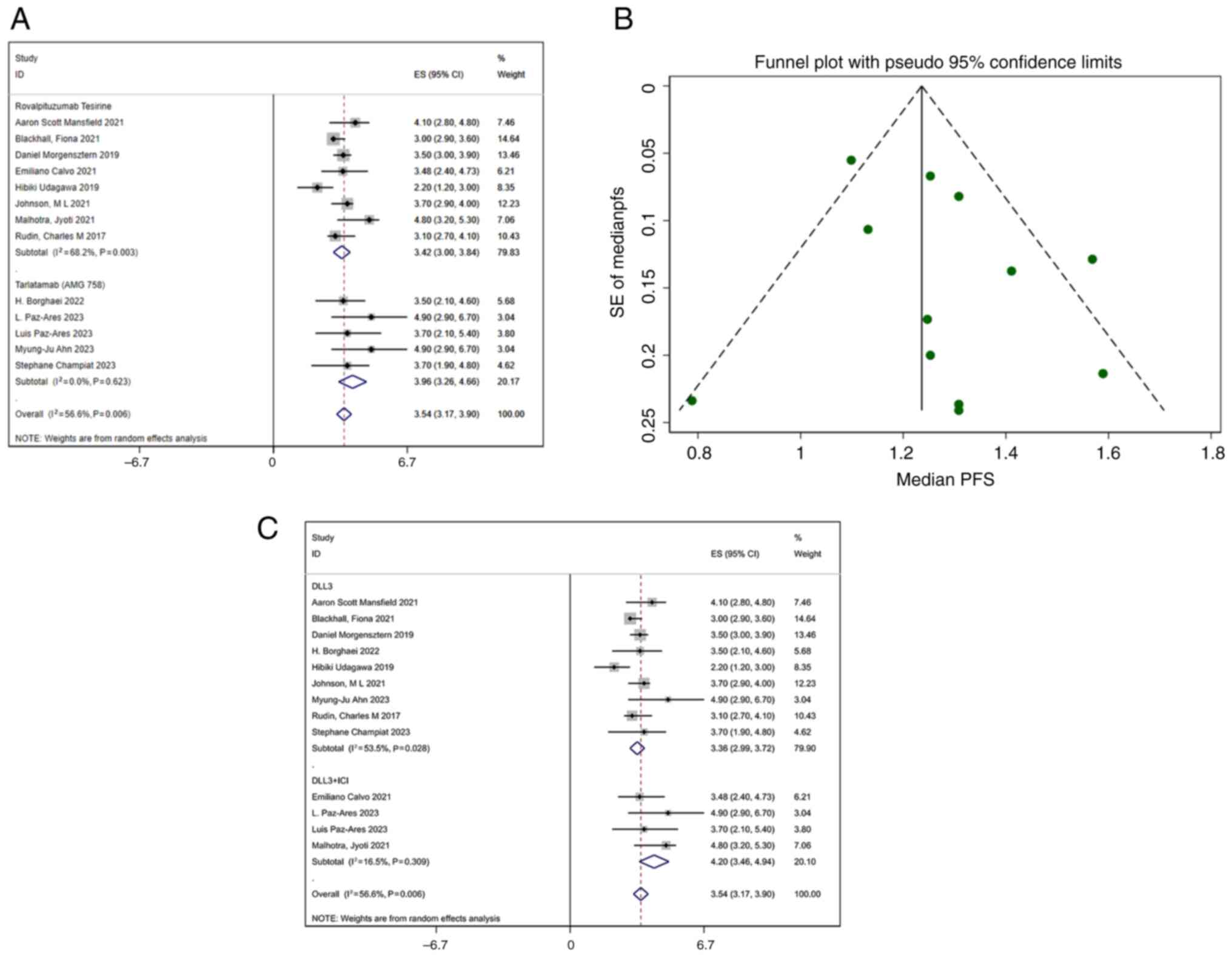
PFS
A total of 13 studies reported PFS. The pooled
median PFS was 3.54 months (95% CI, 3.17–3.90 months), with
moderate heterogeneity (I2=56.6%; P=0.006) (Fig. 4A). The funnel plot (Fig. 4B) and Egger's regression analysis
(Table IV) for PFS indicated no
publication bias, confirming the robustness of the findings.
Meta-regression analysis was performed using eight covariates: DLL3
inhibitor type, year, region, age, sex, combination with immune
checkpoint inhibitors, combination with dexamethasone and study
design. The results indicated no statistically significant
differences among the P-values of these variables (Table V). Subgroup analysis revealed that
the median PFS for patients receiving combined immunotherapy was
4.2 months (95% CI, 3.46–4.94), while the median PFS for patients
receiving monotherapy DLL3 inhibitors was 3.36 months (95% CI,
2.99–3.72) (Fig. 4C).
