Introduction
The incidence and mortality rates of lung cancer are
increasing every year (1), and lung
cancer is one of the most important malignant tumors that threatens
human health (2). Among lung cancer
types, non-small cell lung cancer (NSCLC) is dominant, of which
lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC)
are the main subtypes (3).
Different chemotherapy regimens are required to treat different
molecular subtypes; however, some patients will develop drug
resistance or metastasis after treatment, which ultimately leads to
adverse outcomes (4). The lives of
patients may be prolonged and their quality of life improved by
identifying novel indicators, designing new drugs and providing
novel treatments for patients with drug resistance, poor prognosis
or intolerance to some chemotherapeutic drugs.
Ferroptosis is an iron-dependent mode of cell death,
which is characterized by a large iron-dependent accumulation of
lethal lipid reactive oxygen species (ROS) (5,6).
Numerous studies have focused on ferroptosis in the treatment of
tumors. For example, in hepatocellular carcinoma, the induction of
ferroptosis can lead to the infiltration and activation of
CD8+ T cells, this then leads to the upregulation of
programmed death-ligand 1 in tumor cells (7). In breast cancer, the luminal androgen
receptor (LAR) subtype is highly sensitive to iron-depleting drugs.
The induction of ferroptosis in tumors of the LAR subtype can
inhibit tumor growth, improve the immune microenvironment and
enhance the efficacy of immune checkpoint blockade therapy
(8). In colorectal cancer (CRC),
increased expression of microcapsule triglyceride transfer protein
in exosomes secreted from the adipose tissues of obese patients can
inhibit the occurrence of lipid peroxidation and iron death,
thereby reducing chemotherapeutic sensitivity and promoting CRC
cell resistance to chemotherapeutics (9). The present study aimed to improve the
quality of life of patients and prolong their survival by finding
appropriate indicators to guide clinical medication, reduce
overtreatment, improve drug sensitivity and reduce drug
resistance.
Materials and methods
Data sources
Data from public databases, including The Cancer
Genome Atlas (TCGA; http://portal.gdc.cancer.gov/) and Genotype-Tissue
Expression (GTEx; http://gtexportal.org/home/), were used in the present
study. TCGA data portal was used to download survival data and
tumor staging data for LUAD and LUSC; from a total of 585 cases of
LUAD and 504 cases of LUSC. Of these, RNA sequencing data and
survival data were available for 516 LUAD cases and 501 LUSC cases.
Gene expression profiles were obtained for 59 patients with LUAD
and 49 patients with LUSC. Furthermore, data from 578 lung RNA-seq
profiles were downloaded from GTEx-lung.
Differential expression analysis of
ferroptosis genes
The ferroptosis gene expression profiles of all
samples were extracted using R software (version 4.0.3; The R
Foundation for Statistical Computing) (10). The ferroptosis genes that were
differentially expressed in the tumor group compared with in the
non-tumor group were identified using the tidyverse package
(www.tidyverse.org) and the Deseq2
package (P<0.05) (https://bioconductor.org/packages/release/bioc/html/DESeq2.html).
Subsequently, LUAD and LUSC were divided into three groups
according to tumor stage and the differential expression of
ferroptosis genes between the groups was analyzed. The Wilcoxon
rank-sum test was used for statistical analysis of two groups
(11), whereas the Kruskal-Wallis
test and Dunn's post hoc test was used for multi-group
comparisons.
Survival analysis
Survival data were extracted from survminer
(https://cran.r-project.org/web/packages/survminer/index.html).
Samples were divided into high and low expression groups according
to the median value of gene expression. Outcomes included overall
survival (OS), disease-free survival (DFS) and progression-free
survival (PFS). Kaplan-Meier survival curves (12) were plotted and the log-rank test was
performed to analyze the association between the
ferroptosis-associated differentially expressed genes and
prognostic data. The hazard ratio and 95% CI were used to estimate
OS, DFS and PFS.
Association analysis of tumor stage
and ferroptosis genes
Sankey diagrams were used to generate graphical
representations of the distribution of ferroptosis genes in
different tumor stages, and to indicate the relationship between
gene expression and patient survival. The R software package
ggalluvial was used to construct the Sankey diagram (https://cran.r-project.org/package=ggalluvial). The
association between differentially expressed genes and tumor size,
lymph node metastasis and distant metastasis was determined using
the Kruskal-Wallis test and Dunn's post hoc test.
Immune correlation analysis
The Tumor Immune Estimation Resource (TIMER) score,
EPIC score and CIBERSORT were calculated using the R immunedeconv
package (v4.0.3) (https://omnideconv.org/immunedeconv/). Tumor
immunophenotype (TIP) (https://github.com/dengchunyu/TIP) was calculated
using the R immunedeconv package (v4.0.3). The TIMER score is used
to estimate the number of immune cells present; EPIC provides the
relative proportions of cells; CIBERSORT is used for the assessment
of T-cell characteristics; and the proportion of tumor-infiltrating
immune cells in the cancer immune cycle can be tracked and analyzed
using TIP. The immune correlation network graph was used to
visualize the correlation between gene expression and immune
scores, and the correlation between immune scores themselves.
Pathway analysis
To clarify the relationship between genes and
pathways, the enrichment fraction of each sample in each pathway
was calculated using the Single Sample Gene Set Enrichment Analysis
(ssGSEA) algorithm (13), and the
Spearman correlation between gene expression and the pathway score
was calculated.
Immunohistochemical staining
A total of 99 patients with LUAD who had undergone
surgery at the Central Hospital Affiliated to Shenyang Medical
College (Shenyang, China) and Shengjing Hospital (Shenyang, China)
between January 2015 and December 2018 were included in the present
study. The patients ranged in age from 36 to 81 years(average age,
58.97±9.49 years), including 55 men and 44 women. Sectioning and
immunohistochemical staining were performed using paraffin-embedded
tissue sections from patients with a pathological diagnosis of
LUAD, which were obtained from the Pathology Department, Central
Hospital Affiliated to Shenyang Medical College. The Central
Hospital Affiliated to Shenyang Medical College ethics committee
reviewed and approved the present study [approval no.
Ke-2024-128(02)]. The use of the samples remaining after clinical
diagnosis had no adverse effects on the patients; therefore, the
need for consent was waived.
For immunohistochemistry, paraffin-embedded sections
were collected under Office for Human Research Protections
guidelines. Briefly, the paraffin-embedded sections (3 µm),
underwent antigen retrieval with citric acid under high pressure
for 3 min and were blocked with 3% hydrogen peroxide for 20 min at
24°C. Subsequently, the sections were incubated with primary
antibodies at 4°C for 12 h and with MaxVision™ HRP-Polymer
anti-Mouse/Rabbit secondary antibodies (cat. no. kit5030; Fuzhou
Maixin Biotechnology Development Co., Ltd.) at 4°C for 0.5 h. The
sections then underwent DAB staining for 1 min, after which, they
were counterstained with hematoxylin for 1 min, sealed with neutral
resin and observed under an Olympus BX53 light microscope (Olympus
Corporation). The following primary antibodies were used: CDGSH
iron-sulfur domain-containing protein 1 (CISD1; 1:100; cat. no.
TA500905; Origene Technologies, Inc.), CD4 (1:100; cat. no.
RMA-0620; Fuzhou Maixin Biotechnology Development Co., Ltd.) and
CD20 (1:100; cat. no. Kit0001; Fuzhou Maixin Biotechnology
Development Co., Ltd.)]. Cytoplasmic brown staining was considered
positive for immunohistochemistry. The interpretation of the
results was the responsibility of two senior pathologists. The
degree of CISD1 positivity was categorized as follows: 0, not
positive; 1, weakly positive (light yellow); 2, moderately positive
(yellowish-brown); and 3, strongly positive (tan). Regarding the
CISD1 staining area score, a score of 0–100 was assigned according
to the percentage of positive tumor cells/total tumor cells. The
total staining score was equal to the product of the staining area
score and the degree of positivity of the staining results. A total
score of ≥100 indicated high CISD1 expression, whereas a total
score of <100 indicated low CISD1 expression. For CD4 and CD20
staining, only the percentage of positive cells was calculated.
Statistical analysis
R software (version 4.0.3) was used for both data
analysis and visualization. SPSS 17.0 (IBM Corp.) was used for the
χ2 test. Two groups of specimens were compared using the
Wilcoxon rank-sum test, whereas three groups of specimens were
compared using the Kruskal-Wallis test and Dunn's post hoc test.
Spearman correlation analysis was used to analyze the correlation
between immunohistochemical scores of clinicopathological
indicators, and Kaplan-Meier analysis and log-rank test was used
for survival analysis (SPSS v17.0). P<0.05 was considered to
indicate a statistically significant difference.