OS
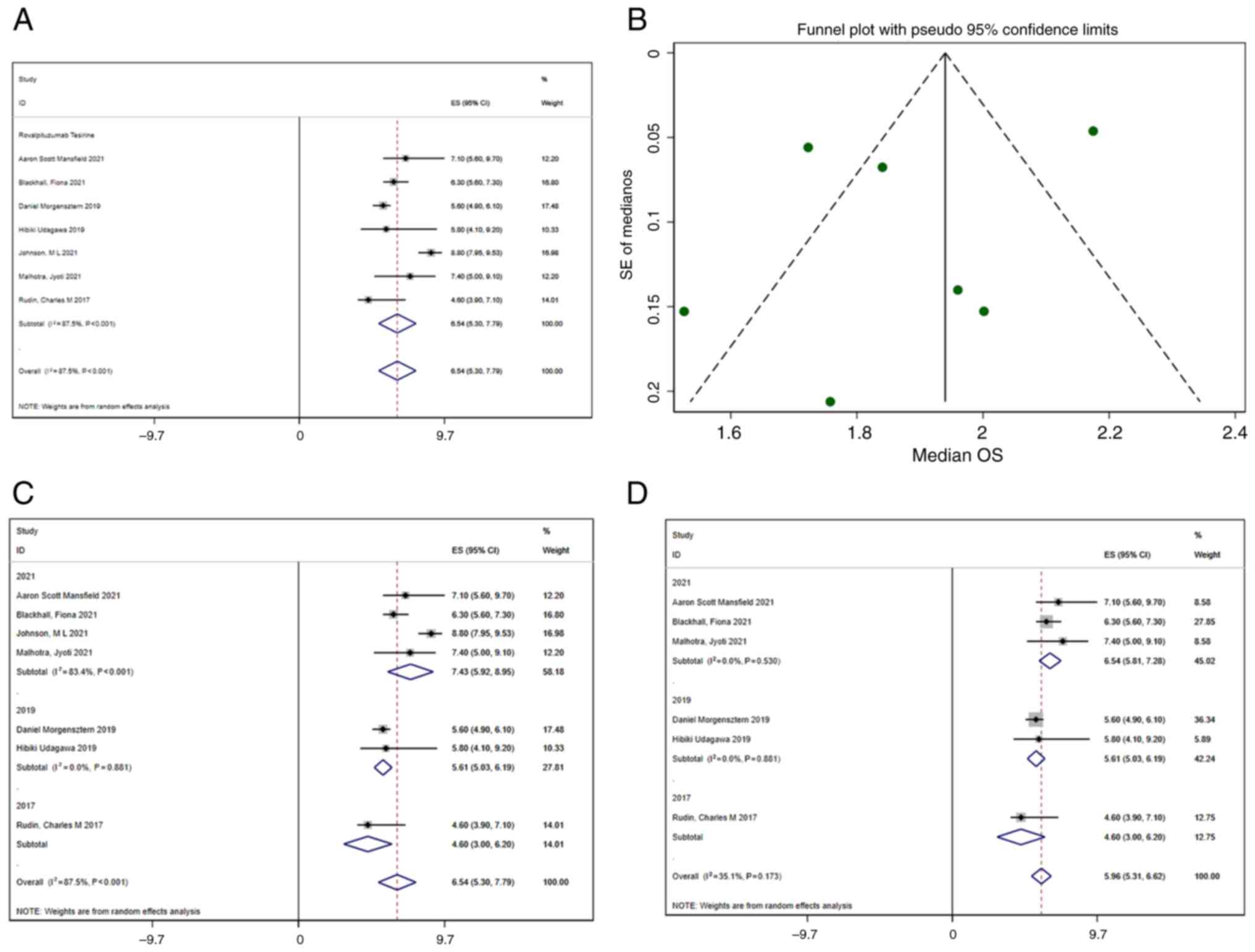
A total of 7 studies reported OS. Using a
random-effects model, the median OS was 6.54 months (95% CI,
5.30–7.79 months) (Fig. 5A).
Significant heterogeneity was present (I2=87.5%),
prompting meta-regression and subgroup analyses to explore
potential sources. In the meta-regression, eight variables were
evaluated: DLL3 inhibitor type, year, region, age, sex, combination
with immune checkpoint inhibitors, combination with dexamethasone
and study design. The meta-regression indicated that the
publication year was statistically significant (Table V). A sensitivity analysis using the
leave-one-out method revealed that excluding the study by Johnson
et al (18) from 2021
significantly reduced heterogeneity, with the I2 value
decreasing from 87.5 to 35.1%. This suggests that the study by
Johnson et al (18)
contributed substantially to the heterogeneity, and its exclusion
enhanced the consistency of the results (Fig. 5D). Although the meta-regression
suggested that publication year might be a source of heterogeneity,
subgroup analysis (Fig. 5C) showed
marked heterogeneity within the 2021 group, indicating that the
difference in publication year may not directly explain the OS
variation. It is more likely that the inherent limitations of
single-arm studies and their designs contributed to the observed
heterogeneity.
Safety and AEs
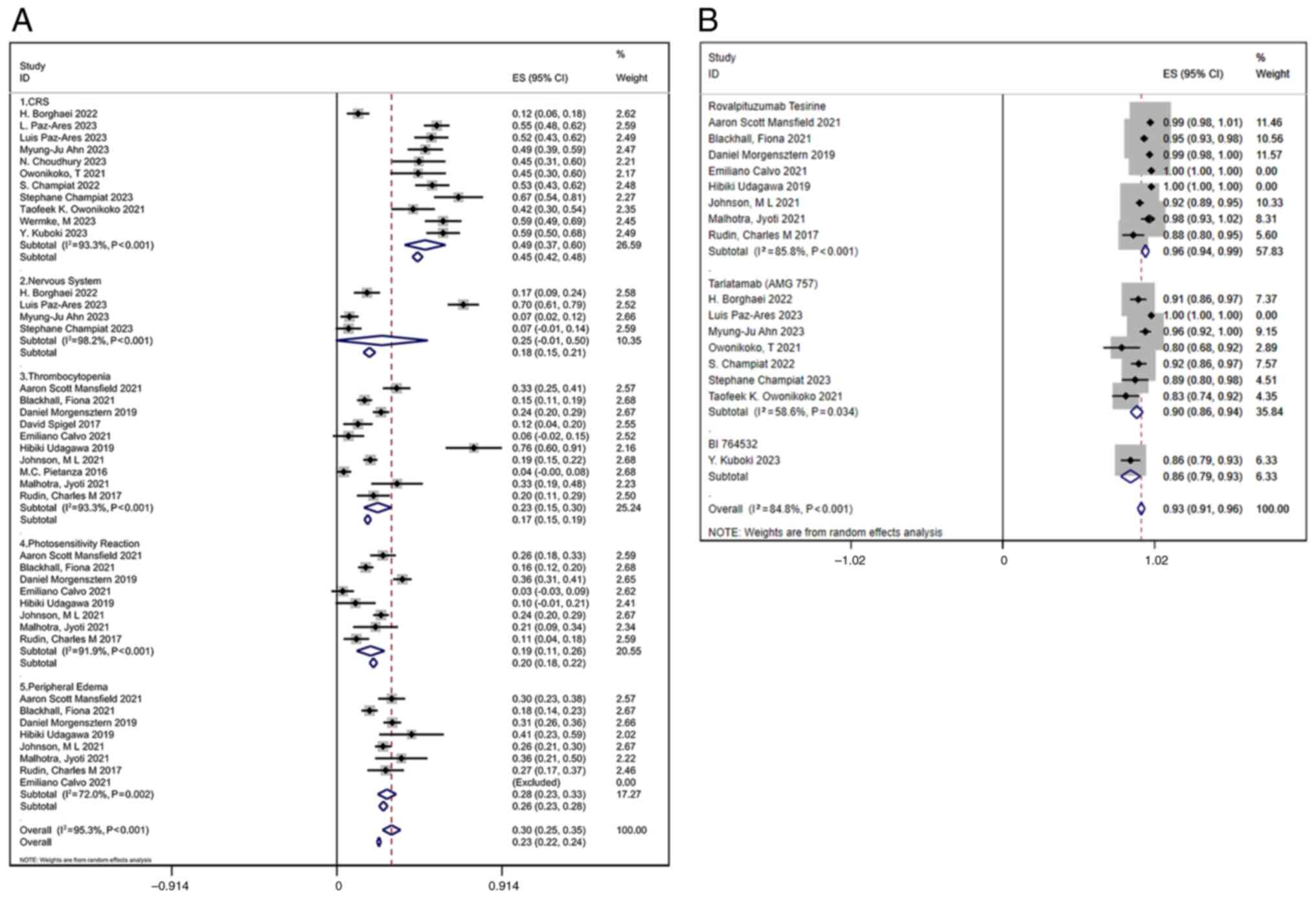
All studies reporting trAEs were reviewed. Common
AEs associated with DLL3 inhibitors included CRS, respiratory
difficulty, pleural effusion, peripheral edema, thrombocytopenia
and bone marrow suppression. In the analysis, 11 studies reported
CRS, with a combined incidence of 0.49 (95% CI, 0.37–0.60); 4
studies documented neurological adverse reactions, with a combined
incidence of 0.25 (95% CI, −0.01–0.50); 10 studies reported
thrombocytopenia, with a combined incidence of 0.23 (95% CI,
0.15–0.30); 8 studies noted photosensitivity reactions, with a
combined incidence of 0.19 (95% CI, 0.11–0.26); and 8 studies
reported peripheral edema, with a combined incidence of 0.28 (95%
CI, 0.23–0.33) (Fig. 6A).