Results
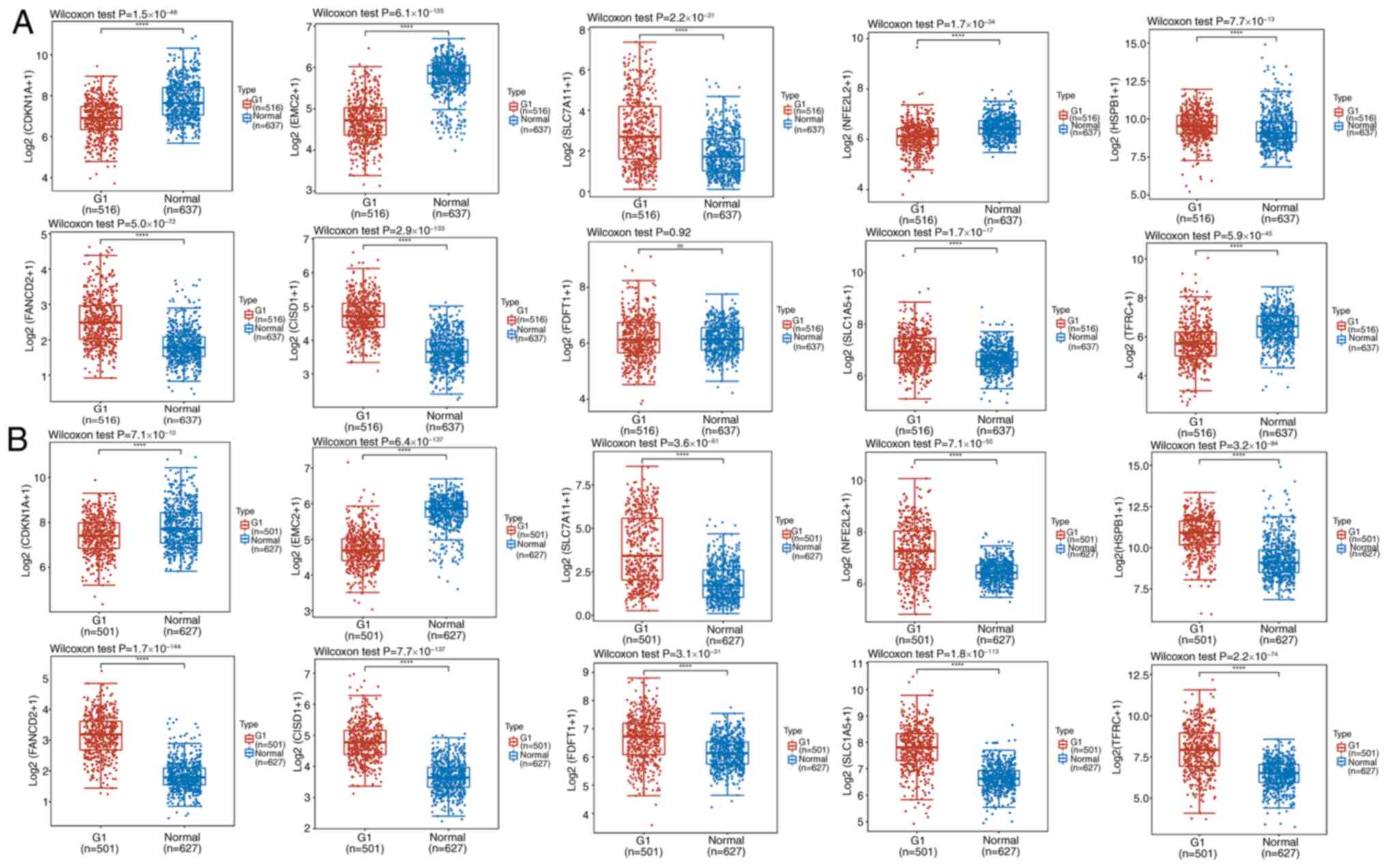
Differential expression of
iron-depleting genes in tumor and non-tumor tissues
Data for 516 cases of LUAD and 59 matched samples of
paracancerous tissues, and 501 cases of LUSC and 49 matched samples
of paracancerous tissues were downloaded from TCGA. A total of 578
lung tissue samples without disease in GTEx were included due to
the small amount of data in the control group. Differences between
the tumor and non-tumor groups were analyzed by extracting the
expression profiles of >20 classical ferroptosis genes. Compared
with those in normal tissues, the expression levels of SLC7A1,
HSPB1, FANCD2, CISD1, HSPA5, NCOA4, GPX4, DPP4, RPL8 and SLC1A5
were upregulated in LUAD cancer tissues, whereas the expression
levels of CDKN1A, EMC2, NFE2L2, MT1G, LPCAT3, GLS2, SAT1, CS,
ALOX1, ACSL4, ATL1 and TFRC were downregulated (Figs. 1A and S1A). In LUSC cancer tissues, the
expression levels of SLC7A1, NFE2L2, HSPB1, FANCD2, CISD1, FDFT1,
SLC1A5, HSPA5, NCOA4, GPX4, RPL8 and TFRC were upregulated, whereas
the expression levels of CDKN1A,LPCAT3, GLS2, SAT1, DPP4, CS,
ACSL4, ATL1 and EMC2 were downregulated (Figs. 1B and S1B).
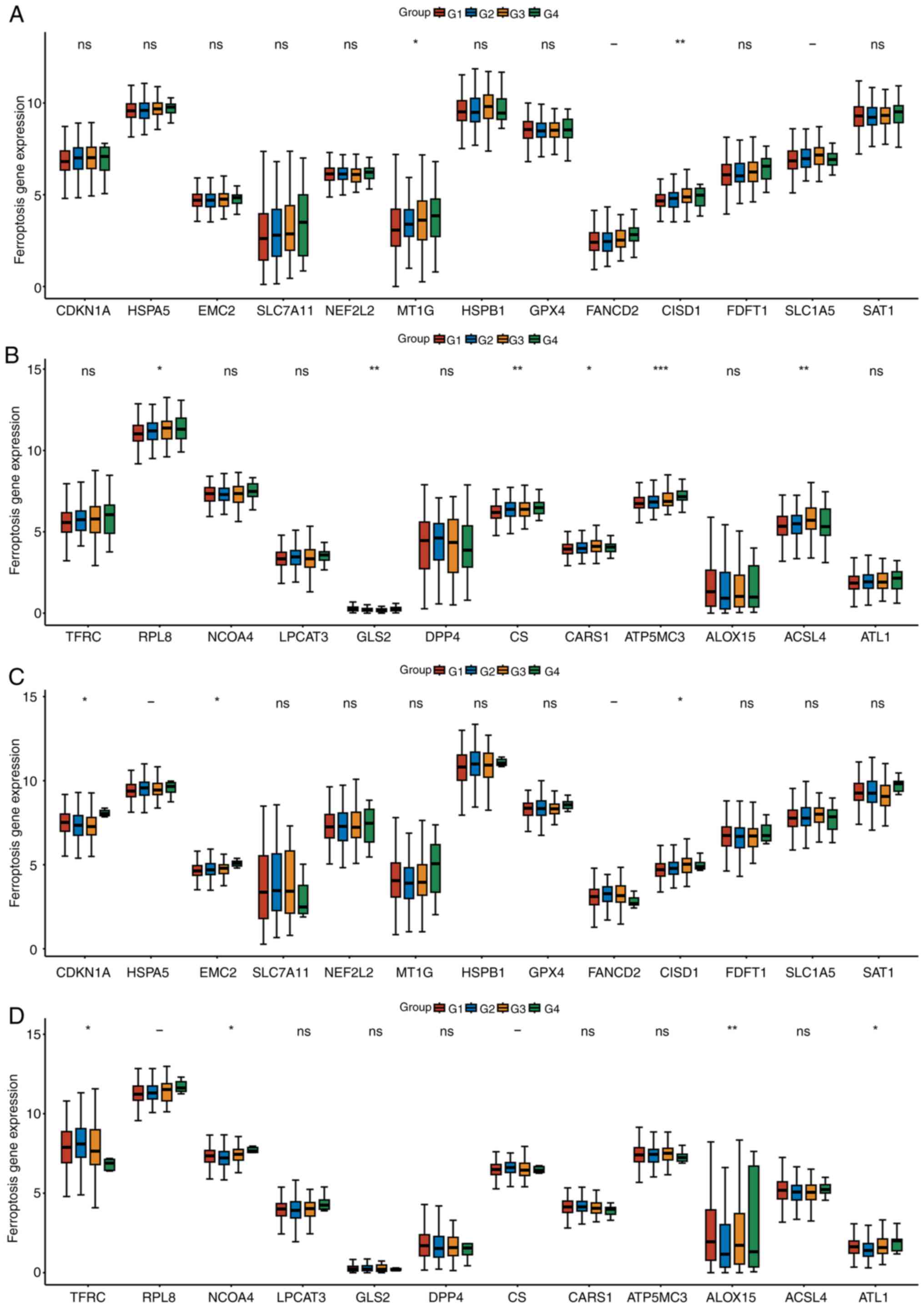
Differential expression of ferroptosis
genes based on tumor clinical stages
Patients with LUAD and LUSC were grouped based on
Tumor-Node-Metastasis (TNM) stage. Patients with LUAD or LUSC were
divided into three groups, G1 corresponds to stage I, G2
corresponds to stage II, G3 corresponds to stage III and G4
corresponds to stage IV. MT1G, CISD1, RPL8, GLS2, CS, CARS1, ATP5M3
and ACSL4 were associated with the TNM stage of LUAD (Fig. 2A and B). CDKN1A, EMC2 CISD1, TFRC,
ALOX15 and ATL1 were associated with the TNM stage of LUSC
(Fig. 2C and D). CISD1 was
identified in both LUAD and LUSC.