For different inhibitor types, the pooled AE
incidence for Rova-T was 0.96 (95% CI, 0.94–0.99) with
I2=85.8%, while for tarlatamab (AMG 757) it was 0.90
(95% CI, 0.86–0.94) with I2=58.6%. BI 764532 had an AE
incidence of 0.86 (95% CI, 0.79–0.93) based on one study (Fig. 6B). These findings suggested
variability in AE rates across different inhibitors, with Rova-T
potentially associated with a higher risk compared with tarlatamab
and BI 764532. The pooled AE incidence for patients with solid
tumors treated with DLL3 inhibitors was 0.93 (95% CI, 0.91–0.96)
(Fig. 6B), with a high
heterogeneity indicator (I2=84.8%). Despite this
heterogeneity, the forest plot suggested that most studies reported
similar AE rates, indicating a consistent safety profile across
trials. Table VI shows the
incidence of AEs by system, with effect sizes, 95% confidence
intervals, I2 values, and P-values. The I2
values indicate heterogeneity, and the small P-values (<0.01)
suggest significant differences across studies. All systems,
including respiratory, skin and immune, nervous, digestive,
circulatory, hematopoietic, and non-specific, exhibited significant
heterogeneity.
 | Table VI.Incidence of adverse events in the
overall estimate with whole body system. |
Table VI.
Incidence of adverse events in the
overall estimate with whole body system.
| Type of adverse
event | Effect sizes (95%
CI) | I2
(%) | P-value |
|---|
| Respiratory | 0.17 (0.11,
0.22) | 95.0 | <0.01 |
| Skin and
immune | 0.31 (0.24,
0.39) | 95.9 | <0.01 |
| Nervous | 0.25 (−0.01,
0.50) | 98.2 | <0.01 |
| Digestive | 0.17 (0.15,
0.20) | 83.4 | <0.01 |
| Circulation | 0.21 (0.16,
0.25) | 91.3 | <0.01 |
| Hematopoietic | 0.20 (0.16,
0.24) | 90.8 | <0.01 |
| Non-specific | 0.22 (0.17,
0.27) | 95.5 | <0.01 |
Sensitivity analysis
The sensitivity analysis assessed the impact of each
study on the combined results by sequentially excluding individual
studies. The findings indicated that no single study significantly
influenced the pooled results within the 95% CI, confirming the
robustness and reliability of the meta-analysis conclusions. The
results of the sensitivity analysis are presented in Fig. S1.
Discussion
The present systematic review and meta-analysis
offers valuable insights into the safety and efficacy of DLL3
inhibitors for treating solid tumors, particularly NECs such as
SCLC. DLL3 is an atypical Notch ligand predominantly expressed in
SCLC (5), making it a promising
therapeutic target. DLL3 inhibitors have demonstrated moderate
efficacy in treating advanced solid tumors, particularly in
relapsed or refractory cases where conventional treatments have
failed (20).
The present analysis indicated that DLL3 inhibitors
had a median OS of 6.54 months, with significant variability. The
median PFS was 3.54 months, with a notably longer duration in
combined immunotherapy (4.2 months) compared with monotherapy (3.36
months). The DCR and ORR were 57 and 21%, respectively, reflecting
substantial heterogeneity, and AEs occurred in 93% of cases. These
findings align with the TRINITY trial (18), which reported a similar PFS for
Rova-T, suggesting a potential improvement over standard treatments
such as topotecan. In the DeLLphi-300 trial, tarlatamab
demonstrated a DCR of 51.4% and a median response duration of 12.3
months (21). The pooled analysis
of AEs showed a high incidence rate of 93% (95% CI, 91–96%), with
variability among inhibitors. Rova-T had a higher rate of AEs (96%)
compared with tarlatamab (90%) and BI 764532 (86%). Despite these
high rates, DLL3 therapies markedly reduce off-target toxicities
compared with conventional chemotherapy (22). This is particularly advantageous for
patients who have undergone multiple lines of therapy and may be
experiencing cumulative toxicities. A recent review demonstrated
that while DLL3 inhibitors have high AE rates, most events are
manageable and classified as grade 1 or 2, in contrast to the
higher-grade toxicities often associated with cytotoxic
chemotherapy (23).
The present findings suggested that DLL3 inhibitors
may offer a comparable or potentially more favorable treatment
option than topotecan, particularly for relapsed SCLC. Topotecan, a
widely used chemotherapeutic agent, demonstrated an ORR of 24.3% in
a clinical trial involving 107 patients, whereas DLL3 inhibitors
showed an ORR of 21% (95% CI, 16–26%). Although the ORR for DLL3
inhibitors was slightly lower, the median PFS and OS for DLL3
inhibitors were 3.54 and 8.73 months, respectively, which
outperformed the median times to progression (13.3 weeks or ~3.1
months) and median survival (25.0 weeks or ~5.8 months) for
topotecan (24).
Furthermore, topotecan is associated with notable
toxicity, including grade 4 neutropenia in 37.8% of courses, and
high rates of thrombocytopenia and anemia (25). By contrast, DLL3 inhibitors exhibit
a more manageable safety profile, with fewer severe hematologic
toxicities compared with topotecan. This distinct safety profile
may provide an advantage for patients who have already undergone
multiple lines of therapy and are at risk for cumulative
toxicities.
Overall, while both treatments have limitations,
DLL3 inhibitors provide a targeted approach that could benefit
patients who may not tolerate the hematologic side effects of
topotecan. The moderate efficacy observed, coupled with a
manageable safety profile, position DLL3 inhibitors as a potential
alternative for patients with relapsed SCLC, particularly those who
are not candidates for intensive chemotherapy regimens such as
topotecan.
The absence of DLL3 in normal tissues minimizes
off-target effects, making DLL3 inhibitors a promising targeted
therapy that reduces damage to healthy tissues compared with
conventional chemotherapy (9,26).
However, the observed variation in response rates across different
studies may be attributed to discrepancies in patient populations,
such as tumor heterogeneity and prior treatment regimens.
Understanding the underlying biological mechanisms that affect DLL3
expression and its role in tumor progression is critical for
optimizing the therapeutic use of DLL3 inhibitors. Previous
research has indicated that adjuvant chemotherapy serves as a
significant independent prognostic factor for patients with
DLL3-negative tumors (HR, 0.05; 95% CI, 0.01–0.41; P<0.01).
However, this factor was reported to not be significant for
patients with DLL3-positive tumors (HR, 0.73; 95% CI, 0.23–2.27;
P=0.58) (27).
Moderate efficacy coupled with a manageable safety
profile suggests that DLL3 inhibitors may be effective in treating
refractory NECs, either alone or in combination with other
therapeutic agents. For example, combining DLL3 inhibitors with
radioimmunotherapy has shown promise in improving treatment
efficacy while maintaining acceptable levels of toxicity (28). Beyond SCLC, DLL3 expression has been
detected in other types of NEC, such as neuroendocrine prostate
cancer, which implies that DLL3 inhibitors could potentially target
a broader spectrum of malignancies (29). Additionally, immunotoxin therapy has
emerged as a promising strategy for cancer treatment. Ataee et
al (30) developed novel
immunotoxins targeting DLL3, which is overexpressed in SCLC. These
recombinant immunotoxins, one fused with granzyme B and the other
with a component from typhoid toxin, demonstrated potential in
preliminary bioinformatics and in vitro analyses, indicating
a promising direction for further experimental research in SCLC
treatment.
Understanding the biological mechanisms that affect
DLL3 expression and its involvement in tumor progression is crucial
for optimizing the therapeutic potential of DLL3 inhibitors.
Identifying DLL3 as a predictive biomarker could aid in selecting
patients most likely to respond to these targeted therapies,
thereby advancing personalized medicine in the context of NEC.
Further research is required to investigate the mechanisms of
resistance to DLL3 inhibitors and to develop strategies for
overcoming this resistance, potentially through combination
therapies that target multiple pathways involved in tumor survival
and progression.
The present analysis is subject to several
limitations, particularly those associated with single-group rate
meta-analyses. The substantial heterogeneity observed in outcomes
such as OS, PFS, DCR, ORR and AEs is a well-recognized limitation
of this method. Unlike comparative analyses, single-group rate
meta-analyses aggregate data from diverse study designs, patient
populations and treatment regimens, which can lead to marked
variability that may lack clinical interpretability. Factors such
as differing outcome definitions, variations in reporting standards
and unmeasured confounding variables contribute to this
heterogeneity, complicating efforts to draw meaningful conclusions
from pooled data.
Future research on DLL3 inhibitors should focus on
several key areas to optimize their clinical impact. Firstly,
exploring combination therapies with immune checkpoint inhibitors,
chemotherapy or other targeted agents is critical to maximize the
potential for synergistic effects. Secondly, biomarker-driven
approaches, such as stratifying patients based on DLL3 expression
levels or tumor mutational burden, are essential for developing
personalized treatment strategies. Thirdly, standardizing clinical
endpoints and reporting practices will enhance the comparability of
studies and strengthen the reliability of pooled analyses.
Furthermore, addressing underrepresented patient populations,
including those with rare tumor subtypes or poor performance
status, is essential for improving the generalizability of
findings. Finally, large-scale RCTs and real-world studies are
crucial for validating results, assessing long-term outcomes and
determining the broader applicability of DLL3-targeted therapies in
clinical practice.
In conclusion, the present meta-analysis highlighted
the promising clinical efficacy and manageable safety profile of
DLL3 inhibitors in treating solid tumors, with encouraging outcomes
in OS, PFS and DCR, particularly for agents such as tarlatamab.
These findings reinforce the potential of DLL3 inhibitors as a
novel therapeutic approach to address unmet needs in oncology.
However, the substantial heterogeneity identified, driven by
variations in inhibitor types, combination with immunotherapy and
treatment regimens, emphasizes the need for more robust and
standardized evidence.
To facilitate the clinical translation of DLL3
inhibitors, future research should focus on developing and
validating predictive biomarkers for better patient selection.