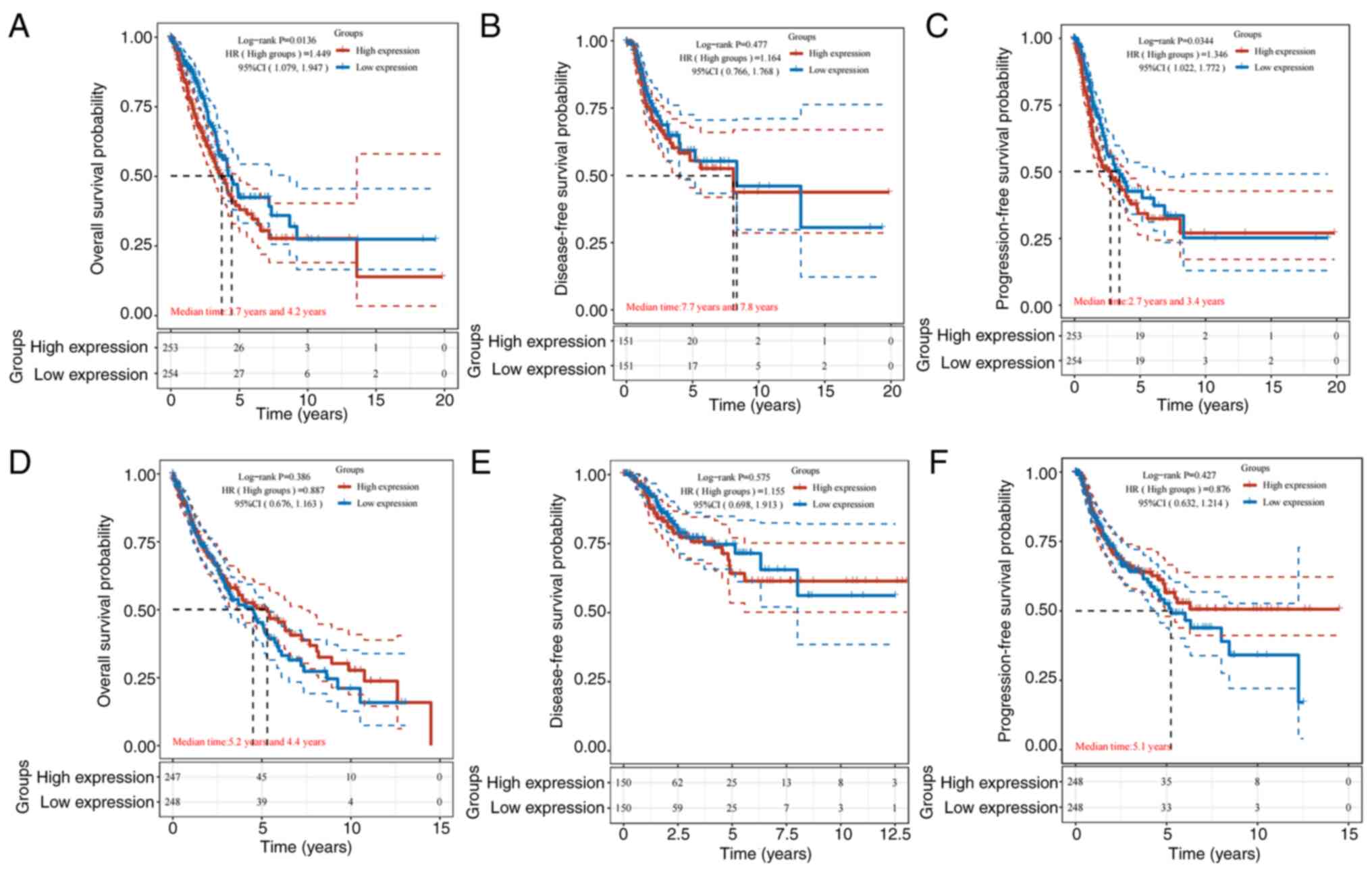
Association between differentially
expressed genes and prognosis
LUAD and LUSC differ in how they are treated and
prognosticated. For the prognosis of LUAD and LUSC, CISD1 was
further analyzed. The results revealed that in LUAD, CISD1 was
associated with prognosis, and high levels were associated with
lower OS and PFS (P<0.05; Fig. 3A
and C). This suggested that high levels of CISD1 were
associated with poor prognosis. In LUSC, CISD1 was not associated
with prognosis (Fig. 3D-F). The
relationship between CISD1 and LUAD was thus focused on.
Analysis of the association between
CISD1 and clinical characteristics
There were 187 deaths and 329 survivors among all
patients with lung cancer from TCGA. Among the patients who died,
there were 69 cases in stage I, 55 cases in stage II, 47 cases in
stage III and 16 cases in stage IV, including 111 cases (58.73%)
with high CISD1 expression and 76 cases (40.64%) with low CISD1
expression. Among the surviving patients, 147 patients (44.68%)
were in the high-level CISD1 group and 182 patients (55.32%) were
in the low-level CISD1 group. Although 58.73% of the dead patients
had high expression of CISD1, it was mainly concentrated in stage I
and II, suggesting that the high expression may delay rather than
completely prevent the disease progression. By contrast, patients
with low expression were more distributed in stages III and IV, and
were associated with 62.5% of stage IV deaths, highlighting its
strong association with aggressive phenotypes. Patients with low
expression of CISD1 accounted for 55.32% of the survival cohort,
which may reflect the effectiveness of early intervention for
patients with low expression, or the existence of other
compensatory pathways to maintain survival (Fig. 4A). Patients with high expression of
CISD1 accounted for 42.0% of patients with T1 cancer, and the
survival rate (67.6%) was lower in the high-expression group than
that in the low-expression group (79.6%). Notably, the survival
rate of patients with high CISD1 expression in T4 stage was low
(16.7%), which was in sharp contrast with the low-expression group
(71.4%) (Fig. 4B). The survival
rate of patients with low CISD1 expression in N0 stage was 76.4%
(139/182), which was significantly higher than that of patients
with high expression (69.3%), suggesting that CISD1 inhibition may
improve the prognosis through immune regulation in the
metastasis-free stage. However, the survival rate of patients with
high expression in N1-N2 stage decreased sharply (N1: 42.4%; N2:
36.4%), and was significantly lower than that of patients with low
expression in the same period, suggesting that CISD1 may drive
malignant progression by enhancing tumor cell adaptability (such as
antioxidant defense) in lymph node metastasis (Fig. 4C). In M0 stage, high CISD1
expression accounted for 52.4%, and its survival rate (56.6%) was
lower than that of the low expression group (67.3%). In M1 stage,
high CISD1 expression accounted for 64.0%, and its survival rate
(31.3%) was lower than that in the low expression group (55.6%)
(Fig. 4D). High CISD1 expression
may exacerbate adverse outcomes by promoting metastasis or
treatment resistance. However, the χ2 test showed that
the level of CISD1 was associated with the outcome; a high level of
CISD1 could indicate a poor outcome (Table I). In order to exclude intra-group
differences, different clinical stages and non-tumor tissues were
also compared. CISD1 expression was positively associated with TNM
stage (Fig. 4E), and TNM stage was
closely associated with tumor prognosis (Fig. 4F). This suggested that, when CISD1
expression was high in patients, the risk of adverse outcomes was
also high. It is recommended that consideration be given to the
addition of ferroptosis-related drugs in the design of treatment
plans for patients.