Exploring combination therapies with immunotherapies or
radiotherapy could improve efficacy and overcome resistance
mechanisms. Additionally, addressing resistance pathways and
standardizing clinical endpoints, reporting practices and trial
designs will be essential to strengthen the evidence base and
improve comparability across studies. By focusing on these
strategies, the clinical application of DLL3 inhibitors could be
refined and expanded, ultimately offering new hope for patients
with solid tumors and advancing the field of precision
oncology.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was supported by Special Funds for Promoting
Scientific And Technological Innovation in Xuzhou in 2022 (grant
no. KC22255).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YS and XL contributed to the conception, design and
writing of the manuscript. TL and YQ were responsible for data
extraction and analysis. The first draft of the manuscript was
written by YS. HC and DY contributed to the statistical analysis
and interpretation of results, ensuring the robustness of the
meta-analysis methodology. Manuscript revision and proofreading
were performed by XL, HC and DY. YS and XL confirm the authenticity
of all the raw data. YX, WX and YG contributed to the study design
and provided critical feedback on the methodology. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guruharsha KG, Kankel MW and
Artavanis-Tsakonas S: The Notch signalling system: Recent insights
into the complexity of a conserved pathway. Nat Rev Genet.
13:654–666. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray SJ: Notch signalling in context. Nat
Rev Mol Cell Bio. 17:722–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meurette O and Mehlen P: Notch signaling
in the tumor microenvironment. Cancer Cell. 34:536–548. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Radtke F and Raj K: The role of Notch in
tumorigenesis: Oncogene or tumour suppressor? Nat Rev Cancer.
3:756–767. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chapman G, Sparrow DB, Kremmer E and
Dunwoodie SL: Notch inhibition by the ligand DELTA-LIKE 3 defines
the mechanism of abnormal vertebral segmentation in spondylocostal
dysostosis. Hum Mol Genet. 20:905–916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabari JK, Lok BH, Laird JH, Poirier JT
and Rudin CM: Unravelling the biology of SCLC: Implications for
therapy. Nat Rev Clin Oncol. 14:549–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saunders LR, Bankovich AJ, Anderson WC,
Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang
A, et al: A DLL3-targeted antibody-drug conjugate eradicates
high-grade pulmonary neuroendocrine tumor-initiating cells in vivo.
Sci Transl Med. 7:302ra1362015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao J, Bergsland E, Aggarwal R, Aparicio
A, Beltran H, Crabtree JS, Hann CL, Ibrahim T, Byers LA, Sasano H,
et al: DLL3 as an emerging target for the treatment of
neuroendocrine neoplasms. Oncologist. 27:940–951. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Owen DH, Giffin MJ, Bailis JM, Smit MD,
Carbone DP and He K: DLL3: An emerging target in small cell lung
cancer. J Hematol Oncol. 12:612019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rudin CM, Pietanza MC, Bauer TM, Ready N,
Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA III,
Robert F, et al: Rovalpituzumab tesirine, a DLL3-targeted
antibody-drug conjugate, in recurrent small-cell lung cancer: A
first-in-human, first-in-class, open-label, phase 1 study. Lancet
Oncol. 18:42–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giffin MJ, Cooke K, Lobenhofer EK, Estrada
J, Zhan J, Deegen P, Thomas M, Murawsky CM, Werner J, Liu S, et al:
AMG 757, a half-life extended, DLL3-targeted bispecific T-cell
engager, shows high potency and sensitivity in preclinical models
of small-cell lung cancer. Clin Cancer Res. 27:1526–1537. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Champiat S, Boyer MJ, Govindan R, Paz-Ares
LG, Owonikoko TK, Borghaei H, Izumi H, Steeghs N, Helen Blackhall
F, Terbuch A, et al: Tarlatamab in small cell lung cancer (SCLC):
Safety and efficacy analyzed by baseline brain metastasis. J Clin
Oncol. 41 (16 Suppl):S85822023. View Article : Google Scholar
|
|
13
|
Ahn MJ, Cho BC, Felip E, Korantzis I,
Ohashi K, Majem M, Juan-Vidal O, Handzhiev S, Izumi H, Lee JS, et
al: Tarlatamab for patients with previously treated small-cell lung
cancer. New Engl J Med. 389:2063–2075. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choudhury N, Jain P, Dowlati A, Thompson
J, Johnson ML, Mamdani H, Sanborn RE, Schenk EL, Aggarwal R, Sankar
K, et al: 698P Interim results from a phase I/II study of HPN328, a
tri-specific, half-life (T1/2) extended DLL3-targeting T cell
engager in patients (pts) with small cell lung cancer (SCLC) and
other neuroendocrine neoplasms (NEN). Ann Oncol. 34 (Suppl
2):S4862023. View Article : Google Scholar
|
|
15
|
Owonikoko TK, Champiat S, Johnson ML,
Govindan R, Izumi H, Lai WVV, Borghaei H, Boyer MJ, Boosman RJ,
Hummel HD, et al: Updated results from a phase 1 study of AMG 757,
a half-life extended bispecific T-cell engager (BiTE)
immuno-oncology therapy against delta-like ligand 3 (DLL3), in
small cell lung cancer (SCLC). J Clin Oncol. 39 (15
Suppl):S85102021. View Article : Google Scholar
|
|
16
|
Wermke M, Kuboki Y, Felip E, Alese OB,
Morgensztern D, Sayehli C, Arriola E, Sanmamed MF, Hamed ZO, Song
E, et al: OA01.05 Phase I dose escalation trial of the DLL3/CD3
Igg-like T cell engager BI 764532 in patients with DLL3+ tumors:
Focus on SCLC. J Thorac Oncol. 18 (Suppl):S45–S46. 2023. View Article : Google Scholar
|
|
17
|
Blackhall F, Jao K, Greillier L, Cho BC,
Penkov K, Reguart N, Majem M, Nackaerts K, Syrigos K, Hansen K, et
al: Efficacy and safety of rovalpituzumab tesirine compared with
topotecan as second-line therapy in DLL3-high SCLC: Results from
the phase 3 TAHOE study. J Thorac Oncol. 16:1547–1558. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson ML, Zvirbule Z, Laktionov K,
Helland A, Cho BC, Gutierrez V, Colinet B, Lena H, Wolf M,
Gottfried M, et al: Rovalpituzumab tesirine as a maintenance
therapy after first-line platinum-based chemotherapy in patients
with extensive-stage-SCLC: Results from the phase 3 MERU study. J
Thorac Oncol. 16:1570–1581. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morgensztern D, Besse B, Greillier L,
Santana-Davila R, Ready N, Hann CL, Glisson BS, Farago AF, Dowlati
A, Rudin CM, et al: Efficacy and safety of rovalpituzumab tesirine
in third-line and beyond patients with DLL3-expressing,
relapsed/refractory small-cell lung cancer: Results from the phase
II TRINITY study. Clin Cancer Res. 25:6958–6966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lovely B and Hollasch M: DLL3-targeting
agents are poised to fill unmet needs in SCLC. Oncol
Live®. 24:2023.
|
|
22
|
Lin S, Zhang Y, Yao J, Yang J, Qiu Y, Zhu
Z and Hua H: DB-1314, a novel DLL3-targeting ADC with DNA
topoisomerase I inhibitor, exhibits promising safety profile and
therapeutic efficacy in preclinical small cell lung cancer models.
J Transl Med. 22:7662024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sands JM, Champiat S, Hummel H, Paulson
KG, Borghaei H, Bustamante Alvarez J, Carbone DP, Carlisle JW,
Choudhury NJ, Clarke JM, et al: Practical management of adverse
events in patients receiving tarlatamab, a DLL3-targeted bispecific
T-cell engager immunotherapy, for previously treated small cell
lung cancer. medRxiv. 2024.
|
|
24
|
von Pawel J, Schiller JH, Shepherd FA,
Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer
MC, Depierre A, et al: Topotecan versus cyclophosphamide,
doxorubicin, and vincristine for the treatment of recurrent
small-cell lung cancer. J Clin Oncol. 17:658–6567. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armstrong D and O'Reilly S: Clinical
guidelines for managing topotecan-related hematologic toxicity.
Oncologist. 3:4–10. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding J and Yeong C: Advances in
DLL3-targeted therapies for small cell lung cancer: Challenges,
opportunities, and future directions. Front Oncol. 14:15041392024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ogawa H, Sakai Y, Nishio W, Fujibayashi Y,
Nishikubo M, Nishioka Y, Tane S, Kitamura Y, Sudo T, Sakuma T and
Yoshimura M: DLL3 expression is a predictive marker of sensitivity
to adjuvant chemotherapy for pulmonary LCNEC. Thorac Cancer.