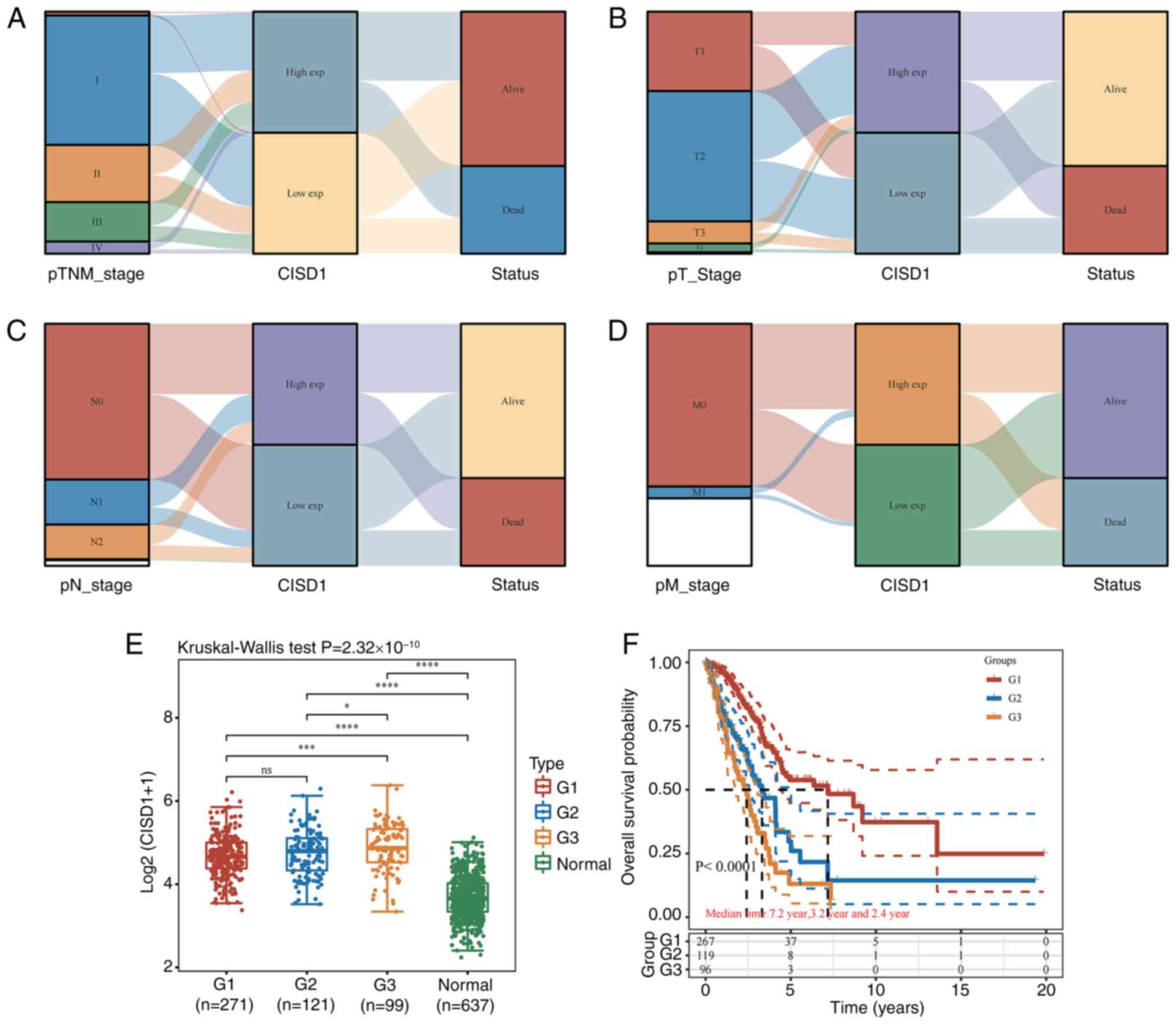 | Figure 4.Association between CISD1 and the
clinical features in lung adenocarcinoma. (A) Distribution trend of
CISD1 and patient outcomes for different clinical stages. (B)
Distribution trend of CISD1 and patient outcomes for different
tumor sizes. (C) Distribution trend of CISD1 and patient outcomes
for lymph node metastasis. (D) Distribution trend of CISD1 and
patient outcomes for distant metastasis. (E) Association between
CISD1 expression and different clinical stages. *P<0.05,
***P<0.001,****P<0.0001. (F) Relationship between different
tumor stages and the prognosis of lung adenocarcinoma. CISD1, CDGSH
iron-sulfur domain-containing protein 1; M0, no distant metastasis;
M1, distant metastasis; N0, no lymph node metastasis; N1,
ipsilateral pulmonary lymph node metastasis; N2, lymph node
metastasis within the mediastinum or below the protuberance on the
same side; T1, ≤3 cm; T2, >3-5 cm; T3, >5-7 cm; T4, >7 cm;
ns, not significant. |
 | Table I.χ2 test of the
relationship between CISD1 expression level and clinical
outcome. |
Table I.
χ2 test of the
relationship between CISD1 expression level and clinical
outcome.
|
| CISD1
expression |
|
|
|---|
|
|
|
|
|
|---|
| Characteristic | High | Low | Pearson
χ2 value | P-value |
|---|
| Clinical
outcome |
|
|
|
|
|
Alive | 147 | 182 | 10.274 | 0.001 |
|
Dead | 111 | 76 |
|
|
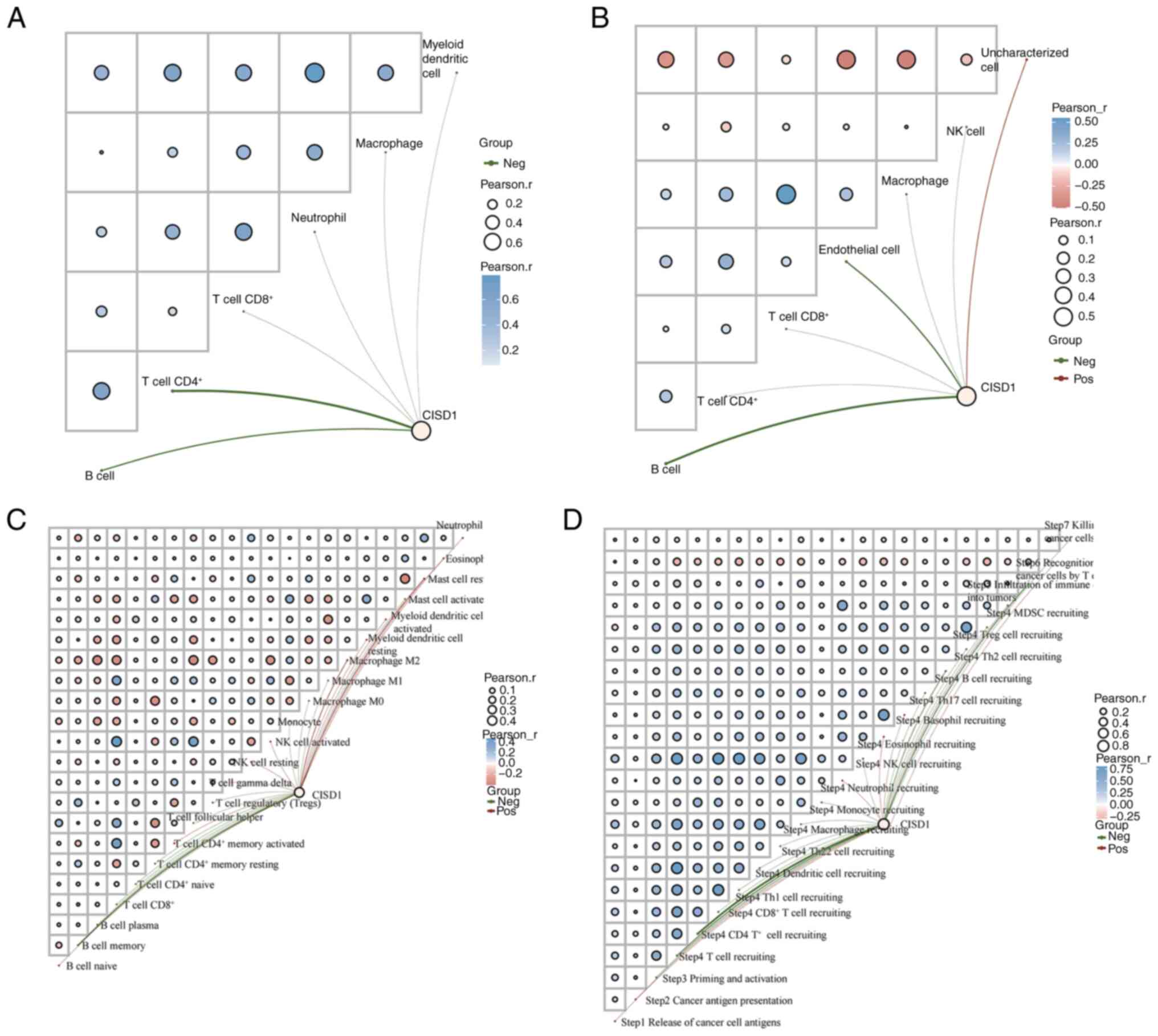
Association between CISD1 and
immunity
The present study further analyzed the association
between CISD1 and immunity to explore the potential application
value of targeting ferroptosis in the immunotherapy of LUAD. The
network connection diagram and heat map were used to visualize the
association between gene expression and the immune score (Fig. 5). The more red or blue, the stronger
the correlation between both, and the larger the ring, the stronger
the correlation. The red line represents a positive correlation
between the model score or the gene expression and the immune
score, and the green line represents a negative correlation between
the two. The TIMER score (Fig. 5A),
EPIC score (Fig. 5B), CIBERSORT
score (Fig. 5C) and TIP score
(Fig. 5D) showed that CISD1 was
negatively correlated with CD4+ T cells and B cells.
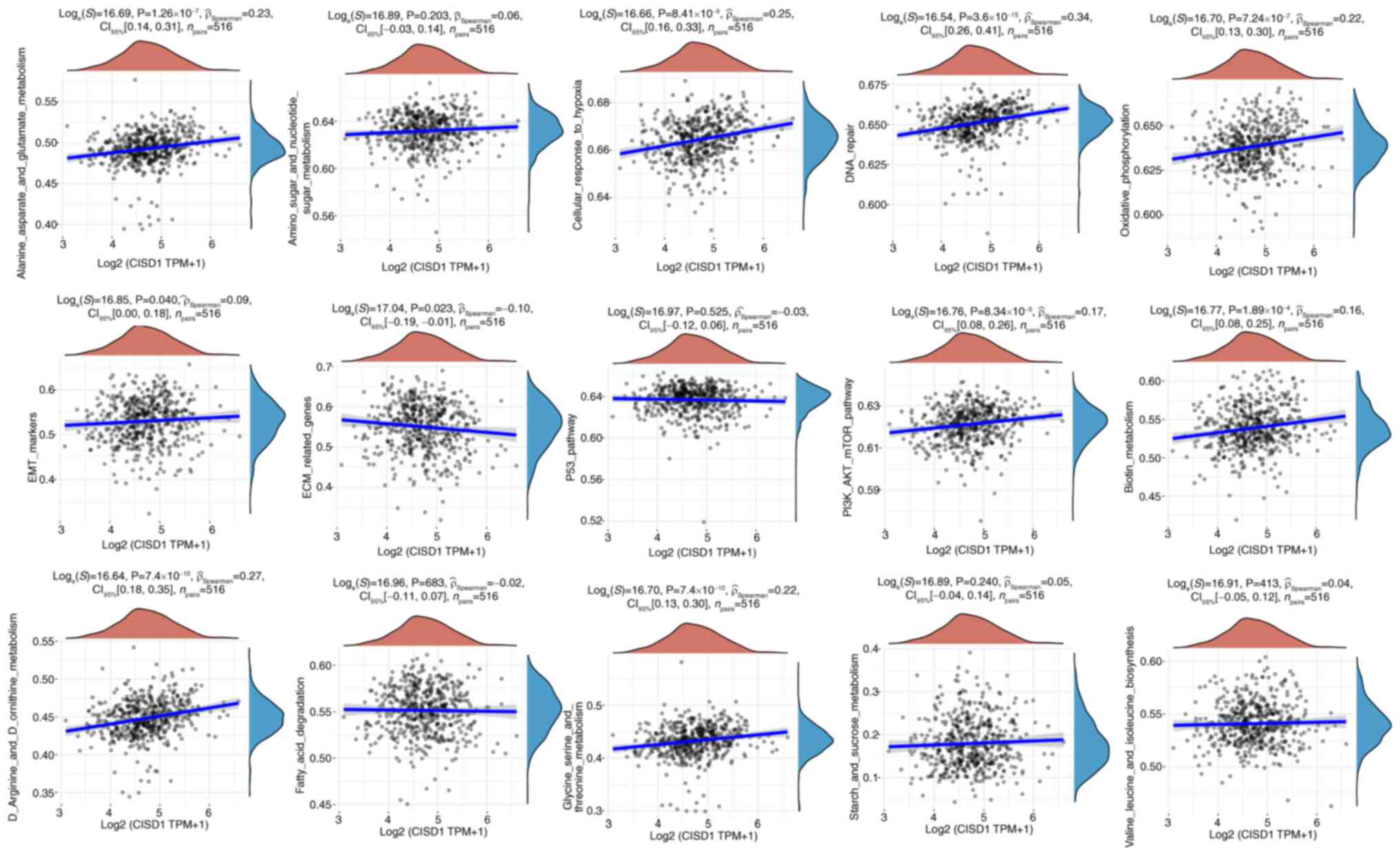
CISD1 and pathways
The ssGSEA algorithm was used to calculate the
enrichment fraction of each sample for a given pathway to
investigate the relationship between samples and pathways. These
calculated enrichment fractions provided an intuitive way to show
how samples and pathways interacted and were associated. The
results suggested that CISD1 may be involved in the cellular
response to hypoxia, DNA repair, extracellular matrix-related
genes, epithelial-mesenchymal transition markers, oxidative
phosphorylation, PI3K-AKT-mTOR signaling pathway and metabolic
pathways (glycine, serine and threonine metabolism; D arginine and
D ornithine metabolism; alanine, aspartate and glutamate
metabolism; biotin metabolism) in LUAD (Fig. 6).
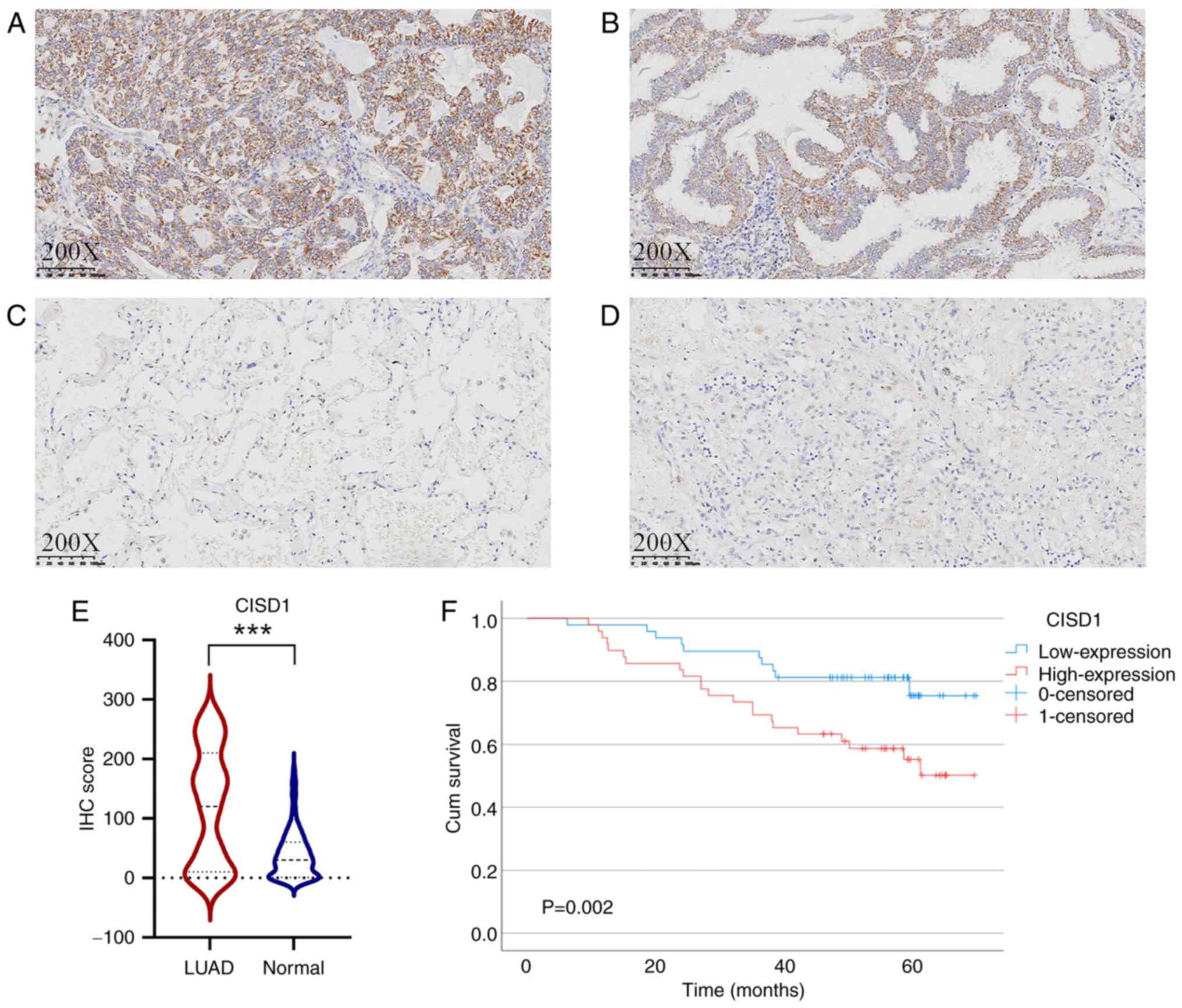
CISD1 expression in cancer tissues and
prognosis
CISD1 expression in LUAD cancer tissues (Fig. 7A and B) was significantly higher
than that in adjacent tissues (Fig. 7C
and D), as determined using Wilcoxon rank-sum test (P<0.001;
Z=−7.471; Fig. 7E). In LUAD, the
χ2 test indicated that CISD1 was positively associated
with lymph node metastasis (P=0.010; χ2 =6.559), and
Ki67 (P=0.005; χ2 =7.932) (Table II). By contrast, CISD1 was not
associated with sex, tumor differentiation, tumor size and TNM in
patients with LUAD. Furthermore, high CISD1 expression indicated
poor prognosis and a shorter DFS (P=0.002; Fig. 7F).