11:2561–2569. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tully KM, Tendler S, Carter LM, Sharma SK,
Samuels ZV, Mandleywala K, Korsen JA, Delos Reyes AM, Piersigilli
A, Travis WD, et al: Radioimmunotherapy targeting delta-like ligand
3 in small cell lung cancer exhibits antitumor efficacy with low
toxicity. Clin Cancer Res. 28:1391–1401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aggarwal RR, Rottey S, Bernard-Tessier A,
Mellado-Gonzalez B, Kosaka T, Stadler WM, Sandhu S, Yu B, Shaw C,
Ju CH, et al: Phase 1b study of tarlatamab in de novo or
treatment-emergent neuroendocrine prostate cancer (NEPC). J Clin
Oncol. 42 (16 Suppl):S50122024. View Article : Google Scholar
|
|
30
|
Ataee MH, Mirhosseini SA, Mirnejad R,
Rezaie E, Hosseini HM and Amani J: Design of two immunotoxins based
rovalpituzumab antibody against DLL3 receptor; a promising
potential opportunity. Res Pharm Sci. 17:428–444. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mansfield AS, Hong DS, Hann CL, Farago AF,
Beltran H, Waqar SN, Hendifar AE, Anthony LB, Taylor MH, Bryce AH,
et al: A phase I/II study of rovalpituzumab tesirine in delta-like
3-expressing, advanced solid tumors. NPJ Precis Oncol. 5:742021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spigel D, Pietanza MC, Bauer T, Ready N,
Morgensztern D, Glisson BS, Byers LA, Johnson M, Burris H, Robert
F, et al: OA05.03 Single-agent rovalpituzumab tesirine, a
delta-like protein 3 (DLL3)-targeted antibody-drug conjugate (ADC),
in small-cell lung cancer (SCLC). J Thorac Oncol. 12
(Suppl):S260–S261. 2017. View Article : Google Scholar
|
|
33
|
Calvo E, Spira A, Miguel MD, Kondo S,
Gazzah A, Millward M, Prenen H, Rottey S, Warburton L, Alanko T, et
al: Safety, pharmacokinetics, and efficacy of budigalimab with
rovalpituzumab tesirine in patients with small cell lung cancer.
Cancer Treat Res Commun. 28:1004052021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borghaei H, Paz-Ares L, Johnson M,
Champiat S, Owonikoko T, Lai V, Boyer M, Hummel HD, Govindan R,
Steeghs N, et al: OA12.05 phase 1 updated exploration and first
expansion data for DLL3-targeted T-cell engager tarlatamab in small
cell lung cancer. J Thorac Oncol. 17 (Suppl):S332022. View Article : Google Scholar
|
|
35
|
Udagawa H, Akamatsu H, Tanaka K, Takeda M,
Kanda S, Kirita K, Teraoka S, Nakagawa K, Fujiwara Y, Yasuda I, et
al: Phase I safety and pharmacokinetics study of rovalpituzumab
tesirine in Japanese patients with advanced, recurrent small cell
lung cancer. Lung Cancer. 135:145–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paz-Ares L, Ahn M, Felip E, Handzhiev S,
Korantzis I, Izumi H, Ohashi K, Tarruella MM, Wolf J, Reck M, et
al: 508MO Tarlatamab for patients (pts) with previously treated
small cell lung cancer (SCLC): Primary analysis of the phase II
DeLLphi-301 study. Ann Oncol. 34:S1664–S1665. 2023. View Article : Google Scholar
|
|
37
|
Paz-Ares L, Champiat S, Lai WV, Izumi H,
Govindan R, Boyer M, Hummel HD, Borghaei H, Johnson ML, Steeghs N,
et al: Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell
engager, in recurrent small-cell lung cancer: An open-label, phase
I study. J Clin Oncol. 41:2893–2903. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pietanza MC, Spigel D, Bauer TM, Ready NE,
Glisson BS, Morgensztern D, Robert F, Salgia R, Kochendorfer M,
Patel M, et al: 7LBA Safety, activity, and response durability
assessment of single agent rovalpituzumab tesirine, a delta-like
protein 3 (DLL3)-targeted antibody drug conjugate (ADC), in small
cell lung cancer (SCLC). Eur J Cancer. 51:S7122015. View Article : Google Scholar
|
|
39
|
Malhotra J, Nikolinakos P, Leal T, Lehman
J, Morgensztern D, Patel JD, Wrangle JM, Curigliano G, Greillier L,
Johnson ML, et al: A phase 1–2 study of rovalpituzumab tesirine in
combination with nivolumab plus or minus ipilimumab in patients
with previously treated extensive-stage SCLC. J Thorac Oncol.
16:1559–1569. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Owonikoko T, Boyer M, Johnson M, Govindan
R, Rodrigues L, Blackhall F, Boosman R, Champiat S, Hummel HD, Lai
WV, et al: OA11.03 A phase 1 study of AMG 757, half-life extended
bispecific T-cell engager (BiTE®)Immune therapy against
DLL3, in SCLC. J Thorac Oncol. 16:S1262021. View Article : Google Scholar
|
|
41
|
Champiat S, Boyer M, Paz-Ares L,
Schoenfeld A, Izumi H, Govindan R, Carlisle J, Borghaei H, Johnson
ML, Steeghs N, et al: 147P Characterizing CRS in phase I study of
DLL3-targeted T cell engager tarlatamab in small cell lung cancer.
Immun Oncol Technol. 16 (Suppl 1):S1002592022. View Article : Google Scholar
|
|
42
|
Kuboki Y, Gambardella V, Capdevila
Castillon J, Alese OB, Morgensztern D, Sayehli CM, Sanmamed MF,
Arriola E, Wolf J, Owonikoko TK, et al: 75MO Phase I trial of the
DLL3/CD3 IgG-like T cell engager BI 764532 in patients (pts) with
DLL3+ tumors: Focus on Asian pts. Ann Oncol. 34 (Suppl
4):S14952023. View Article : Google Scholar
|