 | Table II.χ2 test of the
relationship between CISD1 expression and clinicopathological
parameters. |
Table II.
χ2 test of the
relationship between CISD1 expression and clinicopathological
parameters.
|
|
| CISD1 |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | n | High expression
(n=57) | Low expression
(n=42) | Pearson
χ2 value | P-value |
|---|
| Sex |
|
|
|
|
|
|
Male | 44 | 26 | 18 | 0.074 | 0.785 |
|
Female | 55 | 31 | 24 |
|
|
| Tumor
differentiation |
|
|
|
|
|
|
Well | 36 | 19 | 17 | 1.053 | 0.591 |
|
Moderate | 52 | 24 | 18 |
|
|
|
Poor | 21 | 14 | 7 |
|
|
| Tumor size |
|
|
|
|
|
| ≤3.0
cm | 59 | 32 | 27 | 1.819 | 0.403 |
| >3
cm and ≤5 cm | 25 | 14 | 11 |
|
|
| >5
cm | 15 | 11 | 4 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
Positive | 43 | 31 | 12 | 6.559 | 0.010 |
|
Negative | 56 | 26 | 30 |
|
|
| TNM |
|
|
|
|
|
| I | 43 | 19 | 24 | 5.887 | 0.053 |
| II | 46 | 32 | 14 |
|
|
|
III | 10 | 6 | 4 |
|
|
| Ki-67 |
|
|
|
|
|
|
>10% | 70 | 34 | 36 | 7.932 | 0.005 |
|
≤10% | 29 | 23 | 6 |
|
|
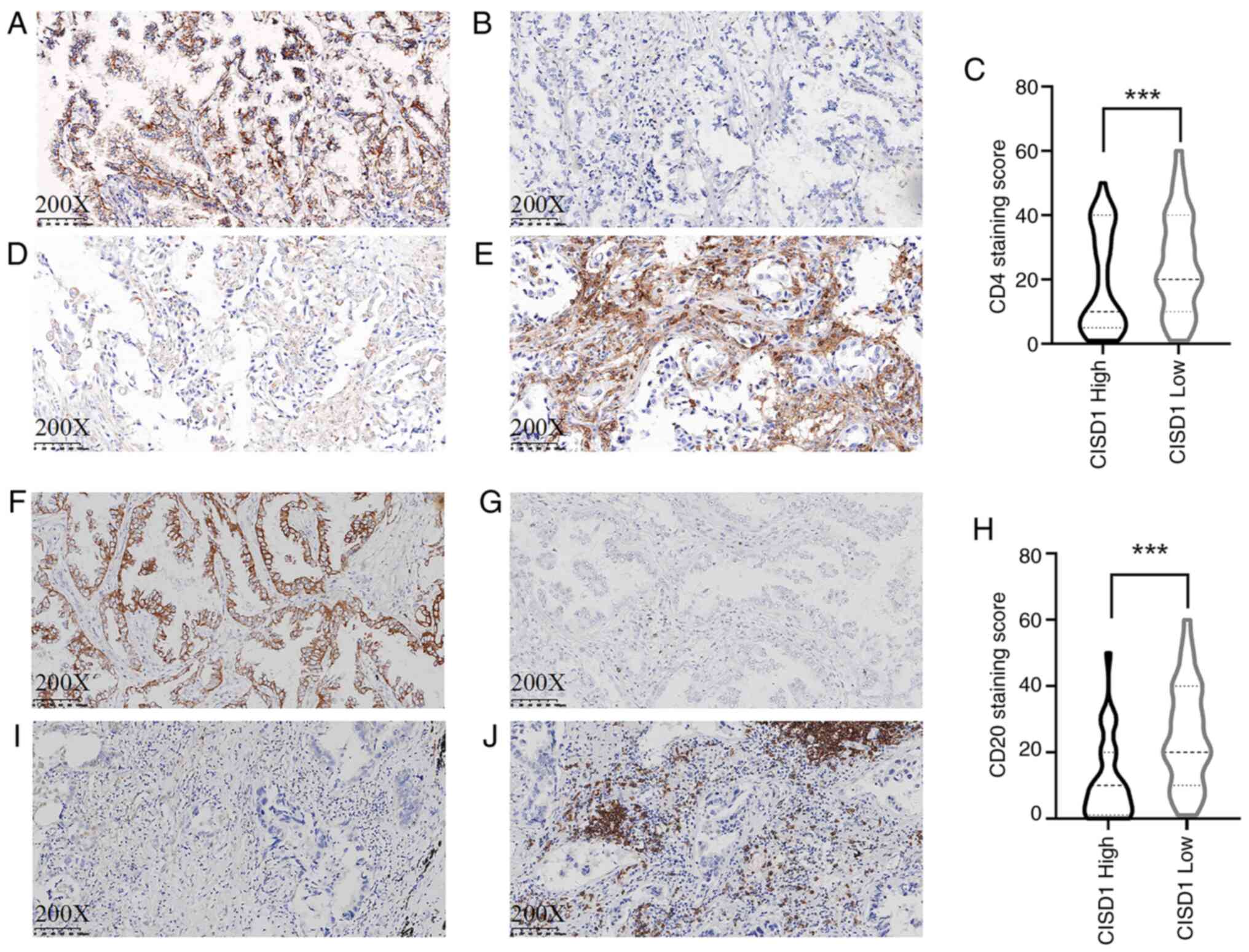
A total of 60 cases were randomly selected for CD4
and CD20 staining. The results demonstrated that the expression
levels of CISD1 in LUAD were negatively associated with the
infiltration of immune cells. The number of CD4 cells was lower in
cases with high CISD1 expression (Fig.
8A and B), and the number of CD20 cells was also lower
(Fig. 8F and G). The number of CD4
and CD20 immune cells was higher in cases with low CISD1 expression
(Fig. 8D, E, I and J). CISD1 was
negatively associated with the immune infiltration of CD4
(P<0.001; Z=−6.575) and CD20 (P<0.001; Z=−5.970) cells using
Wilcoxon signed-rank test (Fig. 8C and
H).
Discussion
Early detection and treatment are important tools
for the improvement of the survival rate of patients with lung
cancer. The advent of personalized and targeted therapies has
served a positive role in prolonging patient survival, improving
the treatment response and quality of life of patients. However,
the correct application of these novel regimens and methods is
highly dependent on appropriate molecular typing. For NSCLC,
detecting EGFR, ALK and ROS1 gene mutations can guide targeted
medication (14). The in-depth
analysis of the molecular characteristics of lung cancer genes and
proteins can provide an important basis for the development of
targeted treatments.
The incidence of LUAD is generally higher than that
of LUSC (15,16). CISD1 is a potential gene that is
related to the stage of the tumor; however, the present study
revealed that CISD1 was not associated with prognosis in LUSC.
Therefore, the study focused on the role of CISD1 in LUAD. CISD1 is
known to belong to the mitoNEET family, other members of which
include CISD2 and CISD3 (17,18).
The CISD1 gene is located at chromosome 10q21.1 and the encoded
protein is located in the outer membrane of mitochondria. CISD1 is
an L-cysteine aminotransferase that catalyzes the reversible
transfer of amino groups from L-cysteine to α-keto acids and
2-oxoglutarate to form 2-oxo-3-thiopropionate and L-glutamic acid,
respectively (19). CISD1 was
originally considered to be targeted by pioglitazone, a drug used
to treat diabetes (20). A previous
study demonstrated that in breast cancer, CISD1 expression is
higher in tumor tissues than in normal tissues, and that it is
positively associated with poor OS (21). Liang et al (22) constructed a prognostic model
including CISD1 for bladder cancer and proposed that this model was
independently associated with OS. This is consistent with the
present findings. The present results demonstrated that CISD1 was
positively associated with poor OS and PFS in LUAD. However, Yang
et al (23) constructed
pancreatic adenocarcinoma prognosis models including CISD1 and
proposed that CISD1 was a tumor suppressor gene, thus suggesting
that CISD1 may serve different roles in different tumors.
CISD1 is closely related to the immune
microenvironment. Notably, there is a negative association between
CISD1 and regulatory T cells or plasma cells in patients with
asthma, and CISD1 has been reported to be upregulated in mild to
moderate asthma and severe asthma (24). Furthermore, the changes of the
immune microenvironment in psoriasis may be related to PRKAA2,
PEBP1, CISD1 and acsf2 (25). A
previous study reported that cigarette smoke can induce the
reduction of CISD1 in macrophages, and promotes M1 polarization and
mitochondrial dysfunction by activating the autophagy pathway, thus
promoting the occurrence and development of chronic obstructive
pulmonary disease (26). In
addition, ferroptosis is associated with immune processes such as
T-cell function, immune checkpoints, human leukocyte antigen and
T-cell co-stimulation (27). CISD1
has been shown to be associated with the prognosis of patients with
hepatocellular carcinoma, and the immune infiltration levels of
CD8+ T cells, macrophages, neutrophils and dendritic
cells (28). Higher CISD1
expression in gastric cancer tissues is also related to an increase
in tumor size, differentiation, depth of invasion and lymph node
metastasis. Furthermore, CISD1 upregulation indicates poor OS of
patients with gastric cancer, and it is expected to participate in
the regulation of gastric cancer immune cell infiltration as an
ferroptosis inhibitor (29). In the
present study, immunohistochemical staining indicated that CISD1
was associated with immune infiltration, and CISD1 was negatively
associated with CD4+ T cells and CD20+ B
cells.
Ferroptosis and immunity have potential applications
(30). In the process of
ferroptosis, ferrous sulfate can be used as a source of iron for
participation in the Fenton reaction and production of excessive
ROS, thus inducing ferroptosis (31). Ferrous sulfate can interfere with
normal iron metabolism by combining with iron; this leads to iron
accumulation in cells, which then causes lipid peroxidation and
cell death (32). CISD1 is closely
related to the aging of organisms and mitochondria. A previous
study reported that CISD1 regulates the longevity of
Caenorhabditis elegans by participating in autophagy and
mitochondrial internal apoptosis pathways, and regulates protein
homeostasis and aging (33).
Furthermore, CISD1 deficiency can lead to iron transport imbalance
and proton leakage, and can reduce ATP production by interrupting
the mitochondrial electron transport chain (34). CISD1 expression has been reported to
be specifically downregulated in the heart and kidney of aging
mice, which may cause the heart mitochondria to gradually lose
their integrity in the process of natural aging (35). Furthermore, CISD1 accumulates in
Drosophila melanogaster during the aging process. By
contrast, reducing the levels of CISD1 can improve the pathological
phenotype of the movement, life span and neurodegeneration of D.
melanogaster; therefore, inhibition of CISD1 may provide a
potential target for therapeutic intervention (36).
Based on the research of aging-related diseases,
PTEN induced kinase 1 and parkin regulate estrogen receptor calcium
release through CISD1 and inositol 1,4,5-trisphosphate receptor,
and are feasible targets for the treatment of Parkinson's disease
(37). Additionally, in the field
of neurological research, targeting CISD1 signaling is considered
to be a novel therapeutic strategy for the prevention and treatment
of neonatal hypoxic-ischemic injury caused by perinatal asphyxia
(38). Furthermore, CISD1 is a
mediator of ferroptosis in neural stem cells, and its expression is
enhanced due to high fructose levels, driving the death of
hippocampal neural stem cells, resulting in a decreased sweet taste
preference (39). Gastrodin can
alleviate CISD1-mediated ferroptosis (39). In a lung injury-related study, CISD1
inhibition by mitoNEET ligand-1 was shown to reduce
lipopolysaccharide-induced iron death in vivo and in
vitro, reduce pathological injury and pulmonary edema, and
reduce the levels of IL-6, IL-1β and TNF-α in the lungs and
bronchoalveolar lavage fluid of mice with acute lung injury
(40).
The GCN5L1/CSID1 axis is crucial for oxidative
stress and ethanol-induced ferroptosis (41). Early iron-dependent cell death
releases a number of damage-related molecular patterns, which can
effectively activate bone marrow-derived dendritic cells by
inhibiting glutathione peroxidase 4-induced cancer cell
ferroptosis, and subsequently induce antitumor adaptive immunity,
which can activate the immune system, aiding the treatment of
tumors (42). In a tumor-related
study, targeted inhibition of CISD1 has been shown to reduce ROS
accumulation, mitochondrial dysfunction and apoptosis through PI3K
and MAPK pathways (43). CISD1
knockout also leads to changes in mitochondrial ultrastructure and
function, increased ROS and decreased cell proliferation. In
addition, CISD1 may be used as a drug target for the treatment of
minimal residual leukemia (44). In
the present study, increased CISD1 was detected in LUAD tissues,
and was associated with worse staging and prognosis; therefore,
CISD1 may be an effect target for the treatment of LUAD.
In conclusion, the prognosis of LUAD was associated
with CISD1, a ferroptosis gene. Through the regulation of CISD1, it
may be possible to influence the immune microenvironment of LUAD to
a certain extent. The development of safe and effective targeted
drugs against CISD1 is a direction worthy of future consideration
and exploration.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the 2022 Shenyang Science and
Technology Plan (grant no. 22-321-33-94) and the 2023 Liaoning
Provincial Department of Education (grant no. JYTMS20231387).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author
Authors' contributions
XL designed the present study. TW conducted the
experiments and wrote the manuscript. XL, XX, ZZ and TW performed
the experiments, collected data and confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Central Hospital Affiliated to Shenyang Medical
College [approval no. Ke-2024-128(02)]. A waiver of informed
consent was granted under 45 CFR 46.104 due to the use of
de-identified retrospective data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Lei S, Ding L, Xu Y, Wu X, Wang H,
Zhang Z, Gao T, Zhang Y and Li L: Global burden and trends of lung
cancer incidence and mortality. Chin Med J (Engl). 136:1583–1590.
2023.PubMed/NCBI
|
|
3
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim ZF and Ma PC: Emerging insights of
tumor heterogeneity and drug resistance mechanisms in lung cancer
targeted therapy. J Hematol Oncol. 12:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Iron death: An iron dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park E and Chung SW: Ros-mediated
autophagy increases intracellular iron levels and ferropositis by
ferritin and transferrin receptor regulation. Cell death Dis.
10:8222019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Conche C, Finkelmeier F, PEšIć M, Nicolas
AM, Böttger TW, Kennel KB, Denk D, Ceteci F, Mohs K, Engel E, et
al: Combining osteoporosis induction with MDSC blockade renders
primary tumours and metals in liver sensitive to immune checkpoint
blockade. Gut. 7:1774–1782. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang F, Xiao Y, Ding JH, Jin X, Ma D, Li
DQ, Shi JX, Huang W, Wang YP, Jiang YZ and Shao ZM: Ferroptosis
heterogeneity in triple-negative breast cancer reveals an
innovative immunotherapy combination strategy. Cell Metab.
35:84–100.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Q, Deng T, Zhang H, Zuo D, Zhu Q,
Bai M, Liu R, Ning T, Zhang L, Yu Z, et al: Adipocyte-derived
exosomal MTTP suppresses ferrooposis and promotes chemoresistance
in colorectal cancer. Adv SCI (Weinh). 9:e22033572022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Wang L, Pang Z, Ge Q, Wu Y and Qi
X: Integrated analysis of ferroptosis and immunity-related genes
associated with diabetic kidney disease. Diabetes Metab Syndr Obes.
16:3773–3793. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsieh SS, Cook DA, Inoue A, Gong H, Sudhir
Pillai P, Johnson MP, Leng S, Yu L, Fidler JL, Holmes DR III, et
al: Understanding reader variability: A 25-radiologist study on
liver metastasis detection at CT. Radiology. 306:e2202662023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Messori A: Synthetizing published evidence
on survival by reconstruction of patient-level data and generation
of a multi-trial kaplan-meier curve. Cureus.
13:e194222021.PubMed/NCBI
|
|
13
|
Cao L, Liu M, Ma X, Rong P, Zhang J and
Wang W: Comprehensive scRNA-seq analysis and identification of
CD8_+T cell related gene markers for predicting prognosis and drug
resistance of hepatocellular carcinoma. Curr Med Chem.
31:2414–2430. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalemkerian GP, Narula N, Kennedy EB,
Biermann WA, Donington J, Leighl NB, Lew M, Pantelas J, Ramalingam
SS, Reck M, et al: Molecular testing guideline for the selection of
lung cancer patients for treatment with targeted thyroid kinase
inhibitors: American society of clinical oncology endowment summary
of the college of American physicians/international association for
the study of lung cancer/association for molecular pathology
clinical practice guideline update. J Clin Oncol. 36:911–919. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukui T, Taniguchi T, Kawaguchi K,
Fukumoto K, Nakamura S, Sakao Y and Yokoi K: Comparisons of the
clinicopathological features and survival outcomes between lung
cancer patients with adenocarcinoma and rectangular cell carcinoma.
Gen Thorac Cardiovasc Surg. 63:507–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang BY, Huang JY, Chen HC, Lin CH, Lin
SH, Hung WH and Cheng YF: The comparison between adenocarcinoma and
rectangular cell carcinoma in lung cancer patients. J Cancer Res
Clin Oncol. 146:43–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wiley SE, Murphy AN, Ross SA, van der Geer
P and Dixon JE: Mitoneet is an iron containing outer mitochondrial
membrane protein that regulates oxidative capacity. Proc Natl Acad
SCI USA. 104:5318–5323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inupakutika MA, Sengupta S, Nechushtai R,
Jennings PA, Onuchic JN, Azad RK, Padilla P and Mittler R:
Phylogenetic analysis of eucaryotic neet proteins uncovers a link
between a key gene duplication event and the evolution of
vertebrates. Sci Rep. 7:425712017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kunk C, Kruger J, Mendoza G, Markitan J,
Bias T, Mann A, Nath A, Geldenhuys WJ, Menze MA and Konkle ME:
Mitoneet's reactivity of lys55 toward pyrooxidative phosphate
demonstrations its activity as a transaminase enzyme. ACS Chem
Biol. 1:2716–2722. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colca JR, McDonald WG, Waldon DJ, Leone
JW, Lull JM, Bannow CA, Lund ET and Mathews WR: Identification of a
novel mitochondrial protein (‘mitoNEET’) cross linked specifically
by a thiazolidinedione photoprobe. Am J Physical Endocrinol Metab.
286:e252–e260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu F, Dong Y, Zhong F, Guo H and Dong P:
CISD1 is a breast cancer diagnostic biomarker associated with
diabetes mellitus. Biomolecules. 13:372022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang Y, Ye F, Xu C, Zou L, Hu Y, Hu J and
Jiang H: A novel survival model based on a paradox related gene
signature for predicting overall survival in bladder cancer. BMC
Cancer. 2:9432021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Wei X, Hu F, Dong W and Sun L:
Development and validation of a novel 3-gene diagnostic model for
pancreatic adenocarcinoma based on ferrosis related genes. Cancer
Cell Int. 22:212022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Jia Y, Gu J, Chen O and Yue S:
Iron death-related genes are involved in ashma and regulate the
immune microenvironment. Front Pharmacol. 14:10875572023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu MN, Zhou DM, Jiang CY, Chen WW, Chen
JC, Zou YM, Han T and Zhou LJ: Genetic analysis of potential
biomarkers and therapeutic targets in ferroptosis from psoriasis.
Front Immunol. 13:11044622023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao J, Dong M, Tian W, Xia J, Qian Y,
Jiang Z, Chen Z and Shen Y: The role of CISD1 reduction in
macrophages in promoting COPD development through M1 polarization
and mitochondrial dysfunction. Eur J Med Res. 29:5412024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu ZH, Tang Y, Yu H and Li HD: The role of
ferroptosis in breast cancer patients: A comprehensive analysis.
Cell Death Discov. 7:932021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu T, Li C, Xiang C, Gong Y, Peng W and
Chen C: Overexpression of CISD1 predicts worse survival in
hepatocarcinoma patients. Biomed Res Int. 2022:78231912022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zang J, Cui M, Xiao L, Zhang J and Jing R:
Overexpression of ferroptosis-related genes FSP1 and CISD1 is
related to prognosis and tumor immune infiltration in gastric
cancer. Clin Transl Oncol. 25:2532–2544. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Igarashi K, Shoji Y, Sekine Suzuki E, Ueno
M, Matsumoto KI, Nakanishi I and Fukui K: Importance of locations
of iron ions to elicit cytotoxicity induced by a fenton type
reaction. Cancers (Basel). 14:36422022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Searle AJ and Wilson RL: Stimulation of
microscopic lipid peroxidation by iron and cysteine
characterization and the role of free radios. Biochem J.
21:549–554. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ploumi C, Kyriakakis E and Tavernarakis N:
Coupling of autophagy and the mitochondrial intrinsic apoptosis
pathway modulates proteostasis and ageing in Caenorhabditis
elegans. Cell Death Dis. 14:1102023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsiung KC, Tang HY, Cheng ML, Hung LM,
Chin-Ming Tan B and Lo SJ: Mitochondrial bioenergetics deficiency
in CISD-1 mutants is linked to AMPK-mediated lipid metabolism.
Biomed J. 7:1008062024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Furihata T, Takada S, Kakutani N, Maekawa
S, Tsuda M, Matsumoto J, Mizushima W, Fukushima A, Yokota T, Enzan
N, et al: Cardiac-specific loss of mitoNEET expression is linked
with age-related heart failure. Commun Biol. 4:1382021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martinez A, Sanchez-Martinez A, Pickering
JT, Twyning MJ, Terriente-Felix A, Chen PL, Chen CH and Whitworth
AJ: Mitochondrial CISD1/CISD accumulation blocks mitophagy and
genetic or pharmacological inhibition rescues neurodegenerative
phenotypes in Pink1/parkin models. Mol Neurodegener. 19:122024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ham SJ, Yoo H, Woo D, Lee DH, Park KS and
Chung J: PINK1 and Parkin regulate IP3R-mediated ER calcium
release. Nat Commun. 14:52022023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang ZB, Xiong LL, Xue LL, Deng YP, Du
RL, Hu Q, Xu Y, Yang SJ and Wang TH: MiR-127-3p targeting CISD1
regulates autophagy in hypoxic-ischemic cortex. Cell Death Dis.
12:2792021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang CF, Ding H, Wu YQ, Miao ZA, Wang ZX,
Wang WX, Pan Y and Kong LD: Gastrodin attenuates high
fructose-induced sweet taste preference decrease by inhibiting
hippocampal neural stem cell ferroptosis. J Adv Res. Sep
29–2024.(Epub ahead of print). View Article : Google Scholar
|
|
40
|
Zhang X, Peng T, Li C, Ai C, Wang X, Lei
X, Li G and Li T: Inhibition of CISD1 alleviates mitochondrial
dysfunction and ferroptosis in mice with acute lung injury. Int
Immunopharmacol. 130:1116852024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang C, Yang Y, Hu X, Tang Q, Zhang J,
Zhang P, Lu X, Xu J, Li S, Dong Z, et al: Loss of GCN5L1
exacerbates damage in alcoholic liver disease through ferroptosis
activation. Liver Int. 44:1924–1936. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dong W, Jiang Y, Yao Q, Xu M, Jin Y, Dong
L, Li Z and Yu D: Inhibition of CISD1 attenuates cisplatin-induced
hearing loss in mice via the PI3K and MAPK pathways. Biochem
Pharmacol. 223:1161322024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Geldenhuys WJ, Piktel D, Moore JC, Rellick
SL, Meadows E, Pinti MV, Hollander JM, Ammer AG, Martin KH and
Gibson LF: Loss of the redox mitochondrial protein mitoNEET leads
to mitochondrial dysfunction in B-cell acute lymphoblastic
leukemia. Free Radic Biol Med. 175:226–235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Efimova I, Catanzaro E, Van der Meeren L,
Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C,
Bachert C, Coppieters F, et al: Vaccination with early atrophic
cancer cells induce effective antitumor immunity. J Immunother
Cancer. 8:e0013692020. View Article : Google Scholar : PubMed/NCBI
|